- 1Department of Orthopedics, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Orthopedic Laboratory of Chongqing Medical University, Chongqing, China
Background: Synovial fluid biomarkers have been found to improve the diagnosis of chronic periprosthetic joint infection (PJI); however, no “gold standard” exists yet. Interleukin-4 (IL-4) and polymorphonuclear cell (neutrophil) count in the synovial fluid are crucial in mediating local inflammation during bacterial infections and could be valuable biomarkers for PJI.
Methods: This prospective study was conducted to investigate the diagnostic potential of synovial fluid IL-4 (SF-IL4) and polymorphonuclear cell percentage (SF-PMN%) for chronic PJI. A total of 110 patients who underwent revision arthroplasty between January 2019 and October 2020 were enrolled, and 11 patients were excluded. Of 99 patients, 43 were classified as having PJI and 56 as having aseptic failures according to the 2013 Musculoskeletal Infections Society criteria. In all patients, SF-IL4, SF-PMN%, serum C-reactive protein (CRP), and serum erythrocyte sedimentation rate (ESR) were quantified preoperatively. The diagnostic value for each biomarker was analyzed, and optimal cutoff values were calculated.
Results: The patient demographics did not significantly vary. The area under the curve of SF-IL4 and SF-PMN% was 0.97 and 0.89, respectively, higher than that for serum ESR (0.72) and serum CRP (0.83). The combination of SF-IL4 and SF-PMN% provided higher specificity (97.0%) and accuracy (96.0%) when the cut-off values were 1.7 pg/mL and 75%, respectively.
Conclusion: SF-IL4 is a valuable biomarker for chronic PJI detection, and the combination of SF-IL4 and SF-PMN% improved the diagnostic value of chronic PJI, and further studies are needed until its clinical application.
Introduction
Periprosthetic joint infection (PJI) is a catastrophic complication after joint replacement surgery. Total knee arthroplasty (TKA) and total hip arthroplasty (THA) have low incidence rates of 0.3–2.4% and 0.8–2%, respectively (1, 2); however, PJI is the most common cause of revision for failed TKA and the third most common cause for revision for failed THA (3, 4). The cost of treatment of a PJI is three to four times higher than that for primary surgery (5). It is important to preoperatively distinguish between PJI and aseptic failure because it will determine the course of antibiotic therapy and surgical strategies.
Currently, there is no “gold standard” for PJI diagnosis (6). The diagnostic standards for PJI have been refined over the past decades (7–9). Serum C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are highly sensitive markers of PJI, but their lack of specificity limits their diagnostic accuracy (10–12). In recent years, the use of synovial fluid (SF) biomarkers to diagnose PJI has attracted attention because of their low cost, ease of interpretation, and high accuracy (13, 14). Neutrophils are the first cells to produce a defensive response to bacterial infections; therefore, they are suitable candidates to achieve high sensitivity and specificity for PJI detection (15). SF-PMN% has been the basic indicator of SF analysis, but the cut-off value is still controversial (14, 16). Therefore, more valuable biomarkers to detect chronic PJI are needed.
IL-4 is involved in acute and chronic bacterial defense inflammatory responses, but the diagnostic value for chronic PJI is not yet known. IL-4 promotes immunoglobulin isotype switching and regulates the function of macrophages mainly via the Stat6 pathway (17). Serum IL-4 level is upregulated in bacterial infection-induced systemic inflammatory response syndrome and can be a good predictor of infection-related mortality risk (18). Serum IL-4 can be used as an immunological marker for diagnosing active tuberculosis and for monitoring the efficacy of antituberculosis therapy (19). Local administration or expression of IL-4 enhanced the pulmonary clearance of Pseudomonas aeruginosa in vivo and decreased mortality following infection (20). In joint aspiration, IL-4 was first secreted by synovial mast cells and then by T helper 2 (Th2) cells (21). Previous studies have demonstrated that the level of IL-4 is elevated in the SF during bacterial infection (6, 22). Therefore, we hypothesized that SF-IL4 may be used as a useful biomarker for chronic PJI detection.
This study was conducted to (1) explore and set an optimal cut-off value for serum CRP, ESR, SF-IL4, and SF-PMN% for chronic PJI diagnosis and (2) improve the diagnostic efficiency of chronic PJI by combining SF-IL4 with other biomarkers.
