
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 15 July 2022
Sec. Obstetrics and Gynecological Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.855314
This article is part of the Research Topic Women in Obstetrics and Gynecology View all 32 articles
Background: Lung metastasis (LM) is an independent risk factor for survival in patients with endometrial cancer (EC).
Methods: We reviewed data on patients diagnosed with EC between 2010 and 2015 from the Surveillance, Epidemiology, and End Results (SEER) database. The independent predictors of LM in patients with EC were identified using univariate and multivariate logistic regression analyses. A nomogram for predicting LM in patients with EC was developed, and the predictive model was evaluated using calibration and receiver operating characteristic (ROC) curves.
Results: Univariate and multivariate logistic regression analyses showed that high grade; specific histological type; high tumor and node stages; larger tumor size; and liver, brain, and bone metastases were positively associated with LM risk. A new nomogram was developed by combining these factors to predict LM in patients newly diagnosed with EC. Internal and external verification of the calibration charts showed that the nomogram was well calibrated. The areas under the ROC curves for the training and validation cohorts were 0.924 and 0.913, respectively.
Conclusion: We performed a retrospective analysis of 42,073 patients with EC using the SEER database, established a new nomogram for predicting LM based on eight independent risk factors, and visualized the model using a nomogram for the first time.
Endometrial cancer (EC) is one of the most serious epithelial malignancies that threatens women's health, ranked fourth in terms of incidence among malignancies affecting women (1). Although surgery and adjuvant radiation therapy have significantly improved disease-free and overall survival in patients with early EC (2), the prognosis in patients with metastases is poor, with a 5-year survival rate of only 17% (1, 3), respectively. EC mainly spreads via intra-abdominal and lymph node metastases (4), while distant organ metastasis is rare. The most common distant metastatic sites of EC are the lungs, followed by the liver, bone, and brain (5). However, compared with other gynecological malignancies, such as cervical cancer and ovarian cancer, EC has the highest frequency of lung metastasis (LM), with a 20%–25% incidence in patients with relapse (6). Although distant metastasis is a rare event in EC (7), it has a significant impact on patient survival. The survival time of patients with EC with LM is only 11 months (8). Especially for early-stage patients, doctors' insufficient judgment on the risk of distant metastasis affects the formulation of treatment and follow-up plans, resulting in decreased patient survival time. Although analysis and modeling of risk factors affecting survival and distant metastasis in EC patients have been carried out, the visualization and evaluation of predictive models for metastasis risk factors are still lacking, which leads to inconvenience in clinical application. In this study, we aimed to evaluate patients with EC registered in the SEER database from 2010 to 2015, and to develop a validated LM nomogram with high accuracy for the first time. In this way, the prognosis can be accurately made and appropriate management strategies can be selected, making it more convenient for clinical management.
Data were obtained from the Surveillance, Epidemiology, and End Results (SEER) database. SEER *Stat 8.3.5 software (https://seer.cancer.gov/data/) was used to access the database. As details of metastases were not recorded before 2010, patients with primary EC who were ≥18 years of age at diagnosis and between 2010 and 2015 were analyzed. The site code ICD-O-3 (International Classification of Diseases Oncology-3) is limited to C54.0–C54.9 and C55.9, and the exclusion criteria for patient selection were: unknown histological type classification, unknown American Joint Committee on Cancer (AJCC) tumor (T) and node (N) staging, unknown exact tumor size, and unknown metastatic information. The patient selection flowchart is shown in Figure 1. Histological types of ECs were classified according to ICD-0-3 site/histology (endometrioid: 8380–8383/3, 8140/3, 8210/3, 8211/3, 8560/ 3, 8260/3, 8262/3, 8263/3, 8570/3, 8261/3, 8480–8482/3; serous: 8441/3, 8460/3, 8461/3; carcinosarcoma: 8950/3, 8951/3, 8980/3, 8981/3; clear cells: 8310/3 and mixed epithelial cells: 8323/3, 8255/3).
Data on clinical and pathological characteristics, including age, ethnicity, marital status at diagnosis, histological type, grade, AJCC T and N stage, tumor size, and metastatic status, were collected from the SEER database. Based on the inclusion and exclusion criteria, 42,073 EC patients were enrolled in this study. We split all populations into training and validation cohorts in a 7/3 ratio. Because all personally identifiable information in the SEER database was withheld, informed consent was not required for the use of SEER data. This research project complied with the 1964 Declaration of Helsinki and its subsequent amendments or similar ethical standards.
