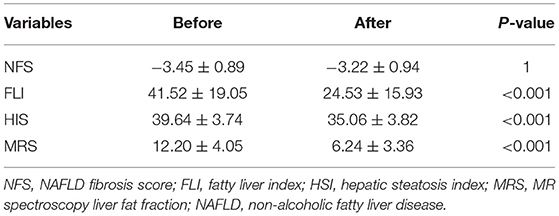- Department of Surgery, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, South Korea
Background: A short-term weight reduction program for potential living donors was introduced to reduce the extent of hepatic steatosis prior to liver transplantation. We aimed to investigate changes in non-invasive hepatic steatosis and fibrosis indices among those who completed the program.
Methods: Among 1,950 potential living liver donors between January 2011 and May 2019, 160 living donors joined the weight reduction program. The prospectively collected clinical data of these potential liver donors were analyzed retrospectively. Hepatic steatosis and fibrosis scores were determined using the fatty liver index (FLI), hepatic steatosis index (HSI), and NAFLD fibrosis score (NFS) and compared to MR spectroscopy (MRS) fat fraction results before and after weight reduction.
Results: Thirty-nine potential living donors who had undergone MRS both before and after weight reduction were included in the analysis. Their body weight decreased from 78.02 ± 10.89 kg to 72.36 ± 10.38 kg over a mean of 71.74 ± 58.11 days. FLI, HSI, and MRS values decreased significantly from 41.52 ± 19.05 to 24.53 ± 15.93, 39.64 ± 3.74 to 35.06 ± 3.82, and 12.20 ± 4.05 to 6.24 ± 3.36, respectively. No significant decreases in NFS were observed. There was a significant correlation between the extent of HSI change and the extent of MRS change (R2 value = 0.69, P < 0.001), although there was no correlation between MRS and FLI.
Conclusion: The weight reduction program significantly improved non-invasive indices of hepatic steatosis over a short period. HSI may allow for prediction of simple decreases in hepatic steatosis.
Introduction
The prevalence of non-alcoholic fatty liver disease (NAFLD) in the general population ranges from 20 to 30% in Europe (1, 2), and is as high as 46% in the United States (3). In Korea, the prevalence of NAFLD among potential donors is also increasing due to increasing adoption of a sedentary and Westernized lifestyle (4–6). However, NAFLD—which is related to metabolic syndrome—has an adverse effect on liver transplantation. Indeed, NAFLD has been associated with postoperative morbidity and mortality in patients with liver grafts (7, 8).
Although NAFLD in the donor is an exclusion criterion for living donor liver transplantation (LDLT) (9), donors with NAFLD are considered marginal donors because the supply of deceased donors has not met the demand for liver transplantation in Korea. Previous studies have indicated that weight reduction and lifestyle modification can reduce hepatic steatosis in such marginal donors (10–14).
Education on weight reduction and lifestyle modification is being provided to potential living donors with NAFLD to improve outcomes. Potential donors who have successfully reduced weight prior to liver transplantation exhibit comparable outcomes to donors from whom liver transplantation is completed using liver grafts with no NAFLD (11, 15, 16).
Hepatic steatosis can be assessed either via liver biopsy or MR spectroscopy (MRS). However, liver biopsy can cause procedure-related complications, and MRS is expensive. Therefore, there is a need for a simple index that can predict the degree of tissue change following weight reduction.
In this retrospective study, we investigated whether indices of hepatic steatosis and fibrosis, which are already in use and can be calculated simply from the donor's data, can predict the extent of NAFLD after weight loss.
Materials and Methods
Study Design
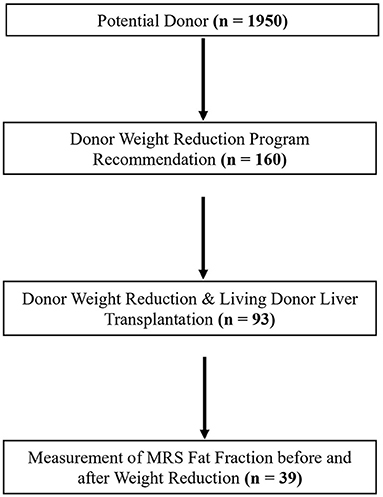
This study involved a single-center retrospective analysis of the electronic medical records of 1,950 potential donors between January 2011 and May 2019. Of the 1,950 potential donors, 160 potential donors with hepatic steatosis received recommendations for weight reduction and lifestyle modification programs. Ninety-three of 160 potential donors lost body weight and underwent LDLT. In this study, the fat fraction of MRS was used as the reference value for the extent of hepatic steatosis (17–19). MRS acquired as previous described (20).
