
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg., 25 April 2022
Sec. Neurosurgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.850050
 Brittany M. Stopa1
Brittany M. Stopa1 Joshua A. Cuoco1,2,3
Joshua A. Cuoco1,2,3 Srijan Adhikari1,2,3
Srijan Adhikari1,2,3 Douglas J. Grider1,4
Douglas J. Grider1,4 Cara M. Rogers1,2,3
Cara M. Rogers1,2,3 Eric A. Marvin1,2,3*
Eric A. Marvin1,2,3*Metastasis of ovarian carcinoma to the central nervous system occurs in <2% of cases and classically localizes within the brain parenchyma. Moreover, leptomeningeal spread of these tumors is an exceedingly rare phenomenon. Here, we conduct a systematic review of the current literature on the natural history, treatment options, and proposed pathogenic mechanisms of leptomeningeal carcinomatosis in ovarian carcinoma. We also report a case of a 67-year-old female with stage IV metastatic ovarian serous carcinoma initially confined to the peritoneal cavity with a stable disease burden over the course of three years. Follow-up imaging demonstrated an intracranial lesion, which was resected via craniotomy, and pathology was consistent with the original diagnosis. Three months after surgery, she developed rapidly progressive dizziness, generalized weakness, fatigue, and ataxia. Repeat MRI demonstrated interval development of extensive and diffusely enhancing dural nodularity, numerous avidly enhancing supratentorial and infratentorial lesions, enhancement of the bilateral trigeminal nerves, internal auditory canals, and exit wound from the surgical site into the posterior aspect of the right-sided neck musculature consistent with diffuse leptomeningeal dissemination. The present case highlights that leptomeningeal dissemination of ovarian carcinoma is a potential yet rare consequence following surgical resection of an ovarian parenchymal metastasis. Progressive clinical symptomatology that develops postoperatively in this patient population should prompt urgent workup to rule out leptomeningeal disease and an expedited radiation oncology consultation if identified.
Ovarian cancer is the second most common gynecologic malignancy as well as the fifth leading cause of cancer deaths in women (1). Diagnosis typically occurs late in the disease course with patients presenting with stage III or IV disease due to a lack of effective screening methodologies and nonspecific presenting symptomatology (1). Specifically, high-grade serous ovarian carcinoma is associated with poor clinical outcomes. More than 70% of patients present with an advanced disease burden and distant metastases (2). Disease dissemination mainly involves the peritoneal cavity, with hematogenous spread rarely seen (2). Ovarian metastases to the brain are a known phenomenon, although they represent less than 2% of cases (3–8). Moreover, leptomeningeal seeding of ovarian carcinoma is an exceedingly rare entity with most cases published as case series or individual case reports (5, 9–11). As such, the literature on the natural history and effective treatment strategies of this phenomenon remains sparse.
Despite adjuvant treatment, the five-year survival for patients with ovarian carcinoma is 48%, which is attributable to disease relapse and drug resistance (2). Moreover, the five-year survival for patients with distant lesions is 29%, compared to up to a 5-year survival rate of 92% in patients with localized disease (2). Traditionally, clinicopathologic prognostic factors have not been sufficient in predicting distant spread as no molecular markers have shown to be associated with a tendency to metastasize (2, 12, 13). However, several recent studies have shown promise in identifying various biomarkers for distinguishing distant metastases in patients with high-grade serous ovarian carcinoma (2, 12, 13). Romani et al. (12) demonstrated reduced expression of claudin-7, a major component of the apical tight junction complex, to be associated with the burden of distant metastases. Moreover, Wu et al. (13) found soluble mesothelin-related peptide to be a promising biomarker of high-grade serous ovarian carcinoma with a higher specificity, omission diagnostic rate, and positive predictive value but lower sensitivity and negative predictive value compared to CA-125. Although these studies have begun to lay the groundwork for biomarker detection of distant metastases, the recognition of intracranial involvement remains a challenge due to the permeability of the blood–brain barrier. Here, we report a case of leptomeningeal carcinomatosis of ovarian serous carcinoma three months following craniotomy for resection of a metastatic intracranial lesion. We conduct a systematic review of the current literature on the natural history, treatment options, and proposed pathogenic mechanisms of leptomeningeal carcinomatosis in ovarian carcinoma.
A 67-year-old female presented to the emergency department with several days of progressively worsening headaches, dizziness, and confusion. Past medical history was remarkable for stage IV high-grade serous ovarian carcinoma diagnosed three years prior. At that time, she underwent five cycles of neoadjuvant chemotherapy followed by diagnostic laparoscopy, exploratory laparotomy, total abdominal hysterectomy, bilateral salpingo-oophorectomy, infragastric omentectomy, bilateral pelvic and para-aortic lymphadenectomy, argon bean coagulation of peritoneal implants, and optimal interval reductive surgery to no gross residual disease. She was dispositioned to postoperative chemotherapy with three cycles of paclitaxel and carboplatin. She completed the aforementioned treatment course three years prior to her presentation with no evidence of recurrent disease in the interim based on radiographic imaging and CA-125 serum levels. Given the nature of her symptomatology, there was a concern for disease recurrence with metastases to the brain prompting MRI, which demonstrated a large right posterior temporal heterogeneously enhancing lesion with scattered calcifications and surrounding vasogenic edema that appeared to arise from the right tentorium (Figures 1A,B). Repeat CT of the chest, abdomen, and pelvis was without evidence of recurrence of her primary disease or additional metastatic burden. The intracranial lesion was suspected to be metastatic in etiology rather than a new primary lesion, given her oncologic history.

Figure 1. (A,B) Initial T1-weighted MRI with contrast demonstrates a large right posterior temporal heterogeneously enhancing lesion with scattered calcifications and surrounding vasogenic edema that appeared to arise from the right tentorium. (C,D) Postoperative T1-weighted MRI with contrast demonstrates expected postoperative changes with minimal residual along the tentorium.
