- 1Department of Clinical Medicine, School of Medicine, Yangzhou University, Yangzhou, China
- 2Department of Clinical Medicine, Dalian Medical University, Dalian, China
- 3Department of Neurosurgery, Clinical Medical College of Yangzhou University, Yangzhou, China
Objective: This study was designed to compare the safety and efficacy of unilateral hemilaminectomy conducted under complete neuroendoscopic visualization (UHNV) relative to unilateral hemilaminectomy under total microscopic visualization (UHMV) for the treatment of patients diagnosed with intraspinal tumors.
Methods: In total, 41 patients undergoing intraspinal tumor resection at Northern Jiangsu People's Hospital were included in this study, including 20 and 21 patients in the UHNV and UHMV groups, respectively. Intraoperative parameters including incision length, operative duration, number of vertebral laminae removed and intraoperative blood loss, as well as indicators of curative efficacy such as total tumor resection rates and postoperative symptom improvement rates, and safety indicators including complication rates, recurrence rates, spinal deformity rates, spinal instability incidence, and length of stay (LOS), were compared between the two groups.
Results: In contrast to the UHMV group, patients in the UHNV group had a significantly shorter incision length and decreased intraoperative blood loss (P < 0.05), while the operative duration (P > 0.05) showed no statistical difference. Although the postoperative improvement and total tumor resection rates were enhanced, the difference was not statistically significant (P > 0.05). In comparison, the bedridden time and length of stay (LOS) were significantly shortened (P < 0.05) in the UHNV group. However, there were no significant differences in recurrence, incidence of complications, spinal deformity, and spinal instability (P > 0.05).
Conclusion: Collectively, our findings indicate that UHNV is not inferior to the UHMV approach. Moreover, due to its safe and minimally invasive nature, UHNV represents a promising alternative to UHMV as a treatment for patients with intradural extramedullary tumors.
Introduction
Only 15% of central nervous system tumors are intraspinal tumors, also called spinal cord tumors, which include intramedullary (around 5%) and extramedullary (approximately 95%) tumors that can occur in any part of the spinal canal (1). These are predominantly benign tumors that cause pain and various sensory, motor, reflex, and sphincter deficits (2). Surgical resection remains the most effective treatment option for the majority of intraspinal tumors (3). There are three commonly used surgical methods to date: total microscopic laminectomy, unilateral microscopic hemilaminectomy, and split microscopic laminotomy (4). Laminotomy is considered a secondary option due to its limited indications, which include dorsal midline intradural and extramedullary lesions, as well as the majority of intramedullary lesions. The studies by Weber et al., Lei et al., and Mobbs et al. (2, 5, 6). suggest that unilateral hemilaminectomy is safer and more effective than total laminectomy because it is minimally invasive and associated with a less negative impact on spinal stability. Furthermore, there is mounting evidence that unilateral hemilaminectomy should be the first-line clinical intervention (5–7). However, because only half of the vertebral laminae are removed during this procedure, the operating space is severely constrained, obstructing or obscuring microscopic visualization. Direct and adequate visualization is critical for safe dissection of tumors located ventrally, lesions that extend bilaterally in the spinal canal, and tumors densely adherent to the cord surface. In such cases, choosing a microscopic hemilaminectomy approach is inappropriate. As a result, microscopic hemilaminectomy is generally reserved for tumors with narrow width and situated on one side of the spine only (8). In contrast, the use of neuroendoscopy can offer superior visualization, overcoming the deficiencies associated with the microscopic approach (9). Therefore, its application value in minimally invasive skull base and pituitary tumor surgery is superior. However, whether this advantage can be demonstrated in spinal cord surgery is unknown. To that end, we compared the relative safety and efficacy of hemilaminectomy procedures performed to remove intraspinal tumors conducted under either microscopy or neuroendoscopy.
