
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 16 June 2022
Sec. Otorhinolaryngology - Head and Neck Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.876340
Objectives: There is currently no established objective diagnostic indicator for the differentiation of sinus fungal ball (SFB) from unilateral nonfungal chronic sinusitis (UCRS). This study evaluated whether computed tomography (CT) attenuation values relative to those of the brainstem (relative CT number) are useful for differentiating SFB from UCRS.
Materials and Methods: Consecutive patients who were pathologically diagnosed with SFB or UCRS between 2013 and 2021 were retrospectively identified. The relative CT numbers of region of interest (ROIs) within the sinuses were compared between the two patient groups. Factors with predictive power for differentiating SFBs from UCRSs were identified by uni/multivariable logistic regression analyses.
Results: One hundred and eighty-three patients with unilateral chronic sinusitis were finally analyzed (SFB, 86 cases; UCRS, 97 cases). Regardless of the presence or absence of calcified lesions, the relative CT numbers in SFB were significantly higher than those in UCRS. ROIs showing high relative CT numbers were those where fungal hyphae were present. In the uni/multivariable logistic regression analysis, age (p < 0.001), relative CT number (p < 0.001), and calcification (p = 0.002) had predictive value for distinguishing SFB from UCRS. Within those cases not showing calcification, age (p = 0.004) and relative CT number (p < 0.001) were predictive factors for differentiating SFB from UCRS. A relative CT number >1.5 was significantly associated with SFB (sensitivity, 70%; specificity, 91%), with a significantly larger area under the receiver operating characteristics curve than age.
Conclusions: High relative CT numbers within the sinus are strongly associated with the presence of fungal hyphae, and measurement of relative CT number is a powerful adjunctive diagnostic method for distinguishing between SFB and UCRS.
The sinus fungal ball (SFB) is the most frequent form of non-invasive fungal rhinosinusitis and is characterized by accumulation of fungal hyphae within the sinus cavity without microscopic evidence of tissue invasion. SFB has appeared with increasing frequency in recent years (1–3) and was reported in about 10% of patients requiring endoscopic sinus surgery (4, 5). SFB can be eradicated completely with surgical treatment, which usually achieves good outcomes including low recurrence and high symptom-free rates (5–7). However, as host immunity deteriorates, SFB may shift to an invasive form with extension to the sinus mucosa, bone, or blood vessels (8). Therefore, prompt and accurate diagnosis of SFB is critical to avoid unnecessary medical therapy and treatment delays.
Among the currently available diagnostic tools, computed tomography (CT) is the optimal imaging technique for the preoperative detection of SFB. One of the most common CT findings is the presence of intralesional hyperdensity with metal-like calcified materials, and this intralesional hyperdensity is known to be a highly predictive radiological parameter for SFB (4, 9). However, this finding involves a subjective assessment, which may lead to variability in results across evaluators. In addition, calcified lesions are often not detected on CT scans, with 18%–50% of patients with SFB not showing them (1, 2, 10–12). Therefore, in cases where calcification does not occur, it may be difficult to distinguish SFB from unilateral chronic rhinosinusitis (UCRS) on the basis of presurgical CT findings (13). Thus, it would be very useful to have a new objective indicator for the detection of fungal hyphae.
The CT attenuation value represents the radiodensity of a material in respect to water and is expressed in Hounsfield units (14). We previously reported on the utility of CT attenuation values of individual sinonasal tumors relative to those of the brainstem (defined by relative CT number) as a possible tool for differentiating individual tumor types (15). We hypothesized that because of the presence of dense matted fungal hyphae, SFB would show high relative CT numbers in comparison with those of the brainstem. The purpose of this study was to investigate whether relative CT number can be used as an objective adjunct diagnostic indicator for diagnosing SFB, and to verify whether relative CT numbers can predict the presence of fungal hyphae even in patients without calcification. We thus performed a retrospective cohort study on patients with SFB and patients with UCRS.
The current study was approved by the Institutional Review Board of the University of Tokyo Hospital (approval no. 2487). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national committee, and with the Helsinki Declaration and its later amendments or comparable ethical standards.
Consecutive patients who were pathologically diagnosed with SFB or UCRS at the University of Tokyo Hospital between 2013 and 2021 were identified by reviewing the medical database. Recurrent cases and cases in which regions of interest (ROIs) could not be accurately defined on film CT or contrast-enhanced CT were excluded from the analysis. In some cases, MRI was performed to assess internal features. The following demographic and clinical information was also recorded: age, sex, side, disease duration, nasal symptom (NO, nasal obstruction; NB, nasal bleeding; ND, nasal discharge; PND, postnasal discharge; HD, headache), body mass index (BMI), and Brinkman index (BI). Disease duration was defined as the interval from the onset of symptoms to the date of the CT acquisition and was recorded as months. The BI is the number of cigarettes smoked per day multiplied by the number of years of smoking.
The following lesion characteristics were analyzed on unenhanced CT: the size of the region of interest (ROI) within the sinus (sinus ROI, mm2), the size of the brainstem ROI (mm2), CT attenuation values (HU), affected sinus, bony changes, and presence of complete opacification. At least six image slices (for both SFBs and UCRSs) were selected from axial unenhanced CT images, and the mean lesion CT attenuation values were measured using ROI settings. At least six slices with the same slice thickness were selected for measurement of the brainstem values (15). Surrounding bony structures and aerated areas inside the sinuses were excluded from the ROI measurements. It was difficult to accurately distinguish the mucus region from mucosal thickening. Therefore, the sinus ROIs were defined as the sinus areas located at least a few millimeters medial to the sinus bony wall, while taking into account the thickness of the sinus mucosal lining. The full sinus ROIs were defined as the sinus regions along the sub-sinus ostium when the sinuses were completely filled with soft tissue density material (presence of complete opacification; SFB, 50 cases; UCRS, 45 cases, Table 2).
The fungal ROIs were defined as regions of low intensity on T2-weighted images, and were drawn directly on the CT images using the MRI scans as a reference, because the absence of free water within fungal hyphae results in marked hypointensity on MRI while calcifications and paramagnetic substances from fungal organisms can contribute to T2 shortening (8). The mucus ROIs were defined as the sinus regions excluding the area with low (fungal regions) or/and high intensity (mucosal thickening region) on T2-weighted images.
