
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 06 April 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.842016
This article is part of the Research Topic Gastrointestinal Surgery: Emerging techniques, controversies and state of art View all 14 articles
 Jiongdi Lu1,2†
Jiongdi Lu1,2† Feng Cao1,2†
Feng Cao1,2† Zhi Zheng1,2†
Zhi Zheng1,2† Yixuan Ding1,2
Yixuan Ding1,2 Yuanxu Qu1,2
Yuanxu Qu1,2 Wentong Mei1,2
Wentong Mei1,2 Yulin Guo1,2
Yulin Guo1,2 Yu-Lu Feng3
Yu-Lu Feng3 Fei Li1,2*
Fei Li1,2*Aim: To explore the indications for early intervention in patients with acute necrotizing pancreatitis (ANP) and evaluate the effect of early intervention on the prognosis of ANP patients.
Methods: The clinical data of patients with ANP who underwent general surgery at Xuanwu Hospital of Capital Medical University from January 1, 2014, to December 31, 2020, were collected retrospectively. The patients were followed-up every 6 months after discharge, and the last follow-up date was June 30, 2021.
Results: A total of 98 patients with ANP were included in the study. They were divided into an early group (n= 43) and a delayed group (n = 55) according to the first percutaneous drainage (PCD) intervention time (≤ 4 weeks or > 4 weeks). Body temperature, inflammatory factor levels, and the number of patients with persistent organ failure (POF) were higher in the early group than in the delayed group. After the minimally invasive intervention, the body temperature and inflammatory factors of the two groups decreased significantly, most patients with POF improved, and the number of patients with reversal of POF in the early group was higher than that in the delayed group. Although the patients in the early group required more surgical intervention than those in the delayed group, there was no significant difference in mortality, incidence of postoperative complications, total length of hospital stay, or operation cost between the two groups. During long-term follow-up, there was no significant difference in the incidence of short-term and long-term complications and overall survival between the two groups.
Conclusions: Compared to patients in the delayed group, early intervention did not affect the prognosis of patients with ANP. It may be more suitable for patients with ANP with deterioration [such as POF or infected pancreatic necrosis (IPN)].
Acute pancreatitis (AP) is a common acute surgical condition of the abdomen. Although 80% of AP patients have mild self-limited disease, 20% of patients develop pancreatic necrosis and progress to acute necrotizing pancreatitis (ANP), and approximately one-third of patients with infected pancreatic necrosis (IPN) have significantly increased mortality (1, 2).
After the Dutch pancreatitis study group proposed and confirmed the effectiveness and safety of “step-up” minimally invasive intervention in the treatment of IPN (3), this strategy has become the preferred intervention recommended by current guidelines (4, 5). Specific measures were as follows: (1) for patients with suspected or confirmed IPN, timely antibiotic treatment should be administered in the early stage (≤4 weeks), and the intervention time should be postponed to 4 weeks after the onset of the disease, when the pancreatic necrosis is encapsulated and the boundary with the surrounding normal tissue is clear; and (2) percutaneous drainage (PCD) or endoscopic drainage (ED) of pancreatic necrotic tissue and effusion were performed to control infection. Video-assisted debridement (VAD) or endoscopic necrosectomy (EN) was performed according to the patient's condition, and laparotomy was performed when necessary.
In a recent international survey on the diagnosis and intervention time of IPN for patients diagnosed with IPN, although 55% of pancreatic experts supported antibiotic treatment first and puncture treatment after necrosis wrapping, but 45% of pancreatic experts still believe that minimally invasive intervention should be performed immediately after the diagnosis of IPN (6). Recently, a multicenter Randomized Controlled Trial (RCT) study published by the Dutch pancreatitis study group found that although early intervention did not benefit IPN patients more than delayed intervention, early intervention was an effective treatment option for IPN patients with clinical deterioration, but there was no clear indication of patients suitable for early intervention (7).
Therefore, by comparing the effects of different timing of PCD intervention on the long-term prognosis of ANP patients, this study clarified the indications for early PCD intervention in ANP patients and provided a reference for clinicians in the treatment of ANP.
This study retrospectively collected the clinical data of patients with AP during general surgery at Xuanwu Hospital of Capital Medical University from January 1, 2014, to December 31, 2020, using the case database of Xuanwu Hospital of Capital Medical University. All patient data were anonymously analyzed using an electronic data acquisition system without informed consent. This study was reviewed and approved by the Ethical Review Committee of Xuanwu Hospital of the Capital Medical University (No. 2020092). A detailed flowchart of the process is shown in Figure 1.
