
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 02 May 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.877040
Objective: To design and develop a disposable superfine catheter system for visual examination of bile and pancreatic ducts and predict its clinical application value.
Methods: The superfine triple-lumen catheter and miniature photography technology were used to design and produce a disposable superfine catheter for visual examination of bile and pancreatic ducts, and animal experiments were conducted to compare said catheter and SpyGlass™.
Results: The designed and developed disposable superfine catheter for visual examination of bile ducts with a diameter of 2.4 mm could enter the third-order and fourth-order bile ducts in the animal liver and also the gallbladder via the cystic duct for observation. The said catheter has a water injection rate of 0.8 mL/s, 0.16 megapixels, a resolution of 400 × 400, a depth of field of 0.3 to 20 mm, and a tilting up angle >90°.
Conclusion: The new disposable catheter for visual examination of bile ducts has a superfine diameter, easier operation, and clearer imaging, and is expected to have a higher clinical practical value.
The hepatobiliary system is an important part of the human digestive system comprising the liver, gallbladder, and a complex network of intrahepatic and extrahepatic bile ducts. These bile ducts, or channels, gather to form a vast three-dimensional structure called the biliary tree (1). According to the anatomical positions, the biliary system is divided into intrahepatic and extrahepatic bile ducts, the common hepatic duct, the cystic duct, and the gallbladder. Common biliary diseases include cholangitis, parasites, cholelithiasis, and bile duct malignancies.
According to statistics, about 20 million people suffer from cholelithiasis in the United States; the overall incidence of gallstone disease is 18.8% in Europe, with 9.5% occurring in males (2). Choledocholithiasis is very common in Asia. Literature shows that the proportion of patients with choledocholithiasis who have clinical symptoms is about 20% (3).
Cholangiocarcinoma (CCA) is the second most common primary hepatic malignancy after hepatocellular carcinoma (HCC), comprising about 15% of all primary liver tumors (4). The slow and silently evolving progression of CCA creates great difficulty for clinical treatment (5). Most of the poor prognoses of CCA result from a late diagnosis, leading to high clinical mortality rate and low postoperative survival. According to research statistics, the five-year survival rate after resection of bile duct malignancies is usually less than 40% and is less than 5% in patients with unresectable bile duct malignancies (6).
The current clinical diagnosis of CCA primarily relies on a combination of multiple imaging techniques, including ultrasound, computed tomography (CT), positron emission computed tomography (PET), and magnetic resonance imaging (MRI). Bile duct pathological tissue is mainly obtained through endoscopic retrograde cholangiopancreatography (ERCP) brush cytology or biopsy, however, bile duct brushing has a low positive rate and easily causes contamination. Bile duct biopsies present problems such as difficulty with biopsy site localization under X-ray and failure to make a section of the sample obtained by the excessively small per-oral choledochoscope biopsy forceps, leading to the difficulty of diagnosing bile duct malignancies (7).
Bakes designed a laryngoscope-like endoscope in 1923, which, for the first time, enabled direct visual observation of the common bile duct (8).
Researchers developed two different types of per-oral cholangioscopy (POCS) techniques in the late 1970s, i.e., the indirect POCS technique proposed by Kawai (9) et al. and the direct POCS technique proposed by Urakami (10) et al. Boston Scientific introduced a single-operator catheter-based POCS technique called SpyGlass™ in 2005 (11). However, according to the related literature, SpyGlass™ presents problems such as a thick diameter, inconvenient operation, low image pixels, a fragile optical fiber, vulnerable steerable wire, and expensive cost (12, 13).
Therefore, we intended to develop and produce a new disposable superfine catheter system for visual examination of bile and pancreatic ducts, which could enter bile and pancreatic ducts for visual observation through catheter insertion during ERCP and whose overall diameter would be minimized on the premise of not affecting the visual observation. The system would be designed to enter the intrahepatic third-order and fourth-order bile ducts and the cystic duct to achieve non-invasive visual observation of intrahepatic bile ducts and the gallbladder.
A disposable superfine catheter system for visual examination of bile and pancreatic ducts was designed and compared with SpyGlass™ in terms of hardware performance and in animal experiments.