Among 86 patients with SFB, the fungal ROIs and the mucus ROIs were determined in 63 patients using MRI. Among 22 patients without calcification, the fungal ROIs and the mucus ROIs were determined in 17 patients using MRI.
Among 97 patients with UCRS, the mucus ROIs were determined in 37 patients with MRI. Among 79 patients without calcification, the mucus ROIs were determined in 29 patients using MRI.
The relative CT number of an individual affected sinus was calculated by dividing the mean CT attenuation value of the ROIs (sinus ROIs, fungal ROIs, or mucus ROIs) by the mean CT attenuation value of the brainstem (15).
Affected sinuses with soft-tissue density within the sinuses were classified as maxillary sinus (MS), ethmoid sinus (ES), frontal sinus (FS), or sphenoid sinus (SS).
On the basis of previous reports (16–19), two categories of bony changes were examined: bone thickening and bone erosion. These changes were analyzed by two independent otolaryngologists who were blinded to the clinical details. Thickening on CT was defined when the cross-section of the bone surrounding the mass showed areas of thickening in three slices compared with the same area on the contralateral side. Erosion on CT was defined as a focal area of cortical loss with sharply defined margins seen on at least three slices. A sinus that was completely filled with material of soft-tissue density was defined as complete opacification, while a sinus with air inclusions was defined as incomplete opacification.
Continuous and categorical variables are expressed as mean ± standard deviation (SD) and number (%), respectively. Relative CT numbers were compared between SFBs and UCRSs using the Mann–Whitney U test or the Steel-Dwass test. A significance test of non-zero values of the Spearman’s correlation coefficient of the data in Figure 2E was performed.
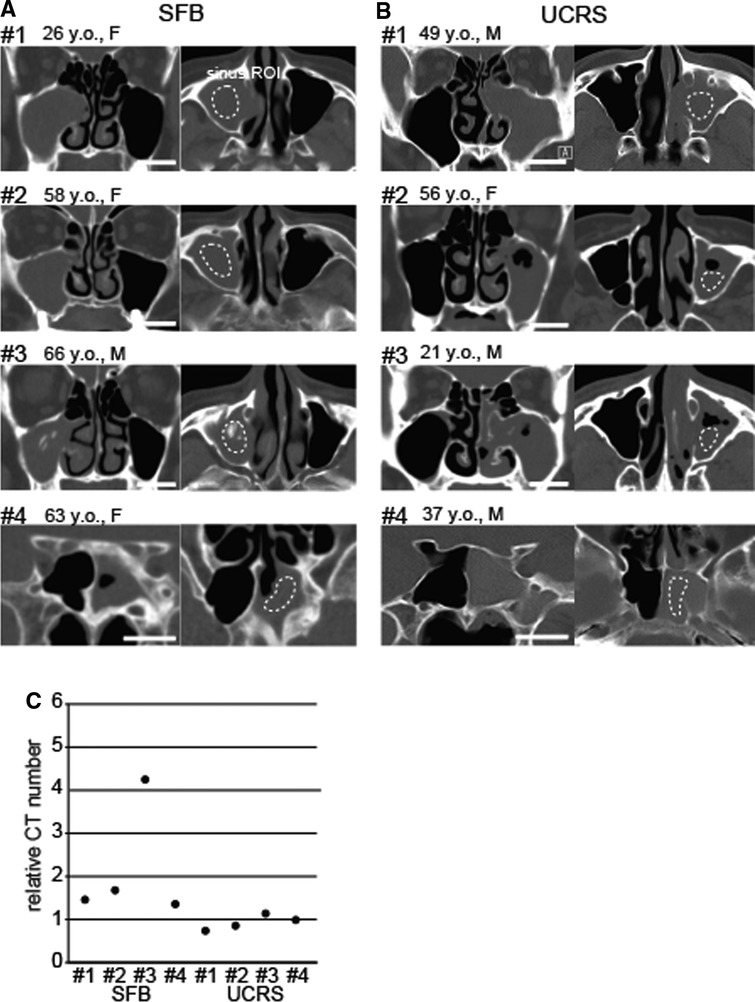
Figure 1. Representative images of sinus fungal ball (SFB) and unilateral nonfungal chronic sinusitis (UCRS), (A) CT images of SFB cases (#1–#4). ROIs were defined in the affected sinus (dotted circle), and the brainstem was used as a control area. ROI, region of interest. Scale bar, 20 mm. (B) CT images of UCRS cases (#1–#4). ROIs were defined in the affected sinus (dotted circle), and the brainstem was used as a control area. Scale bar, 20 mm. (C) Relative CT numbers in SFB and UCRS cases. Each dot represents the relative CT number of an individual case.