Materials and Methods
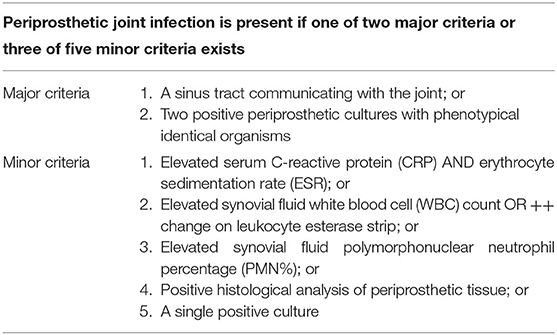
This prospective study protocol was approved by the institutional ethics board of the First Affiliated Hospital of Chongqing Medical University. Informed consent was obtained from every patient. Patients who underwent revision TKA or THA between January 2019 and October 2020 were enrolled in this study. Patients were divided into chronic PJI group and aseptic failure group based on the 2013 Musculoskeletal Infections Society criteria (2013 MSIS) (Table 1) (8). Chronic PJI was defined as the occurrence of PJI symptoms more than 6 weeks after the primary implantation (23, 24). Aseptic failures included aseptic loosening, wear, instability, dislocation, adverse local tissue reactions, and metal allergic reactions (9). To rule out interference with other possible preconditions associated with elevated inflammatory factors, patients with (1) infections of other organs, including pneumonia and urinary tract infection, (2) active rheumatoid arthritis, ankylosing spondylitis, and gouty arthritis, or (3) malignant tumors were excluded.
Data on patient demographics (age, gender, preoperative diagnosis [PJI or aseptic failure and joint involved: hip or knee]) and the survival time of primary implantation were collected. Before revision surgery, peripheral venous blood (3 mL) was withdrawn to test serum ESR and CRP levels. SF (3–4 mL) was sampled for analyzing SF-IL4, SF-PMN%, and culture (48-h routine culture and 14-day prolonged culture; at least three intraoperative tissues were cultured). All biochemical assays were performed at the biochemistry laboratory of the biology technical platform in our institution.
Statistical Analysis
Data were analyzed using SPSS 26.0 (IBM Corporation, TX). Continuous data with a non-normal distribution are presented as the median and interquartile range (IQR). Mann-Whitney U test was used to analyze the statistical significance, and the chi-square test was used to compare the sensitivity and specificity of laboratory test data and categorical data (age, gender, joint involved). P < 0.05 (two-tailed) was considered to indicate a statistically significant difference. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were calculated using MedCalc 19.0.7 (Ostend, Belgium). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were estimated for tested markers. The optimal cut-off value for each maker was computed using the maximized Youden index (sensitivity+specificity-1) method. A higher diagnostic odds ratio (DOR) indicated better discriminatory strength (25).
Results
Baseline Data of the Two Groups Did Not Significantly Differ
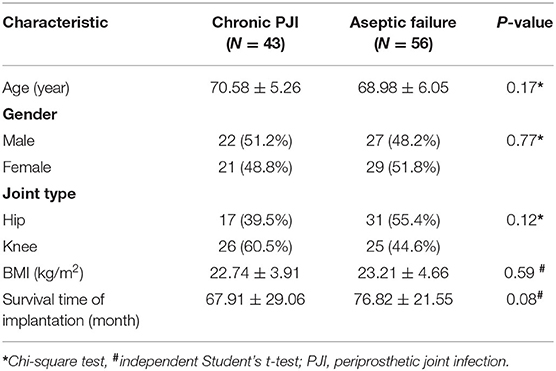
Of the 110 patients initially enrolled, six patients were excluded due to “dry aspiration,” four patients with active rheumatoid arthritis, and one patient with acute PJI. Finally, 99 patients were included in the study, with 43 (43.4%) in the chronic PJI group and 56 (56.6%) in the aseptic failure group. The baseline characteristics of the two groups were similar (Table 2) with no significant difference in the demographics (P > 0.05).
SF-IL4 Had Higher Diagnostic Power Than Serum ESR and CRP Levels
As shown in Table 3, the median value of serum ESR (35.00 mm/h [7.20–120.00 mm/h]) was higher in the chronic PJI group than in the aseptic failure group (22.0 mm/h [2.00–58.00 mm/h]; P = 0.001). Similarly, serum CRP level (21.4 mg/L [5.80–91.20 mg/L]) was higher in the chronic PJI group than in the aseptic failure group (6.75 mg/L [1.25–28.00]; P < 0.001). Results showed that the median level of SF-IL4 in the chronic PJI group is higher than that in the aseptic group (3.30 vs. 1.10 pg/mL, P < 0.0001). Finally, the median SF-PMN% was higher in the PJI group (87.58%) than in the aseptic failure group (56.95%), with statistical significance (P < 0.001).