Given her clinical history and tumor size, surgical resection was recommended. A stereotactic right temporoparietal craniotomy was performed for surgical resection. The dura was opened in a C-shaped fashion toward the transverse sinus, which was skeletonized. Abnormal tissue was immediately identified along the inferior aspect of the craniotomy. The tissue was adherent to the dura and appeared to be somewhat calcified. A circumferential dissection was performed around the tumor with decent white matter planes. The tentorial pedicle was cauterized and cut sharply. There was tumor on the lateral ventricular wall, which was resected. The tumor was resected en bloc. There was no evidence of cerebrospinal fluid egress to suggest gross violation of the ventricular wall. Histopathology showed nests and small papillae of pleomorphic tumor cells, abundant psammoma bodies, and extensive necrosis (Figures 2A–C). Immunohistochemistry demonstrated WT-1, p53, cytokeratin-7, p16, and CA-125 positivity (Figures 2D–H). The intracranial lesion was morphologically similar to her omental biopsy three years prior with an identical immunoprofile upon comparison (Supplementary Figures 1A–H). A diagnosis of metastatic high-grade serous ovarian carcinoma based upon the intracranial lesion's histopathology, immunohistochemical staining profile, and similarity to the prior omental biopsy was made (14–18).
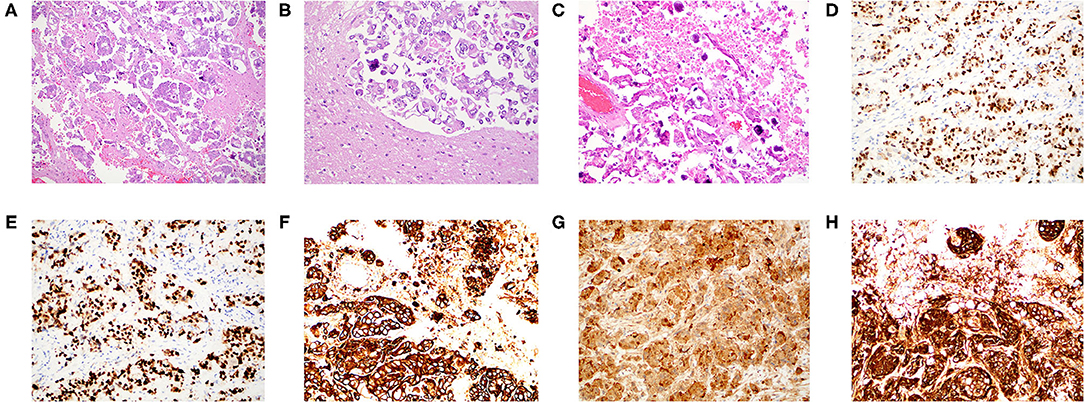
Figure 2. Histopathology and immunohistochemistry of the intracranial metastatic serous carcinoma. (A) H&E stain at 100x magnification with nests and small papillae of pleomorphic tumor cells infiltrating brain parenchyma. (B) H&E stain at 200× magnification demonstrating pleomorphic carcinoma with a well-formed psammoma body. (C) H&E stain at 200× magnification demonstrating numerous psammoma bodies. (D) WT-1 immunostain at 200× magnification demonstrating strong nuclear positivity. (E) p53 immunostain at 200× magnification strongly marking the nuclei of all tumor cells. (F) Cytokeratin-7 immunostain at 200× magnification with strong cytoplasmic reactivity. (G) p16 immunostain at 200× magnification with strong nuclear positivity. (H) CA-125 immunostain at 200× magnification with strong cytoplasmic reactivity.
Postoperative MRI of the brain demonstrated expected changes with minimal residual along the tentorium (Figures 1C,D). Her neurologic symptomatology resolved postoperatively. She completed adjuvant CyberKnife stereotactic radiation to the surgical site. Three months after surgery, she developed rapidly progressive dizziness, generalized weakness, fatigue, and ataxia. Repeat MRI demonstrated interval development of extensive diffusely enhancing dural nodularity, numerous avidly enhancing supratentorial and infratentorial lesions, enhancement of the bilateral trigeminal nerves, internal auditory canals, and exit wound from the surgical site into the posterior aspect of the right-sided neck musculature, consistent with diffuse leptomeningeal dissemination (Figure 3). This repeat MRI was obtained approximately three weeks before the patient expired. Radiation oncology provided expedited whole-brain radiation; however, she was not a candidate for intrathecal chemotherapy given her rapid disease evolution and progressive neurologic symptomatology. She was transitioned to palliative care and expired a few weeks thereafter.
