- 1Department of Urology, Tan Tock Seng Hospital, Singapore, Singapore
- 2Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore
- 3Division of Surgery and Interventional Science, University College London, London, United Kingdom
- 4Department of Urology, University College London Hospital, London, United Kingdom
- 5Department of Urology, NYU Langone Health, New York City, NY, United States
- 6Royal Derby Hospital, University Hospitals of Derby and Burton NHS Foundation Trust, Derby, United Kingdom
- 7Leeds Institute of Medical Research, University of Leeds, Leeds, United Kindgom
- 8Division of Surgery and Interventional Sciences, University College London, United Kingdom
- 9Department of Urology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
- 10Department of Surgery, South Karelian Central Hospital, Lappeenranta, Finland
- 11Department of Urology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 12Urology Department, Eastern Health, Box Hill, Victoria, Australia
- 13Eastern Health Clinical School, Monash University, Box Hill, Victoria, Australia
- 14Department of Urology, University of California San Francisco, San Francisco, CA, United States
- 15Department of Urology, Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria
- 16Department of Urology, Weill Cornell Medical College, New York, New York, USA
- 17Department of Urology, University of Texas Southwestern, Dallas, Texas, USA
- 18Department of Urology, Second Faculty of Medicine, Charles University, Prag, Czech Republic
- 19Hourani Center for Applied Scientific Research, Al-Ahliyya Amman University, Amman, Jordan
- 20Sorbonne University, GRC N 5, Predicitive Onco-uro, AP-HP, Hôpital Pitié-Salpêtriére, Paris, France
- 21Department of Urology, AZ Maria Middelares Hospital, Ghent, Belgium
- 22Department of Urology, Ghent University Hospital, Ghent, Belgium
- 23Department of Human Structure and Repair, Ghent University, Belgium
- 24Department of Urology, Hertfordshire and Bedfordshire Urological Cancer Centre, Lister Hospital Stevenage, School of Medicine and Life Sciences, University of Hertfordshire, Hatfield, United Kingdom
- 25Institute for Urology and Reproductive Health, Sechenov University, Moscow, Russia
- 26Urology Unit, Santa Maria della Misericordia University Hospital, Udine, Italy
- 27Department of Human and Pediatric Pathology “Gaetano Barresi”, Urologic Section, University of Messina, Messina, Italy
- 28S.H. Ho Urology Centre, Department of Surgery, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China
- 29European Association of Urology – Young Academic Urologists Urothelial Carcinoma Working Group (EAU-YAU), Arnhem, Netherlands
Purpose: The COVID-19 pandemic has led to competing strains on hospital resources and healthcare personnel. Patients with newly diagnosed invasive urothelial carcinomas of bladder (UCB) upper tract (UTUC) may experience delays to definitive radical cystectomy (RC) or radical nephro-ureterectomy (RNU) respectively. We evaluate the impact of delaying definitive surgery on survival outcomes for invasive UCB and UTUC.
Methods: We searched for all studies investigating delayed urologic cancer surgery in Medline and Embase up to June 2020. A systematic review and meta-analysis was performed.
Results: We identified a total of 30 studies with 32,591 patients. Across 13 studies (n = 12,201), a delay from diagnosis of bladder cancer/TURBT to RC was associated with poorer overall survival (HR 1.25, 95% CI: 1.09–1.45, p = 0.002). For patients who underwent neoadjuvant chemotherapy before RC, across the 5 studies (n = 4,316 patients), a delay between neoadjuvant chemotherapy and radical cystectomy was not found to be significantly associated with overall survival (pooled HR 1.37, 95% CI: 0.96–1.94, p = 0.08). For UTUC, 6 studies (n = 4,629) found that delay between diagnosis of UTUC to RNU was associated with poorer overall survival (pooled HR 1.55, 95% CI: 1.19–2.02, p = 0.001) and cancer-specific survival (pooled HR of 2.56, 95% CI: 1.50–4.37, p = 0.001). Limitations included between-study heterogeneity, particularly in the definitions of delay cut-off periods between diagnosis to surgery.
Conclusions: A delay from diagnosis of UCB or UTUC to definitive RC or RNU was associated with poorer survival outcomes. This was not the case for patients who received neoadjuvant chemotherapy.
Introduction
Bladder cancer is the 11th most commonly occurring cancer worldwide, with almost 550,000 new cases in 2018 (1, 2). A comprehensive review in 2017 found that bladder cancer ranks 13th in terms of death ranks, with mortality rates decreasing mainly in the most developed countries (3). In comparison, UTUC is much rarer, representing approximately 8.3% of all urothelial carcinoma (4).
At diagnosis, approximately 20% of patients have MIBC (5). One of the factors thought to affect mortality for MIBC is the timing to definitive surgery following diagnosis. The 2020 EAU guidelines cited two studies, with one showing worse clinical outcome and poorer survival in patients who experienced a delay of RC by >3 months while the other showed no survival difference (6, 7). With regards to MIBC patients treated with neoadjuvant chemotherapy, the AUA recommends RC within 6–8 weeks of completion of chemotherapy, unless “medically inadvisable”, while acknowledging that there remains a void of prospective data regarding the optimal timing of RC following NAC (8). Although low grade non-invasive UTUC can be treated endoscopically, RNU remains the treatment of choice for invasive and/or high grade UTUC. The EAU recommends that RNU should not be delayed beyond 12 weeks as this increases the risk of disease progression (9).
This issue of delayed treatment for MIBC and invasive UTUC is especially pertinent in our current ongoing COVID19 pandemic. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic emerged in December 2019 and has resulted in redistribution of healthcare resources to address the pandemic. This has resulted in cancelation of elective surgeries worldwide (10, 11). Many hospitals have deferred elective and non-cancer surgery, while prioritizing emergency cases and select high-risk oncological cases. To provide expert consensus, the EAU Guidelines Office Rapid Reaction Group recommend that RC should be performed within 3 months from MIBC diagnosis and RNU within 6 weeks of high-risk UTUC diagnosis (12).
The impact of the COVID-19 crisis on elective urological cancer surgery has been significant and disruptive worldwide and is compounded by the concerns of a second or third wave of COVID-19 cases. This invariably will result in the deferment of treatment of localized cancers, which may lead to disease progression and worse survival outcomes. In this study, we performed a systematic review and meta-analysis to evaluate the evidence and association of delayed RC and RNU for patients with MIBC and high-risk UTUC. These data should serve as a framework for decision making regarding timelines of definitive therapy in these disease entities.
