- Department of Gastrointestinal Surgery, General Surgery, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
Objectives: To compare the short- and long-term outcomes of totally laparoscopic gastrectomy (TLG) with laparoscopic-assisted gastrectomy (LAG) in gastric cancer (GC) patients and evaluate the efficacy and safety of TLG.
Methods: This retrospective study was based on GC patients who underwent laparoscopic radical gastrectomy in the Qilu Hospital from January 2017 to December 2020. The groups’ variables were balanced by using the propensity score-based inverse probability of treatment weighting (PS-IPTW). The primary outcomes were 3-year relapse-free survival (RFS) and 3-year overall survival (OS). Postoperative recovery and complications were the secondary outcomes.
Results: A total of 250 GC patients were included in the study. There were no significant differences in baseline and pathological features between the TLG and the LAG groups after the PS-IPTW. TLG took around 30 min longer than LAG, while there were more lymph nodes obtained and less blood loss throughout the procedure. TLG patients had less wound discomfort than LAG patients in terms of short-term prognosis. There were no significant differences between groups in the 3-year RFS rate [LAG vs. TLG: 78.86% vs. 78.00%; hazard ratio (HR) = 1.14, 95% confidence interval (CI), 0.55–2.35; p = 0.721] and the 3-year OS rate (LAG vs. TLG: 78.17% vs. 81.48%; HR = 0.98, 95% CI, 0.42–2.27; p = 0.955). The lymph node staging was found to be an independent risk factor for tumor recurrence and mortality in GC patients with laparoscopic surgery. The subgroup analysis revealed similar results of longer operation time, less blood loss, and wound discomfort in totally laparoscopic distal gastrectomy, while the totally laparoscopic total gastrectomy showed benefit only in terms of blood loss.
Conclusion: TLG is effective and safe in terms of short- and long-term outcomes, with well-obtained lymph nodes, decreased intraoperative blood loss, and postoperative wound discomfort, which may be utilized as an alternative to LAG.
Introduction
Gastric cancer is one of the most common and deadly cancers in the world, particularly in East Asia (1). Radical gastrectomy is indispensable for resectable gastric cancer (2, 3). Since Kitano et al. (4) reported the first case of laparoscopic-assisted distal gastrectomy (LADG) in 1994, laparoscopic gastrectomy has developed rapidly and been widely used.
Laparoscopic gastrectomy has two main surgical types: laparoscopic-assisted gastrectomy (LAG) and totally laparoscopic gastrectomy (TLG). The most typical procedure is LAG, which means the stomach and lymph node dissection is performed under laparoscopy, while the stomach resection and anastomosis are performed externally assisted by a 5- to 8-cm abdominal incision. Many studies have shown that there is no significant difference between LAG and open gastrectomy in the long-term prognosis for early or advanced gastric cancer (5–7). Because of the benefits of a smaller incision, less discomfort, and a speedy recovery, laparoscopic gastrectomy has increasingly become a mainstream treatment for gastric cancer (8, 9). In TLG, gastrectomy, lymph node dissection, and gastrointestinal reconstruction are performed under the laparoscopic vision, finally through an approximately 3-cm abdominal incision to take out the resection specimen. TLG eliminates the need for a large abdominal incision and provides apparent benefits in terms of exposure and anatomy (10). However, due to the lack of tactile input and the surgeon’s greater technical requirements, TLG finds it difficult to precisely define the tumor’s border and intracorporeal anastomosis.
Improvements in laparoscopic equipment, gastrointestinal reconstruction methods, and lymph node tracking technologies such as carbon nanoparticle (11) or indocyanine green (ICG) tracer-guided technologies (12, 13) are ushering in a new age of minimally invasive surgery. TLG and new intracorporeal anastomosis have attracted increased attention from scholars (14–16). However, there is currently a paucity of large-scale clinical trials to demonstrate TLG’s safety and efficacy, and the short- and long-term effects require additional medical proof to be proven.
In this study, we retrospectively analyzed TLG and LAG in gastric cancer patients by using the method of the propensity score-based inverse probability of treatment weighting (PS-IPTW) to eliminate the groups’ differences and then evaluating the short- and long-term prognoses to access the safety and effectiveness of TLG.
Methods
Patients
This study was based on gastric cancer patients who received laparoscopic radical gastrectomy in the department of gastrointestinal surgery of the Qilu Hospital from January 2017 to December 2020. The follow-up procedures mainly depended on the hospital’s record system and the telephone. The inclusion criteria were as follows: pathological diagnosis as gastric cancer, no history of other malignancies, and surgical methods of distal or total gastrectomy. The exclusion criteria were as follows: neoadjuvant chemotherapy, palliative surgery, distant metastasis, operation converted to laparotomy, and incomplete clinical data. The data of all patients were approved by the Ethical Review Committee of Qilu Hospital.
Surgical Quality Control
To determine the boundary of the tumor, all TLG patients were endoscopically injected carbon nanoparticles or ICG suspension into the submucosal layer around the tumor 1 day before surgery by the same team of endoscopists. The carbon nanoparticle suspension was 0.5 mm per injection. ICG was prepared with 1.25 mg/ml sterile water and 0.5 milliliters per injection (17). All surgeries were performed by the same surgical team that had previously conducted more than 200 laparotomy and laparoscopic gastric cancer surgeries. According to the Japanese Gastric Cancer Treatment Guidelines (18), all surgeries were performed by radical gastrectomy with D2 lymph node dissection.
The patient was placed in the supine position and given general anesthesia. A subumbilical port was created and used to produce pneumoperitoneum (12–15 mmHg) (1 mmHg = 0.133 KPa). A five-port approach was used for the Trocar position. By utilizing laparoscopic exploration, the gastric resection range and digestive tract rebuilding could be determined. The upper margin should be kept at least 3–5 cm away from the cancer’s edge, while the esophageal junction cancer should be kept as far away from the cancer as feasible when enough room is conserved for esophagojejunal anastomosis, and fast-frozen pathology should be conducted when necessary.
For totally laparoscopic distal gastrectomy (TLDG), the reconstruct method was Billroth-II with Braun anastomosis. After the dissection of gastric lymph nodes, the duodenum and the distal stomach were separated with a linear closure device. A small hole was formed on the greater curvature side of the remnant stomach and on the antimesenteric border of the jejunum, 15–20 cm from the Treitz ligament. Then, a side-to-side gastrojejunostomy was performed by using a linear stapler. The entry hole for the stapler was also closed by stapling, and the anastomosis was continuously reinforced with absorbable sutures. The Braun anastomosis was performed between the input and the output loops of jejunum at 10–15 cm from the gastrojejunum anastomosis with a linear stapler. The resection specimen was put in the endobag and extracted through a small periumbilical incision.
