- 1Department of Clinical Nutrition, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Gastrointestinal Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Purpose: The purpose of the current study was to analyze the effect of intraoperative blood loss (IBL) and intraoperative blood transfusion (IBT) on the short-term outcomes and prognosis for patients who underwent primary colorectal cancer (CRC) surgery.
Methods: We retrospectively collected the patients' information from the database of a teaching hospital from January 2011 to January 2020. IBL and IBT were collected and analyzed, and the propensity score matching (PSM) analysis was performed.
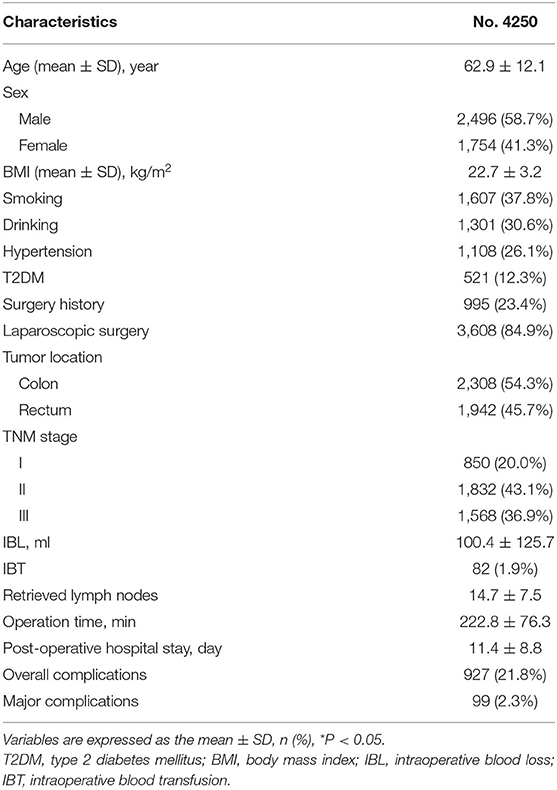
Results: A total of 4,250 patients with CRC were included in this study. There were 1,911 patients in the larger IBL group and 2,339 patients in the smaller IBL group. As for IBT, there were 82 patients in the IBT group and 4,168 patients in the non-IBT group. After 1:1 ratio PSM, there were 82 patients in the IBT group and 82 patients in the non-IBT group. The larger IBL group had longer operation time (p = 0.000 < 0.01), longer post-operative hospital stay (p = 0.000 < 0.01), smaller retrieved lymph nodes (p = 0.000 < 0.01), and higher overall complication (p = 0.000 < 0.01) than the smaller IBL group. The IBT group had longer operation time (p = 0.000 < 0.01), longer hospital stay (p = 0.016 < 0.05), and higher overall complications (p = 0.013 < 0.05) compared with the non-IBT group in terms of short-term outcomes. Larger IBL (p = 0.000, HR = 1.352, 95% CI = 1.142–1.601) and IBT (p = 0.044, HR = 1.487, 95% CI = 1.011–2.188) were independent predictive factors of overall survival (OS). Larger IBL (p = 0.000, HR = 1.338, 95% CI = 1.150–1.558) was an independent predictor of disease-free survival (DFS); however, IBT (p = 0.179, HR = 1.300, 95% CI = 0.886–1.908) was not an independent predictor of DFS.
Conclusion: Based on the short-term outcomes and prognosis of IBL and IBT, surgeons should be cautious during the operation and more careful and proficient surgical skills are required for surgeons.
Introduction
Colorectal cancer (CRC) is one of the most common cancers in the world, with an estimated 1.8 million new cases and 861,000 deaths each year (1). The incidence of CRC is decreasing in western countries including the United States, Canada, and Australia. However, an upward trend has been existing in China; moreover, China is a country with the largest number of new cases and deaths of CRC every year in the world (2). At present, radical surgical treatment of CRC is still the most important and decisive treatment (3–5).
Due to the popularity of laparoscopic surgery and the development of surgical equipment such as electrothermal bipolar activation devices and ultrasound systems, the average intraoperative blood loss (IBL) has decreased (6, 7). However, IBL is still a matter of concern during the operation. Studies have found that larger IBL might affect complications and prognosis in gastrointestinal tumors (8–10).
Perioperative blood transfusion is another concern for surgeons. However, the influence of perioperative blood transfusion on short-term outcomes and prognosis was inconsistent. Some studies reported that perioperative blood transfusion could increase post-operative complications, prolong hospital stay, and affect prognosis (11–13). However, other studies suggested that perioperative blood transfusion did not affect prognosis (14, 15).
The effect of IBL in patients with CRC is still controversial. Some studies reported that larger IBL could increase complications and reduce prognosis (16–18). However, other studies reported that IBL had no effect on complications and prognosis (19, 20). Furthermore, no previous studies reported the intraoperative blood transfusion (IBT) on the outcomes and prognosis of patients with CRC. Thus, the purpose of the current study was to analyze the effect of IBL and IBT on the short-term outcomes and prognosis of patients who underwent primary CRC surgery.
Materials and Methods
We retrospectively collected the patients' information from database of a teaching hospital. The database included patients who underwent primary CRC surgery from Jan 2011 to Jan 2020. This study was conducted in accordance with the World Medical Association Declaration of Helsinki. Ethical approval from the institutional review board of the First Affiliated Hospital of Chongqing Medical University was obtained (2021-540). All patients signed the informed consent.