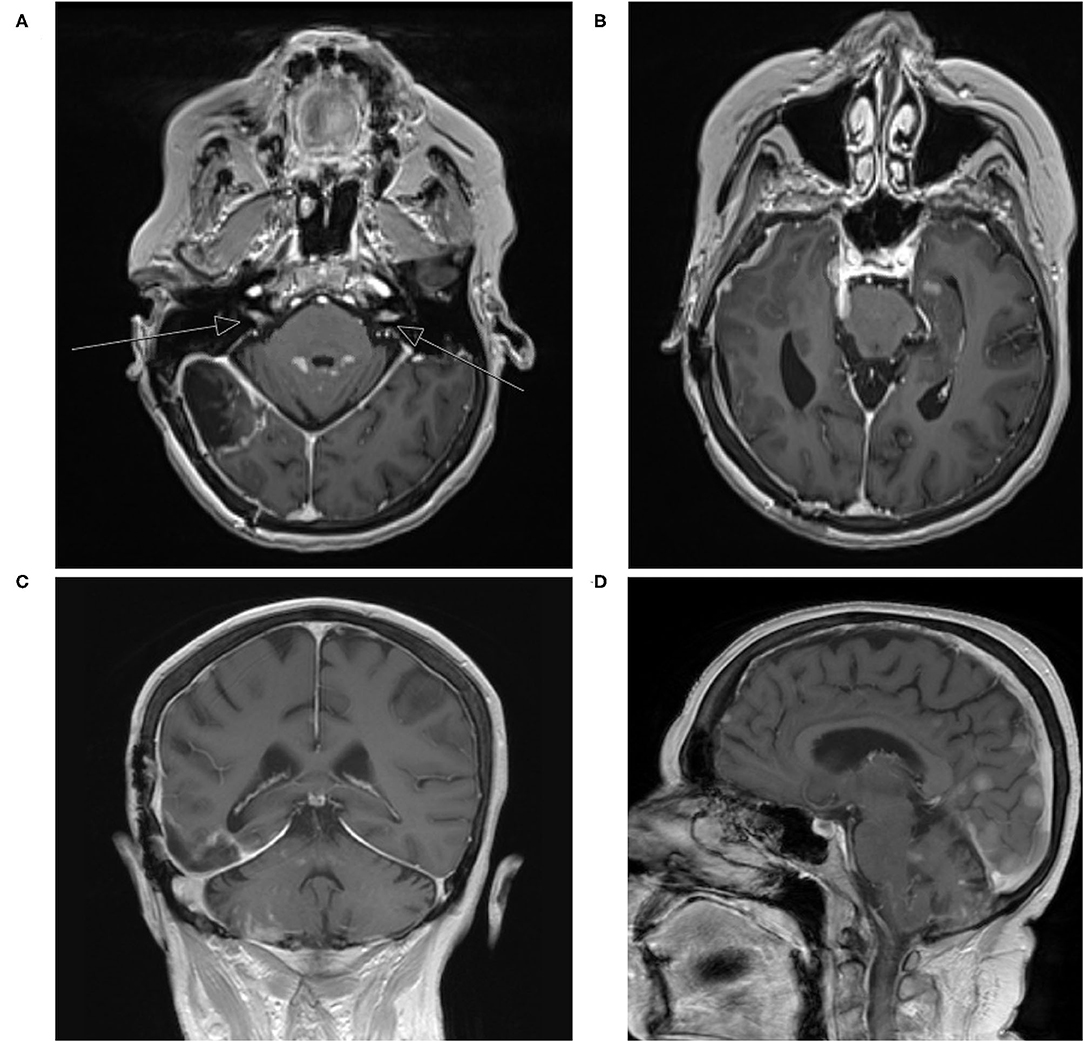
Figure 3. (A–D) Repeat T1-weighted MRI with contrast three years later with interval development of extensive diffuse enhancing dural nodularity, numerous avidly enhancing supratentorial and infratentorial lesions, enhancement of the bilateral trigeminal nerves, internal auditory canals, and exit wound from the surgical site into the posterior aspect of the right-sided neck musculature consistent with diffuse leptomeningeal dissemination (arrows indicate internal auditory canals).
A Siemens 1.5 Tesla MRI was utilized to acquire all of the images. The following machine parameters were utilized for sequences: T1–repetition time: <800, time to echo: <30, flip angle: 90°; T2–repetition time: >2,000, time to echo: >80, flip angle: 90°. Multiplanar, multisequence imaging of the brain was obtained before and after intravenous administration of 8 ml of Gadavist.
Immunohistochemical stains were performed on paraffin tissue sections of the omental biopsy and the right intracranial metastases. The following workflow for WT-1, p53, cytokeratin-7, p16, and CA-125 immunohistochemistry was performed: primary antibody (e.g., p53 or WT-1 antibody), antigen retrieval (e.g., immunohistochemistry-tek epitope retrieval steamer set), blocking (e.g., avidin–biotin blocking kit, streptavidin–biotin blocking kit), detection (e.g., avidin–biotin complex, labeled streptavidin–biotin), chromogen substrate (e.g., 3,3′-diaminobenzidine), counterstain (e.g., Mayer's hematoxylin), and results (e.g., staining patterns).
Catalog numbers and the supplier information for each immunohistochemical stain used for the intracranial metastasis are as follows: WT-1 (Dako, IR055), p53 (Dako, GA616), cytokeratin-7 (Dako, GA619), p16 (BioSB BSB5828), and CA-125 (Dako, GA701). This p53 antibody reacts with wild-type and mutant p53 protein. Catalog numbers and the supplier information for each immunohistochemical stain used for the omental biopsy are as follows: WT-1 (Ventana, 760-4397), p53 (Cell Marque, 453M-96), cytokeratin-7 (Cell Marque, 307M-96), p16 (Ventana, 705-4793), and CA-125 (Nova Castra, NCL-L-CA-125). This p53 antibody also reacts with wild-type and mutant p53 protein.
A systematic literature review of leptomeningeal carcinomatosis in ovarian carcinoma was performed according to PRISMA guidelines. The following databases were queried: PubMed, Ovid, and Web of Science. Search terms included “leptomeningeal” and “ovarian carcinoma.” The following terms were considered to be synonymous with leptomeningeal carcinomatosis: malignant meningitis, diffuse leptomeningeal metastasis, malignant leptomeningeal dissemination, and carcinomatous leptomeningeal metastases. Title and abstract screening and full-text screening were performed by one author (BS) with oversight and input by a second author (JC) as needed. A flowchart of the study selection is depicted in Figure 4. Eligibility criteria comprised the inclusion of case reports, case series, and cohort studies describing presentation, management, and outcomes of leptomeningeal carcinomatosis in ovarian carcinoma. Exclusion criteria included reviews and meta-analyses and reports that provided insufficient details about the clinical course and management, as well as preclinical studies. Cases were included if diagnostic evidence of leptomeningeal carcinomatosis was reported on MRI and/or cerebrospinal fluid cytology. Non-English publications were translated using Google Translate document translation service (translate.google.com) (19). Data extraction was performed by one author (BS). The collected variables included title, author, year, number of patients, primary ovarian lesion, the presence of central nervous system metastasis, time to onset of leptomeningeal carcinomatosis, presenting symptoms, treatment regimen, and survival time after diagnosis of leptomeningeal carcinomatosis. Data are qualitatively synthesized in Table 1.