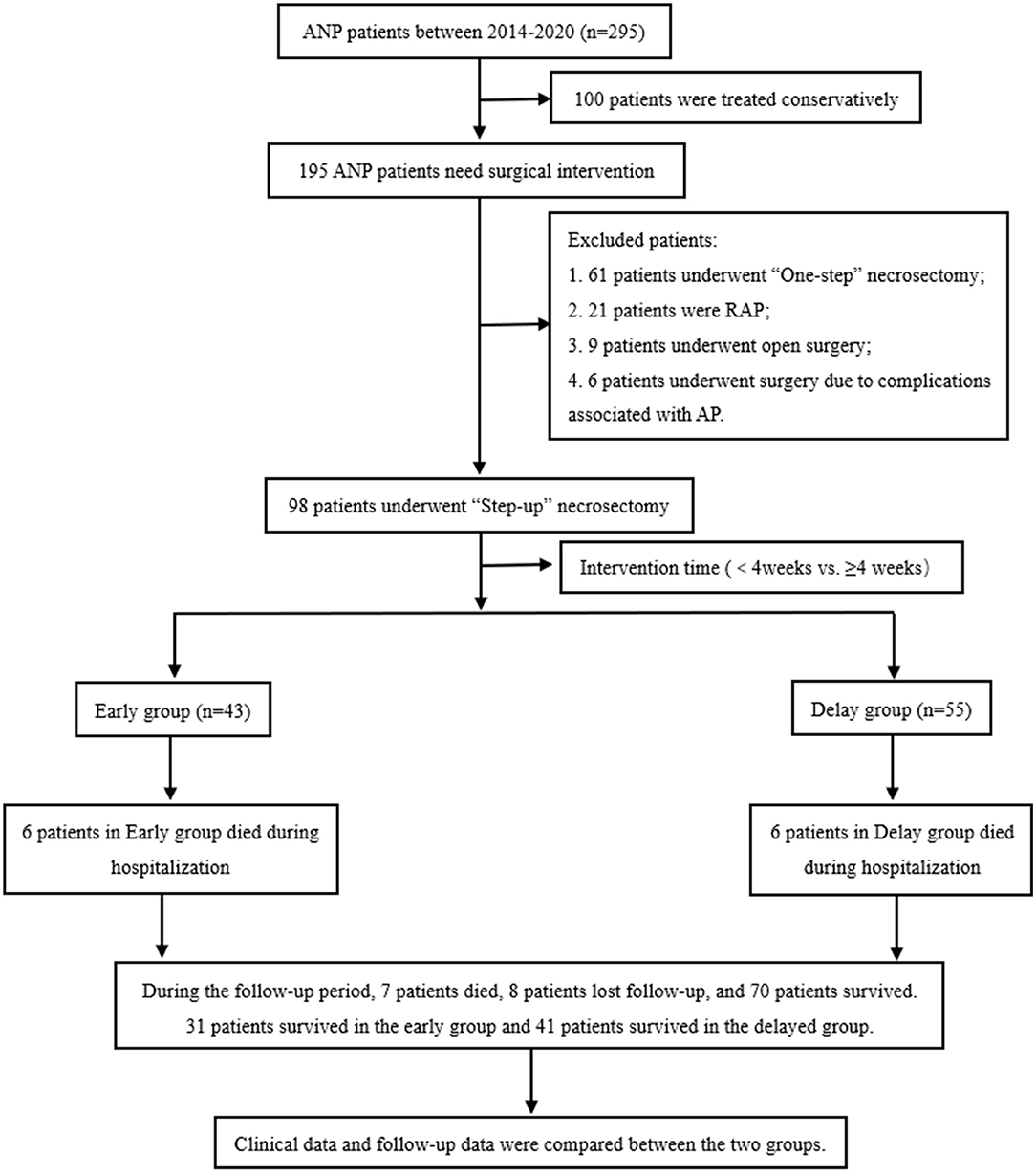
Figure 1. Flow chart of patient enrollment and follow-up. ANP, acute necrotizing pancreatitis; AP, acute pancreatitis; RAP, recurrent acute pancreatitis.
The inclusion criteria of patients were as follows: (1) ANP patients with pancreatic and/or peripancreatic necrosis confirmed by imaging examination (enhanced CT, MRI, etc.); (2) patients were treated with the “step-up” intervention strategy; and (3) the case data and follow-up data are complete.
The exclusion criteria were as follows: (1) mild AP (MAP) without pancreatic necrosis and/or peripancreatic necrosis; (2) ANP patients with conservative treatment or “one-step” intervention strategy; (3) ANP patients requiring emergency surgery; (4) patients with chronic pancreatitis, acute attack, or recurrent acute pancreatitis (RAP); and (5) patients with incomplete case or follow-up data.
The primary outcome of this study was number of surgical interventions and in-hospital mortality in both groups. The secondary outcomes of this study were the number of patients with persistent organ failure (POF), duration of nutritional support, type of nutritional support, operation cost, short-term postoperative complications (such as abdominal bleeding, gastrointestinal obstruction, gastrointestinal fistula, etc.), length of stay in the intensive care unit (ICU), total length of hospital stay, long-term complications during follow-up [incision hernia, pancreatic pseudocyst, RAP, pancreatic exocrine dysfunction (PEI), pancreatic endocrine dysfunction, chronic pancreatitis, pancreatic tumor, other gastrointestinal symptoms, etc.], quality of life score [Short Form-36 (SF-36), Euroqol-5 dimensions (EQ-5D) rating scales], and pain score (Izbicki pain score). The definitions of the relevant observation indicators used in this study are listed in Table 1.
According to current international guidelines (5), patients were given standard treatment measures such as fluid resuscitation, analgesia, inhibition of pancreatic enzyme secretion, and early enteral nutrition after admission. Antibiotic treatment was administered only to patients with suspected or confirmed infections. Laboratory and imaging examinations were performed regularly to observe changes in the patient's condition. If the patient's condition improved, the current treatment was continued. If the patient's condition worsened, a multidisciplinary team (MDT), including pancreatic surgeons, anesthetists, intensivists, and imaging specialists, collaborated and evaluated the patients and took individualized treatment measures. Patients with deterioration organ failure (OF) or new onset OF (NOF) were provided with relevant organ support therapy [continuous pumping of vasoactive drugs, mechanical ventilation therapy (MVT), continuous renal replacement therapy (CRRT), etc.]. Patients with suspected or confirmed IPN were empirically given third-and fourth-generation cephalosporins or carbapenem antibiotics according to previous research results, which were then replaced with sensitive antibiotics according to the results of the pathogen drug sensitivity test (10).
The indications for “step-up” intervention in ANP patients were as follows: (1) after conservative treatment, the patient's condition had no significant improvement or had continued deterioration (NOF or increased temperature and inflammatory indicators, etc.); (2) the presence of IPN was confirmed; and (3) Patients develop pancreatic pseudocyst (PP) or walled-off necrosis (WON), and the range of necrosis in patients was enlarged, resulting in compression symptoms of surrounding organs (such as digestive tract or biliary tract obstruction). Pancreatic surgeons in our center have rich experience in laparoscopic necrotic tissue debridement, “PCD + VAD” was often used for intervention, and the specific intervention steps have been described in detail in previous studies (11).
Patients in the early group received PCD treatment within 4 weeks of onset, and patients in the delayed group received PCD treatment 4 weeks after onset. After PCD intervention, clinicians determined the next treatment strategy by observing whether the patients' clinical symptoms improved (such as reversal of OF, decrease in body temperature, decrease in inflammatory factors, and reduction of pancreatic necrosis on CT). If the patient's condition improved, current treatment was continued. If the patient's condition deteriorated, VAD treatment was performed. Representative images are shown in Figure 2.