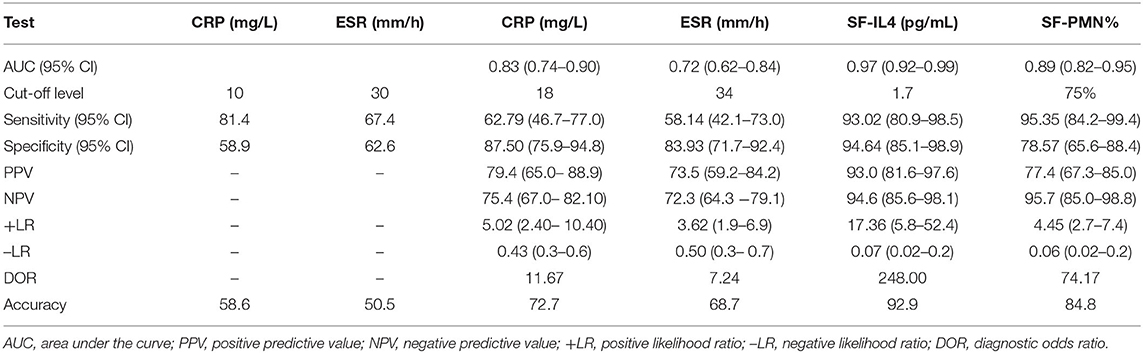
ROC curves were used to measure the discriminatory strength of the indicators (Figures 1A–D). The AUC of SF-IL4 (0.97 [95% CI, 0.92–0.99]) was higher than that of serum ESR (0.72 [95% CI, 0.62–0.84]; P = 0.0004), serum CRP (0.83 [95% CI, 0.74–0.90]; P <0.0001), and SF-PMN% 0.89 [95% CI, 0.82–0.95]; P = 0.053) (Figure 1E). The sensitivity, specificity, PPV, NPV, +LR, –LR, and DOR of these markers are in Table 4. The optimal cut-off value for SF-IL4 of 1.7 pg/mL achieved a sensitivity of 93.02% (95% CI, 80.9–98.5%), specificity of 94.64% (95% CI, 85.1–98.9%), and high PPV (93.0%), NPV (94.6%), and DOR (248).
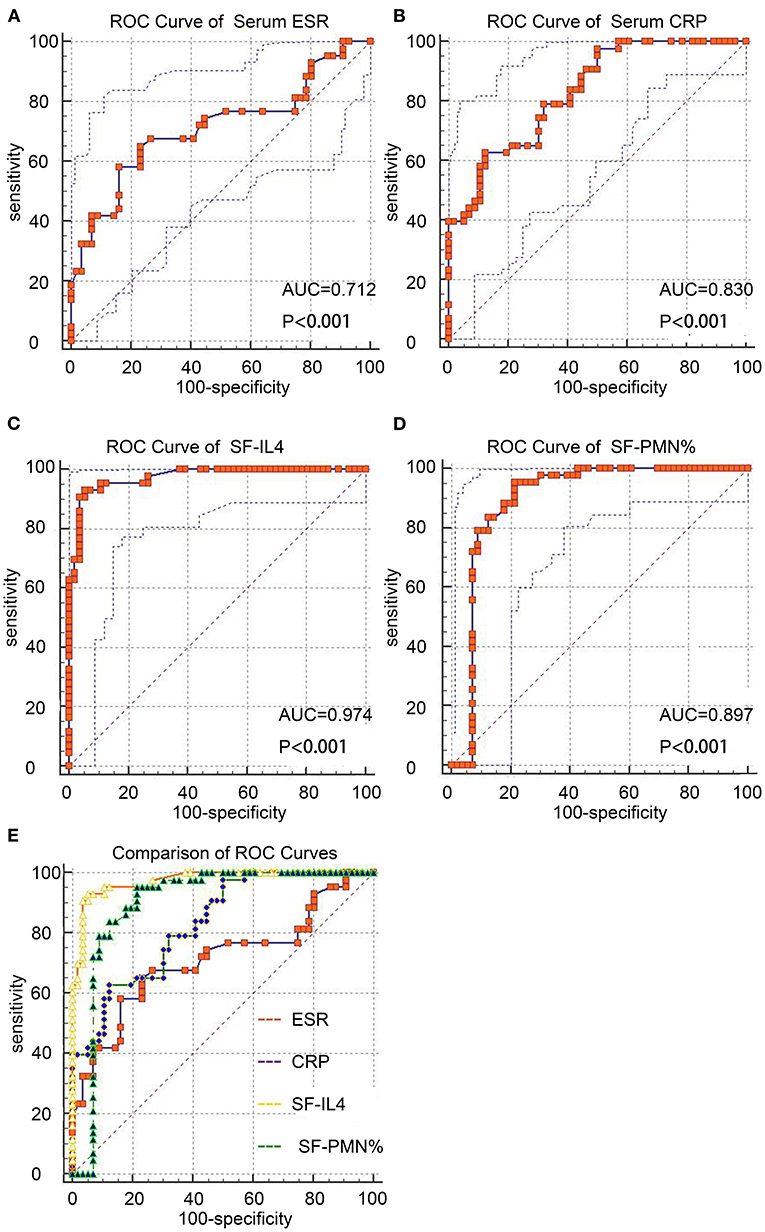
Figure 1. ROC curves of the studied markers for chronic PJI diagnosis. (A–D) ROC curves of serum ESR, CRP, SF-IL4, and SF-PMN%. (E) Comparison of these four markers.
Combination of SF-IL4 With SF-PMN% Had Improved Diagnostic Value for Chronic PJI
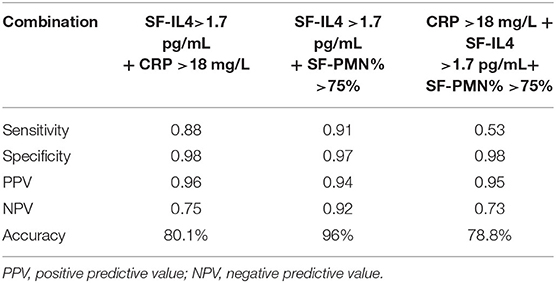
Next, we combined SF-IL4 with other biomarkers (Table 5). When the cut-off values of SF-IL4 and SF-PMN% were met at the same time, the specificity increased to 97% and accuracy increased to 96%, but the sensitivity decreased to 91% for chronic PJI diagnosis.
Discussion
PJI is still a catastrophic complication of arthroplasty. Chronic PJI patients have poorer function score, lower quality of life, and significantly increased risk of short-term mortality (26–28). The diagnosis of chronic PJI relies on clinical symptoms, physical examination, biomarkers examination, and radiological examination. Because chronic PJI is a type of encapsulated and low-grade infection, it usually causes less extensive systemic inflammatory reactions, sometimes resulting in negative laboratory test results (29). Therefore, the diagnosis of chronic PJI is a challenge, especially without a “gold standard” (30).