The catheter system consists of four parts, i.e., the front-end camera part, the examination tube part, the tail-end operation part, and the power supply and data output part. The catheter's front end consists of a miniature camera module and a lighting source. The tube body is in a triple-lumen structure and the tail end is a three-way operating handle.
Description of the specific design and production of the disposable superfine catheter system for visual examination of bile and pancreatic ducts is as follows: the camera part consists of a camera module surrounded by four LED beads and overall constitutes the cylindrical head end, with a diameter of 2.38 mm ± 0.05 mm and a length of 5.0 mm ± 0.03 mm. The LED beads are connected in series to the main control circuit, with the brightness adjustable through the adjustment of resistance. The camera resolution is 160 k (400 × 400), covering a 120° wide-angle range lighted by the front LED beads. The main body is a triple-lumen Teflon catheter, with a total length of 1.8–2.0 m and a circumference of about 2.4 mm, which is molded in one step. The three lumens consist of a superfine guidewire lumen with a diameter of 1.0 mm, allowing the passage of a 0.038-inch yellow zebra guidewire, a data cable transmission channel, with a diameter of about 0.5 mm, and a water injection/suction lumen that can simultaneously operate water injection, air injection, and negative pressure suction (refer to Table 1 for the specific parameters). The tail end is a three-way connector structure, with a water/air inlet/outlet, a superfine guidewire inlet in the middle, and a cable and device inlet. A rubber ring is provided externally, which can tightly wrap around the imaging or photography catheter when said catheter is inserted via the device inlet, forming a sealed lumen that prevents water and air backflow (Figure 1).
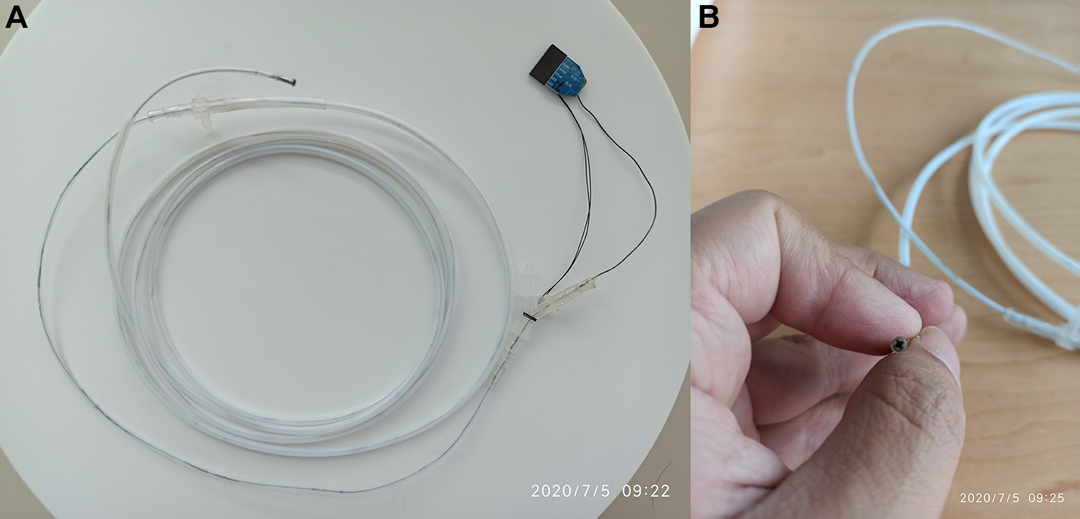
Figure 1. Disposable superfine catheter for visual examination of bile and pancreatic ducts. (A) Overall Catheter, (B) Catheter Front End.

Table 1. Parameters of disposable superfine catheter for visual examination of bile and pancreatic ducts.
Comparison of hardware performance between the disposable superfine catheter for visual examination of bile and pancreatic ducts and SpyGlass™ DS.
Comparison of front-end diameters is shown in Figure 2. The measuring instrument was a micrometer, and the part measured was the camera end (2 mm from the top).