For totally laparoscopic total gastrectomy (TLTG), the reconstruct method was the reverse puncture device reconstruction (19, 20). Following lymph node dissection, duodenum separation, and abdominal esophagus dissociation, a small hole was made on the anterior wall of the esophagus and then a small incision was made in the upper abdomen to enter the abdominal cavity. The anvil of the esophageal stump was inserted into the residual end of the esophagus, tightened, and ligated with a purse string. The linear stapler was used to close the esophagus under the anvil. The main body of the tubular stapler was placed in the distal end of the jejunum. The pneumoperitoneum was then re-established, the tubular stapler was inserted into the distal jejunum, and the central rod was connected with the anvil after penetrating the intestinal wall to complete the esophagojejunal anastomosis. The process for removing the specimen was the same as above.
For the LAG group, the Billroth-II with Braun anastomosis was performed for LADG, and the Roux-en-Y reconstruction was performed for laparoscopic-assisted total gastrectomy (LATG). The surgical methods are detailed in reference (21, 22).
Outcome Measurements
Short-term outcomes were determined as the postoperative recovery during hospitalization. The postoperative complications were defined as the Clavien–Dindo classification ≥II (23). Long-term outcomes were measured using the time from surgery to tumor recurrence (RFS) and the time from surgery to death (OS).
Statistical Analysis
All statistical analyses of the data were performed by using the R software 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) and the SPSS software 25.0 (IBM Corporation, Armonk, NY, USA). The continuous variables were represented by a median or average depending on the normal distribution and were analyzed by using the independent t-test or the Mann–Whitney U-test. The categorical variables were represented by the frequency and its percentage of the total and were analyzed by using the Chi-square test. To make this study closely resemble a randomized clinical trial setting, the method of the PS-IPTW was employed. Multivariable logistic regression was applied to all the baseline and pathological features between the TLG and LAG groups to generate a propensity score. And using the stabilized weights to reduce variability in IPTW models. With the goal of balancing observable characteristics, each patient was weighted by the inverse probability of being in TLG vs. LAG. The univariate and multivariate Cox proportional hazards regression model was used to analyze the independent risk factors of recurrence and mortality. The Kaplan–Meier technique and the log-rank test were used to create survival curves. Statistical significance was set at p < 0.05.
Result
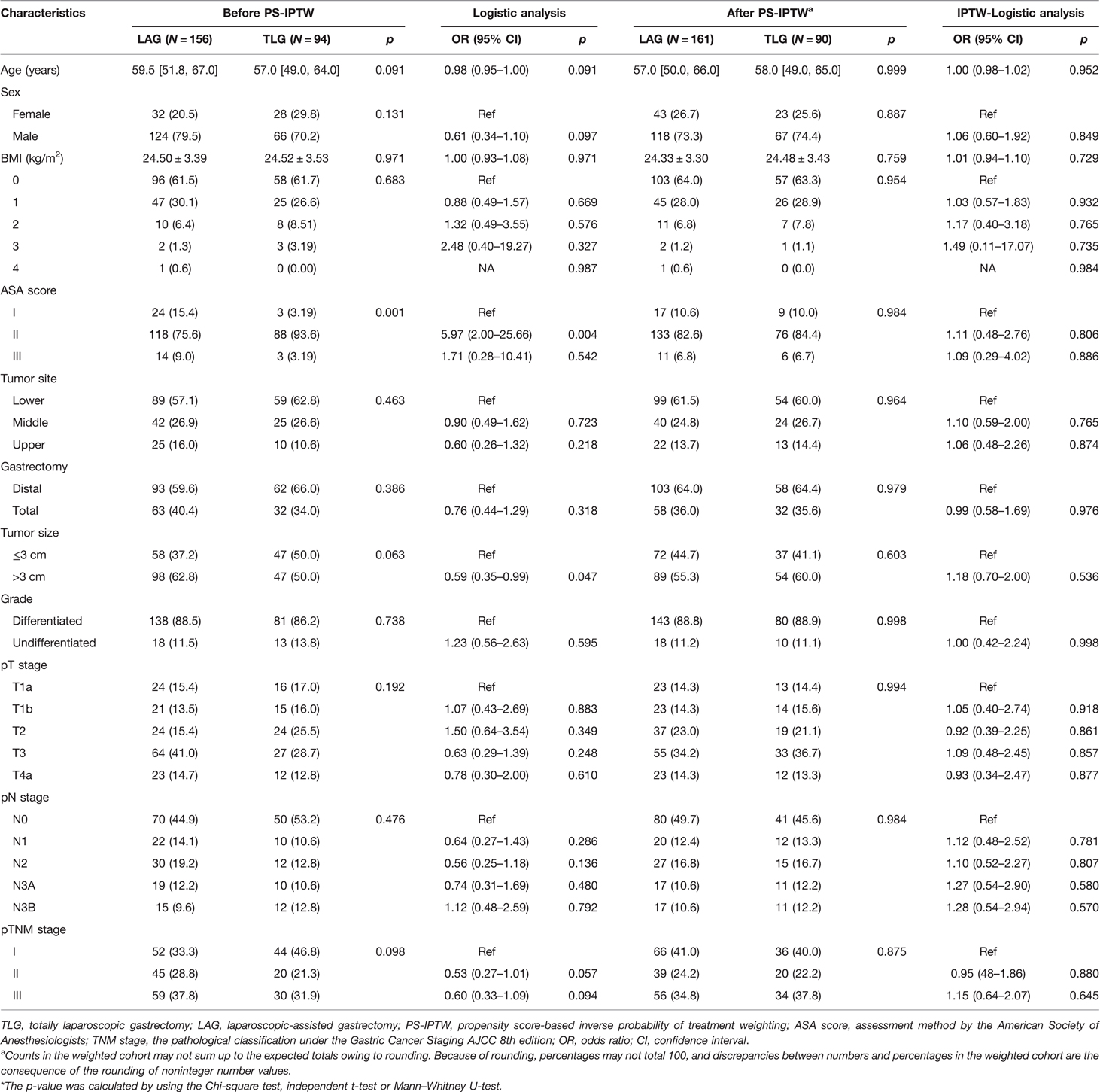
The demographic data and tumor characteristics are shown in Table 1. This research comprised 250 of 314 gastric cancer patients who underwent laparoscopic radical gastrectomy. A total of 156 patients were divided into the LAG group, and 90 patients were divided into the TLG group. In this study, 38 of 250 patients (15.2%) obtained a fast-frozen pathology, with only 3 cases of LAG having a positive margin, and received a second resection. All surgeries completed the R0 resection. The patients’ median age of the total group was 59 (IQR 51–66) years; 190 patients (76.0%) were men, and the average BMI was 24.51 (SD 3.44) kg/m2. For preoperative complications such as hypertension, heart disease, diabetes, or chronic obstructive pulmonary disease, most patients were mostly combined with 0 or 1 chronic disease (90.4%) and graded as the ASA score I/II (93.2%). Advanced gastric cancer (pT1b or above) accounted for 84% of them, and half of the tumors had lymph node metastasis (52.0%).