SF interleukins play an important role in implant-associated infection. TNF-α, IL-1, and IL-6 are pro-inflammatory cytokines essential for initiating an inflammatory response to infection (31). IL-4 is an anti-inflammatory cytokine that participates in regulating chronic infection and immune processes (32). IL-4 regulates the ratio of Th1/Th2 lymphocyte subtypes in chronic infections (17). It can inhibit the development of biofilms in chronic infections of Staphylococcus aureus and promote spontaneous infection clearance (33). IL-4 induces the macrophage switch from the M1 to M2 phenotype, which inhibits osteoclast differentiation (34, 35), and promotes the differentiation of B cells and plasma cells, participates in humoral immunity, and exhibits anti-infective properties (36, 37).
Similarly, SF-IL4 has shown promising potential for PJI diagnosis. Gollwitzer et al. reported that SF-IL4 has 93% sensitivity and 85% specificity in PJI, making it better than other serum markers and SF cytokines such as IL-1β and IL-6 (6). However, their study only enrolled patients with S. aureus infection, which is more virulent. Therefore, the cut-off value for SF-IL4 was 7.79 pg/mL in their study, much higher than our cut-off value (1.7 pg/mL). Fröschen et al. found that combining SF-IL2, SF-IL4, SF-IL5, SF-IL6, SF-IL12, and granulocyte-macrophage colony-stimulating factor can achieve 100% sensitivity and 88.9% specificity, but these are not universal values as it is expensive to quantify all these markers (38). Consistent with previous studies, we found that the median level of SF-IL4 in the chronic PJI group was 3.30 pg/mL. When the cut-off value was set as 1.7 pg/mL for SF-IL4, the highest sensitivity (93.02 [95% CI, 80.9–98.5%]), specificity (94.64% [95% CI, 85.1–98.9%]), and diagnostic accuracy (94.6% [95% CI, 85.1–98.9%]) were obtained compared with values for serum ESR, serum CRP, and SF-PMN%.