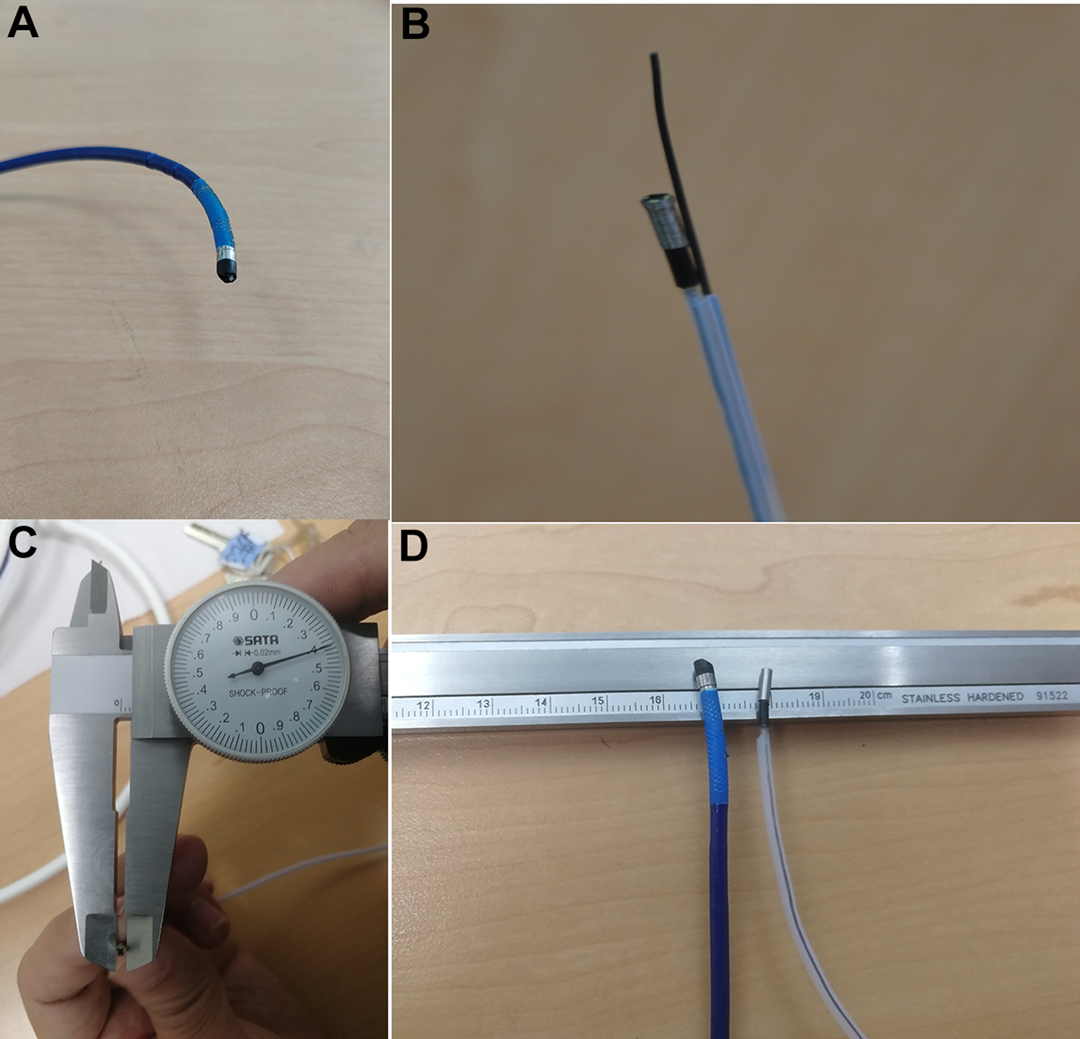
Figure 2. Comparison of front-end diameters. (A) Front End of SpyGlass™ DS, (B) Front End of Disposable Superfine Catheter for Visual Examination of Bile and Pancreatic Ducts, (C) Front-end Diameter of Disposable Superfine Catheter for Visual Examination of Bile and Pancreatic Ducts Measured with Micrometer, (D) Comparison of Front Ends of the Two.