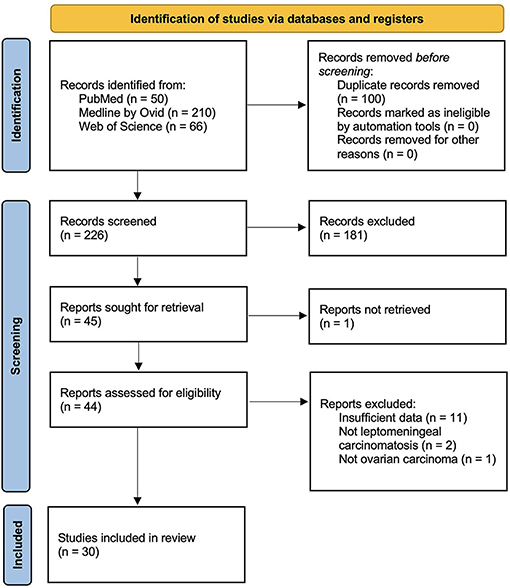
Figure 4. PRISMA study selection flowsheet for systematic review of the literature pertaining to leptomeningeal carcinomatosis of ovarian origin.
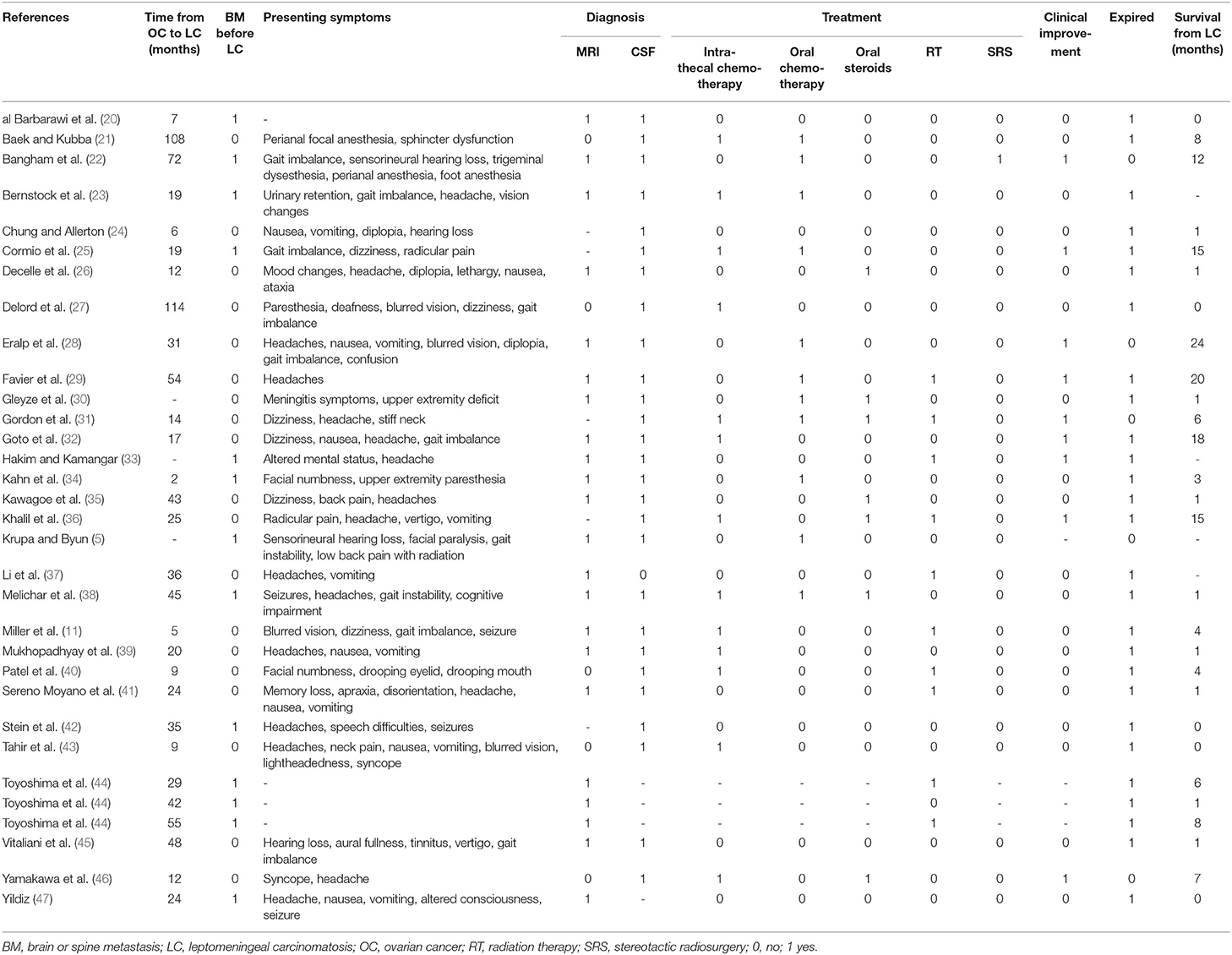
Table 1. A systematic review of the literature pertaining to leptomeningeal carcinomatosis of ovarian origin: case report characteristics.
A systematic review of the literature revealed 30 publications that met criteria for cases of leptomeningeal carcinomatosis in ovarian carcinoma, and a total of 32 patients were included (5, 11, 20–47). The median time to leptomeningeal carcinomatosis from ovarian carcinoma diagnosis was 24 months, and 41% of patients had brain or spine metastases present before or at the time of leptomeningeal carcinomatosis diagnosis. Presenting symptoms varied, but often included headache (17/28, 61%), gait imbalance (10/28, 36%), dizziness (6/28, 21%), and seizure (4/28, 14%) (Table 1). Leptomeningeal carcinomatosis was diagnosed on MRI in 81% (22/27) and based upon cerebrospinal fluid cytology in 96% (27/28) of cases. Treatment included intrathecal chemotherapy in 45% (13/29), oral chemotherapy in 38% (11/29), oral steroids in 24% (7/29), radiation therapy in 31% (10/32), and stereotactic radiosurgery in 3% (1/29). Overall, 32% of patients experienced some degree of clinical improvement, but ultimately 84% expired at the time of publication. The median survival time after the diagnosis of leptomeningeal carcinomatosis was one month.
Leptomeningeal carcinomatosis is defined as the invasion of cancer cells into the cerebrospinal fluid with subsequent seeding of the leptomeninges. Leptomeningeal carcinomatosis, also known as neoplastic meningitis, has been reported in 5–15% of patients with lymphoma and leukemia, 1–5% of patients with solid tumors, and 1–2% of patients with primary intracranial tumors (9–11). Solid tumors with the highest incidence of leptomeningeal dissemination include breast, lung, and melanoma (11). Neurologic symptomatology often includes signs of increased intracranial pressure, which may include headache, nausea, vomiting, or ambulation difficulties. Contrary to infectious meningitis, nuchal rigidity is reported in only 15% of cases with neoplastic meningitis (11).