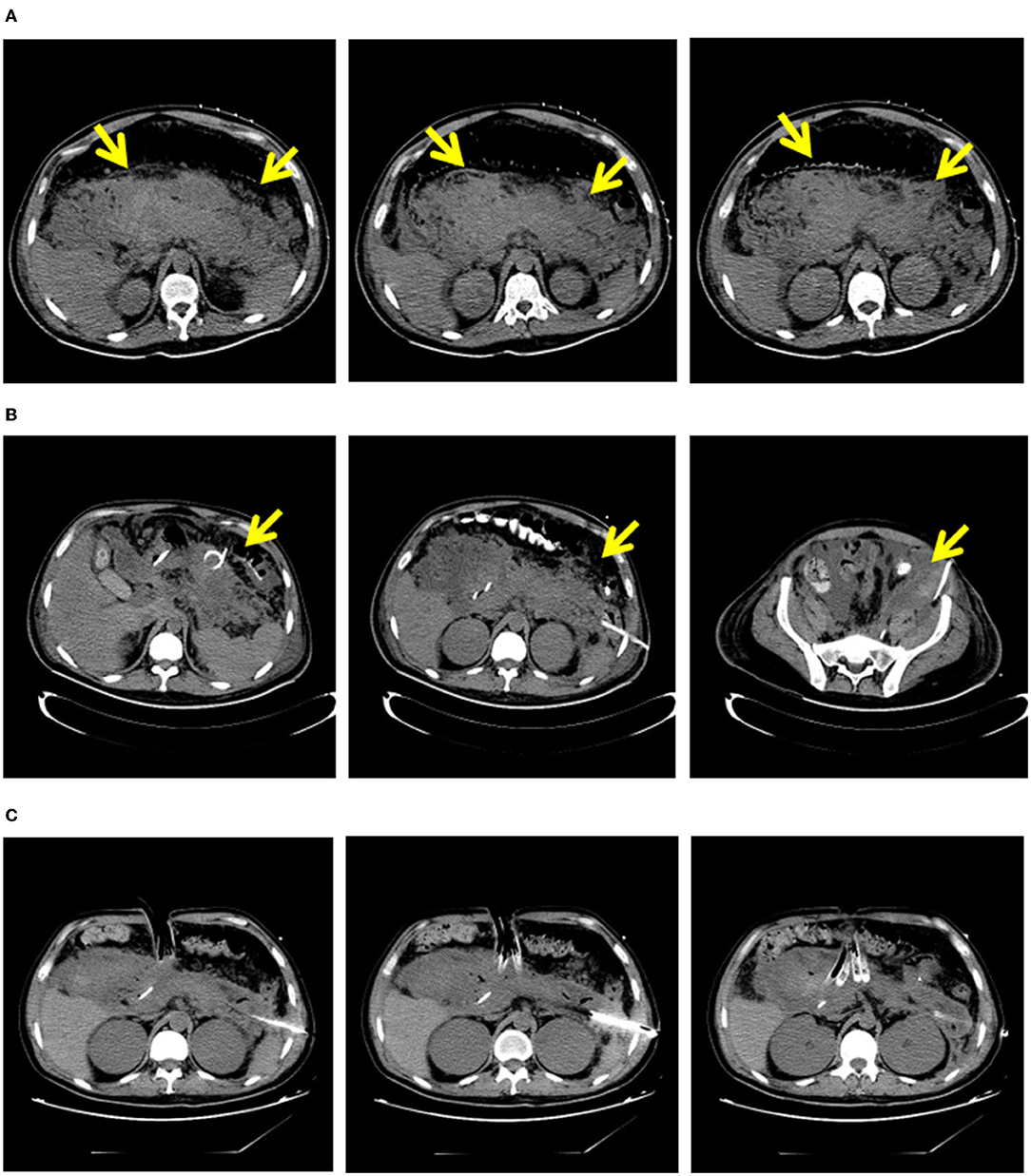
Figure 2. “Step-up” strategy in 32-years-old male with necrosing pancreatitis. (A) In 10 days of the onset of patients with pancreatic necrosis area. (B) Areas of pancreatic necrosis after PCD. (C) Areas of pancreatic necrosis after VAD. PCD, percutaneous catheter drainage; VAD, video assisted debridement.
After the patient was discharged, clinicians mainly followed-up the patients through inpatient visits, outpatient visits, telephone, e-mail, and other means. The follow-up period was 6 months. The follow-up mainly included physical examination (whether there was an incision hernia), laboratory examination (such as routine blood tests, biochemistry, and fecal elastase-1), and imaging examination (enhanced CT to evaluate whether there were morphological changes in the pancreas). In addition, patients completed the SF-36, EQ-5D, and Izbicki pain scales to facilitate the evaluation of their recent quality of life. The last follow-up date in this study was June 30, 2021.
In this study, Excel 2018 (Microsoft, Redmond, CA, USA) was used to record the clinical data of patients (SPSS 23.0, IBM Corp, Armonk, NY, USA), and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis. The Shapiro–Wilk test was used to evaluate whether the study data fit the normal distribution. Data with normal distribution were expressed as mean ± standard deviation (M ± SD), and the differences between groups were analyzed using the independent sample t-test. Data with skewed distribution were presented as median (range), and between-group differences were analyzed using the rank sum test. Quantitative data are presented as rates, and differences between groups were analyzed using the chi-square test or Fisher's exact probability method. The Kaplan-Meier method was used for survival analysis. Statistical significance was set at P < 0.05.
A total of 98 patients with ANP were included in this study, including 69 men and 29 women, with an average age of 45.88 ± 13.95 years. There were 50 cases of biliary pancreatitis, 33 of hyperlipidemic pancreatitis, two of alcoholic pancreatitis, and 13 of other causes (eight of pancreatitis after ERCP, four of unknown cause, and one of traumatic pancreatitis). Patients with ANP were divided into an early group (n = 43) and a delay group (n = 55) according to the time from the onset of ANP to the first intervention (≤ 4 weeks or >4 weeks).