Materials and Methods
Patient Data
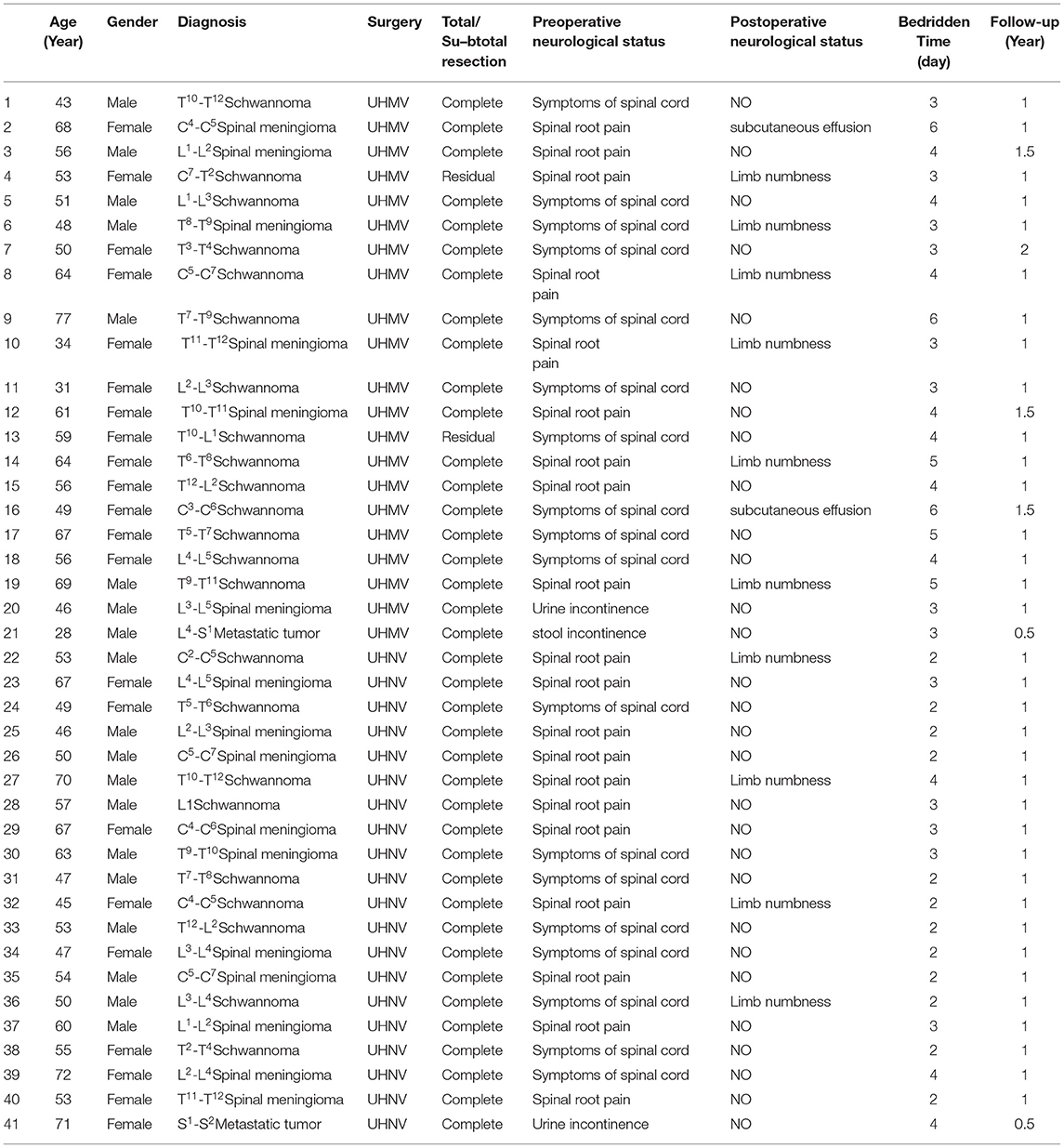
The present study was approved by the Ethics Committee of Northern Jiangsu People's Hospital (2022ky108). This study reviewed previous cases and included 41 intraspinal tumor patients who underwent treatment in the Department of Neurosurgery of Northern Jiangsu People's Hospital via a neuroendoscopic (n = 20) or microscopic (n = 21) approach from June 2018 to September 2020. Patients from June 2018 to June 2019 all received microscopic surgery, and the rest had surgery using the neuroendoscopic approach. Although our medical group consists of five doctors, all surgeries were performed by the same chief physician, while the other doctors offered assistance.