The results of univariate Cox analysis revealed that the tumor site, gastrectomy, tumor size, pN stage, and pTNM stage were all closely related to tumor recurrence (p < 0.05). While with the exception of the tumor site, similar results were shown in the patients’ overall survival (p < 0.05). The pN3b stage had the highest risk factors related to recurrence [OR = 27.85 (9.10–85.30), p < 0.001] and death [OR = 53.05 (11.89–236.60), p < 0.001] in GC patients. However, the operation methods did not show significant differences in patients’ long-term prognosis (p > 0.05).
The PS-IPTW was applied to eliminate group bias, and the results are presented in Table 2. Before the PS-IPTW, the results of the logistic analysis of operation methods revealed that there was a significant difference between LAG and TLG in terms of ASA score and tumor size (p < 0.05), while age, sex, and pTNM stage showed a possible trend toward significance (p < 0.1). After the PS-IPTW, both the Chi-square test and the logistic analysis revealed that all the baseline and pathological variables were well-matched between the two groups (p > 0.1). After rounding, a total of 251 GC patients were selected for this study, of which 161 patients were of LAG and 90 patients were of TLG. In the TLG group, the median age was 58 (IQR 49–65) years, 67 (74.4%) were men, and the average BMI was 24.48 (SD 3.43) kg/m2. Most TLG patients were combined with 0 or 1 chronic disease (92.2%) and ASA I/II (94.4%). In terms of tumor characteristics, the majority of tumors were differentiated adenocarcinoma (88.9%) and located in the lower stomach (60.0%). A total of 77 tumors (85.6%) had invaded the submucosa or deeper regions, and 49 tumors (54.4%) had metastasized to lymph nodes. Stage II and III of pTNM constituted a majority of the TLG group (60.0%).
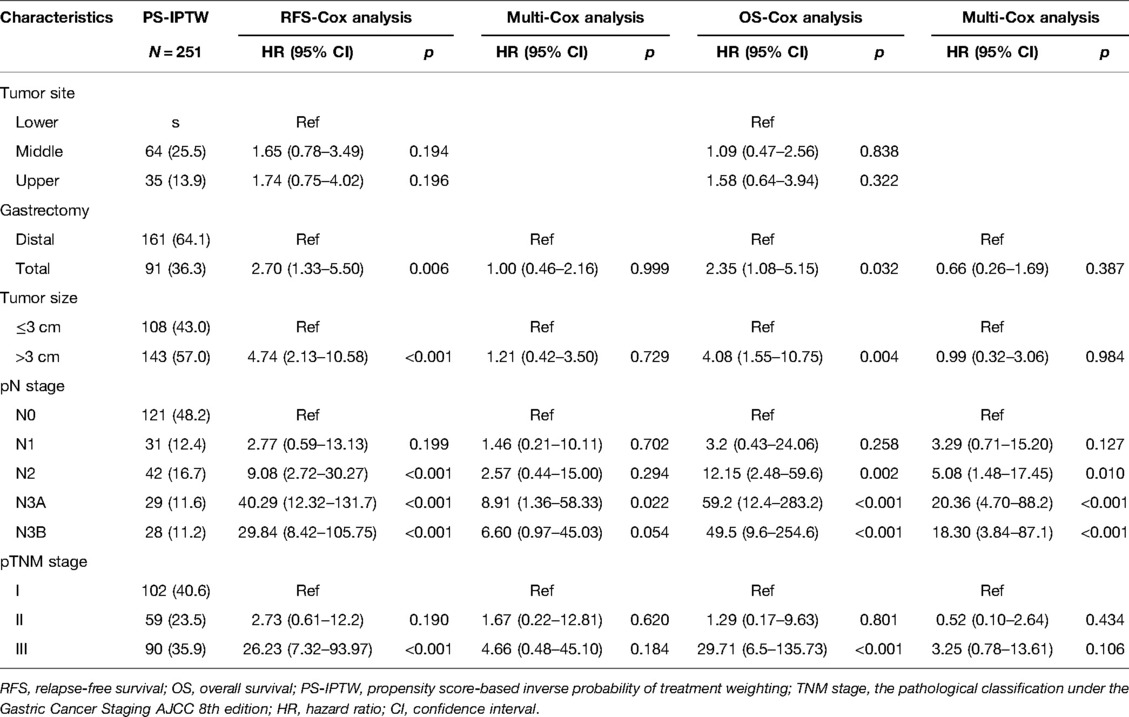
To demonstrate the high-risk factors after the PS-IPTW, the same Cox analysis procedures were used. Those significant high-risk variables before the PS-IPTW that included the tumor site, gastrectomy, tumor size, pN stage, and pTNM stage (p < 0.05) were reanalyzed by using univariate Cox analysis (Table 3). Following the PS-IPTW, those significant variables (p < 0.05) in the univariate Cox analysis were selected and included in the multivariate Cox analysis. The results showed that the pN stage was the only independent risk factor of RFS and OS in laparoscopic surgery (p < 0.05).