Evidence acquisition
Protocol registration
Our study methodology was similar to 2 other papers on prostate cancer (13) and kidney cancer (14), whose protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) registry (CRD42020190882). We performed this study according to the Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (15). Since most of the included studies were retrospective in nature, we also adhered to guidelines from the “Meta-analysis Of Observational Studies in Epidemiology” (MOOSE) group (16).
Literature search
We performed a systematic search of PubMed/MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and Cochrane Database of Systematic Reviews to identify studies up to June 2020. Different variations of key words and MESH terms for urothelial carcinoma were combined with various combinations of survival outcomes in delaying surgery to identify articles that focused on the issue of delayed surgery. Our complete search strategy is shown in Supplementary Table S1.
Objective
The primary objective was to evaluate if delays to RC and RNU would affect the overall survival of patients with MIBC and high-risk UTUC, respectively.
Eligibility criteria, manuscript screening, data abstraction, and study quality
We evaluated studies for inclusion and exclusion based on a pre-defined PICOS approach where the population (P), intervention (I), comparator group (C), outcome (O), and study design (S) were considered. This is summarized in Table 1.
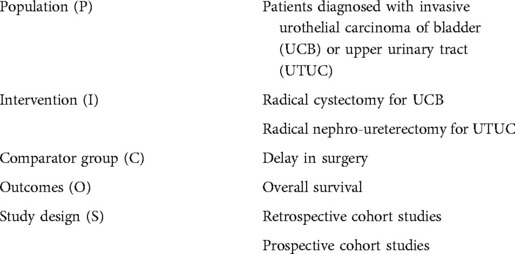
Table 1. Population, intervention group, comparator group, outcomes and study design (PICO) of studies included in this systematic review and meta-analysis.
Screening and data extraction
Search results were screened by two independent reviewers. Any conflicts were resolved by a third reviewer Finally, eligible articles were identified for full text review (Figure 1). Data extraction was then performed by two authors (JJL, JT) with any discrepancy resolved by a third author (WST). Data on the paper (first author, year, center, country, study design), participant demographics and oncologic characteristics, treatment characteristics, and outcomes, and results were extracted.
Statistical methods
Descriptive statistics using median and interquartile range were used to summarize demographic and baseline data of eligible patients. Sample size of individual studies, demographic values were calculated based on percentages and summed up to obtain the values used for this cohort. Pooled averages were estimated using fixed and random-effects model when indicated. The I2 statistic was used to quantify heterogeneity. Statistical analyses were performed using STATA/SE 14.2 (StataCorp, College Station, Texas, USA).
Risk of bias assessment
We performed risk of bias assessment using the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies (Supplementary Table S2) (17).
Evidence synthesis
Search results
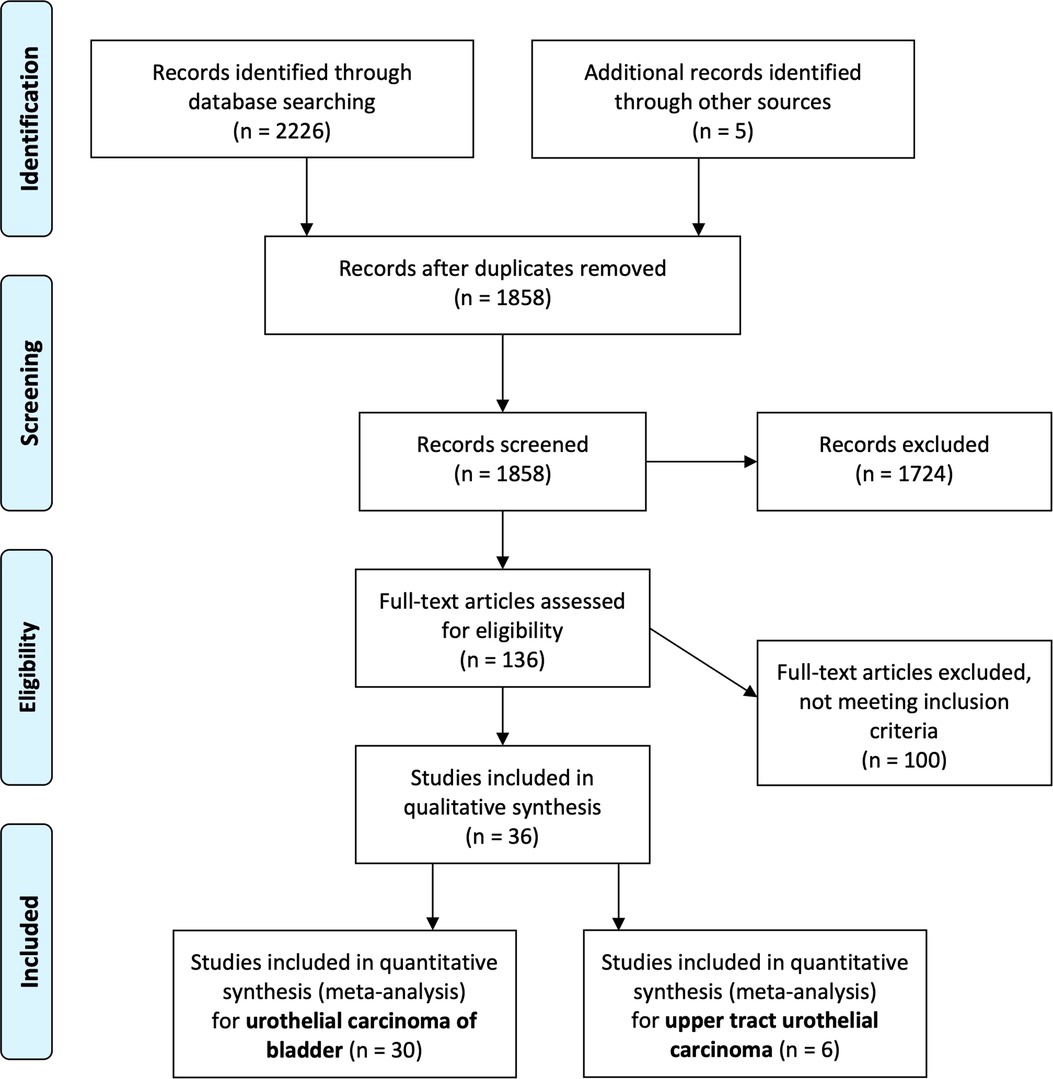
Our literature search initially revealed 1,858 articles after removing duplicates. After screening them based on our pre-defined PICOS criteria, we identified 136 articles which were further reviewed in detail and categorized by type of cancer (Figure 1).