Statistical analysis was performed using the SPSS 21 software. The Kolmogorov–Smirnov test was used to verify the normality of the variables. Categorical data are presented as frequency (%) and analyzed using the chi-square test. Normally distributed variables are expressed as mean ± standard deviation, whereas non-normally distributed variables are expressed as median (interquartile range). Univariate and multivariate logistic regression analyses were used to identify the risk factors. Simultaneously, 95% confidence intervals (CIs) and hazard ratios were calculated. Factors with p values <0.05 were included in the multivariate regression model.
The LM nomogram was also based on the results of a multivariate logistic analysis using the rms package in R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). A receiver operating characteristic (ROC) curve was drawn, and the area under the ROC curve (AUC) was calculated to assess the discriminative ability of the nomogram. Finally, we evaluated the stability of the LM nomogram through internal validation of 1,000 bootstrap samples. A calibration plot was drawn to analyze the agreement between the observed and predicted probabilities.
Based on the selection process, 42,073 patients were included in our study. Specifically, 29,451 patients were included in the training cohort, and the remaining 12,622 patients were included in the test cohort. The incidence of LM was 1.4% (n = 603). The baseline of 42,073 patients are shown in Tables 1, 2. Among patients with and without LM, age (p = 0.002); tumor grade (p < 0.001), AJCC T stage (p < 0.001) and N stage (p < 0.001), and histological type (p < 0.001); race (p < 0.001); insurance status (p = 0.001); tumor size (p < 0.001); and the occurrence of bone (p < 0.001), brain (p = 0.017), and liver metastases (p < 0.001) were significantly different. There were no statistically significant differences in terms of the marital status (p = 0.236).
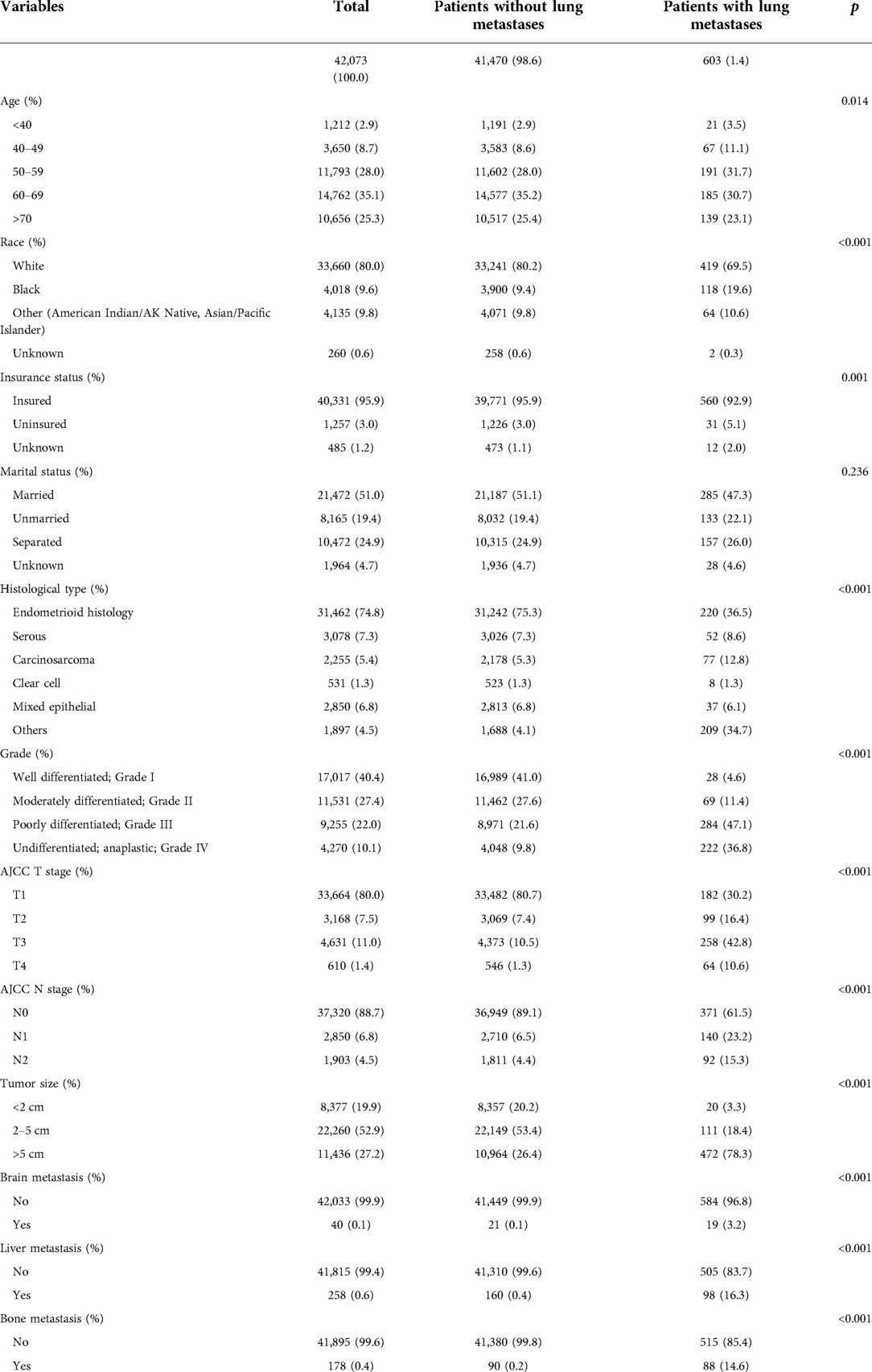
Table 1. Demographical and clinical characteristics between endometrial carcinoma patient with the lung metastases and without the lung metastases.
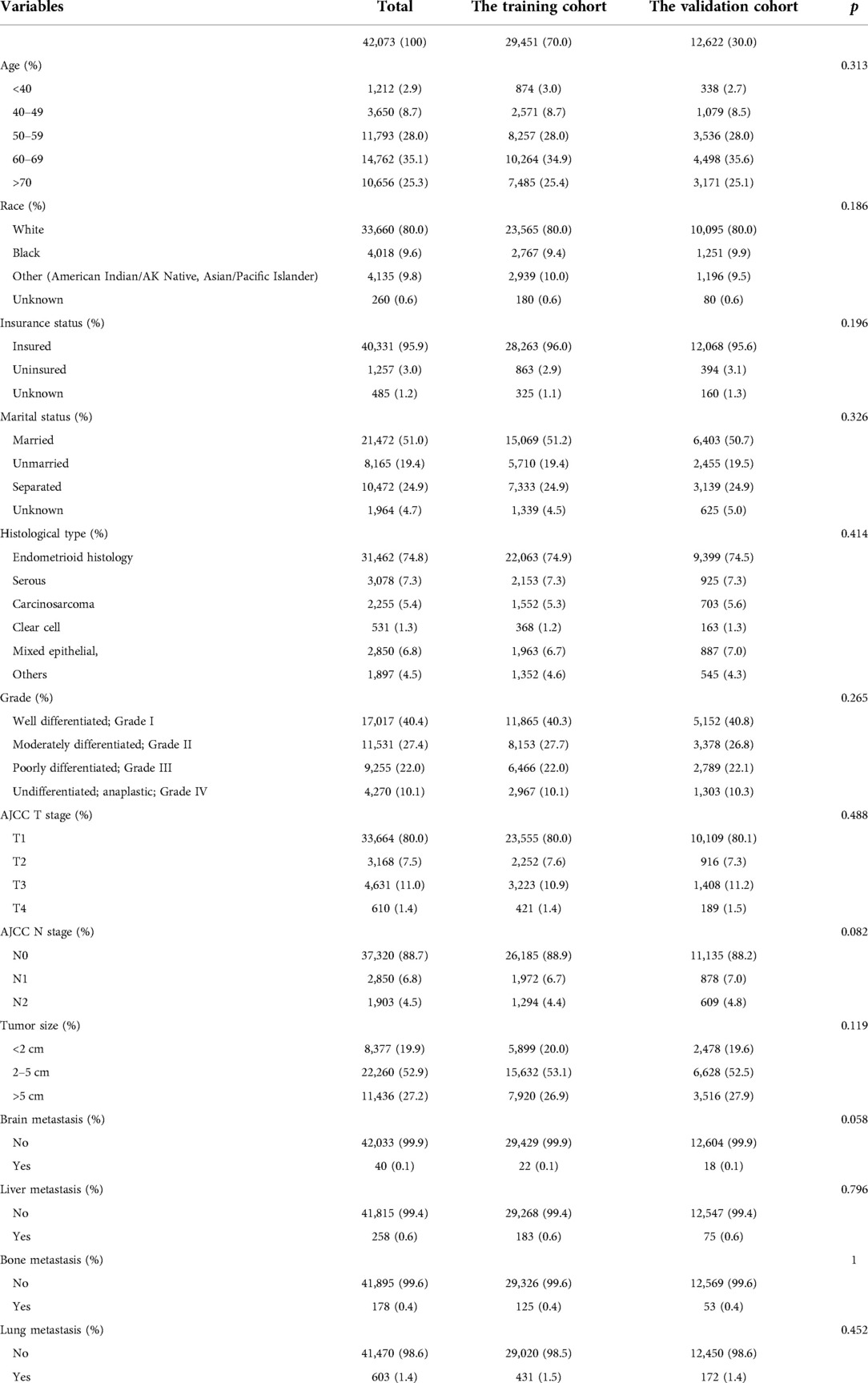
Table 2. Demographical and clinical characteristics between patient with the primary cohort and the validation cohort.
