- 1Department of Neurosurgery, Chang Gung Memorial Hospital, Linkou, Chang Gung University and Medical College, Taoyuan, Taiwan
- 2Department of Neurosurgery, New Taipei Municipal Tucheng Hospital (Built and Operated by Chang Gung Medical Foundation), Taipei, Taiwan
- 3Department of Radiology, Chang Gung Memorial Hospital, Linkou, Chang Gung University and Medical College, Taoyuan, Taiwan
Objective: Delayed progressive mass effect (DPME) after securing an aneurysm is uncommon following microsurgical or endovascular repair and leads to a poor clinical outcome. Patients with ruptured middle cerebral artery (MCA) aneurysms have a high risk of postoperative oedema and mass effect, which may require decompressive treatment. Because few studies have discussed the risk and predictive factors, we focused on ruptured MCA aneurysms and evaluated the outcomes of these patients and the necessity of salvage surgery when DPME presented.
Methods: Data on 891 patients with aneurysmal subarachnoid haemorrhage (aSAH) treated between January 2011 and February 2020 were extracted from the medical database of a tertiary referral centre. A total of 113 patients with aSAH resulting from at least one MCA aneurysm were identified. After excluding patients with several clinical confounders, we enrolled 80 patients with surgically treated aSAH. We examined the characteristics of aneurysms and hematomas, perioperative contrast pooling patterns, presence of distal hematomas, perisylvian low density, occlusive treatment modality, management strategies, the need for salvage surgical decompression, and postoperative 90-day outcomes to identify possible risk factors.
Results: DPME was observed in 27 of the 80 patients (33.7%). The DPME and non-DPME group differed significantly in some respects. The DPME group had a higher risk of salvage surgery (p < 0.001) and poorer outcomes (mRS at day 90; p = 0.0018). The univariate analysis indicated that the presence of hematoma, CTA spot signs, perisylvian low density, and distal hematoma were independent risk factors for DPME. We also noted that DPME remained an independent predictor of a poorer 90-day functional outcome (mRS ≤ 2).
Conclusion: DPME can lead to salvage decompression surgery and directly relates to poor outcomes for patients with a ruptured MCA aneurysm. Distal hematoma, perisylvian low density, and CTA spot signs on preoperative images can predict DPME.
Introduction
Patients who develop the uncommon condition of delayed progressive mass effect (DPME) after an aneurysm is secured generally have poor outcomes (1). Ruptured middle cerebral artery (MCA) aneurysms with a large intraparenchymal or perisylvian hematoma lead to grave prognoses, with mortality rates exceeding 80% in patients who undergo nonoperative management (2–4). Urgent hematoma evacuation and aneurysm clipping have been the primary neurosurgical treatments, and long-term functional neurological outcomes are directly related to known risk factors, such as initial Hunt and Hess (H–H) grade, hematoma size, and hematoma location (2, 5–8). DPME caused by a ruptured aneurysm usually complicates patients’ recovery and may require additional neurosurgical decompression procedures, especially for ruptured MCA aneurysms (9–11). Although articles have indicated that DPME can cause catastrophic outcomes (11–14), few studies have examined the risk factors for DPME.
To our knowledge, this is the first and largest study to examine the development of DPME in patients after securing a ruptured MCA aneurysm. This study focused on patients with ruptured MCA aneurysms and evaluated the outcomes of these patients and the risk of salvage surgery after the development of DPME. We also identified the risk factors for DPME with preoperative computed tomography (CT); we report our experience managing DPME after securing ruptured MCA aneurysms to educate clinicians on the need to conduct secondary decompression procedures before treating ruptured MCA aneurysms.
Methods
Patients and Data Collection
Clinical data on patients surgically treated for aneurysmal subarachnoid haemorrhage (aSAH) between January 2011 and February 2020 were extracted from the medical database of a tertiary referral centre. We identified 113 patients with aSAH resulting from at least one MCA aneurysm.
We excluded patients who lacked early imaging evidence, discontinued treatment because of family matters, were bleeding from an unknown origin because of multiple aneurysms, and had a flow-related aneurysm caused by another vascular anomaly, such as arteriovenous malformation. Eighty patients with surgically treated ruptured MCA aneurysms were included for review.
We compared clinical severity, morphological characteristics of aneurysms and hematomas, perioperative contrast pooling patterns, presence of distal hematoma, perisylvian hypodensity, occlusive treatment modality, management strategies, including the need for salvage surgical decompression for an intracranial mass effect, and postoperative 90-day outcomes to identify possible risk factors for DPME.
Clinical Management
All aneurysms were secured microsurgically or endovascularly within 48 h after presentation. Patients with preoperative clinical or radiographic signs of uncal herniation and patients with large hematomas in whom herniation was deemed imminent underwent emergent hematoma evacuation and aneurysm clipping. If they did not receive immediate surgery, patients with clinical or radiographic hydrocephalus or H–H grades III-V underwent external ventricular drain placement for cerebrospinal fluid (CSF) diversion and intracranial pressure (ICP) monitoring until definitive occlusive treatment for ruptured aneurysm was performed. Whether an additional craniectomy or lobectomy was necessary was determined intraoperatively on the basis of the size of the hematoma, the need for surgical exposure, and the degree of cerebral oedema. All patients underwent postoperative CT angiography (CTA) 1–2 weeks after initial treatment, and these follow-up images were examined for possible vasospasm. All patients were cared for in a dedicated neurovascular intensive care unit according to a standardised aSAH protocol (15). Informed consent was obtained from all patients after informing them in detail about the risks, benefits, and alternatives of the procedures with multidisciplinary decision-making. The study was approved by the Institutional Review Board (IRB no. 201800342B0).