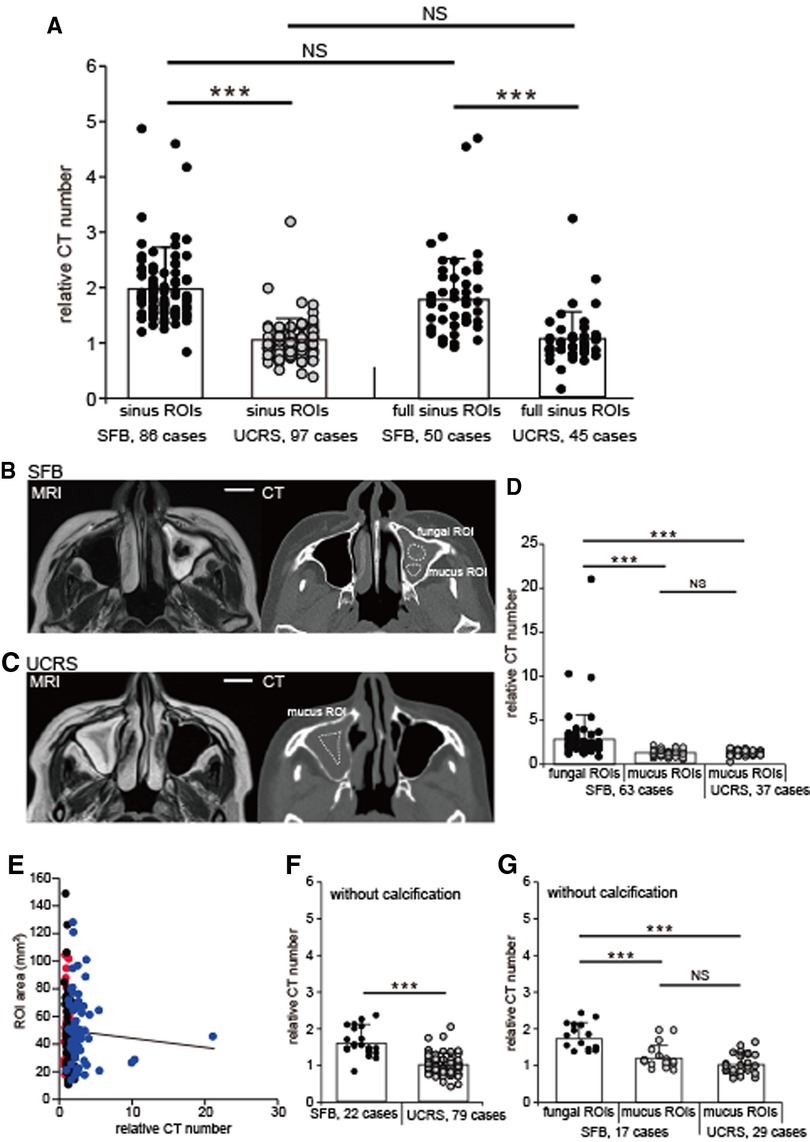
Figure 2. Relative CT numbers of SFB and UCRS cases, (A) Relative CT numbers in the sinus and the full sinus ROIs of SFB and UCRS. Each dot represents the relative CT number of an individual SFB or UCRS. All values are mean ± SD. ***p < 0.001; NS, not significant; Mann–Whitney U test. (B) CT and MRI images of an SFB case. The fungal ROIs were defined as areas with low intensity on T2-weighted images, and were drawn directly on the CT image using the MRI scans as a reference. The mucus ROIs were defined as the sinus ROIs excluding the area with low intensity on T2-weighted images. ROI, region of interest. Scale bar, 20 mm. (C) CT and MRI images of an UCRS case. The mucus ROIs were defined as the sinus ROIs excluding the area with high intensity (mucosal thickening region) on T2-weighted images. ROI, region of interest. Scale bar, 20 mm. (D) Relative CT numbers in fungal and mucus ROIs. Each dot represents the relative CT number of an individual fungal or mucus ROI in SFBs (63 cases) or mucus ROI in UCRSs (37 cases). All values are mean ± SD. ***p < 0.001, Steel-Dwass test. NS, not significant. (E) Correlation between the ROI area and the relative CT numbers. Red, mucus ROIs (UCRS); blue, mucus ROIs (SFB); black, fungal ROIs. (F) Relative CT numbers in SFB and UCRS without calcification. Each dot represents the relative CT number of an individual SFB (22 cases) or UCRS (79 cases) without calcification. All values are mean ± SD. Mann–Whitney U test. NS, not significant. (G) Relative CT numbers in fungal and mucus ROIs without calcification. Each dot represents the relative CT number of an individual fungal or mucus ROI in SFBs (17 cases) or mucus ROI in UCRSs (29 cases) without calcification. All values are mean ± SD. ***p < 0.001, Steel-Dwass test. NS, not significant.
The areas under the receiver operating characteristics curves for relative CT number and age were compared using the χ-square test. Univariable and multivariable logistic regression analyses were performed to identify the factors predictive of disease type (the dependent variable; SFB, 1; UCRS, 0), with the potential predictive factors being age, sex, side, disease duration, nasal symptom (NO, NB, ND, PND, and HD), affected sinus (MS, ES, FS, and SS), relative CT number, sinus ROI size (mm2), bony changes (calcification, bone thickening, bone erosion, and complete opacification), BMI, and BI. Associations between predictive and dependent variables are expressed as odds ratios and their 95% confidence intervals (CIs). Variables were included in the multivariable logistic regression analysis if their univariable p-value was ≤0.05. A p-value of <0.05 was considered statistically significant.
Of 379 patients who underwent unilateral endoscopic sinus surgery, 209 patients with unilateral chronic sinusitis were identified, and 170 patients were excluded from the analysis (tumor, 108 patients; cyst, 49 patients; antrochoanal polyp, six patients; invasive sinus mycosis with bone destruction, two patients; others, five patients). Of the 209 patients with unilateral chronic sinusitis, 26 were excluded because of the following reasons: CT results were unusable because they were only available on film (nine patients); CT results were for recurrent cases (seven patients); CT scans were performed with intravenous contrast enhancement without an unenhanced scan being acquired (five patients); or the ROI could not be determined because of a narrow range of soft-tissue density (five patients). The remaining 183 patients were included in the study (Table 1) and consisted of 86 patients with SFB and 97 patients with UCRS. Among the 86 patients with SFB, 53 were confirmed to have Aspergillus-based SFB according to pathological findings. In the remaining patients, the fungal species could not be identified.
Table 1 lists the demographic and clinical information (age, sex, side, disease duration, nasal symptom, BMI, and BI) of the 183 study patients, and Table 2 summarizes their CT findings (affected sinus, calcification, bone thickness, bone erosion, and complete opacification).
Representative images of four SFB and four UCRS cases are shown in Figures 1A,B. Sinus ROIs were selected from areas within the affected sinus that showed soft-tissue density on non-contrast-enhanced CT. The relative CT numbers for the sinus ROI in each case are shown in Figure 1C (SFB, case #1: 1.5, case #2: 1.7, case #3: 4.2, case #4: 1.3; UCRS, case #1: 0.8, case #2: 0.9, case #3: 1.1, case #4: 1.0).
The relative CT numbers of the affected sinuses are plotted in Figure 2A for all SFBs (86 cases) and all UCRSs (97 cases).