Patients Selection
We included patients who underwent primary CRC surgery in a single teaching hospital (n = 5,473). The exclusion criteria were as follows: 1, incomplete medical records (n = 323); 2, non-R0 resection (n = 25); and 3, stage IV CRC (n = 875). Finally, a total of 4,250 patients with CRC were included in this study (Figure 1).
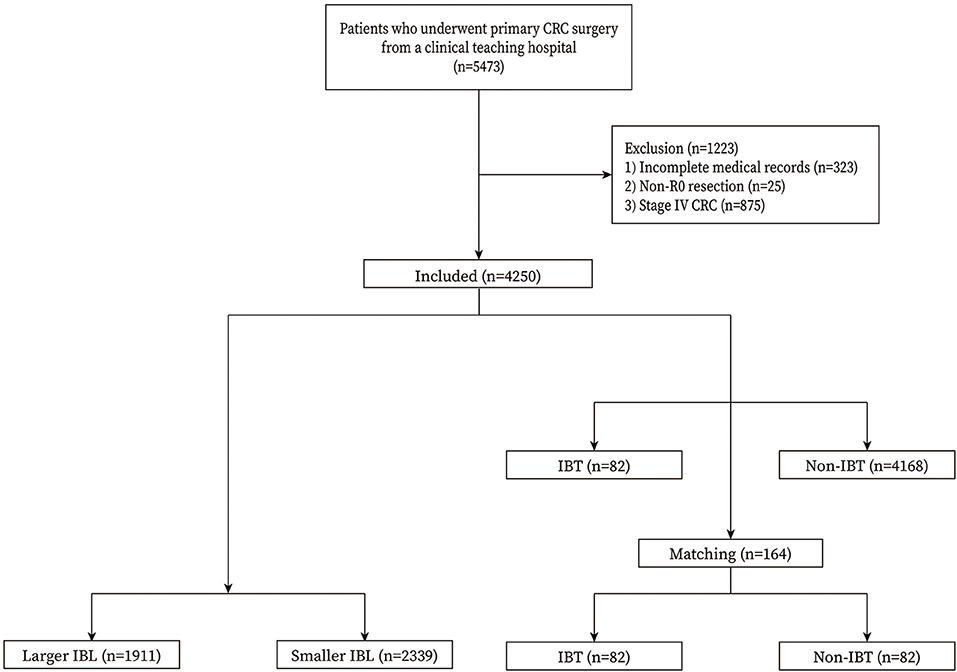
Figure 1. Flow chart of patient selection. IBL, intraoperative blood loss; IBT, intraoperative blood transfusion.
Surgery Management
The CRC surgery was conducted according to the principle of AJCC 8th Edition (16). Radical resection (total mesorectal excision/complete mesocolic excision) was performed, and the pathology confirmed R0 resection.
Definitions
The tumor node metastasis stage was diagnosed according to the AJCC 8th Edition (21). The complications were graded according to the Clavien-Dindo classification (22), and major complications were defined as ≥ III classification complications. Overall survival (OS) was defined as the time from surgery to the last follow-up or death. Disease-free survival (DFS) was defined as the time from surgery to recurrence, death, or last follow-up. IBL was defined as estimated blood loss during CRC surgery, and IBL was divided into two groups including the larger IBL and the smaller IBL group. The cut-off value of IBL was the 75th percentile of IBL (100 ml), larger IBL group was defined as IBL ≥ 100 ml, smaller IBL group was defined as IBL < 100 ml. IBT was defined as patients who underwent blood transfusion during CRC surgery, and they were divided into two groups including IBT group and non-IBT group.
Outcomes
The primary outcome was the prognosis including OS and DFS. The second outcome was the short-term outcomes including operation time, retrieved lymph nodes, overall complications, major complications, and post-operative hospital stay.
Data Collection
The baseline information of patients with CRC was collected retrospectively including sex, age, body mass index (BMI), smoking, drinking, hypertension, type 2 diabetes mellitus (T2DM), tumor location, surgery history, surgical methods (open surgery or laparoscopic surgery), and tumor stage. The short-term outcomes were collected through inpatient medical system. The follow-up information was collected through out-patient system and telephone interview.
Propensity Score Matching
Propensity score matching (PSM) is a method that could minimize the bias of baseline information (23, 24). In this study, we compared the short-term outcomes and prognosis of IBT on patients with CRC using PSM. Nearest neighbor matching was performed without replacement at a 1:1 ratio and a caliper width with a 0.01 standard deviation was specified. The matched baseline information was as follows: age, sex, BMI, smoking, drinking, hypertension, T2DM, tumor location, and tumor stage.
Statistical Analysis
Continuous variables are expressed as the mean ± standard deviation (SD) and independent-sample t-test was analyzed. Frequency variables are expressed as n (%) and Chi-square test or Fisher's exact test was used. Pearson's correlation coefficients were used to analyze the correlation between IBL and clinical characteristics (age, BMI, retrieved lymph nodes, operation time, and post-operative hospital stay). The Kaplan–Meier curve was conducted to compare the difference between the larger IBL group and the smaller IBL group, and between the IBT group and the non-IBT group. Cox regression analyses were performed to identify independent predictive factors for OS and DFS. Data were analyzed using SPSS (version 22.0) statistical software. A bilateral p < 0.05 was considered statistically significant.
Results
Patients
A total of 4,250 patients with CRC were included in this study. There were 1,911 patients in the larger IBL group and 2,339 patients in the smaller IBL group. As for IBT, there were 82 patients in the IBT group and 4,168 patients in the non-IBT group. After 1:1 ratio PSM, there were 82 patients in the IBT group and 82 patients in the non-IBT group. The inclusion and exclusion criteria, and patients with CRC before and after PSM are shown in Figure 1.