Meta-analysis for bladder cancer studies
We identified a total of 30 studies with 32,591 patients (Table 2). There were varied definitions of delay to RC, with 11 studies identifying the “start point” as “diagnosis of bladder cancer” (18–28), while another 10 used “time of transurethral resection of bladder tumour” (TURBT) (6, 7, 29–36). Five studies evaluated the delay between neoadjuvant chemotherapy and RC (29, 37–40). Four other studies evaluated delay from time of diagnosis prompting BCG therapy to RC (41), time from RC to starting adjuvant chemotherapy (42), time from referral to first treatment (43), and time from first clinic appointment to definitive treatment (radiotherapy or RC) (44).
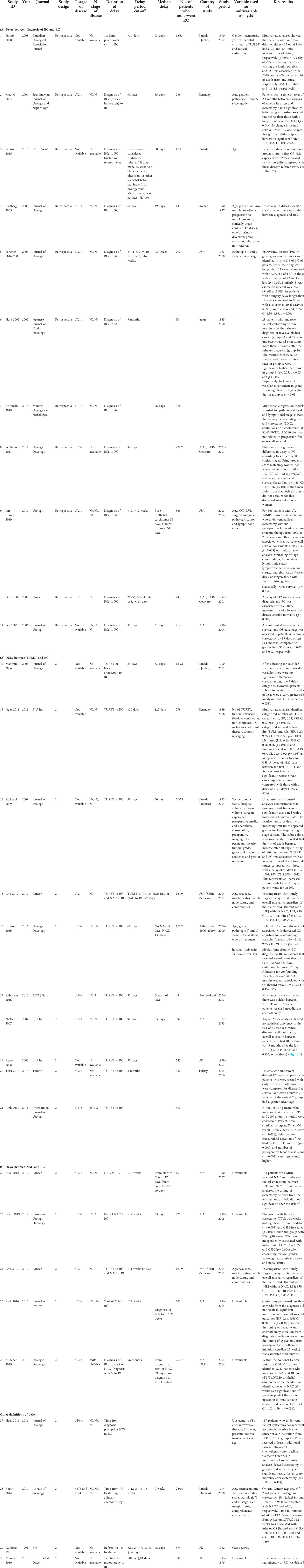
Table 2. Characteristics of included studies evaluating delayed radical cystectomy on survival in bladder cancer and upper tract urothelial carcinoma, based on various definitions of delay: (A) delay between diagnosis of BC and RC; (B) delay between NAC and RC; (C) other definitions of delay.
Given that the diagnosis of bladder cancer is confirmed upon histology obtained from TURBT, it can be safe to assume that these two “events” are synonymous. Although each study's exact cut-off duration varies from 60 to 90 days, we considered this “delay” the exposure variable for our meta-analysis. Across 13 studies (n = 12,201), a delay from diagnosis of bladder cancer/TURBT to RC was associated with poorer overall survival (HR 1.25, 95% CI: 1.09–1.45, p = 0.002) (Figure 2). There was substantial heterogeneity with an I2 value of 76.9% (Cochrane p-value <0.001), so a random-effects model was used. Influence analysis showed that the two most influential studies (38, 44) had the greatest effects on the pooled HR if omitted.
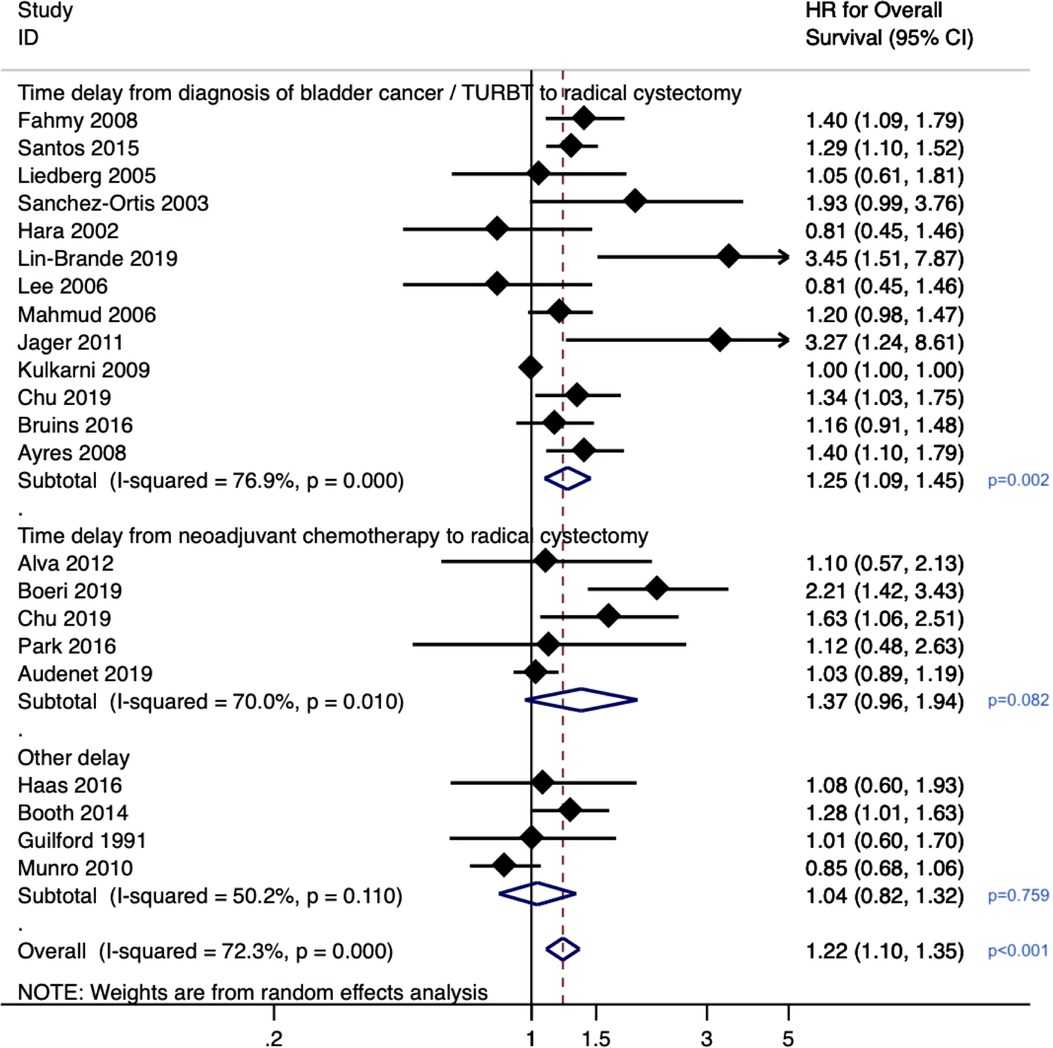
Figure 2. Forrest plot for meta-analysis on effect of delayed radical cystectomy on overall survival in bladder cancer.