Among the 42,073 patients, 603 (1.4%) had LM at the first visit, and 41,470 (98.6%) did not. Univariate logistic analysis was used to analyze 11 predictors to determine LM-related variables in patients with EC. The results showed that eight predictors were related to LM in patients with EC: tumor size (p < 0.001), grade (p < 0.001), histological type (p < 0.001), and AJCC T stage (p < 0.001) and N stage (p < 0.001); and bone (p < 0.001), liver (p < 0.001), and brain (p < 0.001) metastases (Table 3). Then, statistically significant factors of the univariate logistic analysis were included in the multivariate logistic regression analysis, and the results showed that higher grade (p < 0.001); larger tumor (p < 0.001); higher T stage (p < 0.001); higher N stage (p < 0.001); specific histological type (p < 0.001); and the occurrence of bone (p < 0.001), liver (p < 0.001), and brain metastases (p < 0.001) were risk factors for LM. These predictors were evaluated for newly diagnosed patients with EC (Table 3).
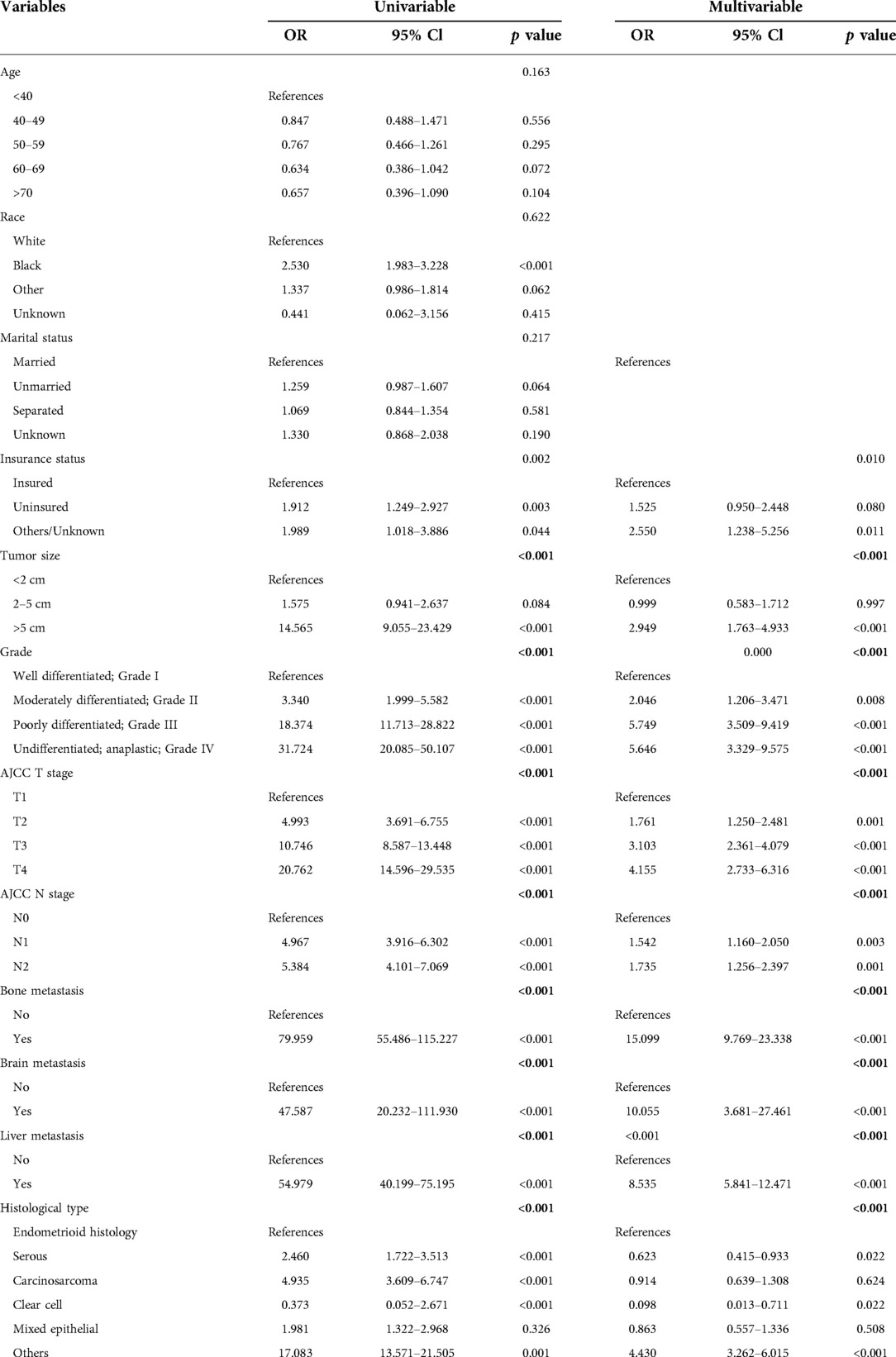
Table 3. Univariable and multivariable logistic regression for analyzing the associated factors for developing liver metastases in endometrial carcinoma cancer patients.