Synovial leukocyte analysis is the basis of the SF test, Synovial fluid leukocyte counts and differentiation ratios have been widely proposed and discussed as secondary diagnostic criteria for chronic PJI. Differences in thresholds exist between different institutions and counting methods. Leukocyte counts are more strongly affected by many factors, especially the use of antibiotics before joint aspiration (15, 39–41) and metallosis, while neutrophil percentages are more stable with infection An SF-PMN% more than 80% has been recommended for the diagnosis of chronic PJI (>6 weeks after surgery) in the 2013 MSIS consensus (8). Zahar et al. found that an SF-PMN% cut-off of 66.1% achieved a sensitivity of 80.6% and specificity of 83.3% for chronic PJI (42). Higuera et al. used an SF-PMN% cut-off of 80% and found the sensitivity and specificity of chronic hip PJI as 92.1 and 85.8%, respectively (16). In the present study, when the optimal cut-off value of SF-PMN% was 75%, the AUC of diagnosing chronic PJI was 0.89 (95% CI, 0.82–0.95), with higher sensitivity of 95.35% (95% CI, 84.2–99.4%) but relatively decreased specificity of 78.57% (95% CI, 65.6–88.4%), respectively.
The 2013 MSIS consensus recommends a CRP cut-off value of 10 mg/L, and at this cut-off, the sensitivity and specificity were 81.4 and 58.9%, respectively, and the diagnostic accuracy was only 58.6% in our study. When the cut-off value of serum ESR was 30 mm/h, the sensitivity, specificity, and accuracy were 67.4, 62.6, and 50.5%, respectively. The unacceptably low sensitivity was coupled with a high number of false negatives. When we set the cut-off values of serum CRP and ESR levels as 18 mg/L and 34 mm/h, respectively, the detection accuracy for chronic PJI improved to 72.7 and 68.7%, respectively.
No single test could provide 100% accuracy for PJI diagnosis. Therefore, a combination of different indicators, with different sensitivity and specificity values, should be used to confirm or rule out the infection under high clinical suspicion (43–46). We found that when the SF-IL4 is >1.7 pg/mL and the SF-PMN% was more than 75%, the specificity and accuracy improved to 97 and 96%, respectively, for chronic PJI diagnosis. However, the combined diagnosis had high specificity but reduced diagnostic sensitivity, which may lead to missed diagnoses in some patients.
This study had some limitations. First, we did not adopt the latest 2018 ICM modified PJI diagnostic criteria, which includes new markers such as serum D-dimer and a scoring system, which is validated to have a higher sensitivity of 97.7% and specificity of 99.5% (47); however, the application of D-dimer for PJI is still controversial (48, 49). Second, we excluded patients diagnosed with active inflammatory arthritis at the time of admission, including rheumatoid arthritis, ankylosing spondylitis, and gouty arthritis. As these patients usually have higher serum ESR, serum CRP, SF-WBC count, and SF-PMN%, the inclusion of this group of patients will affect the accuracy of our results (39), and excluding these patients limits the clinical application of our results due to the limited sample size. Therefore, multicenter studies with larger sample sizes are required to produce more reliable results.
Conclusion
When we set the cut-off value as 1.7 pg/mL, SF-IL4 achieved a higher sensitivity of 93.02 and specificity of 94.64% than serum ESR, serum CRP, and SF-PMN%. The combined measurement of SF-IL4 and SF-PMN% improved the specificity to 97% and the diagnostic accuracy for chronic PJI to 96%. However, the cut-off value of SF-IL4 for chronic PJI detection is not yet consensual, requiring more research data to support our conclusion.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Ethics Board of the First Affiliated Hospital of Chongqing Medical University (Ethics approved number: 20187101). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
NH and WH conceived and designed the study. JH, JW, and BZ analyzed and interpreted the data. JH drafted the article. NH and WH critically revised the paper. All of the authors approved the final submitted version.