For patients who underwent neoadjuvant chemotherapy prior to radical cystectomy, across the five studies (n = 4,316 patients), a delay between neoadjuvant chemotherapy and radical cystectomy was not found to be significantly associated with overall survival (pooled HR 1.37, 95% CI: 0.96–1.94, p = 0.08). There was substantial heterogeneity with an I2 value of 70% (Cochrane p-value 0.01), so a random-effects model was used. Three studies representing patients treated at Johns Hopkins (40), Michigan (37) (ref) and Mayo (39) reported 3 cycles of neoadjuvant chemotherapy administered and received by patients. The other 2 studies did not have such granular data as they were analyses of the National Cancer Data Base (records only whether patients received single or multi-agent chemotherapy) (38) and SEER-Medicare database (provider billing data utilized to determine receipt and timing chemotherapy) (29).
Meta-analysis for upper tract urothelial carcinoma studies
There were six studies evaluating the effect of delay to radical nephroureterectomy on survival for UTUC with a total of 4,629 patients (45–50). When evaluating the delay between diagnosis of UTUC and RNU, the meta-analysis revealed a pooled HR of 1.55 (95% CI: 1.19–2.02, p = 0.001) for overall survival (Figure 3) and a pooled HR of 2.56 (95% CI: 1.50–4.37, p = 0.001) for cancer-specific survival (Figure 4). There was no evidence of heterogeneity so fixed-effects models were used. Influence analysis showed that Alva et al. (37) had the greatest effect on the result if omitted.
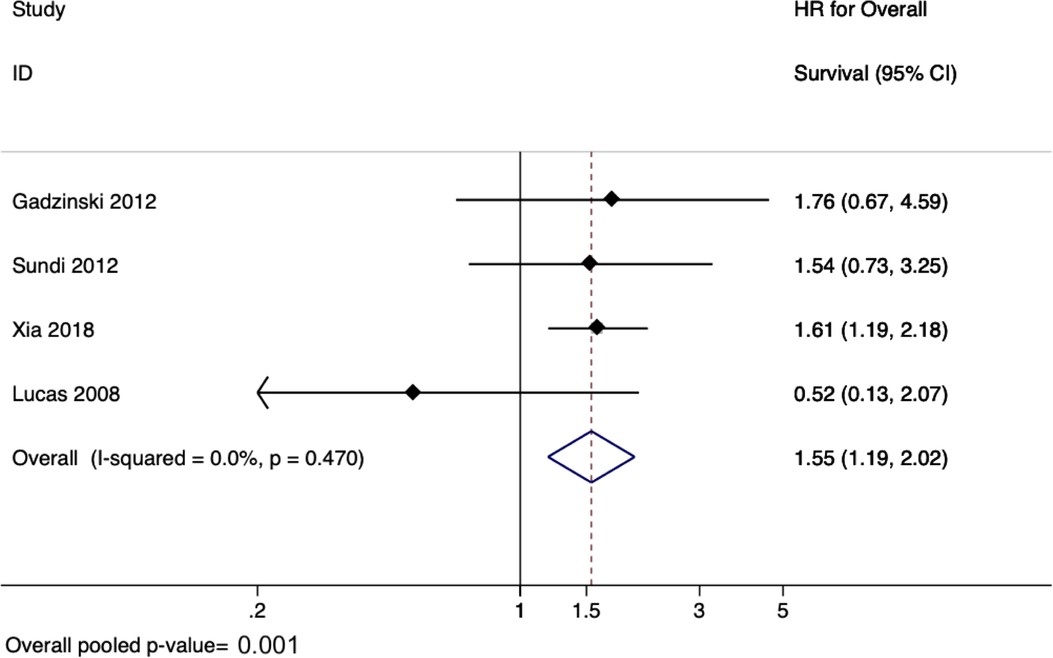
Figure 3. Forrest plot for meta-analysis on effect of delayed radical nephro-ureterectomy on overall survival in upper tract urothelial carcinoma.
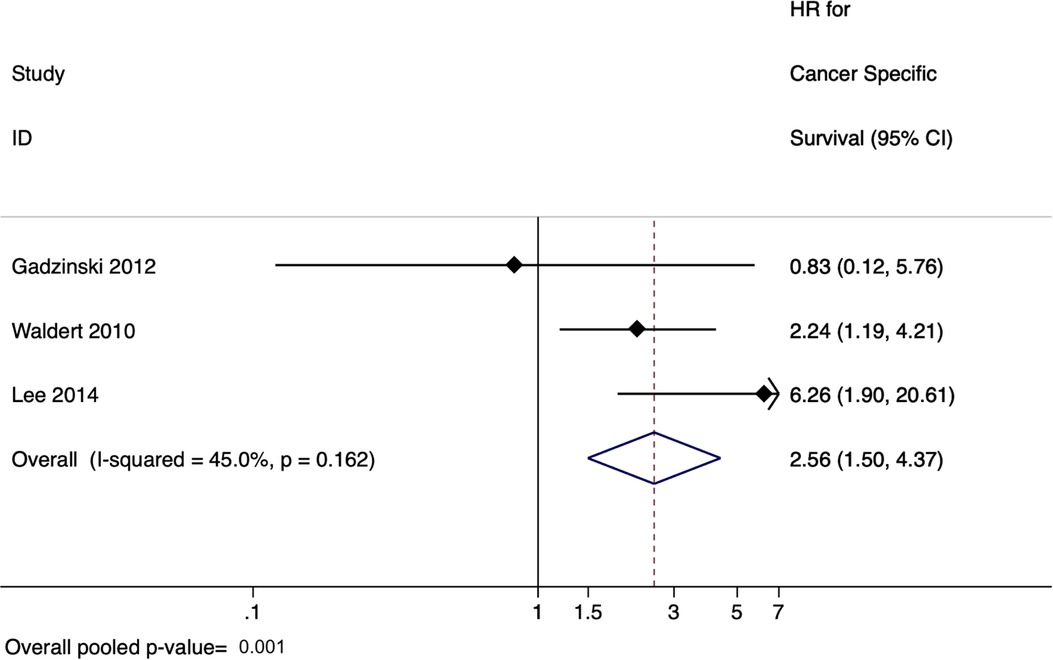
Figure 4. Forrest plot for meta-analysis on effect of delayed radical nephro-ureterectomy on cancer-specific survival in upper tract urothelial carcinoma.