The relative CT numbers of the patients with SFB were significantly higher than those with UCRS (SFBs, 2.0 ± 0.7; UCRS, 1.1 ± 0.4; Figure 2A; Mann–Whitney test, p < 0.001). However, the relative CT number may be affected by the ROI settings. Therefore, in addition to the sinus ROIs, the full sinus ROIs were defined and the influence of both ROIs on the relative CT numbers was investigated. In terms of the full sinus ROIs, the relative CT numbers in SFB were significantly higher than those in UCRS (SFBs, 1.9 ± 1.1; UCRS, 1.0 ± 0.5; Mann–Whitney test, p < 0.001; Figure 2A). Furthermore, there were no significant differences in the relative CT numbers between the sinus ROIs and the full sinus ROIs in patients with SFBs and UCRSs (sinus ROIs in SFB vs. full sinus ROIs in SFB, p = 0.19; sinus ROIs in UCRS vs. full sinus ROIs in UCRS; Mann–Whitney test, p = 0.07; Figure 2A). These results suggest that, regardless of the ROI enclosure, the relative CT numbers of the patients with SFB were significantly higher than those with UCRS.
To investigate whether the high relative CT numbers were caused by fungal hyphae inside the sinus, the relative CT numbers were calculated separately for fungal and mucus ROIs (Figures 2B,C). In patients with an SFB, the relative CT numbers of the fungal ROIs were significantly higher than those of the mucus ROIs (fungal ROIs, 2.9 ± 2.9; mucus ROIs, 1.2 ± 0.3; Steel-Dwass test, p < 0.001; Figure 2D), and were also significantly higher than the relative CT numbers of mucus ROIs in patients with UCRSs (1.1 ± 0.4; Steel-Dwass test, p < 0.001; Figure 2D). However, no significant difference was detected in the relative CT numbers of the mucus ROIs of patients with SFB in comparison with patients with UCRS (Steel-Dwass test, p = 0.94; Figure 2D). These results indicate that ROIs with high relative CT numbers coincided with regions where fungal hyphae were likely to be localized. To rule out the possibility that differences in the ROI area affected the relative CT numbers, the correlations between the area of each ROI and relative CT numbers were examined for the sinus ROIs, fungal ROIs, and mucus ROIs. The results showed no significant correlation between the area of each ROI and relative CT numbers (Spearman’s rank correlation coefficient, r = 0.04; p = 0.84; Figure 2E). Therefore, the relative CT numbers depend on the internal properties within the ROIs, not on ROI area.
We next investigated whether the relative CT number is a meaningful parameter for detecting SFB without calcification. The relative CT numbers of the sinus in cases without calcification (SFB, 22 cases; UCRS, 79 cases) are shown in Figure 2F. The relative CT numbers in SFB without calcification were significantly higher than those in UCRS without calcification (SFB, 1.6 ± 0.4; UCRS, 1.0 ± 0.3, Mann–Whitney test, p < 0.001; Figure 2F). Furthermore, in patients with SFB, fungal ROIs without calcification showed significantly higher relative CT values than mucus ROIs (fungal ROIs, 1.8 ± 0.4; mucus ROIs, 1.2 ± 0.3; Steel-Dwass test, p < 0.001; Figure 2G), as well as significantly higher relative CT values than mucus ROIs in UCRS without calcification (UCRS mucus ROIs, 1.0 ± 0.3; Steel-Dwass test, p < 0.001; Figure 2G). However, no significant difference was detected between the relative CT numbers of mucus ROIs in SFB without calcification and mucus ROIs in UCRS without calcification (Steel-Dwass test, p = 0.95, Figure 2G). These results suggest that measurement of relative CT numbers could be useful for detecting fungal hyphae, even in cases without calcification.
We next examined the predictive factors for distinguishing between SFB and UCRS by conducting univariable logistic regression analysis to select appropriate parameters, followed by a multivariable logistic regression analysis. In the univariable analysis, age, sex, nasal symptom (ND), affected sinus (MS, ES, FS), relative CT number, and calcification had predictive value for differentiating between SFB and UCRS (age: OR, 1.07, 95% CI, 1.05–1.1; sex: OR, 0.43, 95% CI, 0.24–0.79; ND: OR, 0.39, 95% CI, 0.21–0.71; MS: OR, 0.28, 95% CI, 0.09–0.81; ES: OR, 0.5, 95% CI, 0.28–0.91; FS: OR, 0.24, 95% CI, 0.09–0.59; relative CT number: OR, 33.78, 95% CI, 11.64–98.01; calcification: OR, 12.77, 95% CI, 6.31–25.83; Table 3). In the multivariable logistic regression analysis including the factors of age, sex, nasal symptom (ND), affected sinus (MS, ES, and FS), relative CT number, and calcification, SFB was significantly associated with old age, high relative CT number, and the presence of calcification (age: OR, 1.07, 95% CI, 1.03–1.1, p < 0.001; relative CT number: OR, 17.88, 95% CI, 5.4–59.2, p < 0.001; calcification: OR, 5.18, 95% CI, 1.87–14.32, p = 0.002; Table 3).
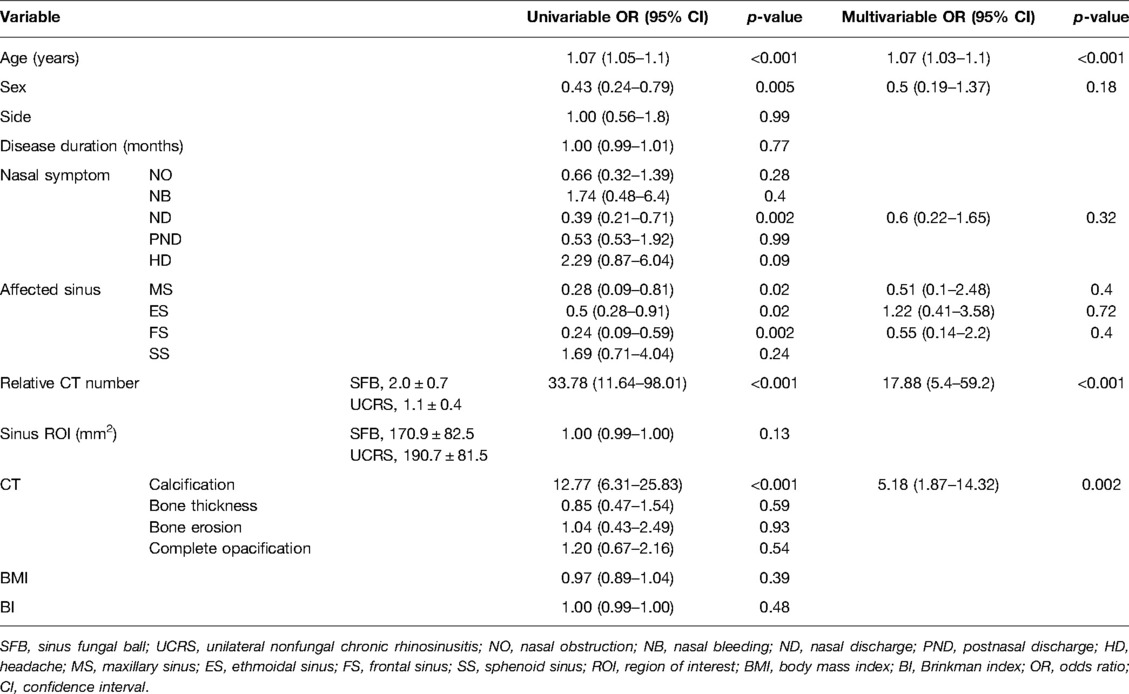
Table 3. Univariable and multivariable analyses of factors predictive of SFB (SFB, 86 cases; UCRS, 97 cases; N = 183).