Based on the eight independent LM-related variables, a diagnostic nomogram was established for LM risk assessment in newly diagnosed patients with EC (Figure 2). Simultaneously, the ROC curves of the training and validation cohorts were established. The AUC of the nomogram in the training cohort was 0.924, sensitivity was 0.903, and specificity was 0.792 (Figure 3A). The AUC of the nomogram in the validation cohort was 0.913, with a sensitivity of 0.892 and specificity of 0.779 (Figure 3B). In addition, to test the performance of the nomogram, 1,000 bootstrap resampling cycles were performed for internal verification. The calibration curves showed good agreement between the training and validation cohorts (Figures 3C,D).
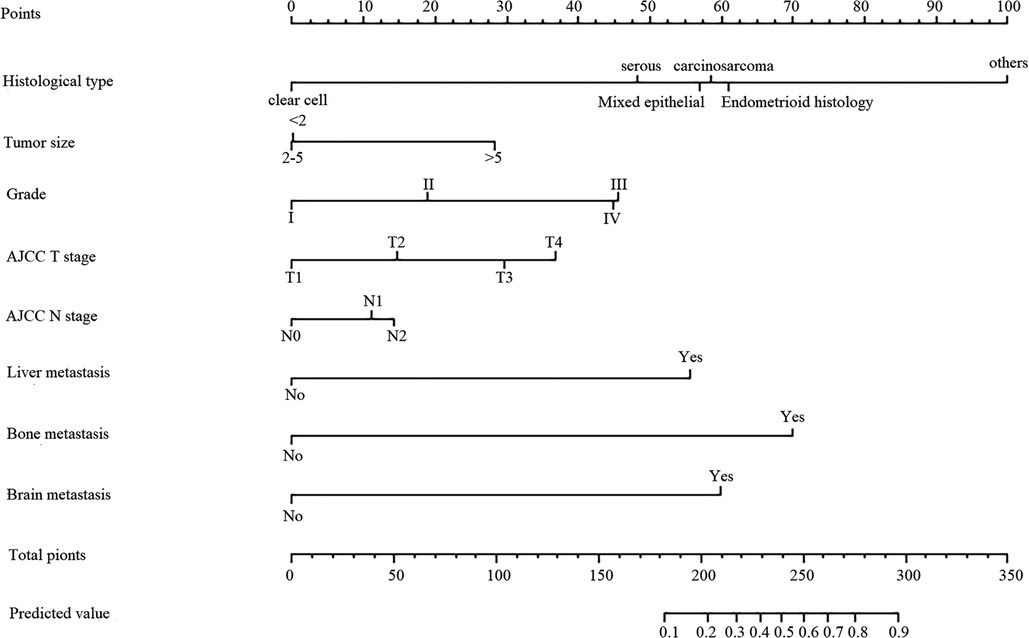
Figure 2. Nomogram predicting the probability of LM. The first line shows the point assignment of each variable. Lines 2–9 show the variables included in the model. When using a nomogram for a single patient, a point is assigned to each variable based on clinicopathological characteristics, and all points are added. Each score in the total score in row 9 will correspond to the probability of risk in the last row.