Operative and Prognosis Outcomes
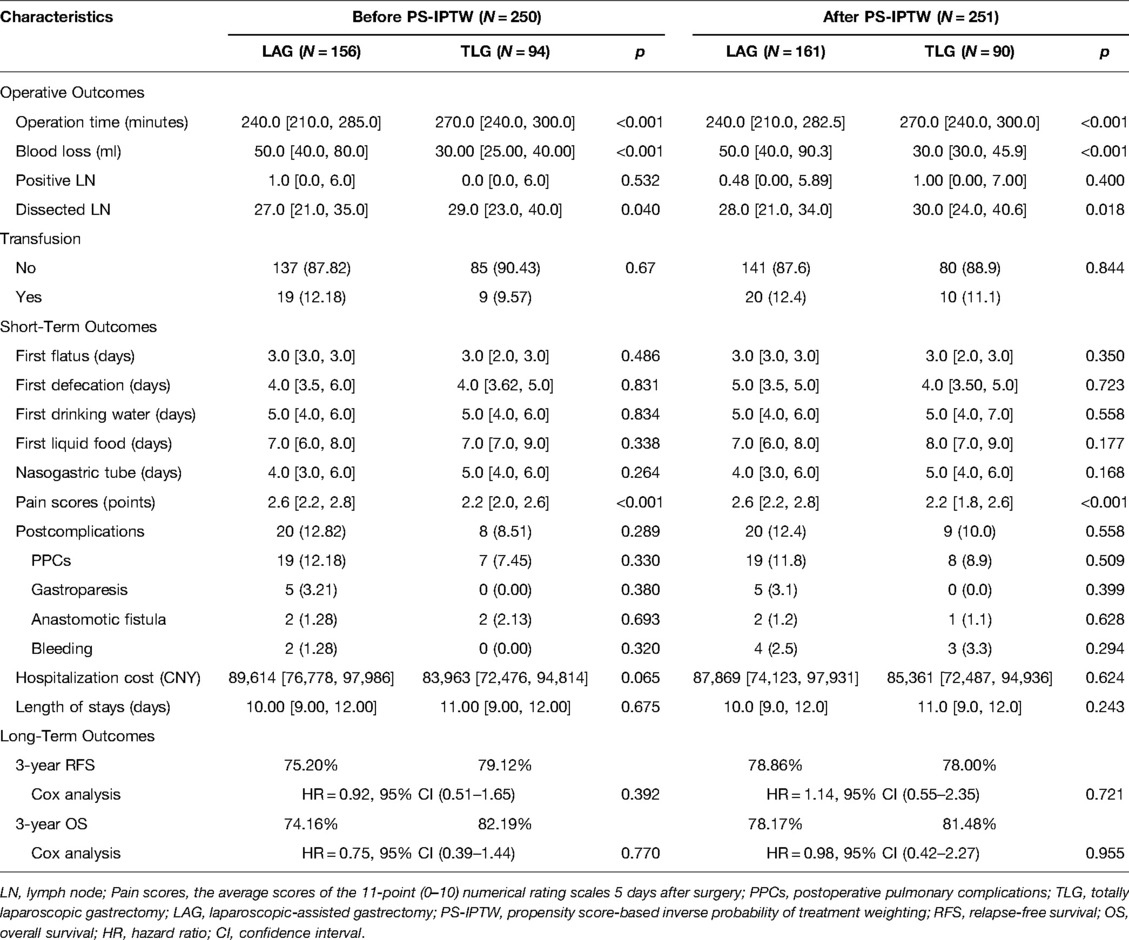
The characteristics of operative and prognosis outcomes are presented in Table 4. Similar outcomes could be found during the PS-IPTW procedures. Following the operative outcomes, both LAG and TLG groups showed a significant difference in operation time, blood loss, and the number of lymph nodes dissected (p < 0.05). TLG took 30 min more than LAG (LAG vs. TLG: 240 min vs. 270 min, p < 0.001) but resulted in 20 ml less blood loss (LAG vs. TLG: 50 ml vs. 30 ml, p < 0.001). In lymph node dissection, both surgeries obtained a good number of lymph nodes (more than 16 lymph nodes), but TLG performed better (LAG vs. TLG: 28 vs. 30, p = 0.018).
In terms of short-term outcomes, the gastrointestinal function recovery of TLG, which included the median time of the first flatus, and first defecation were about 3 and 4 days, respectively. The median times of first drinking water, first liquid food, and removal of the nasogastric tube were 5, 8, and 5 days, respectively. According to Clavien–Dindo classification, the most common postoperative complications were postoperative pulmonary complications (PPCs) (8.9%). The median hospitalization cost was 85,361 (IQR 72,487, 94,936) CNY, and the median length of stay was 11 (9, 12) days. Among them, TLG showed that its short-term outcomes were not significantly different from those of LAG (p > 0.05). Although TLG showed a benefit in reducing wound discomfort, which the median pain score was 0.4 points lower than LAG (LAG vs. TLG: 2.6 vs. 2.2, p < 0.001).
In terms of long-term outcomes, all 250 patients had completed follow-up by September 2021, and the median follow-up time was 25.1 (IQR 21.3–29.0) months. During the follow-up period, 48 patients relapsed after surgery, and 39 died. After the PS-IPTW, there were no significant differences between groups in the 3-year RFS rate (LAG vs. TLG: 78.86% vs. 78.00%; HR = 1.14, 95% CI, 0.55–2.35; p = 0.721) and the 3-year OS rate (LAG vs. TLG: 78.17% vs. 81.48%; HR = 0.98, 95% CI, 0.42–2.27; p = 0.955). Figure 1 depicts the Kaplan–Meier survival curves and log-rank tests, showing that TLG has similar survival outcomes to LAG.
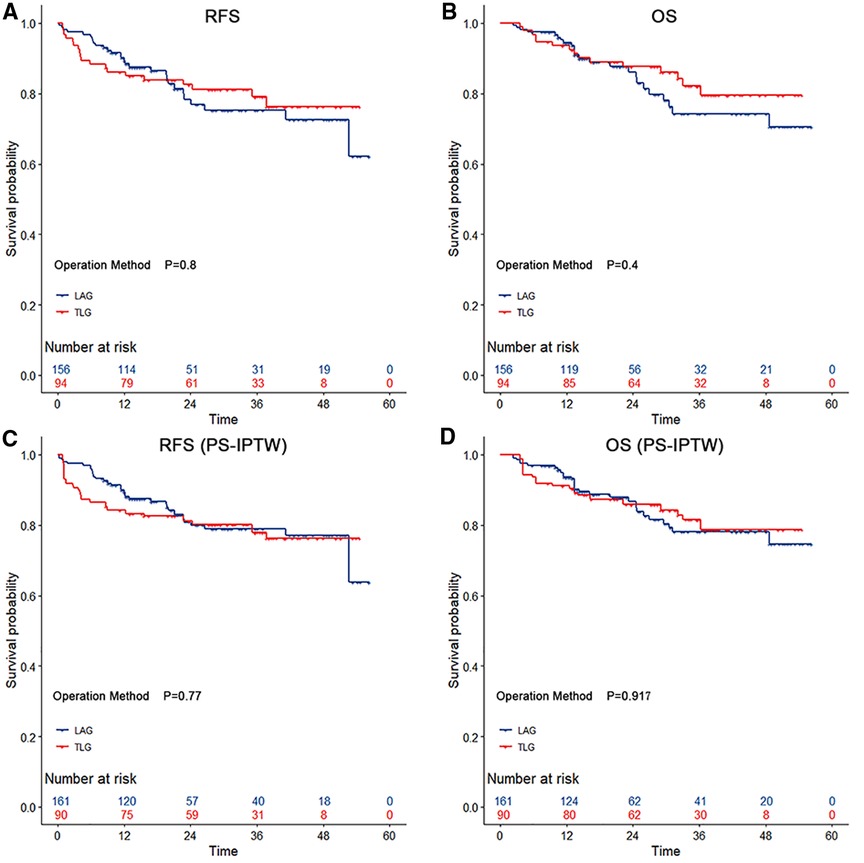
Figure 1. The survival curves among gastric cancer with operation methods during the propensity score-based inverse probability of treatment weighting. According to the type of surgery, both totally laparoscopic gastrectomy and laparoscopic-assisted gastrectomy showed no significant differencess in relapse-free survival and overall survival (p > 0.05 by the log-rank test). The risk tables show the actual number of patients with operation methods.