We further examined the predictive factors for distinguishing between SFB and UCRS in cases without calcification by conducting univariable logistic regression analysis to select appropriate parameters, followed by a multivariable logistic regression analysis. In the univariable analysis, age, sex, affected sinus (MS and SS), and relative CT number had predictive value for differentiating between SFB and UCRS (age: OR, 1.06, 95% CI, 1.02–1.11; sex: OR, 0.37, 95% CI, 0.14–0.98; MS: OR, 0.09, 95% CI, 0.02–0.35; SS: OR, 4.14, 95% CI, 1.3–13.18; relative CT number: OR, 15.58, 95% CI, 4.28–56.67; Table 4). In the multivariable logistic regression analysis including the factors of age, sex, affected sinus (MS and SS), and relative CT number, SFB was significantly associated with old age and high relative CT numbers (age: OR, 1.08, 95% CI, 1.02–1.14, p = 0.004; relative CT number: OR, 17.58, 95% CI, 3.66–84.34, p < 0.001; Table 4).
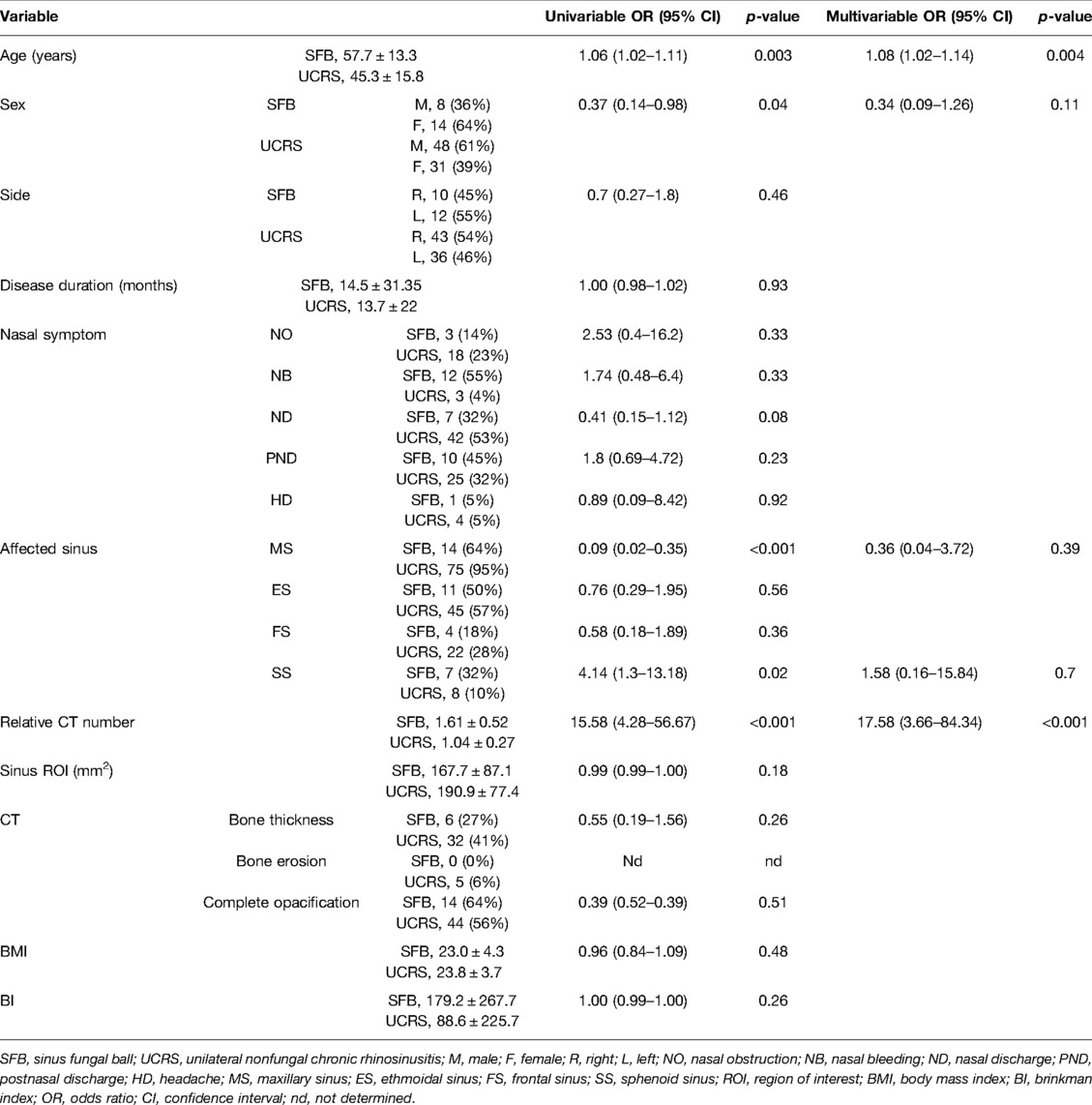
Table 4. Univariable and multivariable analyses of factors predictive of SFB without calcification (SFB, 22 cases; UCRS, 79 cases; N = 101).