Term Definition
We studied the possible deterioration of a regional mass effect, whether haemorrhagic or ischemic; such an effect is rarely observed in patients after initial treatment for ruptured MCA aneurysms. DPME of a ruptured aneurysm is defined as the regional progression between the initial diagnostic CT and postoperative follow-up CTA. Potential predicted image parameters were documented on the basis of the image of the initial brain CT (Figure 1). The parameters comprised distal hematoma (intrasylvian hematoma at or cranially above the level of the third ventricle (Figure 1A)), perisylvian low density (>5 mm Low density area near the Sylvian fissure, detected through CT (Figure 1B)), and CTA spot signs (spot-like contrast extravasation noted on CTA (Figure 1C)).
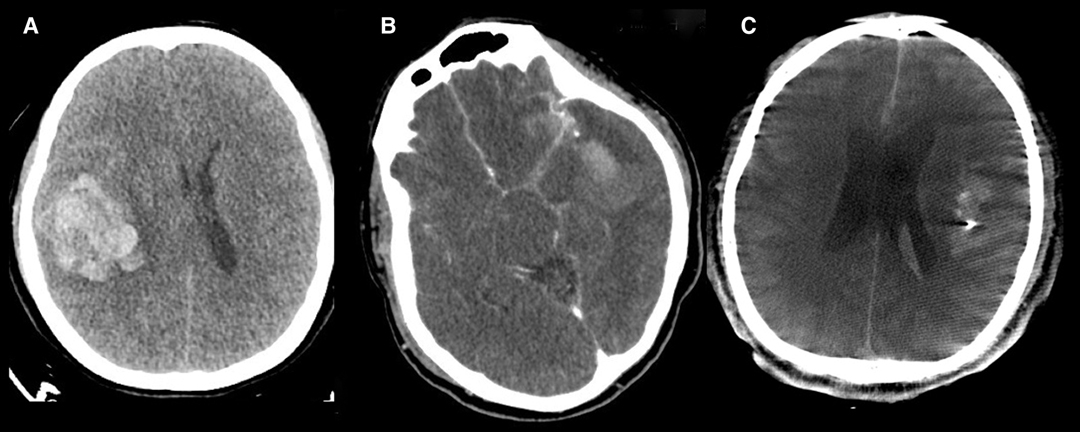
Figure 1. Image parameters documented on the initial brain CTA. (A) Distal hematoma. (B) Perisylvian low density. (C) CTA spot signs.
Statistical Analysis
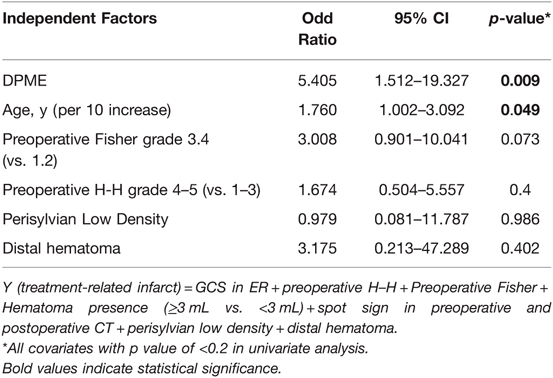
Descriptive statistics were calculated for clinical and radiographic factors using the mean as a measure of central tendency. A univariate analysis and multivariate analysis of clinical characteristics and outcomes were conducted. We included some common outcome-related factors and some specific factors from our theory in our univariate analysis. Multivariate analysis was done by unconditional logistic regression: Association between factors and poor-functional outcome. All covariates with p value <0.2 in the univariate analysis, Y (poor-functional outcome) = PMERA + Age + pre-op Fisher + CTA spot sign + Perisylvian Low Density + Distal hematoma as reference). Contingency statistical analysis on categorical variables was performed with Fisher’s exact test. All statistical tests were two-sided, and p < .05 was prospectively determined to establish statistical significance. All analyses were performed using SPSS Statistics version 21.
Results
Patient Demographics and Radiographic Characteristics
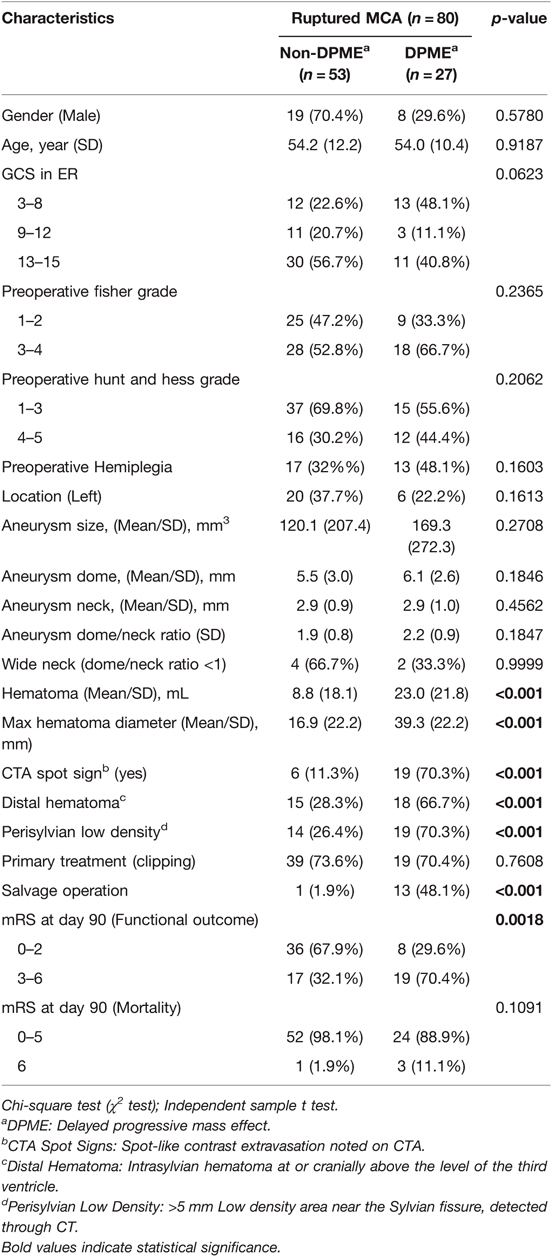
The clinical data of 80 patients with aSAH caused by a ruptured MCA aneurysm were retrospectively analysed, and the detailed demographic and clinical characteristics are summarised in Table 1. The participants’ mean age was 54.2 years, and 31.2% were men. All patients were deemed functionally independent according to a medical record before presentation. The mean aneurysm size was 136.7 mm3, with a dome of 5.7 mm and neck of 2.9 mm, and all