Discussion
The SARS-CoV-2 epidemic has resulted in the cancelation of elective cancer surgeries worldwide, resulting in delay of cares for patients with invasive urothelial carcinoma. We performed a systematic review and meta-analysis to evaluate the evidence and the effect of delayed RC and RNU for patients with MIBC and high risk UTUC. Our study suggests that for patients who underwent upfront RC, a delay between bladder cancer diagnosis and undergoing definitive RC was associated with significantly poorer overall survival. Similarly, for UTUC, a delay between UTUC diagnosis to RNU was associated with worse overall and cancer-specific survival.
On the contrary, we found that a delay in RC following neoadjuvant chemotherapy did not impact survival outcomes. This finding is particularly pertinent because increasingly more patients with MIBC are receiving neoadjuvant chemotherapy, backed by level one evidence (51). This provides some reassurance to patients who face treatment delays due to chemotherapy related adverse events. Even among a relatively healthy study population in the SWOG-8710 trial, 33% of patients had grade 4 (severe) granulocytopaenia, and 17% had grade 3 (moderate) nausea, vomiting, stomatitis, diarrhoea, or constipation after neoadjuvant chemotherapy (52). However, during the COVID-19 pandemic it is important to acknowledge the theoretical competing risk of succumbing to COVID-19 due to an impaired immune system secondary to chemotherapy (53), particularly among the unvaccinated. This may lead to patients or clinicians electing to avoid peri-operative chemotherapy despite guideline recommendations.
Guidelines and societies have risen to the challenge during the COVID pandemic and came up with suggestions on how to overcome and reduce delay in definitive surgery for urology patients. The Urology Research Network from Italy has strategized how best to reorganize routine urologic practice and recommended how to facilitate the process of rescheduling both surgical and outpatient activities during the COVID-19 pandemic, and in subsequent phases (54). For muscle-invasive bladder cancer, radical cystectomy was categorized in the list of urological surgical procedures strongly recommended to continue during the pandemic, as delay can jeopardise cancer-related outcomes. Caution is advised in case of bowel resection due to high prevalence of high virus load in stool. Preoperative staging is suggested to be simplified to CT chest, abdomen and pelvis, omitting diagnostic ureteroscopy which was optional with weak strength rating in the 2020 EAU guidelines (54, 55). For high-risk UTUC, radical nephro-ureterectomy with template-based lymphadenectomy is also strongly recommended to continue, with preoperative staging simplified to CT urogram and flexible urethrocystoscopy alone, omitting diagnostic ureteroscopy (54, 55). These recommendations are a key referendum for all to resume routine urologic practice and can help as this pandemic evolves with time. Another helpful strategy to improve access for patients with haematuria is to use telehealth services to expedite workup with upper tract imaging and flexible cystoscopy, as described in more detail in a review article highlighting practical ways of how telehealth services can be useful during and after the COVID pandemic (56).
The effect of delays in RC has been investigated previously for MIBC. A recent systematic review (19 studies) and meta-analysis (10 studies) was performed for papers up to August 2019, although we found that there were some methodological errors (e.g., hazard ratio for progression-free survival used in overall survival meta-analysis) (57). Our study has updated the literature search up to June 2020 and includes a total of 30 studies in all, representing the latest available evidence for this topic.
Established dogma would suggest that delays in radical surgery for localised cancer carries the risk of disease progression, resulting in patients missing the opportunity to be cured of their cancer (58). Efforts to minimise treatment delays have led to countries such as the United Kingdom establishing cancer targets for providers to initiate treatment within 31 days from the time decision to treat is established (59). However, it is worth bearing in mind that not all cancer types have the same natural history and prognosis, and in the era of the COVID-19 pandemic, a tailored approached based on cancer disease risk should be adopted in terms of prioritising the urgency of each case. Invasive urothelial carcinoma, in the absence of treatment, progresses quickly. Those who decline treatment with curative intent have a 75% chance of dying from bladder cancer and a 40%–50% chance of doing so within 1 year (60). It may also be possible that delay in surgery could lead to more advanced disease, and could lead to more postoperative complications.
The question of what constitutes an “acceptable” time to treatment delay is often a subject of investigation. A SEER-Medicare analysis of patients with T2 bladder cancer who underwent RC between 1992 and 2001 identified 441 patients. Patients who experienced a delay of 8–12 weeks had a similar mortality risk compared to those who underwent RC within 4–8 weeks of diagnosis. However, patients who experienced a delay of 12–24 weeks had significantly worse mortality (HR 2.0) (27). Similar findings were demonstrated in an analysis of 2,535 patients who underwent RC for bladder cancer in Ontario, Canada between 1992 and 2004 where the hazard ratio of death gradually increased in a step-wise manner with an increase in waiting times. The risk of death exponentially increased when time to treatment was more than 150 days (32).
Causes of treatment delays can be multifactorial. Patients undergoing RC or RNU are often elderly and may have cardiovascular and respiratory comorbidities following years of exposure to cigarette smoking (1, 2). Hence, it is likely this patient cohort requires a multidisciplinary evaluation and a period of “prehabilitation” prior to radical surgery which may result in a delay in time to treatment (61). Patients initially diagnosed in community hospitals may also experience delays when referred to a tertiary unit if referral pathways are not efficient. This is increasingly encountered due to the centralisation of complex cancer surgery. These factors add to the complexities of treatment delays secondary to the COVID-19, where limited healthcare personal, availability of intensive care beds and ventilators, and efforts to minimise staff and patients from contracting COVID-19 significantly impair the ability to provide prompt surgical treatment. As the world moves on from the COVID-19 pandemic, healthcare systems can learn from the gaps exposed and put together comprehensive plans to remedy shortcomings in healthcare inefficiencies, particularly those related to delay in definitive treatment for cancer.
For example, delay in time to treatment following cancer diagnosis only represents part of the treatment pathway. In our current study, we could not account for delays between the interval that a patient experiences symptoms suggestive of possible cancer until the time they seek medical care (62). This may be addressed with bladder health awareness campaigns such as those from the Bladder Cancer Advocacy Network (BCAN), Action Bladder Cancer UK, or World Bladder Cancer Patient Coalition, just to name a few. In addition, delays exist between the time from initial consultation until the completion of investigations, such as staging tests and histopathological confirmation of cancer. Such delays can also influence cancer outcomes and are likely as important to identify and address.