Receiver operating characteristics analysis was performed to evaluate the accuracy of a statistical model. The areas under the receiver operating characteristic curve (AUC) for relative CT number and age were 0.88 (relative CT number, 95% CI, 0.84–0.94; p < 0.001) and 0.77 (age, 95% CI, 0.7–0.84; p < 0.001), respectively. The AUC for relative CT number was significantly larger than that for aging (age vs. relative CT number, 95% CI, 0.03–0.21; χ-square test, p = 0.008, Figure 3A). These results suggest that the measurement of relative CT number is a more accurate diagnostic method for detecting fungal hyphae than patient age. From the AUC, the optimum relative CT number cut-off value for determining SFB was calculated as 1.5 (AUC, 0.88; OR, 22.6; sensitivity, 70%; specificity, 91%; Figure 3A); most patients with a relative CT number of less than 1.5 had UCRS, while most of those with a value greater than or equal to 1.5 had SFB (Figure 3B, χ2 test, p < 0.001). These results suggest that the relative CT number is a powerful and objective diagnostic tool for differentiating SFB from UCRS.
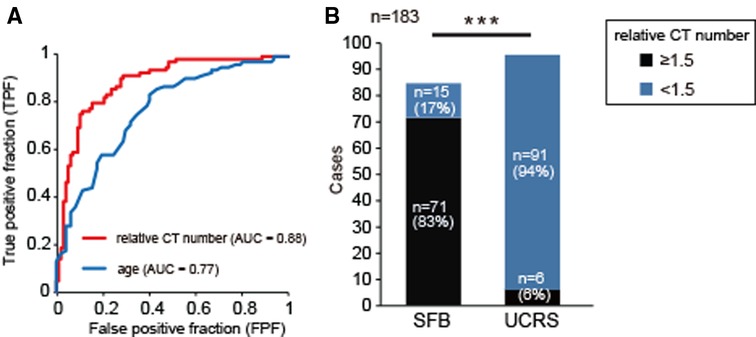
Figure 3. Receiver operator characteristics (ROC) curve analysis for relative CT number and age, (A) ROC curves for application of the classification modeling to the dataset. The curve was created by plotting the true positive fraction (TPF, y-axis; the sensitivity) against the false positive fraction (FPF, x-axis; the specificity) at various threshold settings. The red line indicates the ROC curve for relative CT number, and the blue line indicates the curve for age. The area under the ROC curve for relative CT number was significantly greater than that for age (age vs. relative CT number, 95% CI, 0.03–0.21; χ-square test, p = 0.008). (B) Comparison of relative CT numbers between SFB and UCRS. A relative CT number greater than or equal to 1.5 was significantly associated with the detection of SFB. Chi-square test, n = 183, ***p < 0.001.
SFB is an extramucosal intrasinusal mycosis, which usually occurs as a unilateral lesion in immunocompetent people (20). Univariate analyses have revealed SFB to be more common in elderly and female patients (21–23). Although both factors were significant predictors in our univariable analysis, it is noteworthy that only old age was a significant factor in the multivariable analysis, and old age can therefore be considered a patient characteristic for differentiating SFB from UCRS.
The preoperative diagnosis of unilateral sinusitis can usually be made according to the clinical symptomatology and radiological findings. Nasal obstruction and unilateral nasal discharge are the most frequent complaints (24), although the nasal symptoms of SFB are nonspecific and similar to those of chronic sinusitis because they depend strongly on the location and subsequent involvement of surrounding structures (23, 25, 26).
Various radiological diagnostic signs for SFB are helpful in assessment and clinical decision making. Previous studies have reported well-verified CT features of SFB, including the presence of calcification, complete opacification, and bone thickening (27). In the present study, no bony changes on CT were found to be significant factors in the univariate analysis. The sinus wall is not a static structure: it responds to stimuli such as mechanical stress and inflammation by altering and repairing its structure through a process referred to as remodeling (28). Because most fungal hyphae have little or no invasive ability, it has been speculated that bony changes in SFB are related to the duration of active inflammation within the affected sinus (25, 28). In our analysis, we did not find a significant difference in disease duration between SFB and UCRS, which may be why we did not find any significant difference in the frequency of bony changes between the two diseases.
Our multivariable analysis revealed sinus calcification on CT to be a factor significantly associated with SFB. This finding could represent the presence of heavy metals (iron, zinc, and manganese) and calcium within fungal hyphae, and is considered to be a reliable diagnostic feature of SFB (29, 30). However, the presence of calcified fungal hyphae might not be confirmed in some cases. Aspergillus, which is the organism most commonly involved in sinus SFBs (31), stores metal ions such as zinc in the intracellular vacuole storage system, with the zinc ion known to be an essential nutrient for the organism (32). However, down regulation of the genes related to zinc storage occasionally occurs, and under such conditions, the zinc concentration in the vacuole decreases, which may be reflected by the absence of calcification (33). In agreement with previous reports (1, 2, 10–12), we could not detect calcification within the affected sinus in 26% of the patients with SFB. Other than sinus calcification, objective CT-based indicators strongly predicting the presence of fungal hyphae have not been established.
On MRI, a marked hypointensity on T2-weighted imaging (T2WI) is a significant predictor of SFB (6). However, in clinical practice, marked hypointensity lesions on T2WI are also observed in intra-sinonasal desiccated secretions, air, and even acute clotted hemorrhage (2, 34). Furthermore, because of the high cost of MRI, MRI examinations are not usually performed on all patients. Therefore, it would be clinically meaningful to find a new CT-based diagnostic indicator to detect fungal hyphae.
The CT attenuation values assigned to a voxel represent the average linear attenuation coefficient of that voxel relative to water. They are primarily influenced by the chemical composition of the tissue and organ (e.g., −1,000 HU for air, 0 HU for water, and 1,000 HU for bone) (14). However, CT attenuation values can be affected by the settings used for image reconstruction. In previous studies, we established a method for measuring relative CT numbers in relation to brainstem values, and reported the usefulness of the measurement for the differential diagnosis of individual tumors (15, 19, 35). The measurement of relative CT numbers can be applied to inflammatory sinus diseases, and the evaluation of sinus ROIs can reveal the characteristics of the internal properties of sinuses. In SFB, relative CT numbers could be used to evaluate the concentration of heavy metals inside the affected sinus, which would help to diagnose the presence of fungal hyphae.