aneurysms were located at or proximal to the M1–2 bifurcation. A total of 26 (32.5%) patients had an aneurysm on the left, 28 (35%) patients had an H–H grade of IV or V at admission, and 46 (57.6%) patients had a Fisher grade of III or IV. A total of 46 (57.6%) patients presented with subarachnoid haemorrhage or intracranial haemorrhage. The mean hematoma volume was 13.6 mL, and the hematomas were entirely located on the ipsilateral side of the ruptured aneurysm. The mean of the maximum hematoma diameters was 24.5 mm. Preoperative images detected CTA spot signs in 25 (31.3%) patients, distal hematoma in 33 (41.2%) patients, and perisylvian low density in 33 (41.2%) patients. All patients were treated within 48 h. Microsurgical clipping was performed in 58 (72.5%) patients, and endovascular coiling was performed in 22 (27.5%) patients. Postoperative CT scans indicated 27 (33.7%) patients developed DPME. Of the 14 patients requiring salvage decompression surgery, 13 had DPME. Up to 48.1% patients with DPME required salvage surgery.
DPME and Other Radiographic Characteristics
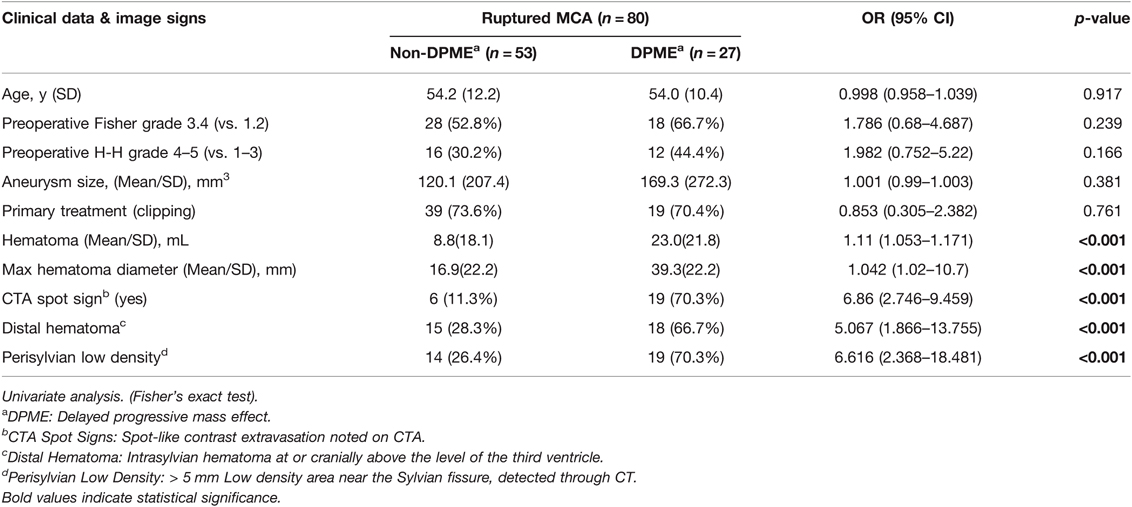
Characteristics of the patients who developed DPME from MCA aSAHs are summarised in Table 2. DPME was observed in 27 of 80 (33.7%) patients with aSAHs caused by ruptured MCA aneurysms. A representative case of DPME is presented in Figure 1. A total of 19 (70.4%) patients who received surgical clipping and 8 (29.6%) patients who received endovascular coiling developed DPME after their primary treatment. The listed radiographic characteristics, including the morphology of the aneurysm, type of therapy (clipping or coiling), and preoperative Fisher and H–H grades, did not differ significantly between the DPME and non-DPME groups. However, some preoperative image factors, including hematoma size, distal hematomas, and perisylvian low density, differed significantly between these two groups (Table 3).
Risk factors predictive of DPME, according to clinical data and images, are presented in Table 3. DPME was not significantly associated with age, primary treatment, preoperative H–H grade, Fisher grade, or aneurysm size. Rather, it was more highly associated with hematoma size (OR 1.11; 95% CI 1.053–1.171; p < 0.001), max diameter of the hematoma (OR 1.042; 95% CI 1.02–10.7; p < 0.001), CTA spot signs (OR 6.86; 95% CI 2.746–9.459; p < 0.001), distal hematoma (OR 5.067; 95% CI 1.866–13.755; p < 0.001), and perisylvian low density (OR 6.616; 95% CI 2.368–18.481; p < 0.001).