There were no significant differences between the two groups in terms of sex, age, etiology, body mass index (BMI), American Society of Anesthesiologists (ASA) score, number of combined systemic diseases, or degree of pancreatic necrosis. In terms of admission laboratory indicators, the percentage of neutrophils (83.96 ± 9.08 vs. 77.99 ± 7.81%, P < 0.05), C-reactive protein (CRP) (306.15 ± 213.85 vs. 175.88 ± 119.01, P < 0.05), procalcitonin (PCT) (1.75 ± 1.35 vs. 1.16 ± 1.01, P < 0.05) and interleukin-6 (IL-6) (326.36 ± 214.14 vs. 203.3 ± 173.34, P < 0.05) in the early group were higher than those in the delay group. In addition, the hemoglobin level in the early group was lower than that in the delay group (88.13 ± 21.79 vs. 109 ± 35.51, P < 0.05) (see Table 2).
The main reason for intervention in the early group was IPN (86.05 vs. 56.36%, P < 0.05), while the main reasons for intervention in the delayed group were IPN and digestive tract obstruction (6.98 vs. 29.09%, P < 0.05). The time of first intervention in early group was earlier than that in delay group (15.26 ± 7.08 days vs. 50.86 ± 19.58 days, P < 0.05). he number of patients who improved after PCD treatment only (20.93 vs. 14.55%, P > 0.05) and the number of patients needing VAD treatment (76.74 vs. 78.18%, P > 0.05) were similar between the two groups. In addition, one patient in the early group and three patients in the delay group required open necrosectomy (2.33 vs. 5.45%, P > 0.05) (Table 3). Although the level of preoperative inflammatory factors (CRP, PCT, IL-6) in the early group was higher than that in the delayed group (P < 0.05), the level of inflammatory factors in both groups decreased significantly after the intervention. The results are presented in Figure 3.
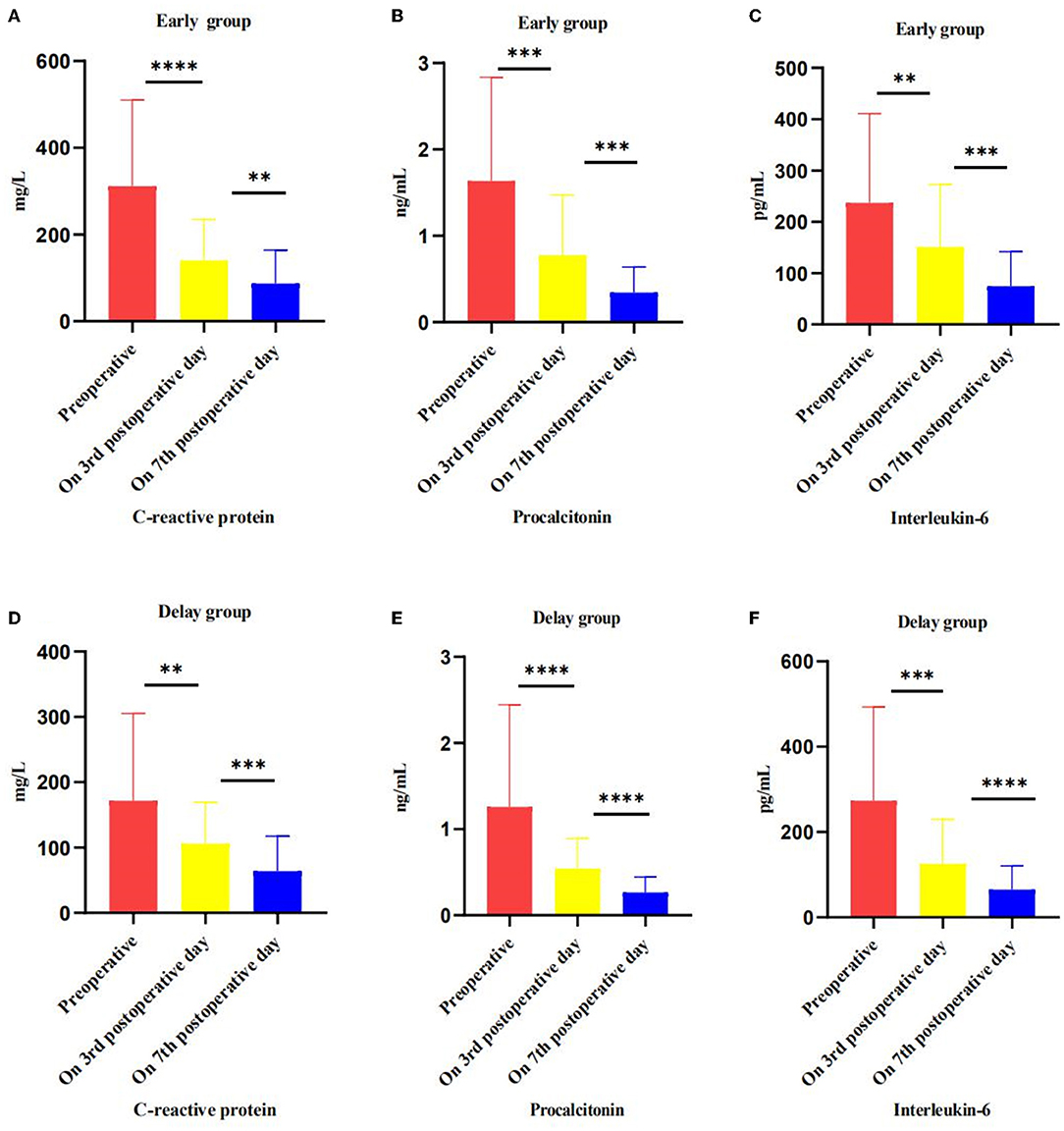
Figure 3. Effect of intervention on inflammatory markers, preoperative, postoperative, and pre-discharge comparison. (A–C) In the early group, the level of CRP, PCT and IL-6 change trend in preoperative, on 3 and 7 postoperative days. (D–F) In the delayed group, the level of CRP, PCT and IL-6 change trend in preoperative, on 3 and 7 postoperative days. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001.