Subgroup Analysis
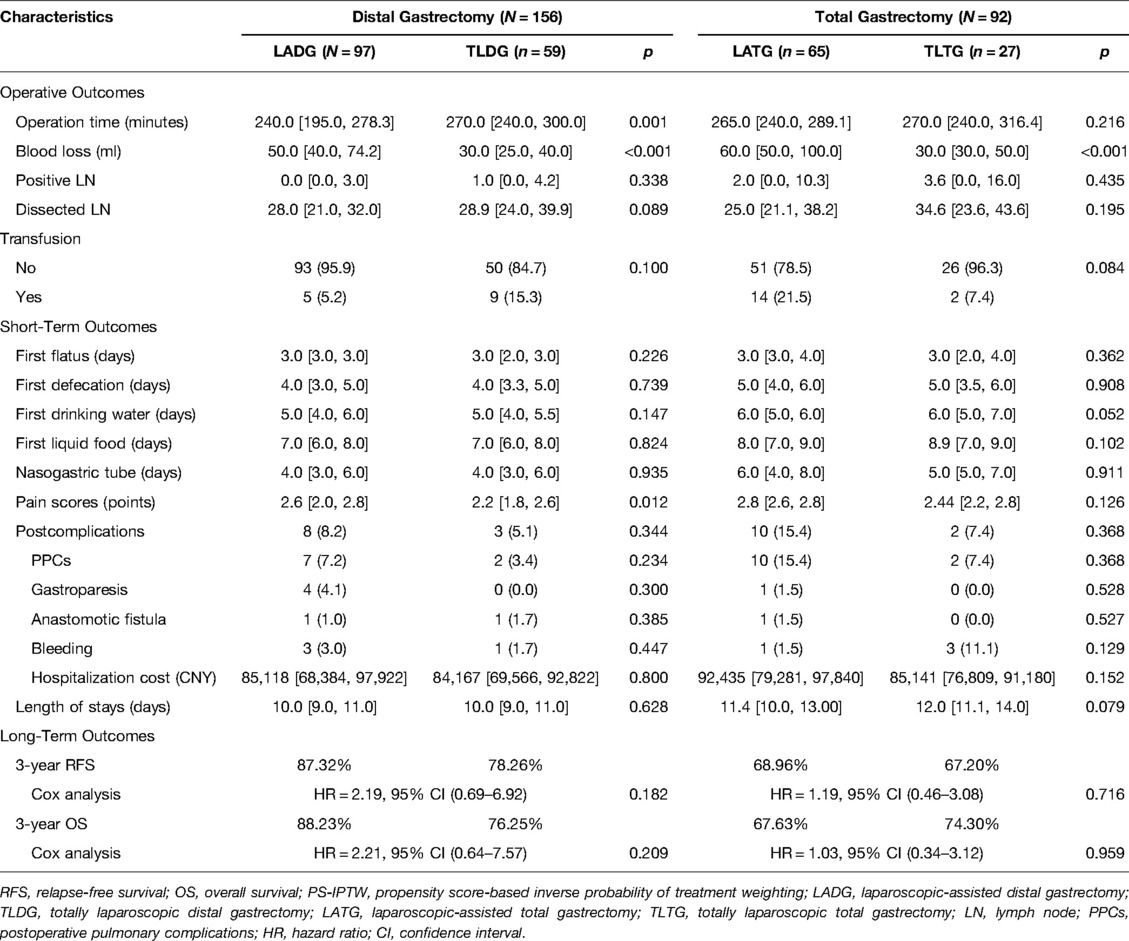
By using the same PS-IPTW procedures to balance the between-group disparities, except for the dissected numbers of lymph nodes, similar prognosis outcomes could be found in the subgroup analysis of LADG and TLDG (Table 5). However, as compared to LATG, TLTG did not increase the operation time (p = 0.216), and the wound pain scores did not indicate a significant advantage (p = 0.126). The 3-year RFS rate (LADG vs. TLDG: 87.32% vs. 78.26%; HR = 2.19, 95% CI, 0.69–6.92; p = 0.182) and the 3-year OS rate (LADG vs. TLDG: 88.23% vs. 76.25%; HR = 2.21, 95% CI, 0.64–7.57; p = 0.209) showed no significant difference in distal gastrectomy. The 3-year RFS rate (LATG vs. TLDG: 68.96% vs. 67.20%; HR = 1.19, 95% CI, 0.46–3.08; p = 0.716) and the 3-year OS rate (LATG vs. TLTG: 67.63% vs. 74.30%; HR = 1.03, 95% CI, 0.34–3.12; p = 0.959) also showed no significant difference in total gastrectomy. Figure 2 demonstrates that TLG has comparable survival outcomes to LAG in both distal and total gastrectomy.