DPME and Functional Outcomes
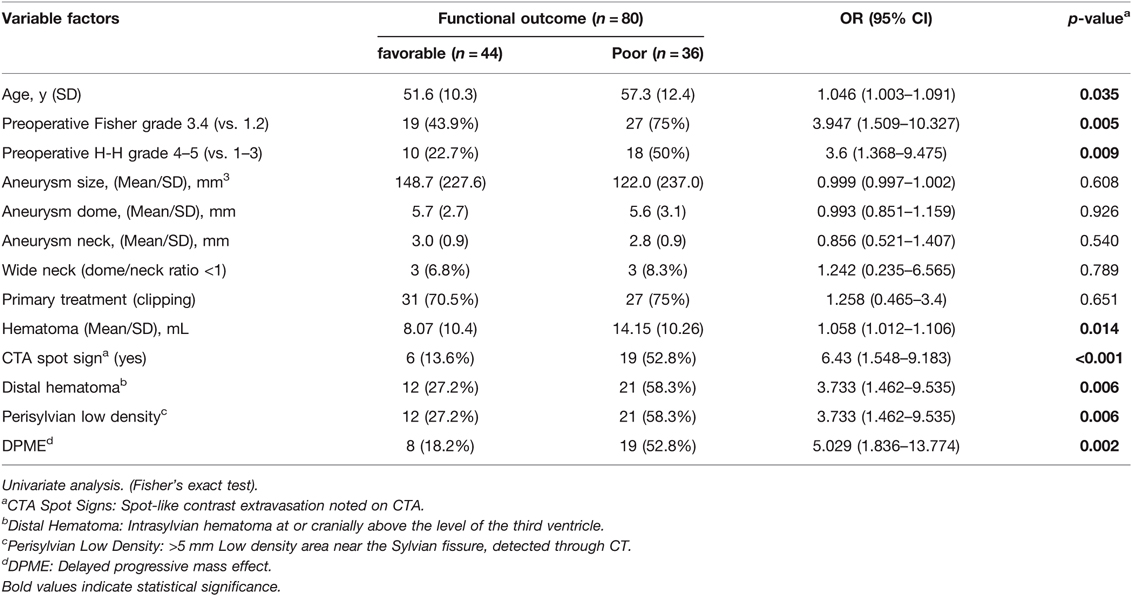
The Modified Rankin Scale (mRS) scores at 3 months after the initial aSAH event in the DPME and non-DPME groups are summarised in Table 2. Favourable functional outcomes (mRS scores of 0–2) were observed in 36 (81.8%) patients without DPME. The non-DPME group had significantly superior functional outcomes (p = 0.0018) to the DPME group.
Univariate logistic regression analysis demonstrated that the DPME group exhibited poorer functional outcomes (OR 5.029; 95% CI 1.836–13.774; p = 0.002; Table 4) than the non-DPME group. Perisylvian low density (OR 3.733; 95% CI 1.462–9.535; p = 0.006), distal hematoma (OR 3.733; 95% CI 1.462–9.535; p = 0.006), CTA spot signs (OR 6.43; 95% CI 1.548–9.183; p = 0.001), Fisher grade 3–4 (vs. 1–2; OR 3.947; 95% CI 1.509–10.327; p = 0.005), H–H grade 4–5 (vs. 1–3; OR 3.6; 95% CI 1.368–9.475; p = 0.009), and age (OR 1.046; 95% CI 1.003–1.091; p = 0.035) were also significant predictors of poor outcomes (Table 4).
Discussion
Ruptured MCA Aneurysm and DPME
Studies have reported that patients with aSAH with intraparenchymal hematomas usually present with higher H–H grades and have poorer functional neurological outcomes than patients with aSAH alone or with smaller hematomas (16). Nonoperative management for aSAH with large parenchymal hematomas and signs of uncal herniation is nearly universally fatal, whereas early hematoma evacuation and aneurysm clipping can improve short-term and long-term outcomes (2, 5, 6, 8). Studies have reported that patients with larger hematomas presented in poorer initial clinical condition and were more likely to require additional neurosurgical interventions involving decompressive craniectomy or hematoma evacuation (17). Smith et al. proposed a pathological process in which brain swelling and ischaemic infarction exacerbate each other in the limited intracranial space (11). Fisher and Ojemann described it as a “feed-forward” cycle of brain injury (9), and Saito et al. reported that patients with SAH with perisylvian hematoma can easily develop delayed postoperative brain swelling approximately 1 week after surgery (18). Perisylvian ICH was deemed to entail greater risk of developing postoperative brain oedema and infarction (Figure 2). A few case reports have identified multifocal extravasation sites from subpial MCA branches resulting in Sylvian hematoma formation. Sylvian hematomas may develop when bleeding occurs in the subpial space rather than in the subarachnoid space, and an expanding hematoma in this location can cause severe brain damage (1, 14). In this study, we identified possible risk factors that can alert neurosurgeons preoperatively and discussed the possible mechanisms resulting in haemorrhagic or ischaemic DPME after securing a ruptured MCA aneurysm.