Our diagnostic process for SFB on CT involves measuring the relative CT number and checking for unilateral opacification of the sinus with calcification. If the relative CT number is greater than 1.5, or if sinus calcification is present, then SFB can be suspected. Indeed, in elderly patients without sinus calcification, high relative CT numbers may be the only basis for strongly suspecting SFB.
The ability to define relative CT numbers in individual sinus diseases may provide meaningful information for differentiating neoplastic from inflammatory lesions within the nasal cavity. For example, the mean relative CT number of inverted papilloma is reported to be 1.3 (35), whereas that of SFB is considerably higher at 2.0. If specific relative CT numbers can be defined for various sinus diseases, it might be possible to broadly classify sinus diseases according to the differences in these relative CT numbers.
The current study has several limitations. First, it is of a retrospective cohort design and is subject to the inherent biases that come with retrospective studies. Second, our study was performed on a limited number of SFB cases, particularly SFB without calcification, and all patients presented at a single institution. Therefore, the generalizability of our findings to other settings is unclear. Third, further studies are needed to determine cut-off values for relative CT numbers if they are to be used for differentiation of the diseases in larger numbers of patients. Nevertheless, this is the first study to show that relative CT numbers can provide useful information for the radiological diagnosis of SFB.
In the present study, we used univariable and multivariable regression analyses to determine the predictive factors for differentiating between SFB and UCRS. The relative CT numbers of SFB were significantly higher than those of UCRS, not only in the analysis of the fungal ROIs but also in the analysis of sinus ROIs (ROIs encompassing the fungus and the mucus). In the multivariable analysis, age, relative CT number, and calcification had predictive value for distinguishing SFB from UCRS. In patients not showing calcification, age and a relative CT number >1.5 were significantly associated with SFB, with relative CT number showing a significantly larger area under the receiver operating characteristics curve than patient age. These results suggest that the measurement of relative CT number can be a useful non-invasive diagnostic tool for the differentiation of SFB from UCRS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the University of Tokyo Hospital (approval no. 2,487). The ethics committee waived the requirement of written informed consent for participation.
SK designed the studies. SK and BH analyzed the data. SK, BH, SY, HN, KK, and TY gave technical support and conceptual advice. SK wrote the manuscript. SK and TY revised and finalized the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by a grant-in-aid for scientific research (C) 17K11354 of the Japan Society for Promotion of Science (SK), the Takeda Science Foundation (SK), and the Cell Science Research Foundation (SK).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yoon YH, Xu J, Park SK, Heo JH, Kim YM, Rha KS. A retrospective analysis of 538 sinonasal fungus ball cases treated at a single tertiary medical center in Korea (1996–2015). Int Forum Allergy Rhinol. (2017) 7(11):1070–5. doi: 10.1002/alr.22007
2. Seo YJ, Kim J, Kim K, Lee JG, Kim CH, Yoon JH. Radiologic characteristics of sinonasal fungus ball: an analysis of 119 cases. Acta Radiol. (2011) 52(7):790–5. doi: 10.1258/ar.2011.110021
3. Kim JS, So SS, Kwon SH. The increasing incidence of paranasal sinus fungus ball: a retrospective cohort study in two hundred forty-five patients for fifteen years. Clin Otolaryngol. (2017) 42(1):175–9. doi: 10.1111/coa.12588
4. Grosjean P, Weber R. Fungus balls of the paranasal sinuses: a review. Eur Arch Otorhinolaryngol. (2007) 264(5):461–70. doi: 10.1007/s00405-007-0281-5
5. Nicolai P, Lombardi D, Tomenzoli D, Villaret AB, Piccioni M, Mensi M, et al. Fungus ball of the paranasal sinuses: experience in 160 patients treated with endoscopic surgery. Laryngoscope. (2009) 119(11):2275–9. doi: 10.1002/lary.20578
6. Aribandi M, McCoy VA, Bazan C 3rd. Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiographics. (2007) 27(5):1283–96. doi: 10.1148/rg.275065189
7. Ledderose GJ, Braun T, Betz CS, Stelter K, Leunig A. Functional endoscopic surgery of paranasal fungus ball: clinical outcome, patient benefit and health-related quality of life. Eur Arch Otorhinolaryngol. (2012) 269(10):2203–8. doi: 10.1007/s00405-012-1925-7
8. Ferguson BJ. Fungus balls of the paranasal sinuses. Otolaryngol Clin North Am. (2000) 33(2):389–98. doi: 10.1016/s0030-6665(00)80013-4
9. deShazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Swain R, Lyons M, et al. Criteria for the diagnosis of sinus mycetoma. J Allergy Clin Immunol. (1997) 99(4):475–85. doi: 10.1016/s0091-6749(97)70073-3
10. Tomazic PV, Dostal E, Magyar M, Lang-Loidolt D, Wolf A, Koele W, et al. Potential correlations of dentogenic factors to the development of clinically verified fungus balls: a retrospective computed tomography-based analysis. Laryngoscope. (2016) 126(1):39–43. doi: 10.1002/lary.25416
11. Chen JC, Ho CY. The significance of computed tomographic findings in the diagnosis of fungus ball in the paranasal sinuses. Am J Rhinol Allergy. (2012) 26(2):117–9. doi: 10.2500/ajra.2012.26.3707
12. Ho CF, Lee TJ, Wu PW, Huang CC, Chang PH, Huang YL, et al. Diagnosis of a maxillary sinus fungus ball without intralesional hyperdensity on computed tomography. Laryngoscope. (2019) 129(5):1041–5. doi: 10.1002/lary.27670