In terms of clinical outcome, POF was more common in patients in the early group (44.19 vs. 18.18%, P < 0.05); however, most of the patients in the two groups had reversed OF after the intervention (Figure 4). Although patients in the early group needed minimally invasive intervention more than patients in the delay group [2 (1–7) vs. 2 (1–5), P < 0.05], the number of patients in the two groups that needed combined nutritional support (74.42 vs. 65.45%, P > 0.05), the duration of enteral nutritional support (22.12 ± 17.30 days vs. 26.87 ± 25.25 days, P > 0.05), length of parenteral nutrition support (27.54 ± 22.35 days vs. 29.61 ± 28.51 days, P > 0.05), operation cost (26,498 ± 9022.98 vs. 27131.92 ± 8918.18, P > 0.05), incidence of postoperative complications (18.60 vs. 18.18%, P > 0.05), length of ICU stay (25.32 ± 24.18 days vs. 30.88 ± 29.51 days, P > 0.05), total length of hospital stay (40.28 ± 27.52 days vs. 47.76 ± 32.51 days, P > 0.05), and mortality during hospitalization (13.95 vs. 10.91%, P > 0.05) were not significantly different (Table 4).
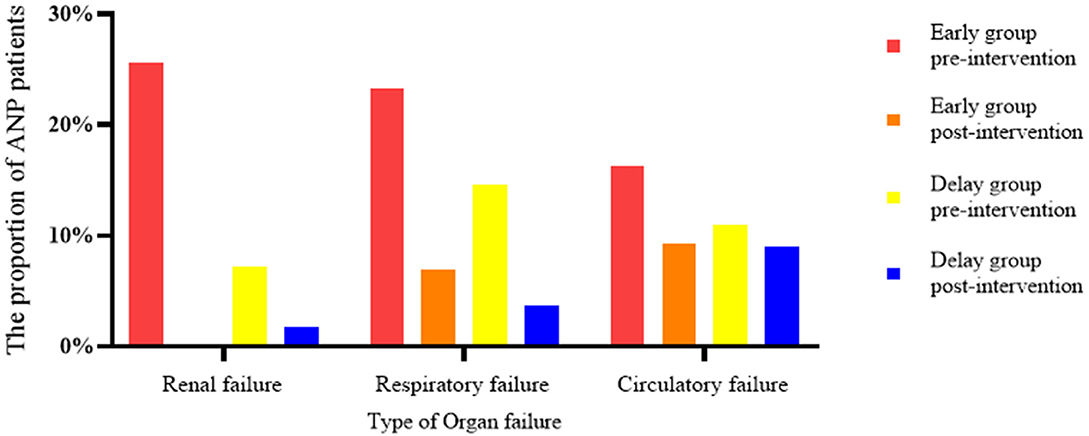
Figure 4. Effect of interventions on organ failure, comparing early vs. delay group. In the early group, the proportion of patients with renal failure decreased from 25.58 to 0%, that of patients with respiratory failure decreased from 23.26 to 6.98%, and that of patients with circulatory failure decreased from 16.28 to 9.30%. In the delayed group, renal failure decreased from 7.27 to 1.82%, respiratory failure decreased from 14.55 to 3.67%, and circulatory failure decreased from 10.91 to 9.09%.
During the follow-up period, seven patients died, eight patients were lost to follow-up, and 71 patients survived. Among them, three patients died, three patients were lost to follow-up, and 31 patients survived in the early group; in the delayed group, four patients died, five patients were lost to follow-up, and 40 patients survived. The overall survival rates of the two groups were 91.18% (31/34) and 90.91% (40/44), respectively (Figure 5).
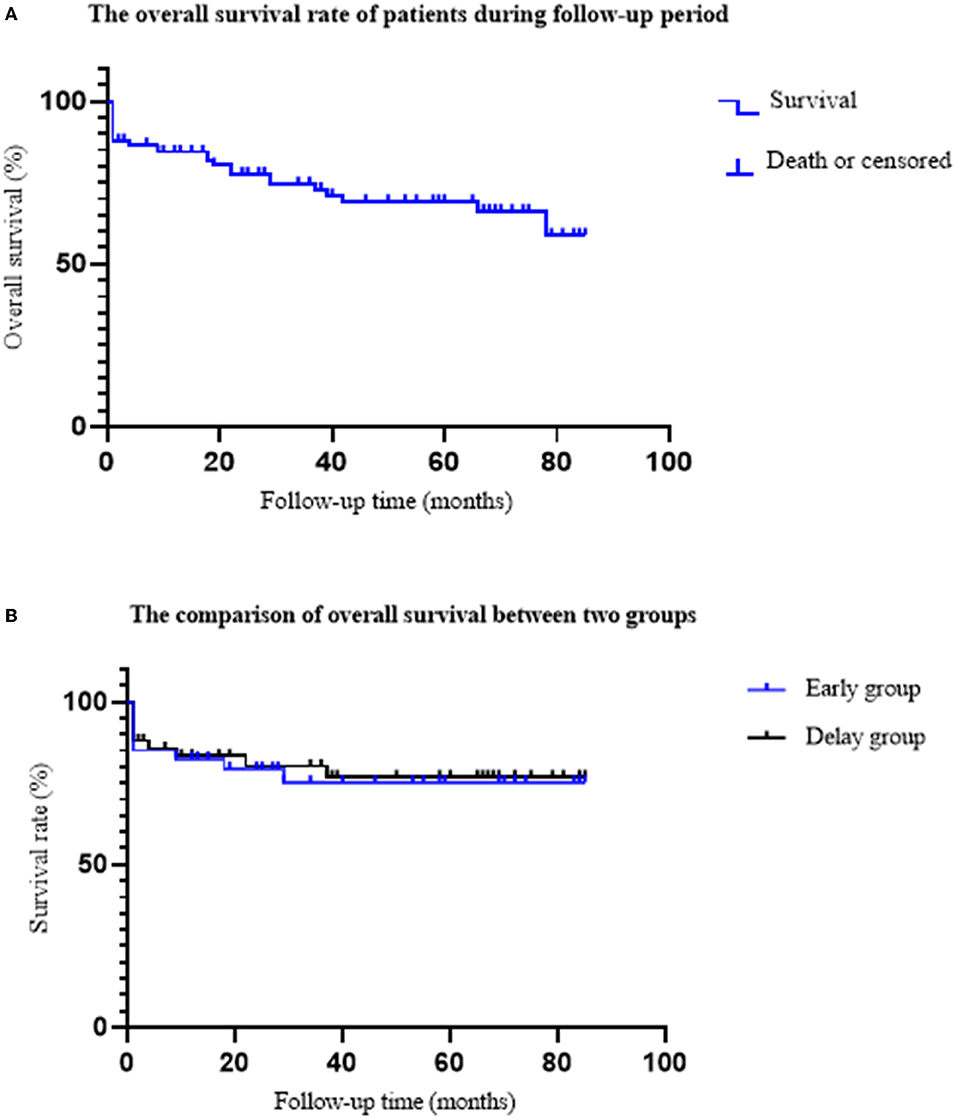
Figure 5. The comparison of overall survival rate between two groups. (A) The overall survival rate of ANP patients. A total of 19 patients died, eight patients were lost follow-up, and 71 patients survived. The average follow-up time was 40.17 ± 26.36 months. (B) The comparison of the overall survival rate between the two groups. In the early group, nine patients died; six patients died during hospitalization, and three patients died during follow-up. In the delayed group, 10 patients died; six patients died during hospitalization and four patients died during follow-up. The overall survival rates of early group and delayed group were 91.18 and 90.91%, respectively (P = 0.967).