Despite the strengths of our study, it is not devoid of limitations. These include the varying definitions and cut-offs used in individual studies’ analysis of delay, with most studies using a cut-off of 84–93 days. Despite the EAU guideline's recommendations of 12 weeks, numerous studies chose to use different cut-offs to define delays. Additionally, there were insufficient granular data from each study, which limited our ability to perform subgroup meta-regression analysis by T or N stages, for example. Additionally, our meta-analysis was limited to studies published up to June 2020. Finally, there was substantial heterogeneity across different studies, although our meta-analysis attempted to overcome this with random effects models.
Conclusion
`Our study revealed that a delay between bladder cancer diagnosis and RC was significantly associated with poorer overall survival outcomes, but this was not the case among patients who underwent neoadjuvant chemotherapy prior to RC. Similarly, a delay between UTUC diagnosis and RNU was significantly associated with worse overall and cancer-specific survival. In the COVID-19 era where hospital resources may be limited, we need to continue to provide prompt definitive treatment for our patients with urothelial cancers in order to achieve the best oncologic outcomes for them.
UroSoMe collaborators
We wish to thank the following UroSoMe collaborators who have helped in the initial phase of the project during the COVID-19 pandemic. William Lay Keat Ong, Pratik Gurung, Giacomo Maria Pirola, Luca Orecchia, Matthew Ping Chao Liew, Hsiang-Ying Lee, Yuding Wang, I-Hsuan Alan Chen, Daniele Castellani, Marcelo Langer Wroclawski, Nikhil Mayor, Niranjan J. Sathianathen, Isaac Braga, Zhenbang Liu, Dora Moon.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
All authors contributed to the drafting, data interpretation and critical revision of manuscript. JL performed the statistical analysis. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.879774/full#supplementary-material.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Teoh JY, Huang J, Ko WY, Lok V, Choi P, Ng CF, et al. Global trends of bladder cancer incidence and mortality, and their associations with tobacco use and gross domestic product per capita. Eur Urol. (2020) 78(6):893–906. doi: 10.1016/j.eururo.2020.09.006
3. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. (2017) 71(1):96–108. doi: 10.1016/j.eururo.2016.06.010
4. Tan WS, Feber A, Sarpong R, Khetrapal P, Rodney S, Jalil R, et al. Who should be investigated for haematuria? Results of a contemporary prospective observational study of 3556 patients. Eur Urol. (2018) 74(1):10–4. doi: 10.1016/j.eururo.2018.03.008
5. David KA, Mallin K, Milowsky MI, Ritchey J, Carroll PR, Nanus DM. Surveillance of urothelial carcinoma: stage and grade migration, 1993-2005 and survival trends, 1993-2000. Cancer. (2009) 115(7):1435–47. doi: 10.1002/cncr.24147
6. Bruins HM, Aben KK, Arends TJ, van der Heijden AG, Witjes AJ. The effect of the time interval between diagnosis of muscle-invasive bladder cancer and radical cystectomy on staging and survival: a Netherlands cancer registry analysis. Urol Oncol. (2016) 34(4):166.e1–6. doi: 10.1016/j.urolonc.2015.11.006
7. Ayres BE, Gillatt D, McPhail S, Cottrell A, McGrath J, Cottier B, et al. A delay in radical cystectomy of >3 months is not associated with a worse clinical outcome. BJU Int. (2008) 102(8):1045.18840144
8. Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. (2017) 198(3):552–9. doi: 10.1016/j.juro.2017.04.086
9. Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. (2020) 79(1):62–79. doi: 10.1016/j.eururo.2020.05.042
10. Teoh JY, Ong WLK, Gonzalez-Padilla D, Castellani D, Dubin JM, Esperto F, et al. A global survey on the impact of COVID-19 on urological services. Eur Urol. (2020) 78(2):265–75. doi: 10.1016/j.eururo.2020.05.025
11. Ong WLK, Lechmiannandan S, Loeb S, Teoh JY. Urological services in public hospitals suffered a greater detriment than private hospitals during the battle of COVID-19. Urology. (2020).
12. Ribal MJ, Cornford P, Briganti A, Knoll T, Gravas S, Babjuk M, et al. European association of urology guidelines office rapid reaction group: an organisation-wide collaborative effort to adapt the European association of urology guidelines recommendations to the coronavirus disease 2019 era. Eur Urol. (2020).
13. Chan VWS, Tan WS, Asif A, et al. Effects of delayed radical prostatectomy and active surveillance on localised prostate cancer-a systematic review and meta-analysis. Cancers (Basel). (2021) 13(13):3274. doi: 10.3390/cancers13133274
14. Chan VWS, Tan WS, Leow JJ, et al. Delayed surgery for localised and metastatic renal cell carcinoma: a systematic review and meta-analysis for the COVID-19 pandemic. World J Urol. (2021) 39(12):4295–303. doi: 10.1007/s00345-021-03734-1
15. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. (2009) 339:b2535. doi: 10.1136/bmj.b2535
16. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. J Am Med Assoc. (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
17. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2020). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed October 20, 2020).