Clinical Characteristics of Patients With CRC
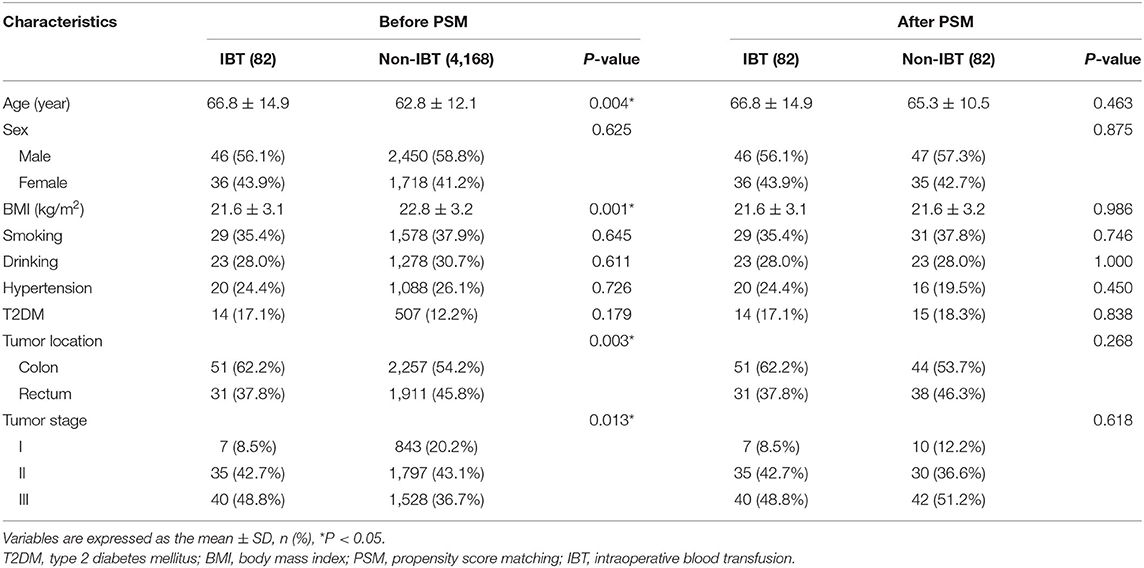
The clinical information and surgery outcomes are summarized in Table 1. There were 2,496 (58.7%) men and 1,754 (41.3%) women. The average IBL was 100.4 ± 125.7 ml and 82 (1.9%) patients underwent IBT.
Baseline Characteristics of IBL
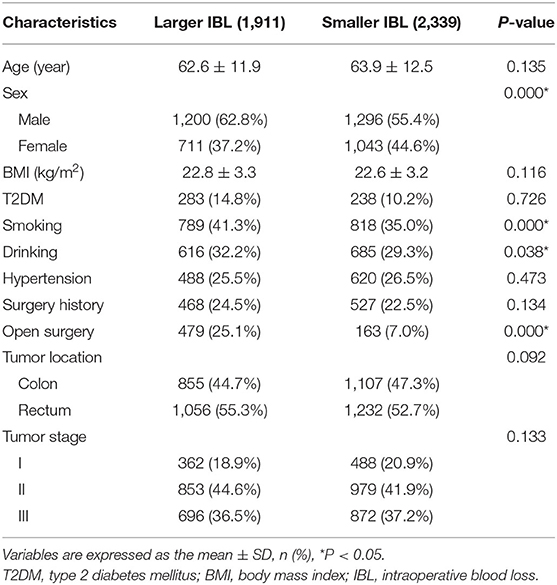
There were 1,911 patients in the larger IBL group and 2,339 patients in the smaller IBL group. The larger IBL group had more men (p = 0.000 < 0.01), higher smoking (p = 0.000 < 0.01), higher drinking (p = 0.038 < 0.05), and more open surgery (p = 0.004 < 0.01) than the smaller IBL group (Table 2).
Short-Term Outcomes of IBL
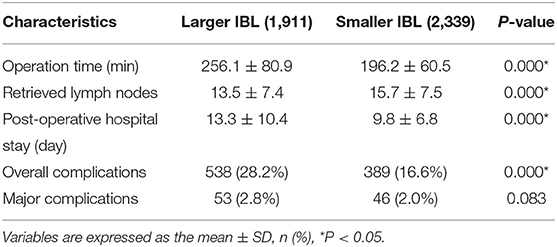
The short-term outcomes were calculated between the larger IBL group and the smaller IBL group. In this study, the larger IBL group had a longer operation time (p = 0.000 < 0.01), longer post-operative hospital stay (p = 0.000 < 0.01), smaller retrieved lymph nodes (p = 0.000 < 0.01), and higher overall complication (p = 0.000 < 0.01) than the smaller IBL group (Table 3).
Correlation of IBL and Clinical Characteristics
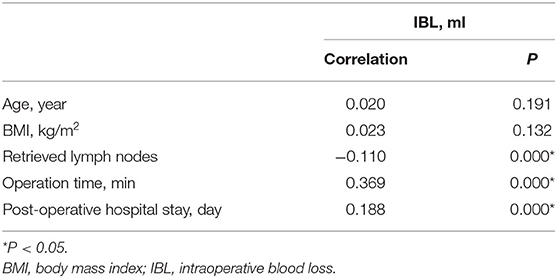
Correlation of IBL and clinical characteristics were analyzed. Retrieved lymph nodes (r = −0.110, p = 0.000 < 0.01), operation time (r = 0.369, p = 0.000 < 0.01), and post-operative stay (r = 0.188, p = 0.000 < 0.01) were significantly correlated with IBL (Table 4).