The most common site of spread of ovarian carcinoma is the abdominal or pelvic cavities; however, metastases may involve the lymph nodes, liver, and lungs (4, 7). Other less common areas of metastatic spread include skin, spleen, thyroid, and bones (4, 7). It is exceedingly rare for primary ovarian malignancy to involve the central nervous system. Indeed, Pectasides et al. (6) found that of 1,450 patients with primary ovarian malignancies, only 17 patients (1.17%) exhibited central nervous system metastases. Moreover, none of the patients in this cohort demonstrated leptomeningeal disease (6). Cormio et al. (3) analyzed a case series of 23 patients with central nervous system metastases and found that one patient (4.34%) exhibited leptomeningeal involvement. Furthermore, Sanderson et al. (8) retrospectively analyzed the radiographic imaging of 1,222 patients with ovarian cancer. The authors reported that 13 patients (1.1%) exhibited central nervous system metastasis and two patients (0.16%) showed leptomeningeal disease (8).
Leptomeningeal seeding of ovarian carcinoma is an exceedingly rare entity with most cases published as a case series or an individual case report (5, 9–11). As such, the literature on the natural history and effective treatment strategies of this phenomenon remains sparse. Krupa et al. (5) reported a 62-year-old female with stage IIC high-grade serous ovarian carcinoma with malignant ascites but without involvement of lymph nodes who underwent bilateral salpino-oophorectomy, omentectomy, and adjuvant chemotherapy. She presented approximately one year thereafter with rapidly progressive bilateral sensorineural hearing loss, right-sided facial paralysis, gait instability, and radicular low back pain in conjunction with a marked rise in her CA-125 level (5). Imaging demonstrated bilateral auditory canal metastases with a mass extending to the right cerebellopontine angle as well as several spinal lesions with leptomeningeal involvement (5). Behnam et al. (9) described a similar rapidly progressive case in a 55-year-old female who, despite appropriate surgical intervention and adjuvant therapies for stage III serous ovarian carcinoma, developed acute onset paraparesis attributable to diffuse cranial and spinal leptomeningeal disease found on autopsy. Similarly, Miller et al. (11) reported a 49-year-old female who developed headache, vertigo, diplopia, seizures, and ataxia five months following initial diagnosis, with imaging demonstrating diffuse cranial and spinal leptomeningeal disease. Here, we report a case of a 67-year-old female who presented with several days of progressively worsening headaches, dizziness, and confusion, three months after craniotomy for resection of an ovarian carcinoma metastasis. Comparatively, the prior cases demonstrate rapidly progressive neurologic symptomatology. Retrospectively, it seems as though the presenting symptomatology is correlated to the location of leptomeningeal spread.
The pathogenic mechanism of leptomeningeal carcinomatosis is a topic of debate. Depending on the histology of the primary tumor, cancer cells may invade the meninges via one of several different mechanisms. Numerous hypotheses, including (i) hematogenous spread to the arachnoid by way of the arterial circulation or seeding of the leptomeninges through Batson's venous plexus via a retrograde venous pathway, (ii) infiltration via perineurium or endoneurium or perivascular lymphatics into dural and arachnoidal sleeves of nerve rootlets, (iii) direct extension of intraparenchymal lesions into the ventricular system or subarachnoid space, (iv) invasion of the central nervous system via the choroid plexus or arachnoidal veins, and (v) seeding of the cerebrospinal fluid from surgical removal of intraparenchymal tumors (9–11, 31, 48–50), have been proposed. Indeed, neurosurgical resection of intraparenchymal lesions permits a route of tumor spread into the cerebrospinal fluid through an iatrogenic pial breach (49). Once tumor cells gain access to the cerebrospinal fluid, they may disperse along the meningeal surface and randomly implant at distant sites forming secondary leptomeningeal metastatic lesions (49). Plaque-like deposits with accumulation in Virchow-Robin spaces and meningeal nodularity are characteristic of solid tumors whereas diffuse leptomeningeal layering is more frequently seen in hematological malignancies (49). Common areas of seeding are typically gravity-dependent anatomic areas with slow cerebrospinal fluid flow, including the posterior fossa, basilar cisterns, and cauda equina (9–11, 31, 48–50).
Although neurosurgical resection of brain metastases has become a cornerstone of oncologic treatment, studies have suggested that resection is associated with a higher risk for developing leptomeningeal dissemination (51–53). In a systematic review and meta-analysis, Tewarie et al. (54) found breast cancer origin and multiple brain metastases increased the risk of leptomeningeal spread following neurosurgical resection. Ahn et al. (55) found that metastatic lesions in proximity to the cerebrospinal fluid pathway, piecemeal resection, and the use of a Cavitron Ultrasonic Surgical Aspirator (CUSA) conferred an increased incidence of leptomeningeal carcinomatosis. Ha et al. (56) also demonstrated an association between the presence of a metastatic deposit adjacent to cerebrospinal fluid and increased risk of leptomeningeal carcinomatosis in breast cancer. Indeed, these data highlight that en bloc resection and avoiding violation of the ventricular wall during surgical resection are critical to circumvent an increased risk of developing leptomeningeal carcinomatosis postoperatively.