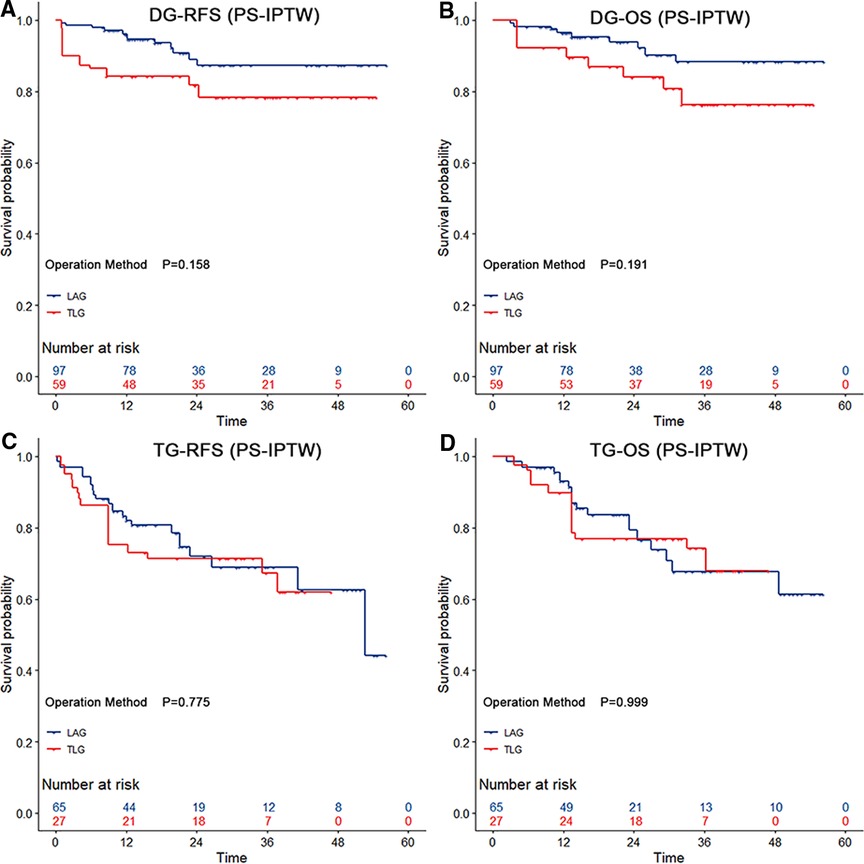
Figure 2. The survival curves among distal and total gastrectomy after the propensity score-based inverse probability of treatment weighting. Both distal gastrectomy and total gastrectomy showed no significant difference in relapse-free survival and overall survival (p > 0.05 by the log-rank test). The risk tables show the actual number of patients with operation methods.
Discussion
The usefulness and effectiveness of intracorporeal vs. extracorporeal approaches in a variety of surgical disciplines are currently a matter of dispute. Many studies have shown that in early or locally advanced gastric cancer, the long-term result of laparoscopic gastrectomy is comparable to that of open gastrectomy (24, 25). A majority of laparoscopic procedures are LADG, and large-scale prospective studies of TLG are still lacking.
This study compared the short- and long-term prognoses of gastric cancer patients who had LAG and TLG. A total of 250 GC patients were included in the study. After using the PS-IPTW to balance the baseline and pathological features of the TLG and LAG groups, we found that TLG took a longer operation time than LAG (p < 0.05) but resulted in more lymph nodes retrieved, less blood loss, and less wound discomfort (p < 0.05). Furthermore, there was no significant difference in long-term prognosis between the two groups (p > 0.05).
For TLDG, Jin et al. (26) reported that a meta-analysis of 25 studies involving 4,562 gastric cancer patients revealed that postoperative complications were comparable for TLDG and LADG. However, TLDG had favorable short-term results such as blood loss, time of liquid feed, and hospital stay (p < 0.05). Besides, Milone et al. (27) reported that a meta-analysis of 3,818 gastric cancer patients under distal gastrectomy showed that the less intraoperative blood loss, the more the harvested lymph nodes and the shorter the length of hospital stay in TLDG than in LADG (p < 0.05). Our study also showed similar results in TLDG. We found that this similarity may be due to the fact that intracorporeal reconstruction proved difficult, and TLDG took 30 min longer operation time than LADG, but there did not seem to be an increased risk of postoperative complications. Despite the longer operation duration, TLG showed benefits in terms of decreased intraoperative blood loss and wound pain, as well as a greater number of lymph node dissections (p < 0.05), without increasing hospital stay or costs (p > 0.05). The possible reasons for these might be that the intracorporeal approaches minimize inadequate surgical field exposure, severe anastomotic tugging, and bleeding produced by laparoscopically assisted small incisions. Besides, the lymph node tracking technologies may result in a more dissected number of lymph nodes. While possibly due to the conservative treatment strategies, the small length of the abdominal incision and pain response of TLG patients did not result in a significant advantage in gastrointestinal function recovery.
Umemura et al. (28) completed a review paper that covered 25 articles on TLTG and demonstrated that it tended to consume more surgical time while having advantages in terms of intraoperative blood loss and postoperative recovery. However, Milone et al. (27) revealed that TLTG was not statistically different from LATG for the above-mentioned outcomes. Our study also showed similar results for TLTG. TLTG revealed no significant difference in prognosis outcomes compared with LATG (p > 0.05), except for blood loss (LATG vs. TLTG: 50 ml vs. 30 ml, p < 0.001). A clearer vision of intracorporeal approaches, particularly in esophageal exposure and esophagojejunal anastomosis, could explain why the operation time of TLTG is not longer than that of TLAG. Furthermore, our research found that TLG had advantages in the sense that less intraoperative traction can prevent subsequent injury caused by excessive traction of the residual stomach, esophagus, and other tissues. This was also more consistent with the principle of a tumor-free operation in which the excision specimens were intracorporeally put into the bag, which could prevent their appearance in the tumor tissue of extrusion.
Studies (29, 30) showed that the occurrence of complications was not determined by the totally laparoscopic approach. Our study also confirmed this, as the Chi-square test revealed no significant difference between the two groups. All 94 TLG patients completed the R0 resection, including 62 TLDG patients (66.0%) and 32 TLTG patients (34.0%), of which only 8 patients (8.51%) suffered from postoperative complications, including 7 cases of PPCs (7.45%) and 2 cases of anastomotic fistula (2.13%) (One patient developed both complications). No gastroparesis and postoperative bleeding occurred in the TLG group. Once the postoperative complications occurred, the same treatments were given in both surgery groups, including conservative and special treatments. Conservative treatments included atomizing, expectorant drugs, antibiotic therapy, dietary abstinence, gastric tube drainage, abdominal drainage, or abdominal double-cannula lavage. Special treatments included chest drainage, trachea cannula, a second surgery, or intensive care. The aforementioned two of seven PPC patients who had respiratory failure were admitted to the Intensive Care Unit (ICU) and treated with a trachea cannula, anti-infection, and other Advanced Cardiac Life Support measures. The two patients with anastomotic fistula were diagnosed with small fistula and were treated with dietary abstinence, anti-infection, continuous gastric tube, and abdominal drainage.