Baseline Characteristics of IBT
The baseline characteristics were compared between the IBT group and the non-IBT group. The IBT group had older age (p = 0.004 < 0.01), lower BMI (p = 0.001 < 0.01), lower portion of rectal cancer (p = 0.003 < 0.01), and lower portion of stage I CRC (p = 0.013 < 0.05) than the non-IBT group before PSM. After 1:1 ratio PSM, there was no significant difference between the two groups (p > 0.05) (Table 5).
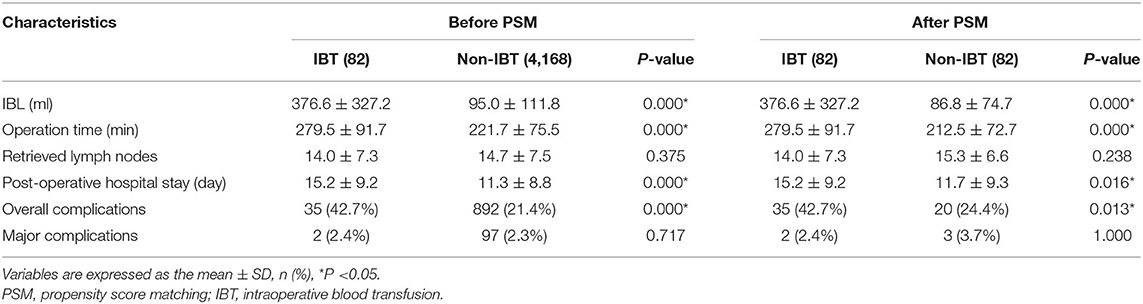
Short-Term Outcomes of IBT
The IBL of the IBT group was 376.6 ± 327.2 ml, which was larger than 95.0 ± 111.8 ml of the non-IBT group before PSM. After 1:1 ratio PSM, the IBL of the IBT group was 376.6 ± 327.2 ml, which was larger than 86.8 ± 74.7 ml of the non-IBT group.
Before 1:1 ratio PSM, the IBT group had longer operation time (p = 0.000 < 0.01), longer hospital stay (p = 0.000 < 0.01), and higher overall complications (p = 0.000 < 0.01) compared with the non-IBT group.
After 1:1 ratio PSM, the IBT group had longer operation time (p = 0.000 < 0.01), longer hospital stay (p = 0.016 < 0.05), and higher overall complications (p = 0.013 < 0.05) compared with the non-IBT group (Table 6).
Prognosis
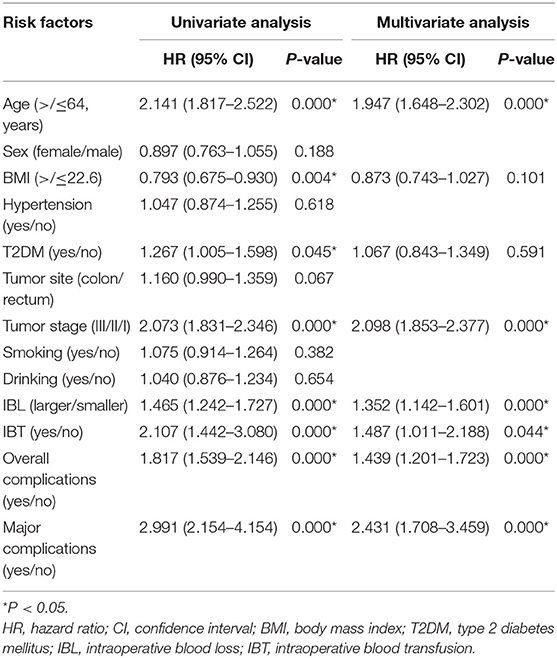
The median follow-up time was 37 (1–114) months. We conducted univariate and multivariate analysis of OS; older age (p = 0.000, HR = 1.947, 95% CI = 1.648–2.302), advanced tumor stage (p = 0.000, HR = 2.098, 95% CI=1.853–2.377), larger IBL (p = 0.000, HR = 1.352, 95% CI = 1.142–1.601), IBT (p = 0.044, HR = 1.487, 95% CI = 1.011–2.188), overall complications (p = 0.000, HR = 1.439, 95% CI = 1.201–1.723) and major complications (p = 0.000, HR = 2.431, 95% CI = 1.708–3.459) were independent predictors of OS (Table 7).
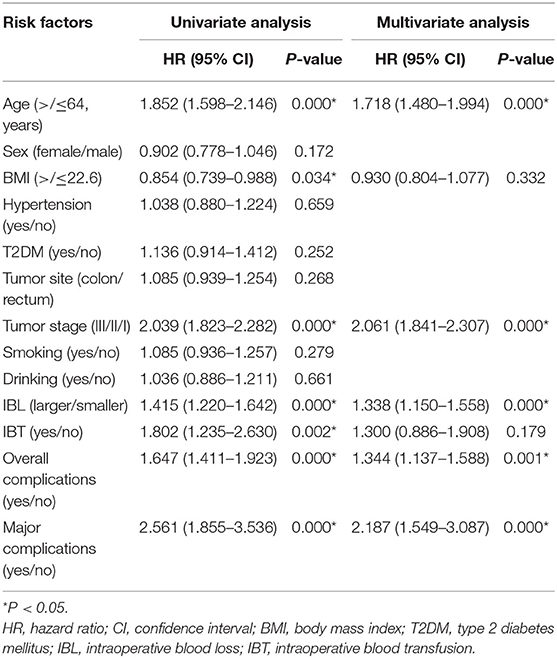
In terms of DFS, older age (p = 0.000, HR = 1.718, 95% CI = 1.480–1.994), advanced tumor stage (p = 0.000, HR = 2.061, 95% CI = 1.841–2.307), overall complications (p = 0.001, HR = 1.344, 95% CI = 1.137–1.588), larger IBL (p = 0.000, HR = 1.338, 95% CI = 1.150–1.558), and major complications (p = 0.000, HR = 2.187, 95% CI = 1.549–3.087) were independent predictors. However, IBT (p = 0.179, HR = 1.300, 95% CI = 0.886–1.908) was not an independent predictor of DFS (Table 8).