Leptomeningeal carcinomatosis has a high morbidity and mortality rate regardless of intervention. The reported median survival of leptomeningeal disease irrespective of primary diagnosis is 60 days from detection (11). Indeed, given the paucity of reported cases of leptomeningeal disease from ovarian carcinoma, there are a few clinical studies to guide treatment options. Gordon et al. (31) reported a patient with leptomeningeal spread from ovarian carcinoma who, at the time of report, survived six months after the detection with whole-brain radiation and intrathecal methotrexate. Ohta et al. (50) reported another case who survived at least 13 months after detection with whole-brain radiation, intrathecal methotrexate, and adjuvant systemic chemotherapy, including cisplatin, paclitaxel, and etoposide. Groves et al. (48) conducted a phase II trial evaluating the therapeutic efficacy of intrathecal topotecan in 62 patients with leptomeningeal carcinomatosis from a variety of primary tumors, including brain, breast, and lungs. However, only one case of ovarian cancer was included in the cohort (48). The authors found clinical improvement in 16% of patients and clinical stability in 29% of patients after a six-week induction period with intrathecal topotecan (0.4 mg two times weekly for six weeks and continued weekly for six doses) (48). Around 65% of the original cohort completed the induction period, of which 21% exhibited cerebrospinal fluid clearance of cancer cells (48). Nevertheless, the median time to progression was only seven weeks, with a median overall survival of 15 weeks (48). Miller et al. (11) utilized this protocol of intrathecal topotecan along with whole-brain radiation to treat their patient with leptomeningeal carcinomatosis of ovarian carcinoma; however, the patient continued to develop progressive symptomatology and expired four months after detection.
In this report, we described a rare case of leptomeningeal carcinomatosis of metastatic ovarian serous carcinoma. The primary disease was stable for three years prior to the identification of a single metastasis to the brain parenchyma. Interestingly, our patient developed progressive neurologic symptomatology three months after craniotomy for resection of the parenchymal lesion and was subsequently found to have leptomeningeal seeding on repeat radiographic imaging. We propose that the inception of her leptomeningeal disease was iatrogenic in nature, and attributable to recent craniotomy three months prior, given the timing of her symptomatology in relation to her recent surgery. However, we do not have direct evidence to prove that leptomeningeal carcinomatosis originated from tumor spillage into the cerebrospinal fluid during neurosurgical resection rather than from systemic metastasis preoperatively. Indeed, microscopic leptomeningeal seeding imperceptible on the initial MRI cannot be definitively ruled out without preoperative cerebrospinal fluid analysis. Nevertheless, the present case highlights that leptomeningeal disease dissemination may be a rare yet potential sequela of craniotomy for the resection of intraparenchymal ovarian serous carcinoma metastases. Progressive clinical symptomatology that develops postoperatively in this patient population should prompt urgent workup to rule out leptomeningeal disease and expedited radiation oncology consultation if identified.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
JC and EM conceived the study idea. SA and CR provided guidance and an oversight. BS and JC made substantial contributions to study planning and data collection, and drafted the manuscript. DG provided pathology images. All authors critically reviewed and revised this manuscript for important intellectual content.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.850050/full#supplementary-material
Supplementary Figure 1. Histopathology and immunohistochemistry of the original omental biopsy confirming a diagnosis of high-grade serous ovarian carcinoma. (A) H&E stain at 100x magnification showing high-grade carcinoma in a desmoplastic stroma. (B) H&E stain at 200× magnification showing a closer view of the malignant carcinoma in nests with a hint of papillae formation in a desmoplastic stroma. (C) H&E stain of the surface of the ovary at 100x magnification demonstrating numerous psammoma bodies. (D) WT-1 immunostain at 200× magnification demonstrating strong nuclear positivity in tumor cells. (E) p53 immunostain at 200× magnification strongly marking all the tumor cell nuclei. (F) Cytokeratin-7 immunostain at 200× magnification with strong cytoplasmic positivity. (G) p16 immunostain at 200× magnification with strong nuclear positivity. (H) CA-125 immunostain at 200× magnification with strong cytoplasmic positivity.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
2. Shen J, Sun X, Zhou J. Insights into the role of mesothelin as a diagnostic and therapeutic target in ovarian carcinoma. Front Oncol. (2020) 10:1263. doi: 10.3389/fonc.2020.01263
3. Cormio G, Maneo A, Parma G, Pittelli MR, Miceli MD, Bonazzi C. Central nervous system metastases in patients with ovarian carcinoma. A report of 23 cases and a literature review. Ann Oncol. (1995) 6:571–4. doi: 10.1093/oxfordjournals.annonc.a059246
4. Cormio G, Rossi C, Cazzolla A, Resta L, Loverro G, Greco P, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. (2003) 13:125–9. doi: 10.1136/ijgc-00009577-200303000-00004
5. Krupa M, Byun K. Leptomeningeal carcinomatosis and bilateral auditory canal metastases from ovarian carcinoma. Radiol Case Rep. (2017) 12:386–90. doi: 10.1016/j.radcr.2017.03.013
6. Pectasides D, Aravantinos G, Fountzilas G, Kalofonos C, Efstathiou E, Karina M, et al. Brain metastases from epithelial ovarian cancer. The Hellenic Cooperative Oncology Group (HeCOG) experience and review of the literature. Anticancer Res. (2005) 25:3553–8.
7. Rose PG, Piver MS, Tsukada Y, Lau TS. Metastatic patterns in histologic variants of ovarian cancer. An autopsy study. Cancer. (1989) 64:1508–153.