Comparison of front-end bendability is shown in Figure 3. The two different products were inserted in the duodenum forceps channel, with the front end extending 2.0 cm from the forceps channel outlet, and the duodenoscope forceps lifter was lifted to the maximum angle, so as to measure the front-end bending angle. The measuring instruments included a ruler and a protractor.

Figure 3. Comparison of front-end tilting up angles. (A) Disposable Superfine Catheter for Visual Examination of Bile and Pancreatic Ducts, (B) SpyGlass™ DS.
The water injection by a water pump in ERCP was simulated to compare the water injection rate (Figure 4). Bubble-free water was used in the experiments, and the water pump was the SpyGlass™ Irrigation Pump that supports SpyGlass™ DS.

Figure 4. Comparison of water injection rates. (A) Water Injection by Disposable Superfine Catheter for Visual Examination of Bile and Pancreatic Ducts, (B) Time of 40 mL Water Injection by Disposable Superfine Catheter for Visual Examination of Bile and Pancreatic Ducts, (C) Water Injection by SpyGlass™ DS, (D) Time of 40 mL Water Injection by SpyGlass™ DS.
After the pump was pressurized (adjusted to the maximum pressure), the time required to inject 40 mL of water into the measuring glass was observed separately for the two products. (Note: The total amount of 40 mL was adopted because repeated observation showed that the pump pressure would change after the pump continued to inject 40 mL of water.) The comparison observation experiment of water injection rates showed that the time required to inject 40 mL of water was 50.31 seconds for the disposal ERCP and photography catheter system (the prototype) and 58.34 s for SpyGlass™ DS.
The animal experiments were approved by the Animal Experimentation Ethics Committee of Lanzhou University Second Hospital.
The duodenum of a miniature pig was dissected to find the duodenal papilla, and the disposable superfine catheter for visual examination of bile and pancreatic ducts was guided into the bile duct by the superfine guidewire and then advanced for observation. After entering the bile duct, the said catheter traveled upward for about 2 to 3 cm, then an independent opening was seen in the left wall of the common bile duct, which was nearly vertical and considered to be the cystic duct opening; the photography catheter and the guidewire were then withdrawn. The guidewire direction was adjusted to enter the gallbladder, and the catheter followed the guidewire into the gallbladder. The shapes of the guidewire and the photography catheter in the gallbladder were observed under X-ray (refer to Figure 5A). Bile was sucked from the water injection lumen of the photography catheter and normal saline was injected into said lumen for rinsing, after which, regular arrangements of fine and dense villi in the inner wall of the gallbladder were observed (refer to Figure 5B). After observation of the gallbladder, the guidewire and the photography catheter were withdrawn to the common bile duct to continue traveling upward through the bile duct for observation, and normal saline was used for rinsing intermittently in the observation process to keep the view clean and the bile duct filled. After traveling upward about 5 to 7 cm, the bile duct was seen to bifurcate into the left and right intrahepatic bile ducts. The guidewire direction was adjusted to enter the intrahepatic bile duct on one side and the photography catheter easily followed the guidewire into the intrahepatic bile duct. After entry, the photography catheter could continue to advance in the liver for visualizing the branch (third-order and fourth-order) bile ducts (refer to Figure 6). The same method was adopted for SpyGlass's entry into the miniature pig's bile duct for observation, and the images were kept for comparison (refer to Figure 7).
We successfully designed, developed, and produced a disposable superfine catheter system for visual examination of bile and pancreatic ducts. The comparison of the hardware performance and the observation of application in animal bile ducts showed the following advantages of the disposable superfine catheter system for visual examination of bile and pancreatic ducts: 1. superfine catheter diameter that allowed the catheter to enter the intrahepatic third-order and fourth-order bile ducts and the gallbladder; 2. efficient water injection; 3. high image resolution; 4. convenient operation (refer to Table 2).
The current choledochoscope products on the market have their respective features and also shortcomings. Our study intended to use miniature photography technology to innovatively develop an ERCP technique-specific superfine high-resolution catheter system in the digestive endoscopy field for visual examination of bile and pancreatic ducts. This system is inserted via the duodenoscope biopsy channel to enter the gallbladder and intrahepatic bile ducts for observation and is a catheter accessory guided by the guidewire to facilitate clinical use.