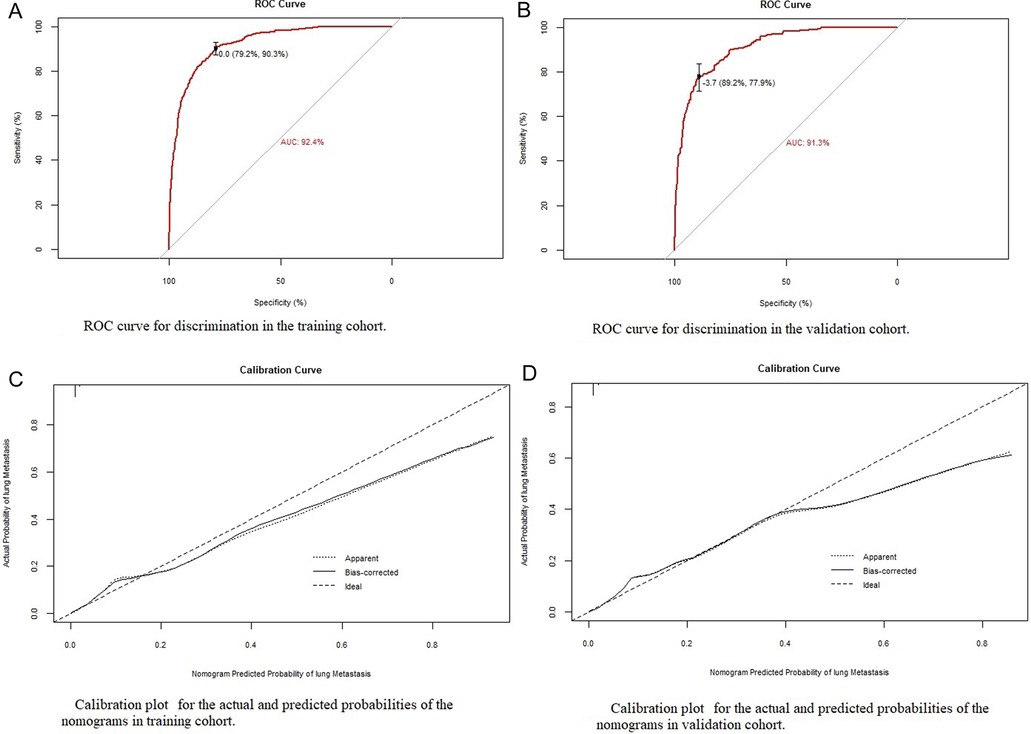
Figure 3. Identification and calibration of the nomogram in the training and validation cohorts. (A,B) ROC curve used to distinguish between training and validation cohorts. The area under the curve (AUC) is 0.924 and 0.913, indicating that the model has good performance. (C,D) Calibration curve of training cohort and validation cohort. The x-axis shows the predicted probability of the model, and the y-axis shows the actual probability.