Following the PS-IPTW in our study, the multivariate Cox regression analysis revealed that the pN stage was an independent risk factor for the recurrence and mortality of laparoscopic surgery, regardless of the operation method. Favorable long-term outcomes have been reported in the limited number of studies comparing LAG with TLG. Moisan Fabrizio et al. (31) reported in a matched cohort study of 31 patients of both open and TLG groups that the 3-year RFS rate and the 3-year OS rate were 79.4% and 82.3%, respectively. Besides, the survival outcomes also showed similar survival rates of LAG and TLG after the PS-IPTW, in which RFS and OS were 78.86% vs. 78.00% and 78.17% vs. 81.48%, respectively. Similar results could be found in the subgroup analysis. TLG did not increase the survival risks in long-term outcomes.
The limitation in our research was that it was a retrospective study, which meant that the treatment strategies were not determined by random assignments, and, therefore, selection bias may have occurred even when using the groups’ balanced method of the PS-IPTW. Secondly, except for the reverse puncture device reconstruction, our surgical team also attempted to perform other intracorporeal endoscopic anastomoses such as overlap (32), isoperistaltic jejunum-later-cut overlap (33), or π-shaped esophagojejunal anastomoses (34) during the study period. Although no serious postoperative complications occurred in these operations, the small number of these surgeries may have resulted in confounding bias, and, therefore, they were not included in this analysis. Besides, because the survival rates in both groups were comparable, the other survival outcomes that were lacking in this study might more substantially guide decisions on the manner of operation.
In conclusion, minimally invasive treatment is a major trend in surgical development (35). However, TLG should be based on the surgeon’s technical skills, the patient’s physical condition, objective economic status, and the features of the equipment used. The following are some of our study’s recommendations: (1) We could endoscopically inject carbon nanoparticles or ICG suspension around the tumor 1 day before TLG to identify the tumor boundaries. (2) For Billroth-II with Braun anastomosis in TLDG, the input loop should not be too long, and the mesenterium should not be twisted. (3) For the reverse puncture device reconstruction in TLTG, place the anvil of the esophageal stump first and then cut the esophagus. It is easy to place the anvil under esophageal traction; (4) Choose a smooth needle thread with high tension resistance, with an appropriate length of around 10 cm, and continuously reinforce the anastomosis under laparoscopy. (5) All should follow the same fundamental principles as an open radical gastrectomy. In case of severe complications that are difficult to manage under laparoscopy, we should switch over to laparotomy. Elaborate considerations should be made to maximize the benefits accrued to patients.
This study showed that TLG for stomach cancer is safe and feasible in both short- and long-term prognoses. Although the surgical procedure is tough to perform, it necessitates greater expertise and coordination on the part of the surgeon. The current long-term efficacy of totally laparoscopic radical gastrectomy still needs evidence-based medical confirmation in the form of large randomized controlled trials.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Review Committee of the Qilu Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
The study concept, statistical analysis, and drafting of the manuscript were developed by XZ. The critical revision of the manuscript and the review and approval of the final manuscript were done by MW and WY. The acquisition and interpretation of the data were done by JO, WC, ZC, YL, YH, and RZ. All authors have read and approved the final manuscript.
Funding
This study was supported by the University Research Project of Shandong University (Grant No. 6010119083).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. (2006) 355(1):11–20. doi: 10.1056/NEJMoa055531
3. Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2016) 14(10):1286–312. doi: 10.6004/jnccn.2016.0137
4. Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. (1994) 4(2):146–8.
5. Wei Y, Yu D, Li Y, Fan C, Li G. Laparoscopic versus open gastrectomy for advanced gastric cancer: a meta-analysis based on high-quality retrospective studies and clinical randomized trials. Clin Res Hepatol Gastroenterol. (2018) 42(6):577–90. doi: 10.1016/j.clinre.2018.04.005
6. Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. (2019) 321(20):1983–92. doi: 10.1001/jama.2019.5359
7. Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, et al. Randomized phase III trial of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer (JCOG0912). J. Clin. Oncol. 375(Suppl. 15):4020. doi: 10.1200/JCO.2019.37.15_suppl.4020
8. Reyes C, Weber K, Gagner M, Divino C. Laparoscopic vs open gastrectomy. Surg Endosc. (2001) 15(9):928–31. doi: 10.1007/s004640080185
9. Jiang L, Yang K-H, Guan Q-L, Cao N, Chen Y, Zhao P, et al. Laparoscopy-assisted gastrectomy versus open gastrectomy for resectable gastric cancer: an update meta-analysis based on randomized controlled trials. Surg Endosc. (2013) 27(7):2466–80. doi: 10.1007/s00464-012-2758-6
10. Okabe H, Obama K, Tsunoda S, Tanaka E, Sakai Y. Advantage of completely laparoscopic gastrectomy with linear stapled reconstruction: a long-term follow-up study. Ann Surg. (2014) 259(1):109–16. doi: 10.1097/SLA.0b013e31828dfa5d
11. Yan J, Zheng X, Liu Z, Yu J, Deng Z, Xue F, et al. A multicenter study of using carbon nanoparticles to show sentinel lymph nodes in early gastric cancer. Surg Endosc. (2016) 30(4):1294–300. doi: 10.1007/s00464-015-4358-8
12. Patti MG, Herbella FA. Indocyanine green tracer-guided lymph node retrieval during radical dissection in gastric cancer surgery. JAMA Surg. (2020) 155(4):312. doi: 10.1001/jamasurg.2019.6034
13. Chen Q-Y, Xie J-W, Zhong Q, Wang J-B, Lin J-X, Lu J, et al. Safety and efficacy of indocyanine green tracer-guided lymph node dissection during laparoscopic radical gastrectomy in patients with gastric cancer: a randomized clinical trial. JAMA Surg. (2020) 155(4):300–11. doi: 10.1001/jamasurg.2019.6033
14. Zhang C, Xiao W, Chen K, Zhang Z, Du G, Jiang E, et al. A new intracorporeal Billroth II stapled anastomosis technique in totally laparoscopic distal gastrectomy. Surg Endosc. (2015) 29(6):1636–42. doi: 10.1007/s00464-014-3825-y
15. Lee HH, Song KY, Lee JS, Park S-M, Kim J-J. Delta-shaped anastomosis, a good substitute for conventional Billroth I technique with comparable long-term functional outcome in totally laparoscopic distal gastrectomy. Surg Endosc. (2015) 29(9):2545–52. doi: 10.1007/s00464-014-3966-z
16. Wang B, Son S-Y, Shin H-J, Hur H, Han S-U. The learning curve of linear-shaped gastroduodenostomy associated with totally laparoscopic distal gastrectomy. J Gastrointest Surg. (2020) 24(8):1770–7. doi: 10.1007/s11605-019-04329-3
17. Baiocchi GL, Molfino S, Molteni B, Quarti L, Arcangeli G, Manenti S, et al. Fluorescence-guided lymphadenectomy in gastric cancer: a prospective western series. Updates Surg. (2020) 72(3):761–72. doi: 10.1007/s13304-020-00836-0
18. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. (2021) 24(1):1–21. doi: 10.1007/s10120-020-01042-y.