The inclusion criteria were as follows: (1) definite diagnosis of intraspinal tumors; and (2) surgical tumor resection using neuroendoscopic or microscopic hemilaminectomy. Exclusion criteria were as follows: (1) neuroendoscope-assisted microscopic surgery, and (2) patients lost to follow-up.
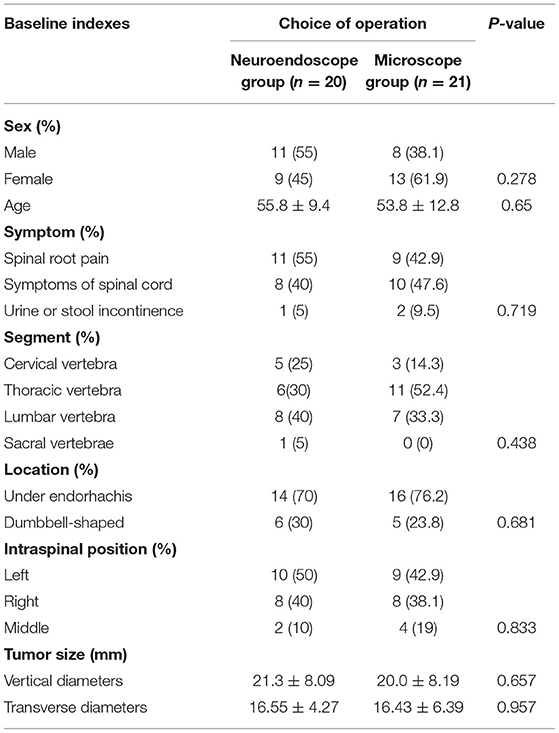
The present study assessed baseline data (age, sex, symptoms, and imaging findings associated with the upper and lower tumor segments, ventral or dorsal position, and position within the spinal canal), surgical approach (microscopic vs. endoscopic), and outcome indicators (incision length, number of vertebral laminae removed, operative duration, intraoperative blood loss, postoperative symptom improvement rates, tumor resection rates, complications, bedridden time, length of stay (LOS), recurrence rate, spinal deformity, and spinal instability (The estimate of spinal stability was based on the variations in sagittal and coronal curvature of the spine's surgical site by comparing preoperative X-ray or CT image data. An angular variation of less than 15 was considered good spinal stability; otherwise, it was defined as spinal instability)). Additionally, in schwannoma, postoperative limb numbness was defined as a postoperative sequela rather than a postoperative complication, while in meningioma, postoperative numbness at follow-up was defined as a postoperative complication to exclude the effects of surgery, anesthesia, and bed rest.
Surgical Approach
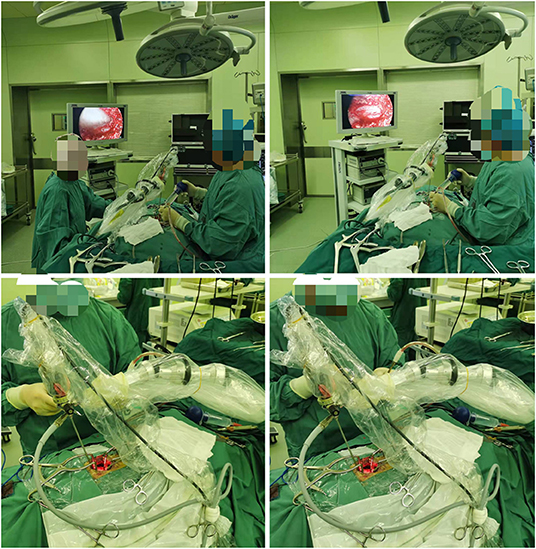
The procedure for patients undergoing unilateral hemilaminectomy under complete neuroendoscopic visualization (UHNV) was performed using a Storz rigid neuroendoscope (4 mm in diameter, 0 or 30-degree lens), a pneumatic mechanical manipulator for endoscope fixation, and a high-definition imaging system. X-ray imaging was used to locate the vertebral segments affected by the intraspinal tumor. Patients adopted the prone position throughout the procedure, and the skin was cut along the posterior median line, with the length of the incision determined by the size of the tumor. The paravertebral muscles on the tumor side were cut and separated, leaving the spinous process and supraspinous ligaments intact, and the paravertebral muscle was manipulated to expose the location of the underlying tumor, creating a working channel for endoscopic visualization. Following neuroendoscopy fixed by a pneumatic mechanical manipulator, the corresponding vertebral laminae were partially removed under neuroendoscope according to location and size, and basal range of tumor, and the window size is suitable for the operation of two instruments such as an aspirator and a detacher under endoscopic visualization. (In the case of smaller lesions, an interlaminar partial hemilaminectomy of the two contiguous laminae was performed, similar to case 1). The endorhachis was cut longitudinally, and adhesions between the tumor envelope and the spinal cord as well as the nerve roots were carefully separated before resection to preserve the nerves and spinal cord. The position of the neuroendoscope was adjusted to avoid intraoperative blind spots caused by bony structures blocking the vertebral canal. Under neuroendoscopic guidance, the spinal dura mater was continuously sutured with non-injury sutures following tumor resection. To avoid the need for a drainage catheter, the paraspinal muscles were sutured to the interspinous ligaments (Figures 1, 2).