There were no significant differences in the follow-up time (42.83 ± 25.74 vs. 41.74 ± 27.09, P > 0.05), pancreatic pseudocyst (8.11 vs. 4.08%, P > 0.05), incisional hernia (5.41 vs. 4.08%, P > 0.05), recurrent acute pancreatitis (24.32 vs. 8.16%, P > 0.05), new onset pancreatic endocrine insufficiency (29.73 vs. 14.28%, P > 0.05), pancreatic exocrine insufficiency (13.52 vs. 16.33%, P > 0.05), and chronic pancreatitis (2.70 vs. 6.12%, P > 0.05) (Table 5).
In the quality-of-life rating scale, there was no statistically significant difference in the SF-36 physical or mental health score, EQ-5D health status score, or Izbicki pain score between the groups (Table 6).
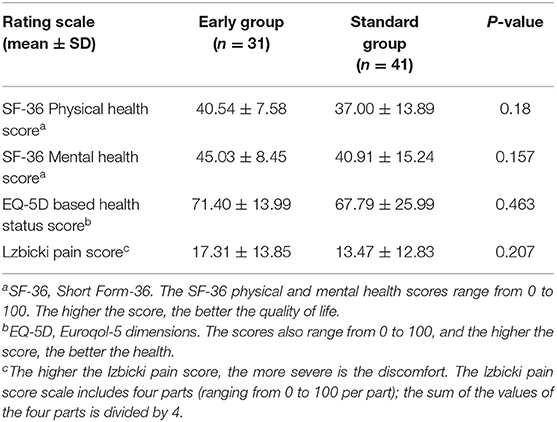
Table 6. Quality of life rating scale during the follow-up period of surviving acute necrotizing pancreatitis patients.
There has always been controversy over the timing of intervention with “step-up” strategies. Some pancreatic experts supported antibiotic treatment first and puncture treatment after necrosis wrapping, these experts believe that: (1) in the early stage of the disease, the boundary between the scope of pancreatic necrosis and normal tissue is blurred. Early intervention causes great trauma to patients and is more prone to postoperative complications. (2) Some patients can improve after conservative treatment with antibiotics without intervention. Other pancreatic experts still believe that minimally invasive intervention should be performed immediately after the diagnosis of IPN (6). They considered that: (1) the concept of “delayed intervention” comes from the era of open surgery, and it is controversial whether it is applicable to the current era of minimally invasive surgery (12); and (2) in the era of minimally invasive approach, PCD is not technically difficult, and in other abdominal cases requiring PCD, drainage before necrotic wrapping has been a very common practice (13). Theoretically, timely drainage of pancreatic necrotic tissue rich in inflammatory factors is conducive to reducing systemic inflammatory response syndrome (SIRS), avoiding further clinical deterioration, and improving the prognosis of patients. In addition, PCD can reduce abdominal pressure and the risk of abdominal compartment syndrome. Furthermore, early PCD intervention can control the source of infection and speed up the encapsulated necrotic tissue (14).
By comparing the clinical data of patients in the early and delayed groups, this study defined the indications for early PCD intervention in patients with ANP. Compared with delayed intervention, early intervention did not increase mortality, incidence of postoperative complications, operation cost, or length of hospital stay. In the long-term follow-up, the overall survival and long-term complication rates of the two groups were similar. It has been further confirmed that early PCD intervention is an effective and safe treatment strategy for ANP patients with deterioration (such as POF or IPN) in the early stages of the disease.
Previous studies have pointed out that 18% of ANP patients were diagnosed with IPN (bubble sign) 3 weeks before the onset, 43% developed package necrosis 3 weeks before the onset, and have intervention indications in the early stage of the disease (15). In a large-scale multicenter study of the Dutch pancreatitis study group, the early group received PCD intervention within 24 h, and the delayed group received PCD intervention as late as 4 weeks after onset on the basis of antibiotics, and 39% of patients in the delayed group improved after conservative treatment with antibiotics, confirming the effectiveness of antibiotics in IPN patients. Although the comprehensive complication index score and mortality of the two groups were similar, it did not prove the superiority of early intervention in the treatment of patients with IPN. It is only suggested that early PCD intervention can be considered for IPN patients with rapid clinical deterioration (7). In this study, patients in the early group received PCD intervention after the failure of conservative antibiotic treatment. The first PCD intervention in most patients in the early group was between 2 and 3 weeks after onset. In addition, only 18.37% of patients with ANP showed improvement after PCD intervention. This is lower than the 35–51% reported by other research institutes (3, 7, 9, 16, 17). It may be that our hospital is one of the largest acute pancreatitis diagnosis and treatment centers in northern China. Approximately 80% of patients were referred from other hospitals, and some patients were referred to our hospital after the PCD intervention failed, and the condition was relatively serious. We believe that for ANP patients with suspected or confirmed IPN, the timing of early intervention should be determined according to the changes in the patients' condition after conservative treatment with antibiotics.