The SpyGlass™ developed by Boston Scientific is full-featured, with the front end adjustable from multiple angles to enable direct-vision biopsy or bile duct stone lithotripsy (14). However, the outer diameter (3.5 mm) of its front end cannot be further reduced because its light source is transmitted to the front end of the camera via an optical fiber. Multiple clinical needs are taken into account and multiple powerful features, such as a continuous injection pump, supporting superfine biopsy forceps, and four-quadrant adjustable steering wire, are integrated. SpyGlass™ thus cannot enter the gallbladder or intrahepatic third-order and fourth-order bile ducts in clinical practice. Furthermore, as the SpyGlass™ DS has a thick front end that contains complex components, the front end is hard and not very flexible. As a result, it requires a big incision or dilatation of the duodenal papilla for insertion during the ERCP, and during the insertion process, the forceps lifter requires great effort to be lifted. Moreover, the optical fiber used by the SpyGlass™ DS can only be bent to a limited extent, and when squeezed, the fiber will become attenuated and affect the imaging, causing fiber damage in cases of any carelessness in the operation process (15).
The disposable ERCP and photography catheter system was designed and developed by us to solve practical problems in clinical practice and meet clinical needs, with features of low cost and single use as an accessory/consumable. The ability of four-quadrant manipulation was given up because the integrated catheter design of the disposable superfine catheter for visual examination of bile and pancreatic ducts enables the in vitro control of the handle to achieve 360° of accurate rotation of the photography catheter so as to effectively adjust the advancing direction of the photography front end. The photography front end can only be bent to a limited extent in the bile ducts with a relative diameter of about 8 mm or less, and inside the narrow space of small-diameter intrahepatic bile ducts, close observation can be achieved without the need for large-angle adjustment. Guided by the superfine guidewire, the disposable superfine catheter for visual examination of bile and pancreatic ducts can enter different bile ducts in reliance on its advantage of a fine outer diameter. Although its overall diameter is only 2.4 mm, it is still superior to SpyGlass™ DS in terms of water injection rate. Furthermore, the 0.16 megapixel camera with a resolution of 400 × 400 ensures the ability to observe the bile duct wall mucosa and blood vessels.
The disposable superfine catheter for visual examination of bile and pancreatic ducts currently does not have direct-vision biopsy ability. We mainly conducted the autopsy by marking the location of the catheter under X-ray and, after withdrawing the catheter, inserting the transnasal gastroscope to use the biopsy forceps. The indirect biopsy method has the disadvantage of repeated changes of accessories; however, it can largely reduce the medical cost.
In conclusion, the focus of clinical medical treatment has gradually shifted toward the microscopic, visual, precise, and super minimally invasive aspects with the rapid development of science and technology and the continuous improvement of the manufacturing level. The fast development of the indirect per-oral visual choledochoscopes allows endoscopist to safely and efficiently “enter” the biliary system to perform diagnosis and treatment and has greatly expanded the clinical indicators of biliary endoscopy. The combination of new materials and imaging technology with special accessories designed and developed by us is expected to address the above aspects and make biliary disease diagnosis and treatment more efficient, convenient, and economical.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by the Animal Experimentation Ethics Committee of Lanzhou University Second Hospital.
Conception and design of the research: J-YH, X-JH. Acquisition of data: J-YH, Y-PZ. Analysis and interpretation of the data: J-YH, Y-PZ. Statistical analysis: J-YH, Y-PZ. Obtaining financing: X-JH. Writing of the manuscript: J-YH. Critical revision of the manuscript for intellectual content: J-YH, X-JH. All authors contributed to the article and approved the submitted version.