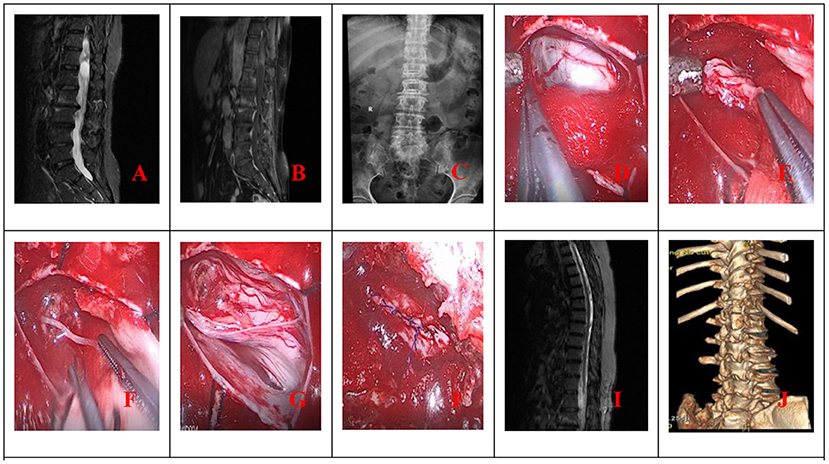
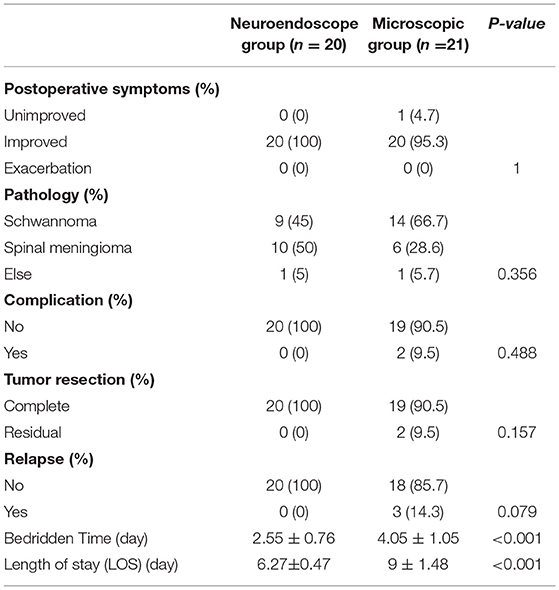
Figure 1. (A) Preoperative MRI; (B) Preoperative MRI enhancement; (C) Position the cone preoperatively; (D) Open the dura mater under endoscope; (E) Separate and pull out the tumor under endoscope; (F) Cut off the tumor-bearing nerve after endoscopic electrocoagulation and remove all the tumor; (G) Endoscope demonstrated good nerve root protection after tumor resection; (H) Continuous suture of spinal dura under endoscope; (I) Postoperative MRI revealed total tumor resection (J). Postoperative 3D CT reconstruction.
The surgical procedure for the UHMV group was the same as described above, but microscopic visualization was used instead.