Funding
This research was supported by the Chinese National Natural Science Foundation (grant number: 82072443) and Chongqing Medical Scientific Research Project (joint project of Chongqing Health Commission and Science and Technology Bureau) (grant number: 2019ZDXM014).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PJI, periprosthetic joint infection; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; SF-IL4, synovial fluid interleukin 4; SF-PMN%, synovial fluid polymorphonuclear cell neutrophil percentage; PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; -LR, negative likelihood ratio; DOR, diagnostic odds ratio; ROC, receiver operating characteristic; AUC, area under the curve.
References
1. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. (2008) 466:1710–5. doi: 10.1007/s11999-008-0209-4
2. Beam E, Osmon D. Prosthetic joint infection update. Infect Dis Clin North Am. (2018) 32:843–59. doi: 10.1016/j.idc.2018.06.005
3. Delanois RE, Mistry JB, Gwam CU, Mohamed NS, Choksi US, Mont MA. Current epidemiology of revision total knee arthroplasty in the United States. J Arthroplasty. (2017) 32:2663–8. doi: 10.1016/j.arth.2017.03.066
4. Gwam CU, Mistry JB, Mohamed NS, Thomas M, Bigart KC, Mont MA, et al. Current epidemiology of revision total hip arthroplasty in the United States: national inpatient sample 2009 to 2013. J Arthroplasty. (2017) 32:2088–92. doi: 10.1016/j.arth.2017.02.046
5. Gomez-Urena EO, Tande AJ, Osmon DR, Berbari EF. Diagnosis of prosthetic joint infection: cultures, biomarker and criteria. Infect Dis Clin North Am. (2017) 31:219–35. doi: 10.1016/j.idc.2017.01.008
6. Gollwitzer H, Dombrowski Y, Prodinger PM, Peric M, Summer B, Hapfelmeier A, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. (2013) 95:644–51. doi: 10.2106/JBJS.L.00205
7. Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J Arthroplasty. (2011) 26:1136–8. doi: 10.1016/j.arth.2011.09.026
8. Parvizi J, Gehrke T. Definition of periprosthetic joint infection. J Arthroplasty. (2014) 29:1331. doi: 10.1016/j.arth.2014.03.009
9. Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. (2018) 33:1309–14.e2. doi: 10.1016/j.arth.2018.02.078
10. Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection. J Bone Joint Surg Am Vol. (2010) 92:2102–9. doi: 10.2106/JBJS.I.01199
11. Pérez-Prieto D, Portillo ME, Puig-Verdié L, Alier A, Martínez S, Sorlí L, et al. C-reactive protein may misdiagnose prosthetic joint infections, particularly chronic and low-grade infections. Int Orthopaed. (2017) 41:1–5. doi: 10.1007/s00264-017-3430-5
12. Mcarthur BA, Abdel MP, Taunton MJ, Osmon DR, Hanssen AD. Seronegative infections in hip and knee arthroplasty: periprosthetic infections with normal erythrocyte sedimentation rate and C-reactive protein level. Bone Joint J. (2015) 97:939–44. doi: 10.1302/0301-620X.97B7.35500
13. Deirmengian C, Hallab N, Tarabishy A, Della Valle C, Jacobs JJ, Lonner J, et al. Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res. (2010) 468:2017–23. doi: 10.1007/s11999-010-1298-4
14. Sousa R, Serrano P, Gomes Dias J, Oliveira JC, Oliveira A. Improving the accuracy of synovial fluid analysis in the diagnosis of prosthetic joint infection with simple and inexpensive biomarkers: C-reactive protein and adenosine deaminase. Bone Joint J. (2017) 99-B:351–7. doi: 10.1302/0301-620X.99B3.BJJ-2016-0684.R1
15. Dinneen A, Guyot A, Clements J, Bradley N. Synovial fluid white cell and differential count in the diagnosis or exclusion of prosthetic joint infection. Bone Joint J. (2013) 95:554–7. doi: 10.1302/0301-620X.95B4.30388
16. Higuera CA, Zmistowski B, Malcom T, Barsoum WK, Sporer SM, Mommsen P, et al. Synovial fluid cell count for diagnosis of chronic periprosthetic hip infection. J Bone Joint Surg Am. (2017) 99:753–9. doi: 10.2106/JBJS.16.00123
17. Ho IC, Miaw SC. Regulation of IL-4 expression in immunity and diseases. Adv Exp Med Biol. (2016) 941:31–77. doi: 10.1007/978-94-024-0921-5_3
18. Torre D, Tambini R, Aristodemo S, Gavazzeni G, Goglio A, Cantamessa C, et al. Anti-inflammatory response of IL-4, IL-10 and TGF-beta in patients with systemic inflammatory response syndrome. Mediat Inflamm. (2000) 9:193–5. doi: 10.1080/09629350020002912
19. Mihret A, Bekele Y, Bobosha K, Kidd M, Aseffa A, Howe R, et al. Plasma cytokines and chemokines differentiate between active disease and non-active tuberculosis infection. J Infect. (2013) 66:357–65. doi: 10.1016/j.jinf.2012.11.005
20. Jain-Vora S, Levine AM, Chroneos Z, Ross GF, Hull WM, Whitsett JA. Interleukin-4 enhances pulmonary clearance of Pseudomonas aeruginosa. Infect Immun. (1998) 66:4229–36. doi: 10.1128/IAI.66.9.4229-4236.1998
21. Hultgren O, Kopf M, Tarkowski A. Staphylococcus aureus-induced septic arthritis and septic death is decreased in IL-4-deficient mice: role of IL-4 as promoter for bacterial growth. J Immunol. (1998) 160:5082–7.