This study was supported by Key research and development plan of Gansu province (20YF8FA076).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhou QB, Lai DM, Yang B, Lin Q, Guo M, Wang QS, et al. Value of modified T staging system in the diagnosis and treatment of hilar cholangiocarcinoma. Chin J Dig Surg. (2012) 11(006):570–3. doi: 10.3760/cma.j.issn.1673-9752.2012.06020
2. Griffin N, Charles-Edwards G, Grant LA. Magnetic resonance cholangiopancreatography: the ABC of MRCP. Insights Imaging. (2012) 3(1):11–21. doi: 10.1007/s13244-011-0129-9
3. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev. Gastroenterol Hepatol. (2016) 13(5):261–80. doi: 10.1038/nrgastro.2016.51
4. Chapman MH, Sandanayake NS, Andreola F, Dhar DK, Webster GJ, Dooley JS, et al. Circulating CYFRA 21-1 is a specific diagnostic and prognostic biomarker in biliary tract cancer. J Clin Exp Hepatol. (2011) 1(1):6–12. doi: 10.1016/S0973-6883(11)60110-2
5. Macias RI. Cholangiocarcinoma: biology, clinical management, and pharmacological perspectives. ISRN Hepatol. (2014) 2014:828074. doi: 10.1155/2014/828074
6. Liu FH, Jiang XZ, Yu Y, Huang B. Comparison of curative effect between ERCP and PTCD in treatment of malignant obstructive jaundice: a Meta-analysi. Chin J Endosc. (2020) 26(3):49–57. doi: 10.3969/j.issn.1007-1989.2020.03.008
7. Hirschowitz BI, Peters CW, Curtiss LE. Preliminary report on a long fiberscope for examination of stomach and duodenum. Med Bull (Ann Arbor. (1957) 23(5):178–80. PMID: 13433948
8. Nimura Y, Shionoya S, Hayakawa N, Kamiya J, Kondo S, Yasui A. Value of percutaneous transhepatic cholangioscopy (PTCS). Surg Endosc. (1988) 2(4):213–9. doi: 10.1007/BF00705323
9. Chen YK. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization, and biopsy. Gastrointest Endosc. (2007) 65(2):303–11. doi: 10.1016/j.gie.2006.07.048
10. Voaklander R, Kim E, Brown WH, Kasmin FE, Siegel JH. An overview of the evolution of direct cholangioscopy techniques for diagnosis and therapy. Gastroenterol Hepatol (NY). (2016) 12(7):433–7. PMID: 27489525; PMCID: PMC4969779
11. Derdeyn J, Laleman W. Current role of endoscopic cholangioscopy. Curr Opin Gastroenterol. (2018) 34(5):301–8. doi: 10.1097/MOG.0000000000000457
12. Nakajima M, Kizu M, Akasaka Y, Kawai K. Five years experience of endoscopic sphincterotomy in Japan: a collective study from 25 centres. Endoscopy. (1979) 11(2):138–41. doi: 10.1055/s-0028-1098339
13. Staritz M, Günther R, Plagwitz R. Endoskopische retrograde Cholangiographie (ERC) mit einem doppellumigen Ballonkathether--eine Verbesserung der konventionellen ERC-Technik [Endoscopic retrograde cholangiography (ERG) with a double-lumen balloon catheter--an improvement on the conventional ERC technic]. Z Gastroenterol. (1983) 21(3):134–8. German. PMID: 6868721
14. Shah RJ, Neuhaus H, Parsi M, Reddy DN, Pleskow DK. Randomized study of digital single-operator cholangioscope compared to fiberoptic single-operator cholangioscope in a novel cholangioscopy bench model. Endosc Int Open. (2018) 6(7):E851–6. doi: 10.1055/a-0584-6458
Keywords: biliary disease, per-oral choledochoscope, visual, disposable, superfine
Citation: Hao J, Zhang Y and Huang X (2022) Design and Development of a Disposable Superfine Catheter for Visual Examination of Bile Ducts and Related Animal Experiments. Front. Surg. 9:877040. doi: 10.3389/fsurg.2022.877040
Received: 16 February 2022; Accepted: 11 April 2022;
Published: 2 May 2022.
Edited by:
Mahesh C. Misra, All India Institute of Medical Sciences, IndiaReviewed by:
Krishna Asuri, All India Institute of Medical Sciences, IndiaCopyright © 2022 Hao, Zhang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Yong Hao aGFvamlueW9uZzUxN0AxNjMuY29tXiao-Jun Huang aHVhbmd4akBsenUuZWR1LmNu
Speciality section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.