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the appropriate Institutional Review Committee (2011-121-1173) at Seoul National University Hospital.
Variable Definitions and Data Collection
Baseline body weight prior to weight loss and final weight after weight loss were measured during the first and last MRI sessions, respectively. Alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl-transpeptidase (GGT), platelet count, albumin, waist circumference, weight loss duration, fat fraction on MRS, and body weight were compared between the two measurement sessions.
Indices
We noninvasively reviewed indices for NAFLD assessment, including six indices [fatty liver index (FLI), hepatic steatosis index (HSI), SteatoTest, lipid accumulation product (LAP), Index of Nash (ION), NAFLD-Liver Fat Score (LFS)] for liver steatosis and eight indices [AST to platelet ratio index (APRI), Fibrosis-4 (FIB-4), FibroTest, Fibrometer NAFLD, Enhanced Liver Fibrosis (ELF), Hepacore, BARD score, NAFLD fibrosis score (NFS)] for liver fibrosis (21, 22). The FLI, HIS, and NFS were selected for this study because these indices can be calculated with only simple laboratory findings, body weight, and circumference using retrospective data.
The FLI is used to predict the degree of hepatic steatosis (23). Scores are derived based on body mass index (BMI), GGT, triglycerides (TG), and waist circumference, as follows: FLI = logistic (0.953 × ln (TG) + 0.139 × BMI + 0.718 × ln (GGT) + 0.053 × waist circumference – 15.745) ×100, where logistic(x) = 1/(1 + e−x) and ln denotes the natural logarithm. Its lower cutoff is 30, while its upper cutoff is 60 (24, 25).
The HSI predicts the degree of hepatic steatosis and is derived using BMI, diabetes mellitus (DM) status, and AST/ALT ratio, as follows: HSI = 8 × ALT/AST + BMI (+ 2, if DM) (+ 2, if female). Its lower cutoff is 30, while its upper cutoff is 36. A prior ultrasonography study revealed that HIS is correlated with the extent of hepatic steatosis (26, 27).
The NFS is used to predict the degree of hepatic fibrosis and is derived based on age, sex, DM status, platelet count, AST/ALT ratio, and albumin levels, as follows. NFS = −1.675 + 0.037 × age + 0.094 × BMI + 1.13 × DM + 0.99 × AST/ALT ratio – 0.013 × platelet (109) – 0.66 × ALB (g/dl). Its lower cutoff is −1.445, while its upper cutoff is 0.676 (28–30).
Statistical Analysis
Statistical analyses were performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA). Continuous variables such as body weight, BMI, AST, ALT, GGT, platelet count, albumin, waist circumference, and indices (NFS, FLI, HSI, MRS) before and after weight loss were compared using Student's t-tests. Variable distributions were subjected to a normality test prior to the Student's t-test. All p-values were one-sided, and P-values <0.05 were considered statistically significant. Quantitative variables are expressed as the mean ± standard deviation. Linear regression analyses were performed to investigate correlations between each index and the fat fraction obtained via MRS. R2 > 0.6 with a p-value <0.05 was considered statistically significant. Arrow graphs were drawn using the R ggplot 2 package.
Results
Study Population
We analyzed data for 39 of 93 living donors who had undergone assessments of fat fraction before and after weight loss via MRS (Figure 1).
Patient Characteristics
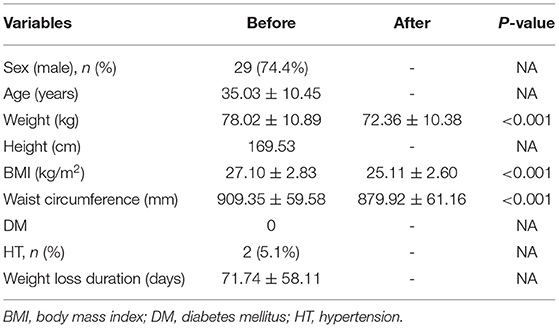
The mean patient age was 35.03 ± 10.45 years (range: 20–59 years), and 29 patients were male (29/39, 74.4%). No patients had DM, although two donors (5.1%) had hypertension. The mean duration of weight reduction was 71.74 ± 58.11 days (range: 11–298 days). Six donors (15.4%) had a body weight reduction period of over 100 days (Table 1).