Approximately 15%–25% of patients with EC are already at an advanced stage at the time of diagnosis. Surgery, chemotherapy, and radiotherapy are the main treatments for patients with EC (9), which can improve the survival rate of patients. Studies have found that the early detection of metastases and surgery at the primary site play an important role in improving the survival rate of patients. The median cancer-specific survival time of patients with solitary LM undergoing surgery and those not undergoing surgery is 23 months and 9 months, respectively (10). LM-directed radiotherapy can control local tumors and improve the survival rate of patients (11). Therefore, the early detection of LM from EC and appropriate clinical measures are critical for patient survival. Regarding LM in EC, there are only studies on its risk factors (12), and no predictive model has been developed to depict the risk of LM more intuitively (8) so that it can be conveniently applied in clinical management. Therefore, a clinical model for predicting LM is urgently required to guide clinical strategies and improve the survival rate of patients with LM. This study was the first to develop a nomogram using the SEER database to predict concurrent LM in patients with EC. The total score can be calculated by obtaining data on several variables on the nomogram for each patient with EC. The risk of LM can then be easily identified using the nomogram, providing guidance for further clinical management.
Our study found that the incidence of LM was 1.4%, which is similar to that in previous studies (8). We found that a high tumor grade; large tumor size; higher T and N stages; specific histological type; and presence of bone, liver (p < 0.001), and brain metastases (p < 0.001) were risk factors for LM and were included in the prediction model. Adachi et al. also found that LM is associated with stage IV disease and deep muscle infiltration (13). Jiang et al. found that large tumor size and deep muscle invasion may be risk factors for LM in patients with stage I endometrioid EC (14). Mao's clinical case study on EC found that tissue type is a risk factor for LM, and carcinosarcoma is more prone to LM (8, 13). We also found that different histological types could affect the occurrence of LM. Compared with endometrioid histology, serous and clear cells are less prone to LM, and carcinosarcoma is more prone to LM (15). In Guo's retrospective study of 730 patients, poor differentiation was an independent high-risk factor for extraperitoneal metastasis and has been widely accepted by clinicians as a feature of high-risk EC (10). Once distant metastasis occurs, it indicates that there has been extraperitoneal diffusion and cervical interstitial infiltration, therefore LM is also more likely to occur. We verified the nomogram internally and externally. There was consistency between the predicted results and those observed during verification. The ROC curve's AUC in the training cohort was 0.924 and the AUC of the line graph was 0.913 in the validation cohort. Through this predictive model, obstetricians and gynecologists will be able to estimate the likelihood of LM in patients with EC. For patients with a higher likelihood of LM, a closer follow-up should be performed. The nomogram can be used to evaluate patients with EC before chest computed tomography (CT) to determine whether the patient requires CT. If the patient's first CT evaluation cannot determine whether metastasis is present, CT follow-up should be encouraged for patients with a high risk of LM shown on the nomogram.
This study had some limitations. First, this was a retrospective study, and there are inherent biases associated with this type of study design. Additionally, only patients with LM at their first visit were analyzed. LMs occurring later in the disease were not analyzed because they may not have been recorded in the SEER database. Third, the nomograms in our study were only validated in the same population, and there may be biases in the validation of the model performance. Therefore, more clinical data should be collected for external validation of this model in the future.
In conclusion, this study performed a retrospective analysis of 42,073 patients with EC using the SEER database and established for the first time a new nomogram for predicting LM based on eight independent risk factors. The verification of the model proved that it has good performance. Although predictive models have certain limitations, nomograms can reveal the relationship between clinicopathological features and LM risk in patients with EC. Through this predictive model, physicians will be able to estimate the likelihood of developing LM in patients with EC, providing guidance for further clinical management.
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/data-software.
YY and RW contributed equally to this manuscript. (I) Conception and design: YY; (II) administrative support: JZ, YY; (III) provision of study materials or patients: RW; (IV) collection and assembly of data: YZ; (V) data analysis and interpretation: RW; (VI) manuscript writing: All authors; (VII) final approval of manuscript: All authors contributed to the article and approved the submitted version.
Shaanxi Province's 2017 Key R&D Plan (2017SF-217).