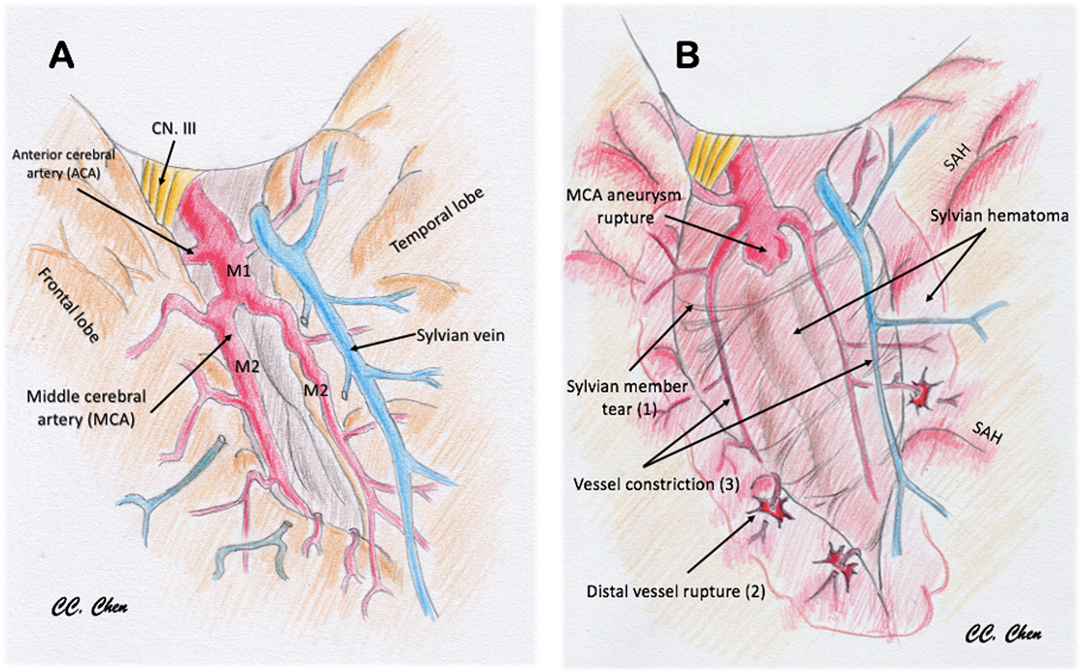
Figure 2. Illustration to demonstrate the pathomechanism of delayed progressive mass effect. (A) Normal vessels and sylvian fissure. (B) Pathological change after MCA aneurysm rupture with sylvian hematoma: (1) sylvian member and pia tear by the stretch of hematoma; (2) cortical artery was straightened and distal vessel rupture (spots sign or distal hematoma); (3) vessel diameter lessening, flow obstruction, and ischemia by hematoma compression (perisylvian low density).
Possible Pathomechanism of DPME
ICH alone is a predictor of poor outcomes in association with ruptured MCA aneurysms (19–25). In such cases, the focal destruction of the functional brain area by an expanding hematoma volume results in possible permanent neurological deficits unrelated to the increased intracranial pressure that can be controlled through hematoma evacuation or other decompression procedures.
In our cohort, the incidence of postoperative brain swelling and infarction was 33% (27/80) in patients with ruptured MCA aneurysms. This incidence is relatively high compared with patients with other types of ruptured anterior circulation aneurysms. Several potential mechanisms have been proposed to explain the development of DPME. First, MCA aneurysms are usually located in a confined space within the Sylvian fissure that is adjacent to both the frontal and temporal lobes (26). Once an aneurysm ruptures, a dense focal hematoma usually forms near the lesion within the Sylvian fissure. When a thickened subarachnoid hematoma forms, small parasylvian cortical arteries are potentially dissected from the parasylvian brain parenchyma. Some studies have suggested that the sudden high pressure resulting from a ruptured aneurysm can damage the pial membrane and the parasylvian cortical arteries, which may result in a subpial extension of the subarachnoid hematoma. This phenomenon could explain the possible ischaemic effect of the blood supply to the parasylvian cortex being halted and the haemorrhagic effect due to punctuate bleeding from the disrupted end of the small cortical arteries. This phenomenon may also result in increased ischaemic or infarct core growth or delayed hematoma formation, as indicated by Yusuke et al. (26). However, we believe that because of the destruction of the microcirculation of the pia mater, this effect should be observed within a limited region of the parasylvian cortex without causing more severe primary vessel territory infarction. Furthermore, van Asch et al. (27) reported that the expansion of the ICH resulting from a ruptured aneurysm without signs of aneurysmal rebleeding is not rare within 48 h after onset, and they speculated that the hematoma expansion was caused by blood from damaged vessels surrounding the hematoma, which is consistent with our observations. These mechanisms were summarized and illustrated in Figure 2.
Second, the development of delayed cerebral ischaemia (DCI), the most critical complication of aSAH, has a severe impact on the outcome of patients; DCI has been widely studied. It accounts for almost 30% of new neurological deficits after an initial haemorrhage (10, 28). Yet, to date, the development of DCI cannot be predicted reliably for individual patients, and some early deterioration of the intracranial mass effect cannot be solely explained through vasospasm.
Third, peri-hematoma cerebral oedema after ICH is secondary to neuroinflammation and oxidative stress from reactions triggered by red blood cell lysis, thrombin production, complement activation, and neuroinflammation, which damage the blood–brain barrier (29–31). This delayed swelling is another possible mechanism of DPME.
Finally, we cannot exclude the possibility that these delayed effects on the brain could be secondary to the requirement for temporary occlusion of the parent artery during treatment of the ruptured aneurysm—the temporary clip used microsurgically or the balloon-assisted technique used endovascularly may be implicated. Therefore, concluding that a single mechanism is responsible for the condition of each patient with DPME is difficult.
Practical Radiographic Predictors of DPME
Figures 3 and 4 demonstrated the contrast results of clipping MCA aneurysm with different preoperative CTA findings. Univariate analysis revealed significant differences in CTA spot signs, distal hematoma, and perisylvian low density between the DPME and non-DPME groups (Table 3). We also demonstrated that hematoma size and maximum hematoma diameter are significant risk factors of DPME. This finding is similar to that of Hilditch et al. (1), who stated that sylvian hematomas may develop secondary to bleeding in the subpial space rather than in the subarachnoid space and an expanding hematoma in the subpial space can cause severe brain damage. When an aneurysm adherent to the pia mater bleeds, the blood may spread below the pia, and the expanding hematoma may rupture the thin arteries that run in the pial space. Suzuki et al. (32) indicated that patients in three studies had either MCA aneurysms or posterior communicating artery aneurysms and a ruptured aneurysm close to the Sylvian fissure may result in blood flowing into the subpial space and triggering events leading to hematoma formation. Therefore, in light of the literature and our findings, we propose that neurosurgeons should note these potential preoperative predictors and prepare for the possible subsequent deterioration of intracranial mass effects and the need for early decompression and extensive hematoma evacuation.