Participation in the program was associated with a significant decrease in weight from 78.02 ± 10.89 kg to 72.36 ± 10.38 kg (P < 0.001), as well as a significant decrease in waist circumference from 909.35 ± 59.58 mm to 879.92 ± 61.16 mm (P < 0.001). Moreover, BMI decreased from 27.10 ± 2.83 kg/m2 to 25.11 ± 2.60 kg/m2 (P < 0.001), and the distribution of BMI changed. Thirty living donors (76.9%) had obesity (BMI > 25 kg/m2), eight donors (20.5%) were overweight (25 kg/m2 > BMI > 23 kg/m2), and one donor' s BMI was 22.89 kg/m2, which is nearly borderline. After completing the weight reduction program, 19 donors (48.8%) had obesity (BMI > 25 kg/m2), 10 donors (25.6%) were overweight (25 kg/m2 > BMI > 23 kg/m2), and 10 donors (25.6%) had normal weight (Table 1).
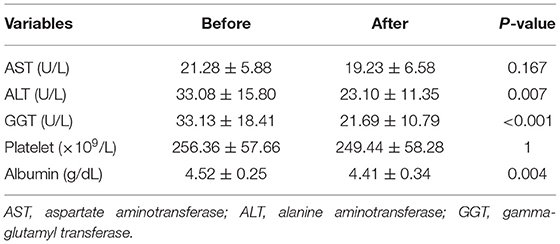
There was no significant difference in AST levels between the baseline and final assessments (21.28 ± 5.88 U/L vs. 19.23 ± 6.58 U/L, P-value = 0.167); however, there were significant decreases in ALT (33.08 ± 15.80 U/L vs. 23.10 ± 11.35 U/L, P = 0.007) and GGT levels (33.13 ± 18.41 U/L vs. 21.69 ± 10.79 U/L, P < 0.001) between the two time points. The number of patients with ALT levels above the normal range (0–40 U/L) changed from 9/39 to 3/39, while the number of patients with GGT levels above the normal range changed from 19/39 to 6/39. There was a significant decrease in albumin levels between the baseline and final assessments (4.52 ± 0.25 g/dl vs. 4.41 ± 0.34 g/dl, P = 0.004). However, platelet count did not significantly differ between the two time points (P = 1.00) (Table 2).
Index Analysis
There was a significant decrease in HSI between the baseline and final assessments (39.64 ± 3.74 vs. 35.06 ± 3.82, P < 0.001). Before weight loss, there were 37 donors (94.9%) in the high HSI group (HSI > 36) and two donors (5.1%) in the intermediate HSI group (36 > HSI > 30). After weight loss, there were 16 donors (41.0%) in the high HSI group (HSI > 36), 21 donors (53.8%) in the intermediate HSI group (36 > HSI > 30), and two donors (5.2%) in the low HSI group (HSI <30) (Table 3).
There was a significant decrease in FLI between the baseline and final assessments (41.52 ± 19.05 vs. 24.53 ± 15.93, P < 0.001). Before weight loss, there were seven donors (17.9%) in the high FLI group (FLI > 60), 27 donors (69.2%) in the intermediate FLI group (60 > FLI > 20), and five donors (12.9%) in the low FLI group (FLI <20). After weight loss, there was one donor (2.6%) in the high FLI group (FLI > 60), while there were 17 donors (43.6%) in the intermediate FLI group (60 > FLI > 20) and 21 donors (53.8%) in the low FLI group (FLI <20).
No significant changes in NFS were observed (P = 1).
Measurements of fat fraction obtained via MRS significantly decreased from 12.20 ± 4.05% to 6.24 ± 3.36% (P < 0.001) between the two periods. Before weight loss, the fat fraction was 10% or higher in 26 donors, and the maximum value was 22.73%. After weight loss, only four donors had fat fractions of 10% or higher (Table 3).
Correlation in the Value of Delta Between HSI and MRS
There were no significant correlations between the extent of change on MRS and indices, except HSI (R2 = 0.69 and P < 0.001) (Figure 2).