19. Omori T, Oyama T, Mizutani S, Tori M, Nakajima K, Akamatsu H, et al. A simple and safe technique for esophagojejunostomy using the hemidouble stapling technique in laparoscopy-assisted total gastrectomy. Am J Surg. (2009) 197(1):e13–17. doi: 10.1016/j.amjsurg.2008.04.019
20. Huang X, Xu L, Peng H, Hu H, Jin Y, Sun D, et al. Reverse puncture device technique: an innovation of esophagojejunostomy in radical laparoscopic total gastrectomy. Future Oncol. (2019) 15(24):2807–17. doi: 10.2217/fon-2018-0837
21. Piessen G, Triboulet J-P, Mariette C. Reconstruction after gastrectomy: which technique is best? J Visc Surg. (2010) 147(5):e273–83. doi: 10.1016/j.jviscsurg.2010.09.004
22. Jones DB. Laparoscopic surgery: principles and procedures, revised and expanded. Boca Raton: CRC Press (2004).
23. Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
24. Lee J, Nam B, Ryu K, Ryu S, Park Y, Kim S, et al. Comparison of outcomes after laparoscopy-assisted and open total gastrectomy for early gastric cancer. J Br Surg. (2015) 102(12):1500–5. doi: 10.1002/bjs.9902
25. Eom BW, Kim Y-W, Lee SE, Ryu KW, Lee JH, Yoon HM, et al. Survival and surgical outcomes after laparoscopy-assisted total gastrectomy for gastric cancer: case–control study. Surg Endosc. (2012) 26(11):3273–81. doi: 10.1007/s00464-012-2338-9
26. Jin HE, Kim MS, Lee CM, Park JH, Choi CI, Lee HH, et al. Meta-analysis and systematic review on laparoscopic-assisted distal gastrectomy (LADG) and totally laparoscopic distal gastrectomy (TLDG) for gastric cancer: Preliminary study for a multicenter prospective KLASS07 trial. Eur J Surg Oncol. (2019) 45(12):2231–40. doi: 10.1016/j.ejso.2019.06.030
27. Milone M, Manigrasso M, Burati M, Elmore U, Gennarelli N, Giglio MC, et al. Intracorporeal versus extracorporeal anastomosis after laparoscopic gastrectomy for gastric cancer. A systematic review with meta-analysis. J Visc Surg. (2019) 156(4):305–18. doi: 10.1016/j.jviscsurg.2019.01.004
28. Umemura A, Koeda K, Sasaki A, Fujiwara H, Kimura Y, Iwaya T, et al. Totally laparoscopic total gastrectomy for gastric cancer: literature review and comparison of the procedure of esophagojejunostomy. Asian J Surg. (2015) 38(2):102–12. doi: 10.1016/j.asjsur.2014.09.006
29. Kim J-H, Jun K-H, Chin H-M. Short-term surgical outcomes of laparoscopy-assisted versus totally laparoscopic Billroth-II gastrectomy for gastric cancer: a matched-cohort study. BMC Surg. (2017) 17(1):1–6. doi: 10.1186/s12893-016-0201-y
30. Topal B, Leys E, Ectors N, Aerts R, Penninckx F. Determinants of complications and adequacy of surgical resection in laparoscopic versus open total gastrectomy for adenocarcinoma. Surg Endosc. (2008) 22(4):980–4. doi: 10.1007/s00464-007-9549-5
31. Moisan F, Norero E, Slako M, Varas J, Palominos G, Crovari F, et al. Completely laparoscopic versus open gastrectomy for early and advanced gastric cancer: a matched cohort study. Surg Endosc. (2012) 26(3):661–72. doi: 10.1007/s00464-011-1933-5
32. Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, et al. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg. (2010) 211(6):e25–9. doi: 10.1016/j.jamcollsurg.2010.09.005
33. Huang C-M, Huang Z-N, Zheng C-H, Li P, Xie J-W, Wang J-B, et al. An isoperistaltic jejunum-later-cut overlap method for esophagojejunostomy anastomosis after totally laparoscopic total gastrectomy: a safe and feasible technique. Ann Surg Oncol. (2017) 24(4):1019–20. doi: 10.1245/s10434-016-5658-5
34. Kwon IG, Son Y-G, Ryu SW. Novel intracorporeal esophagojejunostomy using linear staplers during laparoscopic total gastrectomy: π-shaped esophagojejunostomy, 3-in-1 technique. J Am Coll Surg. (2016) 223(3):e25–9. doi: 10.1016/j.jamcollsurg.2016.06.011
Keywords: totally laparoscopic gastrectomy, laparoscopic-assisted gastrectomy, laparoscopic surgery, gastric cancer, surgery prognosis
Citation: Zhong X, Wei M, Ouyang J, Cao W, Cheng Z, Huang Y, Liang Y, Zhao R and Yu W (2022) Efficacy and Safety of Totally Laparoscopic Gastrectomy Compared with Laparoscopic-Assisted Gastrectomy in Gastric Cancer: A Propensity Score-Weighting Analysis. Front. Surg. 9:868877. doi: 10.3389/fsurg.2022.868877
Received: 3 February 2022; Accepted: 25 April 2022;
Published: 17 May 2022.
Edited by:
Stefano Rausei, ASST Valle Olona, ItalyReviewed by:
Shannon Chan, The Chinese University of Hong Kong, ChinaMichele Manigrasso, University of Naples Federico II, Italy
Copyright © 2022 Zhong, Wei, Ouyang, Cao, Cheng, Huang, Liang, Zhao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Yu d2VuYmluX3l1MjAwM0AxNjMuY29t
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Xin Zhong
Xin Zhong Meng Wei
Meng Wei Jun Ouyang
Jun Ouyang Weibo Cao
Weibo Cao Yadi Huang
Yadi Huang Yize Liang
Yize Liang Wenbin Yu
Wenbin Yu