Although the current guidelines recommend that patients with ANP with suspected or confirmed infection should be treated conservatively with antibiotics, some patients still deteriorate after conservative treatment, suggesting that conservative treatment may not be applicable to all patients with IPN (5). In the expert consensus of the American Gastroenterology Association on the management of pancreatic necrosis, PCD should be considered when patients are suspected of having IPN and conservative treatment fails (18). A retrospective study based on a prospective database pointed out that in ANP patients, although early intervention is conducive to reducing the inflammatory response and improving it compared with delayed intervention, it is considered that early intervention is more suitable for ANP patients with IPN and/or POF (17). A single center RCT study of the Chinese acute pancreatitis clinical trial group found that early intervention may benefit ANP patients with POF (14), and further multicenter studies were conducted to clarify the early intervention indications of ANP patients (19). In this study, the body temperature and inflammatory factor levels of patients in the early group were significantly higher than those in the delayed group, suggesting that patients in the early group had more SIRS caused by ANP. With the extension of SIRS duration, patients are more likely to have OF and IPN, and sepsis caused by IPN may induce or aggravate OF, resulting in an increased risk of disease deterioration and death (20–22). In our study, about 44.19 and 86.05% patients in the early group have POF and IPN, respectively, which were significantly higher than those in the delayed group, also supporting this opinion. After PCD intervention, the inflammatory factor levels of ANP patients decreased significantly, and most patients with POF were successfully separated from organ support treatment. The number of patients with OF remission in the early group was greater than that in the delayed group, suggesting that early PCD intervention is conducive to controlling SIRS and reversing OF.
In terms of clinical outcomes, the overall mortality of patients in this study was 12.24%, which is similar to the mortality reported in endoscopic or surgical early intervention ANP studies in recent years (7.8–30%) (7, 14, 17). Although there was no significant difference between the two groups in terms of the length of nutritional support, incidence of postoperative complications, operation cost, length of ICU stay and mortality. But the number of surgical interventions in the early group was greater than that in the delayed group, combined with the patient's admission condition and the number of patients with POF was more than those in the delayed group, affect the rise of the number of interventions.
Through the long-term follow-up of patients with ANP after discharge, it was found that there was no significant difference in the overall survival rate and the incidence of long-term complications (incision hernia, new pancreatic endocrine insufficiency, PEI, etc.) between the two groups. This further confirms that early PCD intervention is safe and effective for patients with ANP. Previous studies have reported that IPN intervention may cause damage to adjacent pancreatic tissues, resulting in a decline in the pancreatic reserve and secretion function; 21% AP patients have RAP, and ~8% of RAP patients progress to chronic pancreatitis (CP) (23), 27% have PEI, and 37% have new pancreatic endocrine insufficiency during follow-up (24, 25). This aggravates the medical burden on patients and affects their quality of life. A study by Firkins et al. (26) confirmed that age (50–64 years old), male sex, low economic level, Elixhauser comorbidity index ≥ 3 points, components of metabolic syndrome, severe AP (SAP), and RAP are risk factors for pancreatic endocrine dysfunction, while alcoholic etiology, SAP, or ANP are high-risk factors for PEI (23, 25). Sanchez et al. (27) found that triglyceride levels were positively correlated with the risk of RAP by retrospectively collecting clinical data of patients with AP in the United States. Therefore, clinicians should strengthen the publicity and education of AP-related complications, closely monitor AP patients with high-risk factors (such as laboratory examination and imaging evaluation), and follow-up regularly to prevent and delay the occurrence of long-term complications of AP.
However, this study also has some limitations: firstly, this was a retrospective study and some of the patients were referred from other hospitals. The reason for an early or late intervention is not clear, clinical indicators were affected by the details of the clinical data at the time of referral, and there may be some statistical bias. Secondly, the first PCD intervention in some patients was performed in other hospitals. The clinical experience and operation level of pancreatic surgeons in different hospitals may also affect patient prognosis. Third, we did not compare the effect of endoscopic intervention on patients with necrotizing pancreatitis.
Compared to delayed intervention, early intervention did not affect the prognosis of patients with ANP. For ANP patients with deterioration (such as POF) in the early stages of the disease, early intervention may be more suitable than conservative treatment. In view of the complex and changeable condition changes of ANP patients, further multicenter clinical trials with large sample sizes are needed to verify and identify the potential beneficiaries of early intervention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
FL designed and performed the research. YD and Y-LF carried out the studies and participated in collecting data. YG, WM, YQ, and Y-LF performed the statistical analysis and participated in its design. JL, FC, and ZZ wrote the manuscript. FL revised the manuscript. All authors read and approved the final manuscript.
This study supported by Beijing Municipal Science and Technology Commission (Z171100001017077), Beijing Municipal Science and Technology Commission Clinical Diagnosis and Treatment Technology Research and Demonstration Application Project (Z191100006619038), Capital Medical Development and Research Special Project (2020-1-2012 and Z201100005520090), and Construction Project of Clinical Advanced subjects of Capital Medical University (1192070312).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Professor Chun-jing Bian and Professor Ang Li for their guidance.