Furthermore, the Kaplan–Meier curve was conducted to compare the difference between the larger IBL group and the smaller IBL group, and between the IBT group and the non-IBT group. The larger IBL group had worse OS and DFS than the smaller IBL group (p < 0.01) (Figure 2). Moreover, the IBT group had worse OS and DFS before and after PSM than the non-IBT group (p < 0.05) (Figure 3).
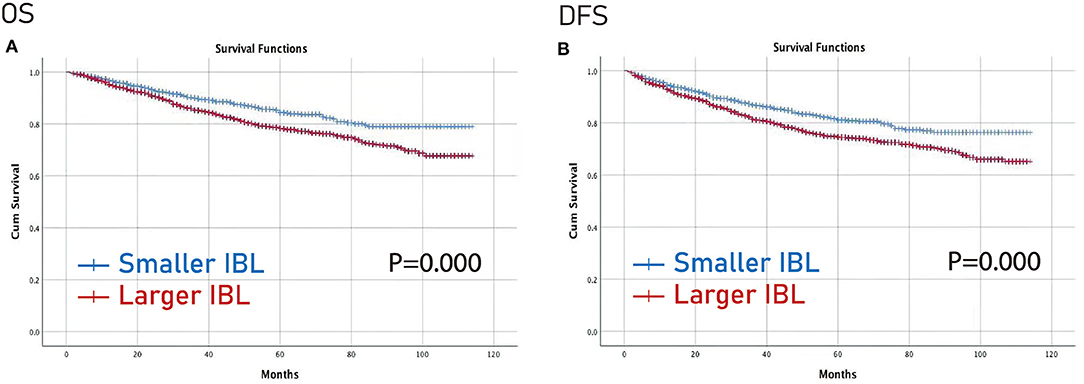
Figure 2. Prognosis of IBL on patients with CRC. (A) OS; (B) DFS. OS, overall survival; DFS, disease-free survival; IBL, intraoperative blood loss; CRC, colorectal cancer.
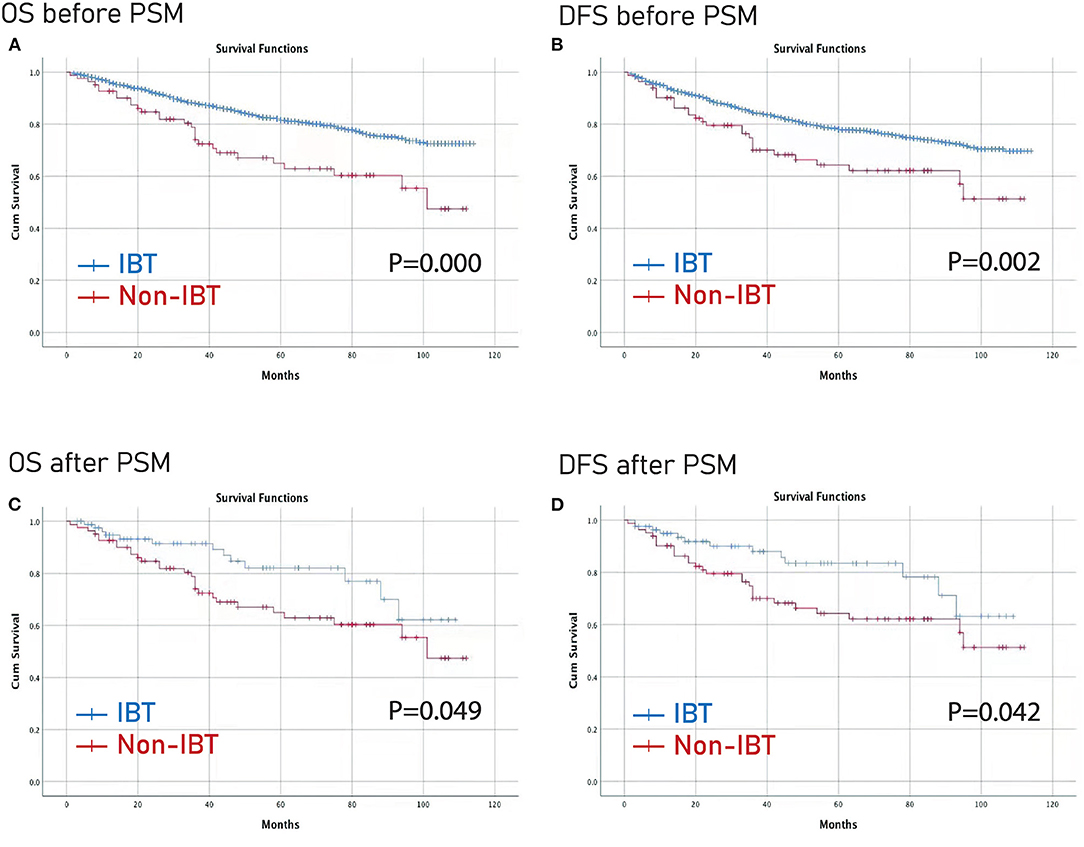
Figure 3. Prognosis of IBT on patients with CRC before and after PSM. (A) OS before PSM; (B) DFS before PSM; (C) OS after PSM; (D) DFS before PSM. OS, overall survival; DFS, disease-free survival; PSM, propensity score matching; IBL, intraoperative blood transfusion; CRC, colorectal cancer.