22. Zhao YX, Ljungdahl Å, Olsson T, Tarkowski A. In situ hybridization analysis of synovial and systemic cytokine messenger RNA expression in superantigen-mediated Staphylococcus aureus arthritis. Arthritis Rheum. (1996) 39:959–67.
23. Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. (2008) 90:1869–75. doi: 10.2106/JBJS.G.01255
24. Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. (2013) 56:e1–25. doi: 10.1093/cid/cis966
25. Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PMM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. (2003) 56:1129–35. doi: 10.1016/S0895-4356(03)00177-X
26. Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am. (2013) 95:2177–84. doi: 10.2106/JBJS.L.00789
27. Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, Mclawhorn AS. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty. (2018) 33:521–6. doi: 10.1016/j.arth.2017.09.021
28. Poulsen NR, Mechlenburg I, Søballe K, Lange J. Patient-reported quality of life and hip function after 2-stage revision of chronic periprosthetic hip joint infection: a cross-sectional study. Hip Int. (2018) 28:407–14. doi: 10.5301/hipint.5000584
29. Akgün D, Müller M, Perka C, Winkler T. The serum level of C-reactive protein alone cannot be used for the diagnosis of prosthetic joint infections, especially in those caused by organisms of low virulence. Bone Joint J. (2018) 100-B:1482–6. doi: 10.1302/0301-620X.100B11.BJJ-2018-0514.R1
30. Goswami K, Parvizi J, Maxwell Courtney P. Current recommendations for the diagnosis of acute and chronic PJI for hip and knee-cell counts, alpha-defensin, leukocyte esterase, next-generation sequencing. Curr Rev Musculoskelet Med. (2018) 11:428–38. doi: 10.1007/s12178-018-9513-0
31. Yoshii T, Magara S, Miyai D, Nishimura H, Kuroki E, Furudoi S, et al. Local levels of interleukin-1beta,−4,−6 and tumor necrosis factor alpha in an experimental model of murine osteomyelitis due to Staphylococcus aureus. Cytokine. (2002) 19:59–65. doi: 10.1006/cyto.2002.1039
32. Chen Z, Andreev D, Oeser K, Krljanac B, Hueber A, Kleyer A, et al. Th2 and eosinophil responses suppress inflammatory arthritis. Nat Commun. (2016) 7:11596. doi: 10.1038/ncomms11596
33. Prabhakara R, Harro JM, Leid JG, Keegan AD, Prior ML, Shirtliff ME. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect Immun. (2011) 79:5010–8. doi: 10.1128/IAI.05571-11
34. Riancho JA, Zarrabeitia MT, Gonzalez-Macias J. Interleukin-4 modulates osteoclast differentiation and inhibits the formation of resorption pits in mouse osteoclast cultures. Biochem Biophys Res Commun. (1993) 196:678–85. doi: 10.1006/bbrc.1993.2303
35. Pesanti EL, Lorenzo JA. Osteoclasts and effects of interleukin 4 in development of chronic osteomyelitis. Clin Orthop Relat Res. (1998) 355:290–9. doi: 10.1097/00003086-199810000-00031
36. Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. (1992) 176:287–92. doi: 10.1084/jem.176.1.287
37. Vazquez MI, Catalan-Dibene J, Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine. (2015) 74:318–26. doi: 10.1016/j.cyto.2015.02.007
38. Fröschen FS, Schell S, Schildberg FA, Klausing A, Kohlhof H, Gravius S, et al. Analysis of synovial biomarkers with a multiplex protein microarray in patients with PJI undergoing revision arthroplasty of the hip or knee joint. Arch Orthop Trauma Surg. (2020) 140:1883–90. doi: 10.1007/s00402-020-03388-5