18. Fahmy N, Kassouf W, Jeyaganth S, Amin M, Mahmud S, Steinberg J, et al. An analysis of preoperative delays prior to radical cystectomy for bladder cancer in Quebec. Can Urol Assoc J. (2008) 2(2):102–8. doi: 10.5489/cuaj.482
19. May M, Nitzke T, Helke C, Vogler H, Hoschke B. Significance of the time period between diagnosis of muscle invasion and radical cystectomy with regard to the prognosis of transitional cell carcinoma of the urothelium in the bladder. Scand J Urol Nephrol. (2004) 38(3):231–5. doi: 10.1080/00365590410029141
20. Santos F, Dragomir A, Kassouf W, Franco E, Aprikian A. Urologist referral delay and its impact on survival after radical cystectomy for bladder cancer. Curr Oncol. (2015) 22(1):e20–6. doi: 10.3747/co.22.2052
21. Liedberg F, Anderson H, Mansson W. Treatment delay and prognosis in invasive bladder cancer. J Urol. (2005) 174(5):1777–81; discussion 81. doi: 10.1097/01.ju.0000177521.72678.61
22. Sanchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. (2003) 169(1):110–5; discussion 5. doi: 10.1016/S0022-5347(05)64047-5
23. Hara I, Miyake H, Hara S, Gotoh A, Okada H, Arakawa S, et al. Optimal timing of radical cystectomy for patients with invasive transitional cell carcinoma of the bladder. Jpn J Clin Oncol. (2002) 32(1):14–8. doi: 10.1093/jjco/hyf002
24. Antonelli A, Zamboni S, Palumbo C, Belotti S, Lattarulo M, Furlan M, et al. Prognostic role of delay before radical cystectomy: retrospective analysis of a single-centre cohort with 376 patients. Minerva Urol Nefrol. (2018) 70(5):494–500. doi: 10.23736/S0393-2249.18.02995-8
25. Williams SB, Huo J, Dafashy TJ, Ghaffary CK, Baillargeon JG, Morales EE, et al. Survival differences among patients with bladder cancer according to sex: critical evaluation of radical cystectomy use and delay to treatment. Urol Oncol. (2017) 35(10):602.e1–e9. doi: 10.1016/j.urolonc.2017.05.022
26. Lin-Brande M, Pearce SMP, Ashrafi AN, Nazemi A, Burg ML, Ghodoussipour S, et al. Assessing the impact of time to cystectomy for variant histology of urothelial bladder cancer. Urology. (2019) 133:157–63. doi: 10.1016/j.urology.2019.07.034
27. Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS, Urologic Diseases in America P. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a surveillance, epidemiology, and end results-medicare analysis. Cancer. (2009) 115(5):988–96. doi: 10.1002/cncr.24052
28. Lee CT, Madii R, Daignault S, Dunn RL, Zhang Y, Montie JE, et al. Cystectomy delay more than 3 months from initial bladder cancer diagnosis results in decreased disease specific and overall survival. J Urol. (2006) 175(4):1262–7; discussion 7. doi: 10.1016/S0022-5347(05)00644-0
29. Chu AT, Holt SK, Wright JL, Ramos JD, Grivas P, Yu EY, et al. Delays in radical cystectomy for muscle-invasive bladder cancer. Cancer. (2019) 125(12):2011–7. doi: 10.1002/cncr.32048
30. Jager W, Thomas C, Haag S, Hampel C, Salzer A, Thuroff JW, et al. Early vs delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: delay of cystectomy reduces cancer-specific survival. BJU Int. (2011) 108(8 Pt 2):E284–8. doi: 10.1111/j.1464-410X.2010.09980.x
31. Kahokehr A, Glasson J, Studd R. Surgical waiting time for radical cystectomy: a New Zealand experience. ANZ J Surg. (2016) 86(12):1042–5. doi: 10.1111/ans.13282
32. Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Longer wait times increase overall mortality in patients with bladder cancer. J Urol. (2009) 182(4):1318–24. doi: 10.1016/j.juro.2009.06.041
33. Mahmud SM, Fong B, Fahmy N, Tanguay S, Aprikian AG. Effect of preoperative delay on survival in patients with bladder cancer undergoing cystectomy in Quebec: a population based study. J Urol. (2006) 175(1):78–83; discussion. doi: 10.1016/S0022-5347(05)00070-4
34. Nielsen ME, Palapattu GS, Karakiewicz PI, Lotan Y, Bastian PJ, Lerner SP, et al. A delay in radical cystectomy of >3 months is not associated with a worse clinical outcome. BJU Int. (2007) 100(5):1015–20. doi: 10.1111/j.1464-410X.2007.07132.x
35. Rink M, Dahlem R, Kluth L, Minner S, Ahyai SA, Eichelberg C, et al. Older patients suffer from adverse histopathological features after radical cystectomy. Int J Urol. (2011) 18(8):576–84. doi: 10.1111/j.1442-2042.2011.02794.x
36. Turk H, Un S, Cinkaya A, Kodaz H, Parvizi M, Zorlu F. Effect of delayed radical cystectomy for invasive bladder tumors on lymph node positivity, cancer-specific survival and total survival. Tumori. (2018) 104(6):434–7. doi: 10.5301/tj.5000626
37. Alva AS, Tallman CT, He C, Hussain MH, Hafez K, Montie JE, et al. Efficient delivery of radical cystectomy after neoadjuvant chemotherapy for muscle-invasive bladder cancer: a multidisciplinary approach. Cancer. (2012) 118(1):44–53. doi: 10.1002/cncr.26240
38. Audenet F, Sfakianos JP, Waingankar N, Ruel NH, Galsky MD, Yuh BE, et al. A delay ≥8 weeks to neoadjuvant chemotherapy before radical cystectomy increases the risk of upstaging. Urol Oncol. (2019) 37(2):116–22. doi: 10.1016/j.urolonc.2018.11.011
39. Boeri L, Soligo M, Frank I, Boorjian SA, Thompson RH, Tollefson M, et al. Delaying radical cystectomy after neoadjuvant chemotherapy for muscle-invasive bladder cancer is associated with adverse survival outcomes. Eur Urol Oncol. (2019) 2(4):390–6. doi: 10.1016/j.euo.2018.09.004
40. Park JC, Gandhi NM, Carducci MA, Eisenberger MA, Baras AS, Netto GJ, et al. A retrospective analysis of the effect on survival of time from diagnosis to neoadjuvant chemotherapy to cystectomy for muscle invasive bladder cancer. J Urol. (2016) 195(4 Pt 1):880–5. doi: 10.1016/j.juro.2015.11.024
41. Haas CR, Barlow LJ, Badalato GM, DeCastro GJ, Benson MC, McKiernan JM. The timing of radical cystectomy for bacillus calmette-guerin failure: comparison of outcomes and risk factors for prognosis. J Urol. (2016) 195(6):1704–9. doi: 10.1016/j.juro.2016.01.087