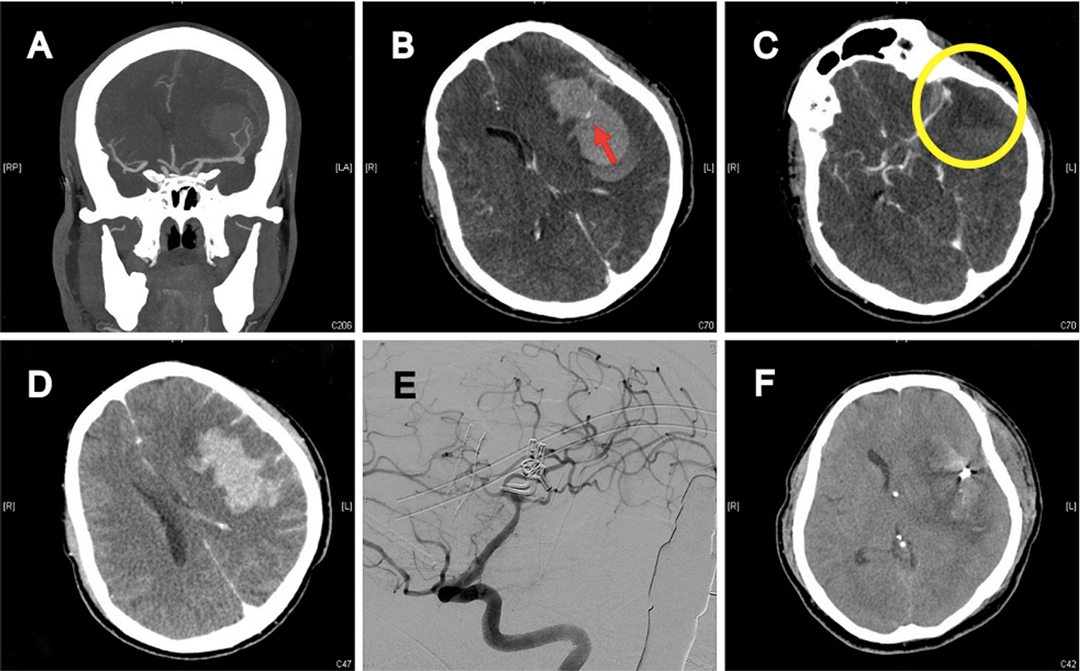
Figure 3. 41-year-old female with high-grade aSAH caused by ruptured left MCA aneurysm. (A) Preoperative brain CTA depicted severe aSAH, ICH, and two MCA aneurysms. (B) Intra-hematoma spot sign (red arrow). (C) Perisylvian low density (yellow circle). (D) Distal hematoma. (E) 5 days after craniotomy to remove hematoma and clip two aneurysms; post-operative angiography revealed complete obliteration of aneurysms, well preservation of all MCA branches, and no vasospasm. (F) Delayed progressive mass effect with midline shift.
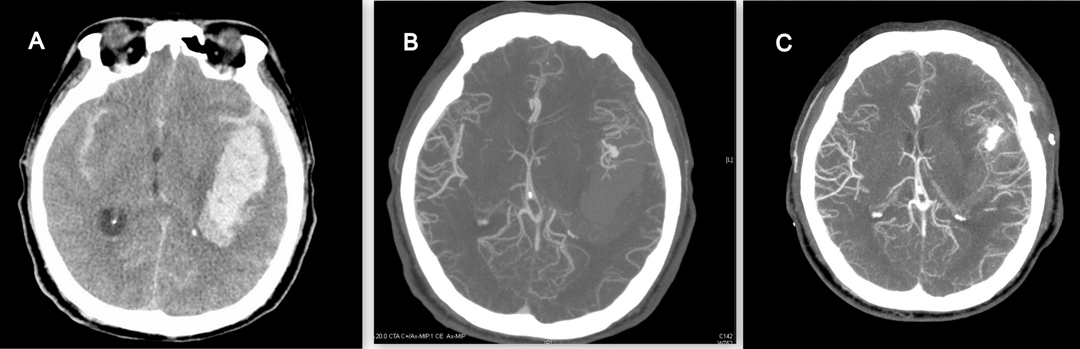
Figure 4. 55-year-old female with ruptured left MCA aneurysm. (A) Large left temporal hematoma, but no perisylvian low density, no spot sign, no distal hematoma. (B) CTA revealed M1 bifurcation aneurysm. (C) After successful clipping, there were no delayed progressive mass effect 7 days after operation.
DPME and Functional Outcomes
The outcome of a patient with a ruptured aneurysm depends heavily on the initial GCS score; however, other factors, such as age, vasospasm, collateral vessel perfusion, and hematoma volume, may alter the outcome (33). In this study, the outcomes between the DPME and non-DPME group differed significantly (p = 0.0018; Table 2); moreover, in our cohort, 14 (17.5%) patients required salvage surgery, but the rate of those requiring salvage surgery was significantly higher (48.1%) in the DPME group (p < 0.001; Table 2). Only one patient in the non-DPME group required salvage surgery because he received the surgery as his level of consciousness diminished. We determined other possible risk factors, including age, initial H–H and Fisher grades, volume of hematoma, CTA spot signs, perisylvian low density, distal hematoma, and DPME, through a univariate logistic regression analysis (Table 5). Neither the morphology of the aneurysm or a wide-neck aneurysm appears to greatly affect patients’ outcomes.