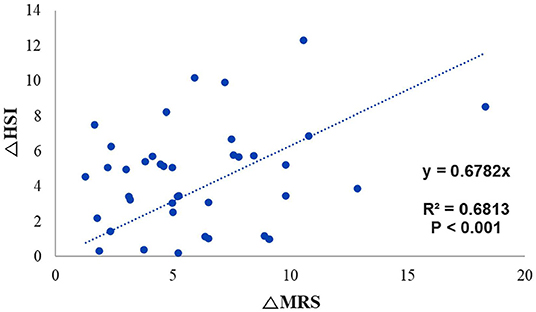
Figure 2. Trend line for identifying the correlation between the change in MRS fat fraction and the change in HSI. MRS, magnetic resonance spectroscopy; HSI, hepatic stenosis index.
The gradient trend of the delta HSI/delta MRS graph was similar to that of the trend line, but there were some exceptional cases (Figure 3). No correlations were observed in the other two graphs (Figures 4, 5).
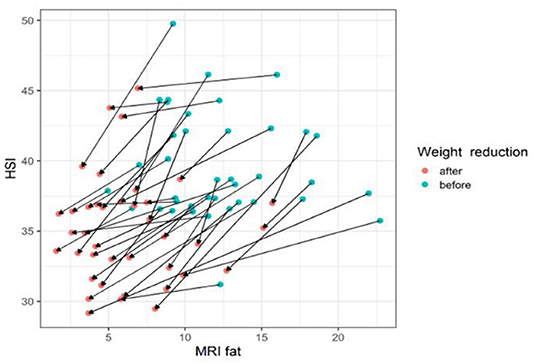
Figure 3. The changes of HIS according to MRI fat before and after body weight reduction. The values of HSI and MRS tend to decrease with weight loss. The line slope representing the change is the ratio of the MRS decrease and the HSI decrease. Therefore, the HSI value changes appropriately according to the change amount of the MRI fat fraction. MRS, magnetic resonance spectroscopy; HSI, hepatic stenosis index. Blue circle, before weight reduction; red circle and arrows, after weight reduction.
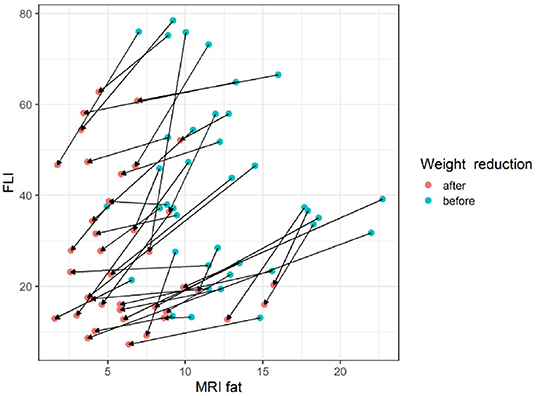
Figure 4. The changes of FLI according to MRI fat before and after body weight reduction. The slope of each line appears in various ways without a particular pattern. Since the amount of change in the FLI value according to the amount of change in the MRS fat fraction is not constant, it appears that the FLI cannot consistently reflect the MRS fat fraction. MRS, magnetic resonance spectroscopy; FLI, fatty liver index. Blue circle, before weight reduction; red circle and arrows, after weight reduction.
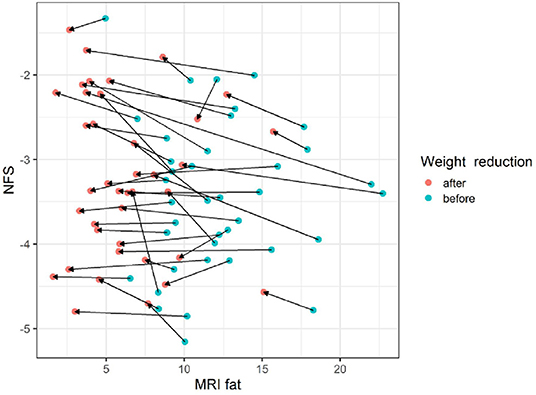
Figure 5. The changes of NFS according to MRI fat before and after body weight reduction. Regardless of the change in the MRS fat fraction according to the change in body weight, there was little change in the NFS value. MRS, magnetic resonance spectroscopy; NFS, NAFLD fibrosis score. Blue circle, before weight reduction; red circle and arrows, after weight reduction.