42. Booth CM, Siemens DR, Peng Y, Tannock IF, Mackillop WJ. Delivery of perioperative chemotherapy for bladder cancer in routine clinical practice. Ann Oncol. (2014) 25(9):1783–8. doi: 10.1093/annonc/mdu204
43. Gulliford MC, Petruckevitch A, Burney PG. Survival with bladder cancer, evaluation of delay in treatment, type of surgeon, and modality of treatment. Br Med J. (1991) 303(6800):437–40. doi: 10.1136/bmj.303.6800.437
44. Munro NP, Sundaram SK, Weston PM, Fairley L, Harrison SC, Forman D, et al. A 10-year retrospective review of a nonrandomized cohort of 458 patients undergoing radical radiotherapy or cystectomy in yorkshire, UK. Int J Radiat Oncol Biol Phys. (2010) 77(1):119–24. doi: 10.1016/j.ijrobp.2009.04.050
45. Lucas SM, Svatek RS, Olgin G, Arriaga Y, Kabbani W, Sagalowsky AI, et al. Conservative management in selected patients with upper tract urothelial carcinoma compares favourably with early radical surgery. BJU Int. (2008) 102(2):172–6. doi: 10.1111/j.1464-410X.2008.07535.x
46. Waldert M, Karakiewicz PI, Raman JD, Remzi M, Isbarn H, Lotan Y, et al. A delay in radical nephroureterectomy can lead to upstaging. BJU Int. (2010) 105(6):812–7. doi: 10.1111/j.464-410X.2009.08821.x
47. Gadzinski AJ, Roberts WW, Faerber GJ, Wolf JS Jr. Long-term outcomes of immediate versus delayed nephroureterectomy for upper tract urothelial carcinoma. J Endourol. (2012) 26(5):566–73. doi: 10.1089/end.2011.0220
48. Sundi D, Svatek RS, Margulis V, Wood CG, Matin SF, Dinney CP, et al. Upper tract urothelial carcinoma: impact of time to surgery. Urol Oncol. (2012) 30(3):266–72. doi: 10.1016/j.urolonc.2010.04.002
49. Lee JN, Kwon SY, Choi GS, Kim HT, Kim TH, Kwon TG, et al. Impact of surgical wait time on oncologic outcomes in upper urinary tract urothelial carcinoma. J Surg Oncol. (2014) 110(4):468–75. doi: 10.1002/jso.23589
50. Xia L, Taylor BL, Pulido JE, Guzzo TJ. Impact of surgical waiting time on survival in patients with upper tract urothelial carcinoma: a national cancer database study. Urol Oncol. (2018) 36(1):10.e5–e22. doi: 10.1016/j.urolonc.2017.09.013
51. Vale C, Collaboration ABCM. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. (2003) 361(9373):1927–34. doi: 10.1016/S0140-6736(03)13580-5
52. Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. (2003) 349(9):859–66. doi: 10.1056/NEJMoa022148
53. Jee J, Foote MB, Lumish M, Stonestrom AJ, Wills B, Narendra V, et al. Chemotherapy and COVID-19 outcomes in patients with cancer. J Clin Oncol. (2020).32795225
54. Ficarra V, Novara G, Abrate A, Bartoletti R, Crestani A, De Nunzio C, et al. Urology practice during the COVID-19 pandemic. Minerva Urol Nefrol. (2020) 72(3):369–75. doi: 10.23736/S0393-2249.20.03846-1
55. Simonato A, Giannarini G, Abrate A, Bartoletti R, Crestani A, De Nunzio C, et al. Clinical pathways for urology patients during the COVID-19 pandemic. Minerva Urol Nefrol. (2020) 72(3):376–83. doi: 10.23736/S0393-2249.20.03861-8
56. Novara G, Checcucci E, Crestani A, Abrate A, Esperto F, Pavan N, et al. Telehealth in urology: a systematic review of the literature. How much can telemedicine be useful during and after the COVID-19 pandemic? Eur Urol. (2020) 78(6):786–811. doi: 10.1016/j.eururo.2020.06.025
57. Russell B, Liedberg F, Khan MS, Nair R, Thurairaja R, Malde S, et al. A systematic review and meta-analysis of delay in radical cystectomy and the effect on survival in bladder cancer patients. Eur Urol Oncol. (2019).31668714
58. Richards M. The national awareness and early diagnosis initiative in England: assembling the evidence. Br J Cancer. (2009) 101(S2):S1. doi: 10.1038/sj.bjc.6605382
59. England N. Achieving world-class cancer outcomes: a strategy for England 2015-2020. London, UK 2015.
60. de Vere White R, Dall'Era M. RE: quality indicators for bladder cancer services: a collaborative review. Eur Urol. (2021) 79(5):700. doi: 10.1016/j.eururo.2020.11.031
61. Psutka SP, Barocas DA, Catto JWF, Gore JL, Lee CT, Morgan TM, et al. Staging the host: personalizing risk assessment for radical cystectomy patients. Eur Urol Oncol. (2018) 1(4):292–304. doi: 10.1016/j.euo.2018.05.010
Keywords: delay in surgery, delayed treatment, time-to-treatment, urinary bladder neoplasms, ureteral neoplasms, urothelial carcinoma, bladder cancer, bladder carcinoma
Citation: Leow JJ, Tan WS, Tan WP, Tan TW, Chan VW-S, Tikkinen KAO, Kamat A, Sengupta S, Meng MV, Shariat S, Roupret M, Decaestecker K, Vasdev N, Chong YL, Enikeev D, Giannarini G, Ficarra V, Teoh JY-C and On behalf of the UroSoMe Collaborators (2022) A systematic review and meta-analysis on delaying surgery for urothelial carcinoma of bladder and upper tract urothelial carcinoma: Implications for the COVID19 pandemic and beyond. Front. Surg. 9:879774. doi: 10.3389/fsurg.2022.879774
Received: 20 February 2022; Accepted: 23 August 2022;
Published: 4 October 2022.
Edited by:
Christian P. Meyer, Ruhr University Bochum, GermanyReviewed by:
Nicola Pavan, Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone, ItalyWei Yu, First Hospital, Peking University, China
© 2022 Leow, Tan, Tan, Tan, Chan, Tikkinen, Kamat, Sengupta, Meng, Shariat, Roupret, Decaestecker, Vasdev, Chong, Enikeev, Giannarini, Ficarra and Teoh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremy Yuen-Chun Teoh amVyZW15dGVvaEBzdXJnZXJ5LmN1aGsuZWR1Lmhr
Specialty Section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
 Jeffrey J. Leow
Jeffrey J. Leow Wei Shen Tan
Wei Shen Tan Wei Phin Tan
Wei Phin Tan Teck Wei Tan
Teck Wei Tan Vinson Wai-Shun Chan
Vinson Wai-Shun Chan Kari A. O. Tikkinen
Kari A. O. Tikkinen Ashish Kamat
Ashish Kamat Shomik Sengupta
Shomik Sengupta Maxwell V. Meng14
Maxwell V. Meng14 Shahrokh Shariat
Shahrokh Shariat Karel Decaestecker
Karel Decaestecker Nikhil Vasdev
Nikhil Vasdev Dmitry Enikeev
Dmitry Enikeev Gianluca Giannarini
Gianluca Giannarini Vincenzo Ficarra
Vincenzo Ficarra Jeremy Yuen-Chun Teoh
Jeremy Yuen-Chun Teoh