Discussion
In this study, there were 1,911 patients in the larger IBL group and 2,339 patients in the smaller IBL group. As for IBT, after 1:1 ratio PSM, there were 82 patients in the IBT group and 82 patients in the non-IBT group. The larger IBL group had longer operation time, longer post-operative hospital stay, smaller retrieved lymph nodes, and higher overall complication than the smaller IBL group. The IBT group had longer operation time, longer hospital stays, and higher overall complications compared with the non-IBT group in terms of short-term outcomes. Larger IBL and IBT were independent predictive factors of OS.
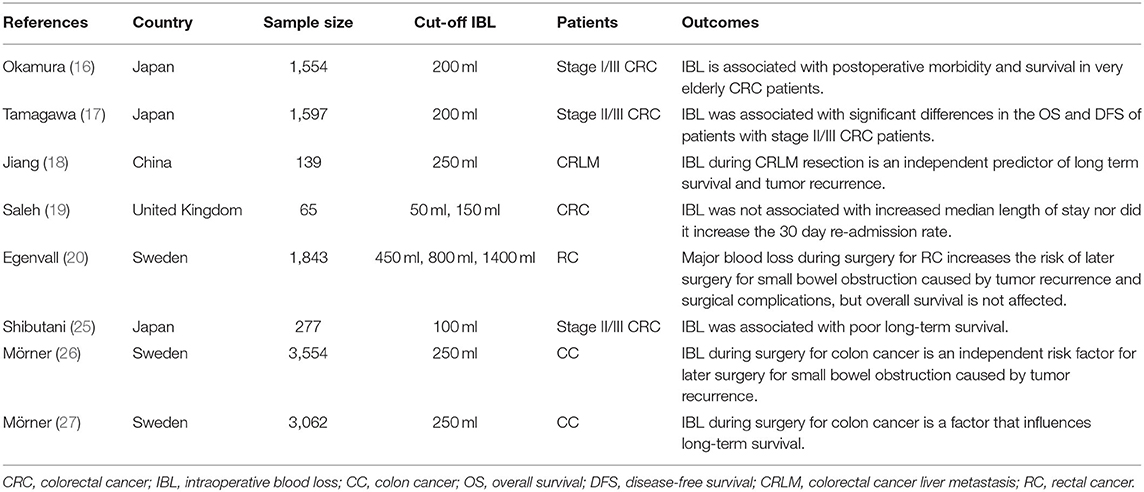
The amount of IBL could reflect the difficulty of surgery. There was a controversy of prognosis regarding IBL for patients with CRC (16–20, 25–27). Egenvall et al. reported that larger IBL increases the risk of later surgery for small bowel obstruction caused by tumor recurrence and surgical complications, but OS was not affected (20). Another study also reported IBL was not associated with increased median length of hospital stay nor did it increase the 30-day readmission rate (19). However, other studies reported that larger IBL was associated with increased complications or poor prognosis (16–18). Therefore, it was necessary to analyze the effect of IBL on the short-term outcomes and prognosis of patients who underwent primary CRC surgery.
However, the cut-off value of IBL was inconsistent in previous studies. We concluded the baseline information, cut-off value of IBL, and outcomes of previous studies in Table 9. The cut-off value of IBL was 50 ml, 100 ml, 200 ml, 250 ml, 450 ml, 800 ml, and 1,400 ml (16–20, 25–27). In this study, the cut-off value of IBL was the 75th percentile of IBL (100 ml), which was according to a previous study (16).
Perioperative blood transfusion might affect the complications, hospital stay, short-term death, and prognosis (11–15). However, no previous studies analyzed the effect of IBT on the short-term outcomes or prognosis. In this study, we analyzed IBT on the outcomes of CRC surgery; furthermore, PSM was conducted to minimize the bias of baseline information and the results would be more robust after PSM.
In terms of short-term outcomes, we found that larger IBL and IBT prolonged operation time and hospital stay, and increased complications. The prolonged hospital stay might be due to post-operative complications, and the recovery of intestinal peristalsis might be affected by larger IBL and IBT, which resulted in prolonged hospital stay. Therefore, surgeons should try to ensure the accuracy of the operation to avoid larger IBL or IBT.
In this study, larger IBL was an independent predictive factor of OS and DFS. The mechanism might be as follows: the animal experiments show that the activity or cytotoxicity of natural killer cells was reduced after blood loss, and the degree of reduction was related to the amount of blood loss (28, 29). IBL might increase the risk of tumor spread through blood during CRC surgery, which might lead to early recurrence and poor prognosis (30). In addition, larger IBL could lead to insufficient tissue perfusion and insufficient oxygenation (18). These events inhibited mitogen-induced lymphocyte proliferation and the production of interleukin-2 (31, 32), thus hindering anti-tumor immunity and upregulating vascular endothelial growth factor, which accelerated tumor angiogenesis (33, 34). Moreover, hypoxia could promote genome instability and lead to various genetic changes, which resulted in more aggressive phenotypes of residual tumor cells (35).