Limitations of the Study
Selection bias and lack of randomisation are two limitations of this retrospective study. The role of vasospasm in the development of DPME is underestimated because our study mandated that a minority of patients who required intra-arterial therapy for vasospasm. Additionally, operator-dependent matters, such as the timing and method of temporary occlusion during the procedure, were still variable. However, in the study, the procedures were all performed by attending neurosurgeons and neurointerventionalists with at least 5-year experience of aneurysm treatment in the single center. Therefore, the general principles and protocols, including postoperative care, image follow up, nimodipine, fluid and blood pressure control for vasospasm, were consistent and coherent. The bias should be minimum. A well-designed prospective validation of independent cohorts is required to determine the exact timing of and indication for extra decompression procedures when DPME is anticipated. Otherwise, a multicenter investigation would be considered to establish more population and better confidence with normal distribution.
Conclusion
DPME is directly related to poor outcomes in patients with a ruptured MCA aneurysm. Furthermore, these patients might have a higher incidence of secondary salvage decompression procedures. Examining preoperative images for prognostic characteristics, such as distal hematoma, perisylvian low density, and CTA spot signs, is recommended to predict DPME.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by IRB no. 201800342B0. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Y-CL: concept and design of the work, drafting the article. C-CC: data analysis and interpretation. C-TC: techniques review and conduct. P-HT: techniques review and conduct. M-CY: techniques review and conduct. Y-MW: techniques review and conduct. Z-HL: techniques review and conduct. T-WC: techniques consultation. Y-JL: techniques consultation. T-WEW: statistical analysis and consultation. P-CH: concept and design of the work, final review and interpretation. All authors: final approval and data collection. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Chen-Ying Liu for assistance in the endovascular procedures and Wallace Academic Editing Services Co. for editing this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hilditch CA, Sonwalkar H, Wuppalapati S. Remote multifocal bleeding points producing a Sylvian subpial hematoma during endovascular coiling of an acutely ruptured cerebral aneurysm. BMJ Case Rep. (2016) 2016. doi: 10.1136/bcr-2016-012715
2. Bohnstedt BN, Nguyen HS, Kulwin CG, Shoja MM, Helbig GM, Leipzig TJ, et al. Outcomes for clip ligation and hematoma evacuation associated with 102 patients with ruptured middle cerebral artery aneurysms. World Neurosurg. (2013) 80:335–41. doi: 10.1016/j.wneu.2012.03.008
3. Heiskanen O, Poranen A, Kuurne T, Valtonen S, Kaste M. Acute surgery for intracerebral haematomas caused by rupture of an intracranial arterial aneurysm. A prospective randomized study. Acta Neurochir (Wien). (1988) 90:81–3. doi: 10.1007/BF01560559
4. Nowak G, Schwachenwald D, Schwachenwald R, Kehler U, Muller H, Arnold H. Intracerebral hematomas caused by aneurysm rupture. Experience with 67 cases. Neurosurg Rev. (1998) 21:5–9. doi: 10.1007/BF01111478
5. Joo SP, Kim TS, Choi JW, Lee JK, Kim YS, Moon KS, et al. Characteristics and management of ruptured distal middle cerebral artery aneurysms. Acta Neurochir (Wien). (2007) 149:661–7. doi: 10.1007/s00701-007-1061-5
6. Shimoda M, Oda S, Mamata Y, Tsugane R, Sato O. Surgical indications in patients with an intracerebral hemorrhage due to ruptured middle cerebral artery aneurysm. J Neurosurg. (1997) 87:170–5. doi: 10.3171/jns.1997.87.2.0170
7. Tapaninaho A, Hernesniemi J, Vapalahti M. Emergency treatment of cerebral aneurysms with large haematomas. Acta Neurochir (Wien). (1988) 91:21–4. doi: 10.1007/BF01400522
8. Yoshimoto Y, Wakai S, Satoh A, Hirose Y. Intraparenchymal and intrasylvian haematomas secondary to ruptured middle cerebral artery aneurysms: prognostic factors and therapeutic considerations. Br J Neurosurg. (1999) 13:18–24. doi: 10.1080/02688699944131
9. Fisher CM, Ojemann RG. Bilateral decompressive craniectomy for worsening coma in acute subarachnoid hemorrhage. Observations in support of the procedure. Surg Neurol. (1994) 41:65–4. doi: 10.1016/0090-3019(94)90210-0
10. Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. (2014) 10:44–58. doi: 10.1038/nrneurol.2013.246
11. Smith ER, Carter BS, Ogilvy CS. Proposed use of prophylactic decompressive craniectomy in poor-grade aneurysmal subarachnoid hemorrhage patients presenting with associated large sylvian hematomas. Neurosurgery. (2002) 51:117–24; discussion 124. doi: 10.1097/00006123-200207000-00018
12. Minami N, Kimura T, Ichikawa Y, Morita A. Emerging sylvian subpial hematoma after the repair of the ruptured anterior cerebral artery aneurysm with interhemispheric approach: case report. Neurol Med Chir (Tokyo). (2014) 54:227–30. doi: 10.2176/nmc.cr2013-0025