8. Sanderson A, Bonington SC, Carrington BM, Alison DL, Spender JA. Cerebral metastasis and other cerebral events in women with ovarian cancer. Clin Radiol. (2002) 57:815–9. doi: 10.1053/crad.2001.0965
9. Behnman K, Aguilera AJ, Kornfeld M, Jordan SW, Hilgers RD. Meningeal carcinomatosis from an ovarian primary: a clinicopathologic study. Gynecol Oncol. (1984) 19:104–9. doi: 10.1016/0090-8258(84)90165-3
10. Chamberlain MC. Neoplastic meningitis. Oncologist. (2008) 13:967–77. doi: 10.1634/theoncologist.2008-0138
11. Miller E, Dy I, Herzog T. Leptomeningeal carcinomatosis from ovarian cancer. Med Oncol. (2012) 29:2010–5. doi: 10.1007/s12032-011-0076-9
12. Romani C, Zizioli V, Silvestri M, Ardighieri L, Bugatti M, Corsini M, et al. Low expression of claudin-7 as potential predictor of distant metastases in high-grade serous ovarian carcinoma patients. Front Oncol. (2020) 10:1287. doi: 10.3389/fonc.2020.01287
13. Wu X, Li D, Liu L, Liu B, Liang H, Yang B. Serum soluble mesothelin-related peptide (SMRP): a potential diagnostic and monitoring marker for epithelial ovarian cancer. Arch Gynecol Obstet. (2014) 289:1309–11. doi: 10.1007/s00404-013-3128-x
14. Hatano Y, Hatano K, Tamada M, Morishige KI, Tomita H, Yanai H, et al. A comprehensive review of ovarian serous carcinoma. Adv Anat Pathol. (2019) 26:329–39. doi: 10.1097/PAP.0000000000000243
15. Kobel M, Luo L, Grevers X, Lee S, Brooks-Wilson A, Gilks CB, et al. Ovarian carcinoma histotype: strenghts and limitations of integrating morphology with immunohistochemical predictions. Int J Gynecol Pathol. (2019) 38:353–62. doi: 10.1097/PGP.0000000000000530
16. Kobel M, Rahimi K, Rambau PF, Naugler C, Le Page C, Meunier L, et al. An immunohistochemical algorithm for ovarian carcinoma typing. Int J Gynecol Pathol. (2016) 35:430–41. doi: 10.1097/PGP.0000000000000274
17. McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. (2011) 43:420–32. doi: 10.1097/PAT.0b013e328348a6e7
18. Rosen DG, Zhang Z, Shan W, Liu J. Morphological and molecular basis of ovarian serous carcinoma. J Biomed Res. (2010) 24:257–63. doi: 10.1016/S1674-8301(10)60036-X
19. Balk EM, Chung M, Hadar N. Accuracy of data extraction of non-english language trials with Google Translate. Rockville, MD: Agency for Healthcare Research and Quality (2012).
20. al Barbarawi M, Smith SF, Qudsieh S, Sekhon LH. Multiple cerebral and leptomeningeal metastases from ovarian carcinoma: unusual early presentation. J Clin Neurosci. (2005) 12:697–9. doi: 10.1016/j.jocn.2004.08.021
21. Baek WS, Kubba SV. Cauda equina syndrome due to leptomeningeal carcinomatosis of the ovary. Gynecol Oncol. (2008) 111:544–5. doi: 10.1016/j.ygyno.2008.03.002
22. Bangham M, Goldstein R, Walton H, Ledermann JA. Olaparib treatment for BRCA-mutant ovarian cancer with leptomeningeal disease. Gynecol Oncol Rep. (2016) 18:22–4. doi: 10.1016/j.gore.2016.10.004
23. Bernstock JD, Ostby S, Fox B, Sotoudeh H, Janssen A, Kang YJ, et al. Cauda equina syndrome in an ovarian malignant-mixed mullerian tumor with leptomeningeal spread. Clin Case Rep. (2019) 7:2341–5. doi: 10.1002/ccr3.2472
24. Chung P, Allerton R. Malignant meningitis secondary to ovarian carcinoma: an unusual occurrence. Clinical Oncology. (2001) 13:112–3. doi: 10.1053/clon.2001.9231
25. Cormio G, Loizzi V, Selvaggi L. Leptomeningeal involvement after remission of brain metastases from ovarian cancer. Int J Gynaecol Obstet. (2007) 99:139. doi: 10.1016/j.ijgo.2007.04.038
26. Decelle L, D'Hondt L, Andre M, Delree P, Calicis B, Lonchay C, et al. Ovarian cancer associated with carcinomatous meningitis: a case report and review of the literature. Int J Gynecol Cancer. (2007) 17:1136–40. doi: 10.1111/j.1525-1438.2007.00911.x
27. Delord JP, Fizazi K, El Hajj M, Pautier P, Lhomme C. Isolated leptomeningeal metastasis from ovarian carcinoma: an unusual event. Eur J Cancer. (1998) 34:758–9. doi: 10.1016/S0959-8049(97)10051-X
28. Eralp Y, Saip P, Aydin Z, Berkman S, Topuz E. Leptomeningeal dissemination of ovarian carcinoma through a ventriculoperitoneal shunt. Gynecol Oncol. (2008) 108:248–50. doi: 10.1016/j.ygyno.2007.09.024
29. Favier L, Truc G, Boidot R, Bengrine-Lefevre L. Long-term response to Olaparib in carcinomatous meningitis of a BRCA2 mutated ovarian cancer: A case report. Mol Clin Oncol. (2020) 13:73–5. doi: 10.3892/mco.2020.2035
30. Gleyze PO, Petit E, Doe A, Nguyen B, Poquet E, Colbert N. Meningeal metastases of ovarian cancer. Contribution of imaging. Presse Med. (2000) 29:593–5.
31. Gordon AN, Kavanagh JJ Jr, Wharton JT, Rutledge FN, Obbens EA, Bodey GP Sr. Successful treatment of leptomeningeal relapse of epithelial ovarian cancer. Gynecol Oncol. (1984) 18:119–24. doi: 10.1016/0090-8258(84)90015-5
32. Goto Y, Katsumata N, Nakai S, Sasajima Y, Yonemori K, Kouno T, et al. Leptomeningeal metastasis from ovarian carcinoma successfully treated by the intraventricular administration of methotrexate. Int J Clin Oncol. (2008) 13:555–8. doi: 10.1007/s10147-008-0782-2
33. Hakim M, Kamangar N. 783: leptomeningeal carcinomatosis in a patient with metastatic ovarian cancer. Crit Care Med. (2020) 48:371. doi: 10.1097/01.ccm.0000626868.45228.db
34. Kahn RM, Gandhi SK, Mvula MR, Li X, Frey MK. Metastatic epithelial ovarian cancer to Meckel's cave with leptomeningeal spread at time of diagnosis. Gynecol Oncol Rep. (2020) 34:100641. doi: 10.1016/j.gore.2020.100641
35. Kawagoe Y, Nakayama T, Matuzawa S, Fukushima K, Onishi J, Sato Y, et al. Epithelial ovarian carcinoma associated with metastases to central nervous system: two case reports. Case Rep Obstet Gynecol. (2018) 2018:4301247. doi: 10.1155/2018/4301247
36. Khalil AM, Yamout BI, Tabbal SD, Salem ZMK, Mroueh AN. Case report and review of the literature: leptomeningeal relapse in epithelial ovarian cancer. Gynecol Oncol. (1994) 54:227–31. doi: 10.1006/gyno.1994.1199
37. Li HK, Harding V, Williamson R, Blagden S, Gabra H, Agarwal R. Cerebral sinus thrombosis and leptomeningeal carcinomatosis in a patient with ovarian cancer. J Clin Oncol. (2012) 30:e19–20. doi: 10.1200/JCO.2011.38.1426
38. Melichar B, Tomsova M, Rehak S, Malirova E, Simonova G. Meningeal carcinomatosis as a late complication of brain metastases of epithelial ovarian carcinoma. Eur J Gynaecol Oncol. (2008) 29:402–4.