Postoperative Management and Follow-Up
Patients were encouraged to leave their beds and engage in functional exercise or other treatments as soon as possible following surgery. A computed tomography (CT) scan of the spinal canal was performed the day after surgery to assess the status of the vertebral laminae and detect any bleeding in the surgical site. Magnetic resonance imaging was used to assess tumor resection status 6 months after treatment (MRI). Annual MRIs and 3D reconstruction CT scans were then performed to assess outcomes such as tumor recurrence, spinal deformity, and spinal instability. All patients were followed up postoperatively via email, phone call, SMS, return visits, to name a few, to assess clinical outcomes such as symptom improvement, complications, and imaging findings.
Statistical Analysis
Continuous data were expressed as means ± standard deviation (x ± s) and compared using Student's t-tests, whereas categorical data were expressed as rates (%) and compared using chi-squared tests and Fisher's exact test. A P-value < 0.05 was deemed statically significant. SPSS version 23.0 was used for all statistical analyses.
Results
Baseline Indicators in the Two Treatment Groups
There were no significant differences in baseline characteristics such as age, gender, or symptoms between patients in the two treatment groups (Tables 1, 2).
Intraoperative Indicators in the Two Treatment Groups
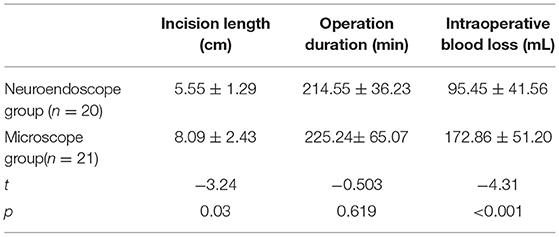
Intraoperative indicators including incision length, operative duration, and intraoperative blood loss were compared between the two groups (Table 3). The surgical incision length was significantly shorter in the UHNV group (5.55 ± 1.29 cm) compared to the UHMV group (8.09 ± 2.43 cm) (P < 0.05). There were no differences in operative duration between these groups (225.24 ± 65.07 vs. 214.55 ± 36.23 min), while intraoperative blood loss was significantly reduced in the UHNV group as compared to the UHMV group (95.45 ± 41.56 vs. 172.86 ± 51.20 mL; P < 0.05). There were no differences in the vertical and horizontal diameters of the tumor between groups. between groups. (Pverticaldiameters > 0.05, Phorizontaldiameters > 0.05).
Indicators of Curative Efficacy in the Two Treatment Groups
Indicators of curative efficacy were then compared between patient groups (Table 4). Postoperative improvement rates were 100% (20/20) in the UHNV group and 95.3% (20/21) in the UHMV group, showing no significant difference (P = 1.000). Following surgery, both groups experienced varying degrees of improvement in preoperative symptoms such as nerve radiculopathy, paresthesia, incontinence, and muscle weakness. However, one patient with urinary incontinence in the UHMV group did not experience symptomatic recovery. The total resection rates did not differ significantly between the UHNV and UHMV groups (100 vs. 90.5%, respectively; P > 0.05). The UHNV group had lower recurrence rates than the UHMV group (0 vs. 14.3%), but the difference was not statistically significant (P > 0.05).
Safety Indicators in the Two Treatment Groups
Next, safety outcomes between patient groups were compared, including complication rates, pathological findings, bedridden time, and length of stay (LOS) (Table 4). There were no reported complications in the UHNV group, while two patients (9.5 percent) from the UHMV group suffered from delayed wound healing due to subcutaneous effusion. However, the difference was not statistically significant (P > 0.05).
Moreover, the results showed that the bedridden time of the UHNV group was significantly shorter by 1.3 days (P < 0.001). Similarly, the mean length of stay (LOS) in the UHNV group (6.27 ± 0.47 d) was significantly shorter than in the UHMV group (9 ± 1.48 d) (P < 0.001). Follow-up MRI and CT radiographs of these patients postoperatively revealed no evidence of spinal instability, spinal stenosis, or recurrence. Kanrnofsky Performation Scale (KPS) and Functional Independence Measurement Scale (FIMTM) were used to assess patients' quality of life after 1 year. It was determined that all patients were able to live and work normally.