13. Roth P, Happold C, Eisele G, Nagele T, Weller M, Luft AR: A series of patients with subpial hemorrhage: clinical manifestation, neuroradiological presentation and therapeutic implications. J Neurol. (2008) 255:1018–22. doi: 10.1007/s00415-008-0824-8
14. Suzuki K, Matsuoka G, Abe K, Okada Y, Sakai S. Subpial hematoma and extravasation in the interhemispheric fissure with subarachnoid hemorrhage. Neuroradiol J. (2015) 28:337–40. doi: 10.1177/1971400915576664
15. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. (2012) 43:1711–37. doi: 10.1161/STR.0b013e3182587839
16. Abbed KM, Ogilvy CS. Intracerebral hematoma from aneurysm rupture. Neurosurg Focus. (2003) 15:E4. doi: 10.3171/foc.2003.15.4.4
17. Darkwah Oppong M, Skowronek V, Pierscianek D, Gembruch O, Herten A, Saban DV, et al. Aneurysmal intracerebral hematoma: risk factors and surgical treatment decisions. Clin Neurol Neurosurg. (2018) 173:1–7. doi: 10.1016/j.clineuro.2018.07.014
18. Saito A, Akamatsu Y, Mikawa S, Sugawara T, Seki H. Comparison of large intrasylvian and subpial hematomas caused by rupture of middle cerebral artery aneurysm. Neurol Med Chir (Tokyo). (2010) 50:281–5. doi: 10.2176/nmc.50.281
19. Fukuda H, Hayashi K, Moriya T, Nakashita S, Lo BW, Yamagata S. Intrasylvian hematoma caused by ruptured middle cerebral artery aneurysms predicts recovery from poor-grade subarachnoid hemorrhage. J Neurosurg. (2015) 123:686–92. doi: 10.3171/2014.10.JNS141658
20. Hauerberg J, Eskesen V, Rosenorn J. The prognostic significance of intracerebral haematoma as shown on CT scanning after aneurysmal subarachnoid haemorrhage. Br J Neurosurg. (1994) 8:333–9. doi: 10.3109/02688699409029622
21. Jabbarli R, Reinhard M, Roelz R, Shah M, Niesen WD, Kaier K, et al. Intracerebral hematoma due to aneurysm rupture: are there risk factors beyond aneurysm location? Neurosurgery. (2016) 78:813–20. doi: 10.1227/NEU.0000000000001136
22. Lee CS, Park JU, Kang JG, Lim YC. The clinical characteristics and treatment outcomes of patients with ruptured middle cerebral artery aneurysms associated with intracerebral hematoma. J Cerebrovasc Endovasc Neurosurg. (2012) 14:181–5. doi: 10.7461/jcen.2012.14.3.181
23. Liu X, Rinkel GJ. Aneurysmal and clinical characteristics as risk factors for intracerebral haematoma from aneurysmal rupture. J Neurol. (2011) 258:862–5. doi: 10.1007/s00415-010-5855-2
24. Pasqualin A, Bazzan A, Cavazzani P, Scienza R, Licata C, Da Pian R. Intracranial hematomas following aneurysmal rupture: experience with 309 cases. Surg Neurol. (1986) 25:6–17. doi: 10.1016/0090-3019(86)90107-2
25. Wan A, Jaja BN, Schweizer TA, Macdonald RL, Collaboration obotS. Clinical characteristics and outcome of aneurysmal subarachnoid hemorrhage with intracerebral hematoma. J Neurosurg. (2016) 125:1344–51. doi: 10.3171/2015.10.JNS151036
26. Egashira Y, Yoshimura S, Enomoto Y, Ishiguro M, Asano T, Iwama T. Ultra-early endovascular embolization of ruptured cerebral aneurysm and the increased risk of hematoma growth unrelated to aneurysmal rebleeding. J Neurosurg. (2013) 118:1003–8. doi: 10.3171/2012.11.JNS12610
27. van Asch CJ, Oudendijk JF, Rinkel GJ, Klijn CJ. Early intracerebral hematoma expansion after aneurysmal rupture. Stroke. (2010) 41:2592–5. doi: 10.1161/STROKEAHA.110.589291
28. Sarrafzadeh AS, Vajkoczy P, Bijlenga P, Schaller K. Monitoring in neurointensive care - the challenge to detect delayed cerebral ischemia in high-grade aneurysmal SAH. Front Neurol. (2014) 21(5):134. doi: 10.3389/fneur.2014.00134
29. Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT. Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg. (1996) 84:91–6. doi: 10.3171/jns.1996.84.1.0091
30. Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke. (2001) 32:2932–8. doi: 10.1161/hs1201.099820
31. Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. (2006) 5:53–63. doi: 10.1016/S1474-4422(05)70283-0
32. Suzuki K, Ueno E, Kasuya H. Origin of sylvian hematoma in patients with subarachnoid hemorrhage: findings of extravasation on multiphase contrast-enhanced computed tomography. World Neurosurg. (2014) 82:e747–51. doi: 10.1016/j.wneu.2013.02.014
Keywords: brain oedema, middle cerebral artery, intracranial aneurysm, aneurysm clip, aneurysm embolization
Citation: Li Y, Chen C, Chen C, Tu P, Yeap M, Wu Y, Liu Z, Chang T, Lin Y, Wu TE and Hsieh P (2022) Delayed Progressive Mass Effect After Secured Ruptured Middle Cerebral Artery Aneurysm: Risk Factors and Outcomes. Front. Surg. 9:852576. doi: 10.3389/fsurg.2022.852576
Received: 11 January 2022; Accepted: 14 April 2022;
Published: 2 May 2022.
Edited by:
Alfio Spina, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Francesco Calvanese, Hôpital Pierre Wertheimer, FranceGianluca Nocera, San Raffaele Hospital (IRCCS), Italy
Copyright © 2022 Li, Chen, Chen, Tu, Yeap, Wu, Liu, Chang, Lin, Wu and Hsieh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Po-Chuan Hsieh cG9jaHVhbi5oc2llaEBnbWFpbC5jb20=
Speciality section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Ying-Ching Li1
Ying-Ching Li1 Ching-Chang Chen
Ching-Chang Chen Zhuo-Hao Liu
Zhuo-Hao Liu Ya-Jui Lin
Ya-Jui Lin