Discussion
Previous studies have reported that MRS can accurately predict steatosis, although it remains controversial to what extent it can predict the degree of fibrosis (25, 31, 32). MRS is also non-invasive and more objective than biopsy assessments. In the present study, we investigated whether indices of hepatic steatosis and fibrosis can predict the extent of NAFLD after weight loss. Our findings indicated that there was no strong correlation between either of the two indices and the degree of steatosis, suggesting that the extent of change in hepatic steatosis due to weight reduction can only be roughly determined based on changes in HSI and FLI values.
Kahl et al. and Cuthbertson et al. reported the similar result that these HSI and FLI would be surrogate parameters for hepatic steatosis clinically, but could not replace MRS (33, 34).
Previous studies have suggested a correlation between HSI and fat fraction on ultrasonography; however, the R2 value was 0.334, and ultrasonography is a user-dependent method. Thus, the results were not clinically significant. Furthermore, the authors did not validate the correlation using liver histology findings (26). Although they attempted to determine the correlation between the index and MRS, no indices exhibited a quantitative correlation. Because we used the objective value for the fat fraction obtained via MRS as the reference value, our trends observed for HSI and FLI may be more reliable. Our regression analysis revealed a correlation for HSI only. Given that one can infer the extent of change on MRS based on the extent of change in HSI, this tool may allow clinicians to predict improvements in liver fat fraction without additional MRI or biopsy.
Liu et al. reported that FLI had a high diagnostic predictive rate for metabolic-associated fatty liver disease, but this may be due to triglyceride, gamma GT, which is highly correlated with metabolic diseases (35).
While NFS values increased, the change was not statistically significant, likely because we observed unexpected decreases in platelet levels, which are used in the calculation of NFS. Therefore, the results may have been clinically insignificant because the extent of change was not large. In addition, the NFS value was significantly smaller than the lower cutoff of −1.455 (−3.45 ± 0.89 before weight loss and −3.22 ± 0.94 after weight loss), and there was no donor with an index value exceeding the lower cutoff at either time point. Such findings indicate a degree of fibrosis without clinical significance. Since the study involved only those patients who had completed liver transplantation, our results were likely influenced by selection bias due to the inclusion of people with relatively healthy livers. For the same reason, most patients may have had low NFS values caused by simple steatosis. However, since this is also an index that suggests the probability of fibrosis, it is limited in that the true extent of fibrosis cannot be determined without a direct biopsy.
Our findings indicate that improvements in NAFLD can be predicted based only on routine laboratory examination results, which may aid in improving outcomes among donors with NAFLD. However, the arrow graph shows cases outside the normal gradient range. For clinical use, an additional understanding of the characteristics of exceptional cases will be required. In this study, we did not perform feature analysis of exceptional cases due to the small number of cases, although such analyses will be possible in larger-scale studies.
Our results also indicated that the fibrosis index was clinically and statistically insignificant; therefore, only steatosis could be identified. Pathological analyses should be performed before and after weight reduction to identify other factors that contribute to NAFLD, such as inflammation, ballooning injury, and fibrosis. The results of this study should also be validated based on pathological results.
Previous studies that have examined pathological findings at 1-year post-operatively have reported that recipient-related factors can influence the risk of recipient NAFLD after liver transplantation (36). To exclude these factors, future studies should examine pathology findings 7 days post-operatively, as this will help to ascertain the effect of weight reduction on liver engraftment in a linear fashion.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, with permission of IRB.
Ethics Statement
The studies involving human participants were reviewed and approved by Seoul National University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JC: data collection, draft, and the final manuscript. YC: initial idea, study conceptualization, critical review, draft, and the final manuscript. SYH, SS, KH, and EH: data collection and draft review. JML: data collection, study conceptualization, critical review, draft, and review. SKH, NJY, KWL, and KSS: study conceptualization, critical review, and draft review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NAFLD, non-alcoholic fatty liver disease; LDLT, living donor liver transplantation; MRS, magnetic resonance spectroscopy; ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma-glutamyl transpeptidase; FLI, fatty liver index; HSI, hepatic steatosis index; LAP, lipid accumulation product; ION, Index of Nash; NAFLD-LFS, NAFLD Liver Fat Score; APRI, AST to platelet ratio index; FIB-4, Fibrosis-4; ELF, Enhanced Liver Fibrosis; NFS, NAFLD Fibrosis Score; BMI, body mass index; TG, triglycerides; DM, diabetes mellitus.