Intraoperative blood transfusion was also an independent predictive factor of OS. The potential hypothesis was that IBT might cause immune disturbances through transfused leukocytes, including changes in circulating lymphocytes, B cell function, suppressor T cell ratios, and helper T cells (12, 36). Another possible interpretation was that the need for IBT was a marker for sicker patients who were more likely to have worse prognosis than non-IBT patients (37).
There were some limitations in this study. First, this was a single-center retrospective study, which might cause selection bias; second, the follow-up time was relatively short and long-term follow-up is needed in the future; third, the information of classification and differentiation of tumors was lacking; fourth, the preoperative hemoglobin, the kind and volume of transfusion were lacking as well. Therefore, larger sample size with detailed information and long-term follow-up should be conducted in the following experiments.
In conclusion, larger IBL and IBT were associated with longer operation time, longer hospital stay, higher overall complications, and poorer OS. Based on the short-term outcomes and prognosis of IBL and IBT, surgeons should be cautious and more careful during the operation, and they require proficient surgical skills.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DP and BK contributed to the conception and design of the study and wrote the first draft of the manuscript. BK organized the database. DP performed the statistical analysis. X-YL, Z-WL, CY, BZ, and Z-QW wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by Chongqing Key Diseases Research and Application Demonstration Program (Colorectal Cancer Prevention and Treatment Technology Research and Application Demonstration [No. 2019ZX003]).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to Xun Lei for the substantial work in the statistical methods and Peng Hong for English editing.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Zhang Y, Zhu M, Wu X, Meng Y, Pu F, Zhang M. Factors associated with returning to work and work ability of colorectal cancer survivors. Support Care Cancer. (2021) 30:2349–57. doi: 10.1007/s00520-021-06638-3
3. Cheng YX, Tao W, Zhang H, Peng D, Wei ZQ. Does liver cirrhosis affect the surgical outcome of primary colorectal cancer surgery? a meta-analysis. World J Surg Oncol. (2021) 19:167. doi: 10.1186/s12957-021-02267-6
4. Ma Y, Shi L, Lu P, Yao S, Xu H, Hu J, et al. Creation of a novel nomogram based on the direct bilirubin-to-indirect bilirubin ratio and lactate dehydrogenase levels in resectable colorectal cancer. Front Mol Biosci. (2021) 8:751506. doi: 10.3389/fmolb.2021.751506
5. Peng D, Cheng YX, Cheng Y. Improved overall survival of colorectal cancer under multidisciplinary team: a meta-analysis. Biomed Res Int. (2021) 2021:5541613. doi: 10.1155/2021/5541613
6. Son IT, Kim JY, Kim MJ, Kim BC, Kang BM, Kim JW. Clinical and oncologic outcomes of laparoscopic versus open surgery in elderly patients with colorectal cancer: a retrospective multicenter study. Int J Clin Oncol. (2021) 26:2237–45. doi: 10.1007/s10147-021-02009-4
7. Bizzoca C, Zupo R, Aquilino F, Castellana F, Fiore F, Sardone R, et al. Video-laparoscopic versus open surgery in obese patients with colorectal cancer: a propensity score matching study. Cancers. (2021) 13:1844. doi: 10.3390/cancers13081844
8. Mizuno A, Kanda M, Kobayashi D, Tanaka C, Iwata N, Yamada S, et al. Adverse effects of intraoperative blood loss on long-term outcomes after curative gastrectomy of patients with stage II/III gastric cancer. Dig Surg. (2016) 33:121–8. doi: 10.1159/000443219
9. Liang YX, Guo HH, Deng JY, Wang BG, Ding XW, Wang XN, et al. Impact of intraoperative blood loss on survival after curative resection for gastric cancer. World J Gastroenterol. (2013) 19:5542–50. doi: 10.3748/wjg.v19.i33.5542
10. Kamei T, Kitayama J, Yamashita H, Nagawa H. Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J Surg. (2009) 33:1240–6. doi: 10.1007/s00268-009-9979-4
11. McSorley ST, Tham A, Dolan RD, Steele CW, Ramsingh J, Roxburgh C, et al. Perioperative blood transfusion is associated with postoperative systemic inflammatory response and poorer outcomes following surgery for colorectal cancer. Ann Surg Oncol. (2020) 27:833–43. doi: 10.1245/s10434-019-07984-7
12. Wu HL, Tai YH, Lin SP, Chan MY, Chen HH, Chang KY. The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: a propensity score analysis of 4,030 patients. Sci Rep. (2018) 8:13345. doi: 10.1038/s41598-018-31662-5
13. Skånberg J, Lundholm K, Haglind E. Effects of blood transfusion with leucocyte depletion on length of hospital stay, respiratory assistance and survival after curative surgery for colorectal cancer. Acta Oncol. (2007) 46:1123–30. doi: 10.1080/02841860701441830
14. Nanji S, Mir ZM, Karim S, Brennan KE, Patel SV, Merchant SJ, et al. Perioperative blood transfusion and resection of colorectal cancer liver metastases: outcomes in routine clinical practice. HPB. (2021) 23:404–12. doi: 10.1016/j.hpb.2020.06.014
15. Qiu L, Wang DR, Zhang XY, Gao S, Li XX, Sun GP, et al. Impact of perioperative blood transfusion on immune function and prognosis in colorectal cancer patients. Transfus Apher Sci. (2016) 54:235–41. doi: 10.1016/j.transci.2015.07.004