The authors acknowledge the great efforts of the Surveillance, Epidemiology, and End Results Program in the creation of the Surveillance, Epidemiology, and End Results database. We would like to thank Emma Clinton for English language editing. Thanks for the funding of Shaanxi Province's 2017 Key R&D Plan (2017SF-217).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66(1):7–30. doi: 10.3322/caac.21332
2. Elshaikh MA, Vance S, Suri JS, Mahan M, Munkarah A. Improved survival endpoints with adjuvant radiation treatment in patients with high-risk early-stage endometrial carcinoma. Int J Radiat Oncol Biol Phys. (2014) 88(2):351–6. doi: 10.1016/j.ijrobp.2013.11.003
3. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. (2005) 366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8
4. Lewin SN. Revised FIGO staging system for endometrial cancer. Clin Obstet Gynecol. (2011) 54(2):215–8. doi: 10.1097/GRF.0b013e3182185baa
5. Liu Y, Chi S, Zhou X, Zhao R, Xiao C, Wang H. Prognostic value of distant metastatic sites in stage IV endometrial cancer: a SEER database study of 2948 women. Int J Gynaecol Obstet. (2020) 149(1):16–23. doi: 10.1002/ijgo.13084
6. Sohaib SA, Houghton SL, Meroni R, Rockall AG, Blake P, Reznek RH. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol. (2007) 62(1):28–34; discussion 5–6. doi: 10.1016/j.crad.2006.06.015
7. Mariani A, Webb MJ, Keeney GL, Podratz KC. Routes of lymphatic spread: a study of 112 consecutive patients with endometrial cancer. Gynecol Oncol. (2001) 81(1):100–4. doi: 10.1006/gyno.2000.6111
8. Mao W, Wei S, Yang H, Yu Q, Xu M, Guo J, et al. Clinicopathological study of organ metastasis in endometrial cancer. Future Oncol. (2020) 16(10):525–40. doi: 10.2217/fon-2020-0017
9. Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. (2019) 69(4):258–79. doi: 10.3322/caac.21561
10. Guo J, Cui X, Zhang X, Qian H, Duan H, Zhang Y. The clinical characteristics of endometrial cancer with extraperitoneal metastasis and the value of surgery in treatment. Technol Cancer Res Treat. (2020) 19, 1533033820945784. doi: 10.1177/1533033820945784
11. Baschnagel AM, Mangona VS, Robertson JM, Welsh RJ, Kestin LL, Grills IS. Lung metastases treated with image-guided stereotactic body radiation therapy. Clin Oncol (R Coll Radiol). (2013) 25(4):236–41. doi: 10.1016/j.clon.2012.12.005
12. Zhu L, Sun X, Bai W. Nomograms for predicting cancer-specific and overall survival among patients with endometrial carcinoma: a SEER based study. Front Oncol. (2020) 10:269. doi: 10.3389/fonc.2020.00269
13. Adachi M, Mizuno M, Mitsui H, Kajiyama H, Suzuki S, Sekiya R, et al. The prognostic impact of pulmonary metastasectomy in recurrent gynecologic cancers: a retrospective single-institution study. Nagoya J Med Sci. (2015) 77(3):363–72. PMID: 26412882; PMCID: PMC4574323
14. Jiang W, Chen J, Tao X, Huang F, Zhu M, Wang C, et al. Possible risk factors of pulmonary metastases in patients with international federation of gynecology and obstetrics stage I endometrioid-type endometrial cancer. Int J Gynecol Cancer. (2017) 27(6):1206–15. doi: 10.1097/IGC.0000000000001002
Keywords: Endometrial carcinoma, lung metastases, nomogram, receiver operating characteristic curve, the surveillance, epidemiology, end results
Citation: Yuan Y, Wang R, Zhang Y, Yang Y and Zhao J (2022) A new nomogram for predicting lung metastasis in newly diagnosed endometrial carcinoma patients: A study based on SEER. Front. Surg. 9:855314. doi: 10.3389/fsurg.2022.855314
Received: 15 January 2022; Accepted: 30 June 2022;
Published: 15 July 2022.
Edited by:
Zaleha Abdullah Mahdy, National University of Malaysia, MalaysiaReviewed by:
Maria Lee, Seoul National University Hospital, South Korea© 2022 Yuan, Wang, Zhang, Yang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhao eGlhbnNpeXVhbnpoYW9qaW5nQDE2My5jb20= Yang Yang eWFuZ3lhbmcxOTg0MDUxNkBzdHUueGp0dS5lZHUuY24=
†Yufei Yuan and Ruoran Wang contributed equally to this manuscript.
Specialty Section: This article was submitted to Obstetrics and Gynecological Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.