References
1. Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. (2005) 42:44–52. doi: 10.1002/hep.20734
2. Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. (2012) 56:234–40. doi: 10.1016/j.jhep.2011.03.020
3. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. (2011) 140:124–31. doi: 10.1053/j.gastro.2010.09.038
4. Lee JY, Kim KM, Lee SG, Yu E, Lim Y-S, Lee HC, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. (2007) 47:239–44. doi: 10.1016/j.jhep.2007.02.007
5. Farrell GC, Wong VW, Chitturi S. NAFLD in Asia–as common and important as in the West. Nat Rev Gastroenterol Hepatol. (2013) 10:307–18. doi: 10.1038/nrgastro.2013.34
6. Jeong EH, Jun DW, Cho YK, Choe YG, Ryu S, Lee SM, et al. Regional prevalence of non-alcoholic fatty liver disease in Seoul and Gyeonggi-do, Korea. Clin Mol Hepatol. (2013) 19:266. doi: 10.3350/cmh.2013.19.3.266
7. Chu MJ, Dare AJ, Phillips AR, Bartlett AS. Donor hepatic steatosis and outcome after liver transplantation: a systematic review. J Gastrointest Surg. (2015) 19:1713–24. doi: 10.1007/s11605-015-2832-1
8. Zhang QY, Zhang QF, Zhang DZ. The impact of steatosis on the outcome of liver transplantation: a meta-analysis. Biomed Res Int. (2019) 2019:3962785. doi: 10.1155/2019/3962785
9. Nugroho A, Kim OK, Lee KW, Song S, Kim H, Hong SK, et al. Evaluation of donor workups and exclusions in a single-center experience of living donor liver transplantation. Liver Transpl. (2017) 23:614–24. doi: 10.1002/lt.24762
10. Mattar SG, Velcu LM, Rabinovitz M, Demetris AJ, Krasinskas AM, Barinas-Mitchell E, et al. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. (2005) 242:610-7; discussion 618–20. doi: 10.1097/01.sla.0000179652.07502.3f
11. Nakamuta M, Morizono S, Soejima Y, Yoshizumi T, Aishima S, Takasugi S, et al. Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation. (2005) 80:608–12. doi: 10.1097/01.tp.0000166009.77444.f3
12. Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. (2010) 51:121–9. doi: 10.1002/hep.23276
13. Sanyal AJ, Chalasani N, Kowdley KV, Mccullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. (2010) 362:1675–85. doi: 10.1056/NEJMoa0907929
14. Jin YJ, Kim KM, Hwang S, Lee SG, Ha TY, Song GW, et al. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: analysis of biopsies of living liver donors. J Gastroenterol Hepatol. (2012) 27:1341–7. doi: 10.1111/j.1440-1746.2012.07165.x
15. Hwang S, Lee SG, Jang SJ, Cho SH, Kim KH, Ahn CS, et al. The effect of donor weight reduction on hepatic steatosis for living donor liver transplantation. Liver Transpl. (2004) 10:721–5. doi: 10.1002/lt.20172
16. Doyle A, Adeyi O, Khalili K, Fischer S, Dib M, Goldaracena N, et al. Treatment with Optifast reduces hepatic steatosis and increases candidacy rates for living donor liver transplantation. Liver Transpl. (2016) 22:1295–300. doi: 10.1002/lt.24495
17. Bohte AE, Van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. (2011) 21:87–97. doi: 10.1007/s00330-010-1905-5
18. Chabanova E, Bille DS, Thisted E, Holm JC, Thomsen HS. (1)H MRS assessment of hepatic steatosis in overweight children and adolescents: comparison between 3T and open 1T MR-systems. Abdom Imaging. (2013) 38:315–9. doi: 10.1007/s00261-012-9930-2
19. Choi Y, Lee JM, Yi NJ, Kim H, Park MS, Hong G, et al. Heterogeneous living donor hepatic fat distribution on MRI chemical shift imaging. Ann Surg Treat Res. (2015) 89:37–42. doi: 10.4174/astr.2015.89.1.37
20. Hwang I, Lee JM, Lee KB, Yoon JH, Kiefer B, Han JK, et al. Hepatic steatosis in living liver donor candidates: preoperative assessment by using breath-hold triple-echo MR imaging and 1H MR spectroscopy. Radiology. (2014) 271:730–8. doi: 10.1148/radiol.14130863
21. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1264-81 e1264. doi: 10.1053/j.gastro.2018.12.036
22. Sahin T, Arikan BT, Serin A, Emek E, Bozkurt B, Server S, et al. The utility of noninvasive scoring systems for prediction of hepatic steatosis in liver transplantation donor candidates. Transplant Proc. (2019) 51:2383–6. doi: 10.1016/j.transproceed.2019.01.177
23. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230X-6-33
24. Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. (2013) 11:1201–4. doi: 10.1016/j.cgh.2012.12.031
25. Khang AR, Lee HW, Yi D, Kang YH, Son SM. The fatty liver index, a simple and useful predictor of metabolic syndrome: analysis of the Korea National Health and Nutrition Examination Survey 2010-2011. Diabetes Metab Syndr Obes. (2019) 12:181–90. doi: 10.2147/DMSO.S189544
26. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. (2010) 42:503–8. doi: 10.1016/j.dld.2009.08.002
27. Sviklane L, Olmane E, Dzerve Z, Kupcs K, Pirags V, Sokolovska J. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J Gastroenterol Hepatol. (2018) 33:270–6. doi: 10.1111/jgh.13814
28. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. (2007) 45:846–54. doi: 10.1002/hep.21496
29. Treeprasertsuk S, Bjornsson E, Enders F, Suwanwalaikorn S, Lindor KD. NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World J Gastroenterol. (2013) 19:1219–29. doi: 10.3748/wjg.v19.i8.1219
30. Jun DW, Kim SG, Park SH, Jin SY, Lee JS, Lee JW, et al. External validation of the non-alcoholic fatty liver disease fibrosis score for assessing advanced fibrosis in Korean patients. J Gastroenterol Hepatol. (2017) 32:1094–9. doi: 10.1111/jgh.13648
31. Cho SG, Kim MY, Kim HJ, Kim YS, Choi W, Shin SH, et al. Chronic hepatitis: in vivo proton MR spectroscopic evaluation of the liver and correlation with histopathologic findings. Radiology. (2001) 221:740–6. doi: 10.1148/radiol.2213010106
32. Bonekamp S, Kamel I, Solga S, Clark J. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol. (2009) 50:17–35. doi: 10.1016/j.jhep.2008.10.016
33. Kahl S, Straßburger K, Nowotny B, Livingstone R, Klüppelholz B, Keßel K, et al. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS One. (2014) 9:e94059. doi: 10.1371/journal.pone.0094059
34. Cuthbertson DJ, Weickert MO, Lythgoe D, Sprung VS, Dobson R, Shoajee-Moradie F, et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur J Endocrinol. (2014) 171:561–9. doi: 10.1530/EJE-14-0112
35. Liu Y, Liu S, Huang J, Zhu Y, Lin S. Validation of five hepatic steatosis algorithms in metabolic- associated fatty liver disease: a population based study. J Gastroenterol Hepatol. (2022). doi: 10.1111/jgh.15799 [Epub ahead of print].
Keywords: hepatic steatosis, hepatic fibrosis, fatty liver, living donor, NAFLD, weight loss
Citation: Choi J, Choi Y, Hong SY, Suh S, Hong K, Han ES, Lee JM, Hong SK, Yi NJ, Lee KW and Suh KS (2022) Changes in Indices of Steatosis and Fibrosis in Liver Grafts of Living Donors After Weight Reduction. Front. Surg. 9:827526. doi: 10.3389/fsurg.2022.827526
Received: 02 December 2021; Accepted: 28 March 2022;
Published: 03 May 2022.
Edited by:
Peter Schemmer, Medical University of Graz, AustriaReviewed by:
Behzad Hatami, Shahid Beheshti University of Medical Sciences, IranMartin Krššák, Medical University of Vienna, Austria
Copyright © 2022 Choi, Choi, Hong, Suh, Hong, Han, Lee, Hong, Yi, Lee and Suh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: YoungRok Choi, Y2hvaXlvdW5ncm9rQGdtYWlsLmNvbQ==
 Jaehyuk Choi
Jaehyuk Choi YoungRok Choi
YoungRok Choi Sanggyun Suh
Sanggyun Suh Kwangpyo Hong
Kwangpyo Hong Jeong-Moo Lee
Jeong-Moo Lee Kwang-Woong Lee
Kwang-Woong Lee