13. Lee DH, Yoon TM, Lee JK, Lim SC. Computed tomography-based differential diagnosis of fungus balls in the maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol. (2020) 129(3):277–81. doi: 10.1016/j.oooo.2019.08.008
14. Birnbaum BA, Hindman N, Lee J, Babb JS. Multi-detector row CT attenuation measurements: assessment of intra- and interscanner variability with an anthropomorphic body CT phantom. Radiology. (2007) 242(1):109–19. doi: 10.1148/radiol.2421052066
15. Nakada T, Kikuta S, Mori H, Shimizu Y, Nishijima H, Kondo K, et al. Low CT attenuation values of sinonasal benign tumours relative to the brainstem identify schwannomas. ORL J Otorhinolaryngol Relat Spec. (2018) 80(1):41–50. doi: 10.1159/000487240
16. Momose KJ, Weber AL, Goodman M, MacMillan AS Jr, Roberson GH. Radiological aspects of inverted papilloma. Radiology. (1980) 134(1):73–9. doi: 10.1148/radiology.134.1.7350638
17. Head CS, Sercarz JA, Luu Q, Collins J, Blackwell KE. Radiographic assessment of inverted papilloma. Acta Otolaryngol. (2007) 127(5):515–20. doi: 10.1080/00016480600895144
18. Vrabec DP. The inverted Schneiderian papilloma: a clinical and pathological study. Laryngoscope. (1975) 85(1):186–220. doi: 10.1288/00005537-197501000-00014
19. Sano N, Kikuta S, Kondo K, Yamasoba T. High CT values relative to the brainstem differentiate inverted papillomas from nasal polyps. Auris Nasus Larynx . (2021) 48(5):905–13. doi: 10.1016/j.anl.2021.02.011
20. Pagella F, Matti E, De Bernardi F, Semino L, Cavanna C, Marone P, et al. Paranasal sinus fungus ball: diagnosis and management. Mycoses. (2007) 50(6):451–6. doi: 10.1111/j.1439-0507.2007.01416.x
21. Nomura K, Asaka D, Nakayama T, Okushi T, Matsuwaki Y, Yoshimura T, et al. Sinus fungus ball in the Japanese population: clinical and imaging characteristics of 104 cases. Int J Otolaryngol. (2013) 2013:731640. doi: 10.1155/2013/731640
22. Jiang RS, Huang WC, Liang KL. Characteristics of sinus fungus ball: a unique form of rhinosinusitis. Clin Med Insights Ear Nose Throat. (2018) 11:1179550618792254. doi: 10.1177/1179550618792254
23. Dufour X, Kauffmann-Lacroix C, Ferrie JC, Goujon JM, Rodier MH, Klossek JM. Paranasal sinus fungus ball: epidemiology, clinical features and diagnosis. A retrospective analysis of 173 cases from a single medical center in France, 1989–2002. Med Mycol. (2006) 44(1):61–7. doi: 10.1080/13693780500235728
24. Paz Silva M, Pinto JM, Corey JP, Mhoon EE, Baroody FM, Naclerio RM. Diagnostic algorithm for unilateral sinus disease: a 15-year retrospective review. Int Forum Allergy Rhinol. (2015) 5(7):590–6. doi: 10.1002/alr.21526
25. Cha H, Song Y, Bae YJ, Won TB, Kim JW, Cho SW, et al. Clinical characteristics other than intralesional hyperdensity may increase the preoperative diagnostic accuracy of maxillary sinus fungal ball. Clin Exp Otorhinolaryngol. (2020) 13(2):157–63. doi: 10.21053/ceo.2019.00836
26. Butler RT, Patel RM, McHugh JB. Head and neck schwannomas: 20-year experience of a single institution excluding cutaneous and acoustic sites. Head Neck Pathol. (2016) 10(3):286–91. doi: 10.1007/s12105-016-0680-2.26747460
27. Dhong HJ, Jung JY, Park JH. Diagnostic accuracy in sinus fungus balls: CT scan and operative findings. Am J Rhinol. (2000) 14(4):227–31. doi: 10.2500/105065800779954446
28. Ma L, Xu R, Shi J, Zhou W, Xu G, Jiang G, et al. Identification of fungi in fungal ball sinusitis: comparison between MUC5B immunohistochemical and Grocott methenamine silver staining. Acta Otolaryngol. (2013) 133(11):1181–7. doi: 10.3109/00016489.2013.814156
29. Jiang Z, Zhang K, Huang W, Yuan Q. A preliminary study on sinus fungus ball with MicroCT and X-ray fluorescence technique. PLoS One. (2016) 11(3):e0148515. doi: 10.1371/journal.pone.0148515
30. Nicolai P, Mensi M, Marsili F, Piccioni M, Salgarello S, Gilberti E, et al. Maxillary fungus ball: zinc-oxide endodontic materials as a risk factor. Acta Otorhinolaryngol Ital. (2015) 35(2):93–6.26019392
31. Willinger B, Obradovic A, Selitsch B, Beck-Mannagetta J, Buzina W, Braun H, et al. Detection and identification of fungi from fungus balls of the maxillary sinus by molecular techniques. J Clin Microbiol. (2003) 41(2):581–5. doi: 10.1128/jcm.41.2.581-585.2003
32. Wilson D, Citiulo F, Hube B. Zinc exploitation by pathogenic fungi. PLoS Pathog. (2012) 8(12):e1003034. doi: 10.1371/journal.ppat.1003034
33. Eide DJ. Homeostatic and adaptive responses to zinc deficiency in Saccharomyces cerevisiae. J Biol Chem. (2009) 284(28):18565–9. doi: 10.1074/jbc.R900014200
34. Som PM, Dillon WP, Curtin HD, Fullerton GD, Lidov M. Hypointense paranasal sinus foci: differential diagnosis with MR imaging and relation to CT findings. Radiology. (1990) 176(3):777–81. doi: 10.1148/radiology.176.3.2389036
Keywords: sinus fungal ball, unilateral chronic sinusitis, computed tomography, diagnosis, brainstem
Citation: Kikuta S, Han B, Yoshihara S, Nishijima H, Kondo K and Yamasoba T (2022) High CT Attenuation Values Relative to the Brainstem Predict Fungal Hyphae Within the Sinus. Front. Surg. 9:876340. doi: 10.3389/fsurg.2022.876340
Received: 15 February 2022; Accepted: 30 May 2022;
Published: 16 June 2022.
Edited by:
Naif H. Alotaibi, King Faisal Specialist Hospital & Research Centre, Saudi ArabiaCopyright © 2022 Kikuta, Han, Yoshihara, Nishijima, Kondo and Yamasoba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Kikuta c2gta2lrdUBtLnUtdG9reW8uYWMuanA=
Specialty section: This article was submitted to Otorhinolaryngology - Head and Neck Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.