1. Trikudanathan G, Wolbrink D, Van Santvoort H, Mallery S, Freeman M, Besselink M. Current concepts in severe acute and necrotizing pancreatitis: an evidence-based approach. Gastroenterology. (2019) 156:1994–2007.e3. doi: 10.1053/j.gastro.2019.01.269
2. Mederos MA, Reber HA, Girgis M. Acute pancreatitis: a review. JAMA. (2021) 325:382–90. doi: 10.1001/jama.2020.20317
3. van Santvoort HC BM, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. New Engl J Med. (2010) 362:1491–502. doi: 10.1056/NEJMoa0908821
4. Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví-Poma E, Petrov MS, et al. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. (2012) 256:875–80. doi: 10.1097/SLA.0b013e318256f778
5. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis-−2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11. doi: 10.1136/gutjnl-2012-302779
6. Grinsven JV, Brunschot SV, Bakker OJ, Bollen TL, Boermeester MA, Bruno MJ, et al. Diagnostic strategy and timing of intervention in infected necrotizing pancreatitis: an international expert survey and case vignette study. HPB. (2016) 18:49–56. doi: 10.1016/j.hpb.2015.07.003
7. Boxhoorn L, van Grinsven J, Verdonk RC, Boermeester MA, Bollen TL, Bouwense SAW, et al. Immediate versus postponed intervention for infected necrotizing pancreatitis. N Engl J Med. (2021) 385:1372–81. doi: 10.1056/NEJMoa2100826
8. Balthazar EJ, Ranson J, Naidich DP, Megibow AJ, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. (1985) 156:767. doi: 10.1148/radiology.156.3.4023241
9. Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J. Chronic pancreatitis. Lancet. (2020) 396:499–512. doi: 10.1016/S0140-6736(20)31318-0
10. Lu JD, Cao F, Ding YX, Wu YD, Guo YL Li F. Timing, distribution, and microbiology of infectious complications after necrotizing pancreatitis. World J Gastroenterol. (2019) 25:5162–73. doi: 10.3748/wjg.v25.i34.5162
11. Li A, Cao F, Li J, Fang Y, Wang X, Liu DG, et al. Step-up mini-invasive surgery for infected pancreatic necrosis: results from prospective cohort study. Pancreatology. (2016) 16:508–14. doi: 10.1016/j.pan.2016.03.014
12. Besselink MG VT, Schoenmaeckers EJ, Buskens E, Ridwan BU, Visser MR, Nieuwenhuijs VB, et al. Timing of Surgical Intervention in Necrotizing Pancreatitis. Arch Surg. (2007) 142:1194–201. doi: 10.1001/archsurg.142.12.1194
13. Van Grinsven J, Van Santvoort HC, Boermeester MA, Dejong CH, Van Eijck CH, Fockens P, et al. Timing of catheter drainage in infected necrotizing pancreatitis. Nat Rev Gastroenterol Hepatol. (2016) 13:306–12. doi: 10.1038/nrgastro.2016.23
14. Ke L DX, Chen T, Doig GS Li G, Ye B, Zhou J, et al. Early on-demand drainage or standard management for acute pancreatitis patients with acute necrotic collections and persistent organ failure: a pilot randomized controlled trial. J Hepatobiliary Pancreat Sci. (2021) 28:387–96. doi: 10.1002/jhbp.915
15. Van Grinsven J, Van Brunschot S, Van Baal MC, Besselink MG, Fockens P, Van Goor H, et al. Natural history of gas configurations and encapsulation in necrotic collections during necrotizing pancreatitis. J Gastroint Surg. (2018) 22:1557–64. doi: 10.1007/s11605-018-3792-z
16. van Brunschot S, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. (2017) 391:51–8. doi: 10.1016/S0140-6736(18)31189-9
17. Trikudanathan G, Tawfik P, Amateau SK, Munigala S, Arain M, Attam R, et al. Early (<4 weeks) versus standard (≥ 4 weeks) endoscopically centered step-up interventions for necrotizing pancreatitis. Am J Gastroenterol. (2018) 113:1550–8. doi: 10.1038/s41395-018-0232-3
18. Baron TH, Dimaio CJ, Wang AY, Morgan KA. American gastroenterological association clinical practice update: management of pancreatic necrosis. Gastroenterology. (2020) 158:67–75.e1. doi: 10.1053/j.gastro.2019.07.064
19. Cheng QA, He ZA, Tao CB, Yin ZC, Qf D, Feng GE, et al. Early on-demand drainage versus standard management among acute necrotizing pancreatitis patients complicated by persistent organ failure: the protocol for an open-label multi-center randomized controlled trial. Pancreatology. (2020) 20:1268–74. doi: 10.1016/j.pan.2020.08.012
20. Petrov MS, Shanbhag S, Chakraborty M, Phillips A, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. (2010) 139:813–20. doi: 10.1053/j.gastro.2010.06.010
21. Tan CYL, Shi F, Hu J, Zhang X, Wang Y, Deng Z, et al. Early systemic inflammatory response syndrome duration predicts infected pancreatic necrosis. J Gastrointest Surg. (2019) 24:590–7. doi: 10.1007/s11605-019-04149-5
22. Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. (2006) 93:738–44. doi: 10.1002/bjs.5290
23. Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. (2018) 16:175–84. doi: 10.1038/s41575-018-0087-5
24. Das S, Singh PP, Phillips A, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. (2014) 63:818–31. doi: 10.1136/gutjnl-2013-305062
25. Hollemans RAHN, Mager DJ, Kelder JC, Besselink MG, Bruno MJ, Verdonk RC, et al. Pancreatic exocrine insufficiency following acute pancreatitis: Systematic review and study level meta-analysis. Pancreatology. (2018) 18:253–62. doi: 10.1016/j.pan.2018.02.009
26. Firkins SA, Hart PA, Papachristou GI, Lara LF, Krishna SG. Identification of a risk profile for new-onset diabetes after acute pancreatitis. Pancreas. (2021) 50:696–703. doi: 10.1097/MPA.0000000000001818
Keywords: acute necrotizing pancreatitis, percutaneous drainage, infected pancreatic necrosis, persistent organ failure, complications
Citation: Lu J, Cao F, Zheng Z, Ding Y, Qu Y, Mei W, Guo Y, Feng Y-L and Li F (2022) How to Identify the Indications for Early Intervention in Acute Necrotizing Pancreatitis Patients: A Long-Term Follow-Up Study. Front. Surg. 9:842016. doi: 10.3389/fsurg.2022.842016
Received: 23 December 2021; Accepted: 02 March 2022;
Published: 06 April 2022.
Edited by:
Stefano Rausei, ASST Valle Olona, ItalyReviewed by:
Marcus Hollenbach, University Hospital Leipzig, GermanyCopyright © 2022 Lu, Cao, Zheng, Ding, Qu, Mei, Guo, Feng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Li, ZmVpbGkzNkBjY211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.