16. Okamura R, Hida K, Hasegawa S, Sakai Y, Hamada M, Yasui M, et al. Impact of intraoperative blood loss on morbidity and survival after radical surgery for colorectal cancer patients aged 80 years or older. Int J Colorectal Dis. (2016) 31:327–34. doi: 10.1007/s00384-015-2405-5
17. Tamagawa H, Numata M, Aoyama T, Kazama K, Atsumi Y, Iguchi K, et al. Impact of intraoperative blood loss on the survival of patients with stage II/III colorectal cancer: a multicenter retrospective study. in vivo. (2021) 35:3483–8. doi: 10.21873/invivo.12649
18. Jiang W, Fang YJ, Wu XJ, Wang FL, Lu ZH, Zhang RX, et al. Intraoperative blood loss independently predicts survival and recurrence after resection of colorectal cancer liver metastasis. PLoS ONE. (2013) 8:e76125. doi: 10.1371/journal.pone.0076125
19. Saleh A, Ihedioha U, Babu B, Evans J, Kang P. Is estimated intra-operative blood loss a reliable predictor of surgical outcomes in laparoscopic colorectal cancer surgery? Scott Med J. (2016) 61:167–70. doi: 10.1177/0036933015597174
20. Egenvall M, Mörner M, Påhlman L, Gunnarsson U. Degree of blood loss during surgery for rectal cancer: a population-based epidemiologic study of surgical complications and survival. Colorectal Dis. (2014) 16:696–702. doi: 10.1111/codi.12630
21. Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. (2018) 25:1454–5. doi: 10.1245/s10434-018-6462-1
22. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick PD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
23. Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. (1996) 52:249–64. doi: 10.2307/2533160
24. Lonjon G, Boutron I, Trinquart L, Ahmad N, Aim F, Nizard R, et al. Comparison of treatment effect estimates from prospective nonrandomized studies with propensity score analysis and randomized controlled trials of surgical procedures. Ann Surg. (2014) 259:18–25. doi: 10.1097/SLA.0000000000000256
25. Shibutani M, Maeda K, Kashiwagi S, Hirakawa K, Ohira M. The impact of intraoperative blood loss on the survival after laparoscopic surgery for colorectal cancer. Anticancer Res. (2021) 41:4529–34. doi: 10.21873/anticanres.15264
26. Mörner M, Gunnarsson U, Jestin P, Egenvall M. Volume of blood loss during surgery for colon cancer is a risk determinant for future small bowel obstruction caused by recurrence–a population-based epidemiological study. Langenbecks Arch Surg. (2015) 400:599–607. doi: 10.1007/s00423-015-1317-8
27. Mörner ME, Gunnarsson U, Jestin P, Svanfeldt M. The importance of blood loss during colon cancer surgery for long-term survival: an epidemiological study based on a population based register. Ann Surg. (2012) 255:1126–8. doi: 10.1097/SLA.0b013e3182512df0
28. Angele MK, Faist E. Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit Care. (2002) 6:298–305. doi: 10.1186/cc1514
29. Hoynck van Papendrecht MA, Busch OR, Jeekel J, Marquet RL. The influence of blood loss on tumour growth: effect and mechanism in an experimental model. Neth J Surg. (1991) 43:85–8.
30. Katz SC, Shia J, Liau KH, Gonen M, Ruo L. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. (2009) 249:617–23. doi: 10.1097/SLA.0b013e31819ed22f
31. Abraham E, Chang YH. Effects of hemorrhage on inflammatory response. Arch Surg. (1984) 119:1154–7. doi: 10.1001/archsurg.1984.01390220040009
32. Abraham E, Lee RJ, Chang YH. The role of interleukin 2 in hemorrhage-induced abnormalities of lymphocyte proliferation. Circ Shock. (1986) 18:205–13.
33. Leo C, Giaccia AJ, Denko NC. The hypoxic tumor microenvironment and gene expression. Semin Radiat Oncol. (2004) 14:207–14. doi: 10.1016/j.semradonc.2004.04.007
34. Shibutani M, Nakao S, Maeda K, Nagahara H, Fukuoka T. Inflammation caused by surgical stress has a negative impact on the long-term survival outcomes in patients with colorectal cancer. Anticancer Res. (2020) 40:3535–42. doi: 10.21873/anticanres.14342
35. Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res. (1996) 56:5754–7.
36. Weber RS, Jabbour N, Martin RC 2nd. Anemia and transfusions in patients undergoing surgery for cancer. Ann Surg Oncol. (2008) 15:34–45. doi: 10.1245/s10434-007-9502-9
Keywords: colorectal cancer, intraoperative blood loss, surgery, prognosis, intraoperative blood transfusion, outcomes
Citation: Kang B, Liu X-Y, Li Z-W, Yuan C, Zhang B, Wei Z-Q and Peng D (2022) The Effect of the Intraoperative Blood Loss and Intraoperative Blood Transfusion on the Short-Term Outcomes and Prognosis of Colorectal Cancer: A Propensity Score Matching Analysis. Front. Surg. 9:837545. doi: 10.3389/fsurg.2022.837545
Received: 16 December 2021; Accepted: 23 February 2022;
Published: 04 April 2022.
Edited by:
Annamaria Auricchio, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Marcel André Schneider, University Hospital of Zürich, SwitzerlandChristina A. Fleming, Centre Hospitalier Universitaire de Bordeaux, France
Copyright © 2022 Kang, Liu, Li, Yuan, Zhang, Wei and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Peng, Y2FycnlfZG9uZ0AxMjYuY29t
†These authors share first authorship
 Bing Kang
Bing Kang Xiao-Yu Liu
Xiao-Yu Liu Zi-Wei Li
Zi-Wei Li Chao Yuan
Chao Yuan Bin Zhang
Bin Zhang Zheng-Qiang Wei
Zheng-Qiang Wei Dong Peng
Dong Peng