39. Mukhopadhyay S, Mukhopadhyay S, El-Zammar O, Khurana KK, Graziano SL. CASE 2. Meningeal metastases from ovarian carcinoma. J Clin Oncol. (2006) 24:1010–1. doi: 10.1200/JCO.2005.01.2427
40. Patel KB, Gaidis A, Stephens A, Thompson TZ, Williams H, Rungruang B, et al. A report of Bell's Palsy triggered by leptomeningeal metastases from recurrent high grade serous ovarian cancer. Gynecol Oncol Rep. (2018) 26:82–6. doi: 10.1016/j.gore.2018.10.006
41. Sereno Moyano M, Casado Saenz E, Belda Iniesta C, Gonzalez Baron M. Subacute dementia as presenting feature of carcinomatous leptomeningeal metastases. Clin Transl Oncol. (2005) 7:29–30. doi: 10.1007/BF02710023
42. Stein M, Steiner M, Ben-Schachar M, Kuten A, Malberger E, Goldsher D, et al. Leptomeningeal involvement by epithelial ovarian carcinoma: a case report. Gynecol Oncol. (1987) 27:241–5. doi: 10.1016/0090-8258(87)90299-X
43. Tahir N, Ram A, Jain N, Vemireddy LP, Zahra F. Leptomeningeal Carcinomatosis in Epithelial Ovarian Cancer: A Diagnostic Challenge. Cureus. (2021) 13:e14440. doi: 10.7759/cureus.14440
44. Toyoshima M, Tsuji K, Shigeta S, Tokunaga H, Ito K, Watanabe Y, et al. Leptomeningeal metastasis from gynecologic cancers diagnosed by brain MRI. Clin Imaging. (2017) 41:42–7. doi: 10.1016/j.clinimag.2016.09.013
45. Vitaliani R, Spinazzi M, Del Mistro AR, Manara R, Tavolato B, Bonifati DM. Subacute onset of deafness and vertigo in a patient with leptomeningeal metastasis from ovarian cancer. Neurol Sci. (2009) 30:65–7. doi: 10.1007/s10072-008-0006-6
46. Yamakawa H, Ariga H, Enomoto A, Netsu S, Suzuki Y, Konno R. Meningeal dissemination from an ovarian carcinoma with effective response to intrathecal chemotherapy. Int J Clin Oncol. (2009) 14:447–51. doi: 10.1007/s10147-008-0846-3
47. Yildiz ÖK. Leptomeningeal Carcinomatosis in a Patient with Ovarian Cancer. Turkish Journal Of Neurology. (2019) 25:179–81. doi: 10.4274/tnd.2019.43799
48. Groves MD, Glantz MJ, Chamberlain MC, Baumgartner KE, Conrad CA, Hsu S, et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol. (2008) 10:208–15. doi: 10.1215/15228517-2007-059
49. Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int. (2013) 4:S265–S88. doi: 10.4103/2152-7806.111304
50. Ohta H, Koyama R, Nagai T, Hirayama Y, Saito S, Yonesaka A. Meningeal carcinomatosis from an ovarian primary with complete response to adjuvant chemotherapy after cranial irradiation. Int J Clin Oncol. (2001) 6:157–62. doi: 10.1007/PL00012100
51. DeAngelis LM, Mandell LR, Thaler HT, Kimmel DW, Galicich JH, Fuks Z, et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. (1989) 24:798–805. doi: 10.1227/00006123-198906000-00002
52. Dosoretz DE, Blitzer PH, Russell AH, Wang CC. Management of solitary metastasis to the brain: the role of elective brain irradiation following complete surgical resection. Int J Radiat Oncol Biol Phys. (1980) 6:1727–30. doi: 10.1016/0360-3016(80)90260-6
53. Seute T, Leffers P, ten Velde GP, Twijnstra A. Leptomeningeal metastases from small cell lung carcinoma. Cancer. (2005) 104:1700–5. doi: 10.1002/cncr.21322
54. Tewarie IA, Jessurun CAC, Hulsbergen AFC, Smith TR, Mekary RA, Broekman MLD. Leptomeningeal disease in neurosurgical brain metastases patients: a systematic review and meta-analysis. Neurooncol Adv. (2021) 3:vdab162. doi: 10.1093/noajnl/vdab162
55. Ahn JH, Lee SH, Kim S, Joo J, Yoo H, Lee SH, et al. Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection. J Neurosurg. (2012) 116:984–93. doi: 10.3171/2012.1.JNS111560
Keywords: leptomeningeal carcinomatosis, neoplastic meningitis, ovarian cancer, craniotomy, metastases, case report, literature review
Citation: Stopa BM, Cuoco JA, Adhikari S, Grider DJ, Rogers CM and Marvin EA (2022) Iatrogenic Leptomeningeal Carcinomatosis Following Craniotomy for Resection of Metastatic Serous Ovarian Carcinoma: A Systematic Literature Review and Case Report. Front. Surg. 9:850050. doi: 10.3389/fsurg.2022.850050
Received: 07 January 2022; Accepted: 14 February 2022;
Published: 25 April 2022.
Edited by:
Davide Croci, University of South Florida, United StatesReviewed by:
Martina Dalolio, Ente Ospedaliero Cantonale (EOC), SwitzerlandCopyright © 2022 Stopa, Cuoco, Adhikari, Grider, Rogers and Marvin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric A. Marvin, ZWFtYXJ2aW5AY2FyaWxpb25jbGluaWMub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.