39. Cipriano CA, Brown NM, Michael AM, Moric M, Della Valle CJ. Serum and synovial fluid analysis for diagnosing chronic periprosthetic infection in patients with inflammatory arthritis. J Bone Joint Surg Am. (2012) 94:594–600. doi: 10.2106/JBJS.J.01318
40. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. (2012) 27:1589–93. doi: 10.1016/j.arth.2012.03.059
41. Shahi A, Deirmengian C, Higuera C, Chen A, Restrepo C, Zmistowski B, et al. Premature therapeutic antimicrobial treatments can compromise the diagnosis of late periprosthetic joint infection. Clin Orthop Relat Res. (2015) 473:2244–9. doi: 10.1007/s11999-015-4142-z
42. Zahar A, Lausmann C, Cavalheiro C, Dhamangaonkar AC, Bonanzinga T, Gehrke T, et al. How reliable is the cell count analysis in the diagnosis of prosthetic joint infection? J Arthroplasty. (2018) 33:3257–62. doi: 10.1016/j.arth.2018.05.018
43. Bauer TW, Bedair H, Creech JD, Deirmengian C, Eriksson H, Fillingham Y, et al. Hip and knee section, diagnosis, laboratory tests: proceedings of international consensus on orthopedic infections. J Arthroplasty. (2019) 34:S351–9. doi: 10.1016/j.arth.2018.09.019
44. Qin L, Li F, Gong X, Wang J, Huang W, Hu N. Combined measurement of D-dimer and C-reactive protein levels: highly accurate for diagnosing chronic periprosthetic joint infection. J Arthroplasty. (2020) 35:229–34. doi: 10.1016/j.arth.2019.08.012
45. Qin L, Li X, Wang J, Gong X, Hu N, Huang W. Improved diagnosis of chronic hip and knee prosthetic joint infection using combined serum and synovial IL-6 tests. Bone Joint Res. (2020) 9:587–92. doi: 10.1302/2046-3758.99.BJR-2020-0095.R1
46. Van Den Kieboom J, Tirumala V, Xiong L, Klemt C, Kwon Y-M. Concomitant hip and knee periprosthetic joint infection in periprosthetic fracture: diagnostic utility of serum and synovial fluid markers. J Arthroplasty. (2021) 36:722–7. doi: 10.1016/j.arth.2020.08.029
47. Parvizi J, Ghanem E, Sharkey P, Aggarwal A, Burnett RS, Barrack RL. Diagnosis of infected total knee: findings of a multicenter database. Clin Orthop Relat Res. (2008) 466:2628–33. doi: 10.1007/s11999-008-0471-5
48. Xu H, Xie J, Huang Q, Lei Y, Zhang S, Pei F. Plasma fibrin degradation product and D-dimer are of limited value for diagnosing periprosthetic joint infection. J Arthroplasty. (2019) 34:2454–60. doi: 10.1016/j.arth.2019.05.009
Keywords: synovial fluid, interleukin 4, polymorphonuclear cell percentage, chronic periprosthetic joint infection, diagnosis
Citation: Huang J, Wang J, Qin L, Zhu B, Huang W and Hu N (2022) Combination of Synovial Fluid IL-4 and Polymorphonuclear Cell Percentage Improves the Diagnostic Accuracy of Chronic Periprosthetic Joint Infection. Front. Surg. 9:843187. doi: 10.3389/fsurg.2022.843187
Received: 25 December 2021; Accepted: 03 February 2022;
Published: 09 March 2022.
Edited by:
Sujit Kumar Tripathy, All India Institute of Medical Sciences Bhubaneswar, IndiaReviewed by:
Jochen Salber, Ruhr-University Bochum, GermanyPasquale Pagliano, University of Salerno, Italy
Copyright © 2022 Huang, Wang, Qin, Zhu, Huang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Hu, aHVuY3Fqb2ludEB5ZWFoLm5ldA==
 Jiaxing Huang
Jiaxing Huang Jiawei Wang1,2
Jiawei Wang1,2 Wei Huang
Wei Huang Ning Hu
Ning Hu