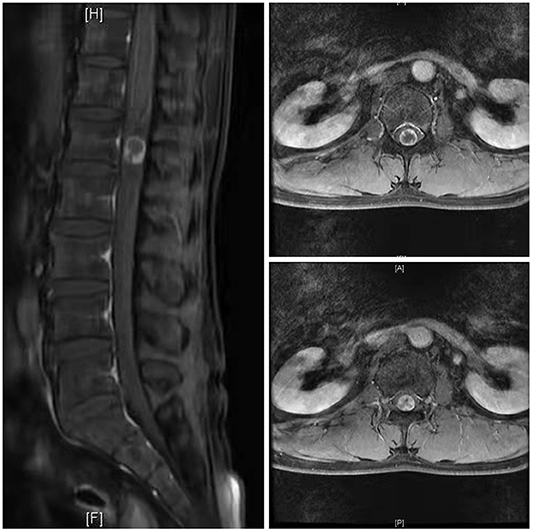
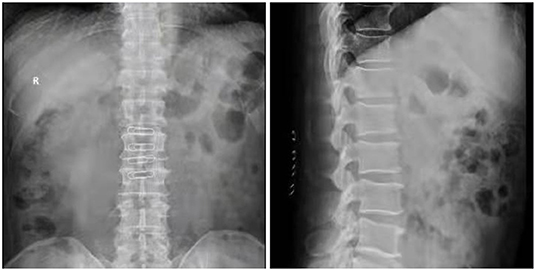
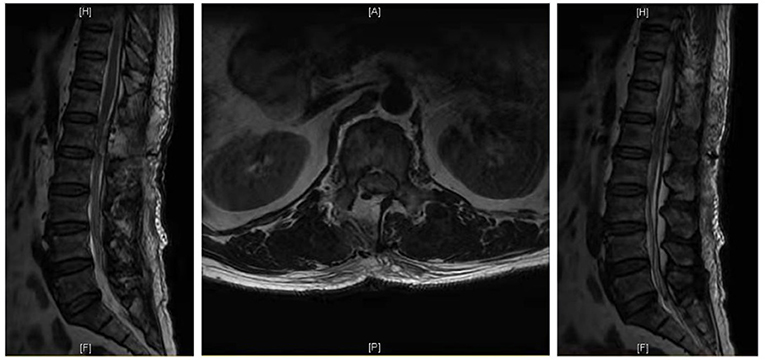
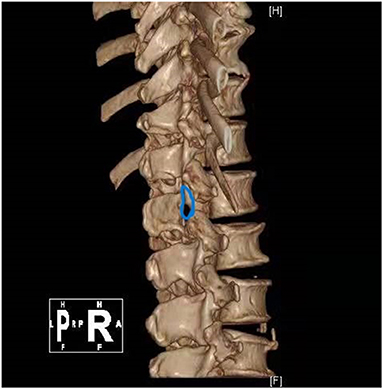
Case 1: A 57-year-old male complaining of pain in his right lower back and leg for one month. Contrast-enhanced magnetic resonance imaging of the spine revealed a lesion posterior to the spinal cord at the L1 level (Figure 3). The patient underwent endoscopic surgery following preoperative positioning (Figure 4). A small amount of bone was removed relative to the size and location of the tumor to enlarge the corresponding laminal space and expose the tumor. An MRI performed postoperatively revealed complete resection of the tumor (Figure 5). Three-dimensional computed tomography confirmed good spinal stability (Figure 6). The pathological examination revealed the presence of schwannoma, and the patient experienced no pain at the follow-up visit.
Discussion
Laminectomy
Total laminectomy, as performed by Gowers et al. in 1888, was the most fundamental and classic surgical technique for intraspinal tumors, from which all subsequent surgical techniques evolved (10). The laminectomy procedure involves the exposure of the tumor via surgical resection of the supraspinous ligament, interspinous ligament, spinous process, and entire vertebral lamina. This approach enables full lesion visualization, thus providing a clear operative field for surgical resection. Thus, total laminectomy under a microscope is appropriate for patients with spinal stenosis and multi-segment massive tumors in the spinal canal, as it safely removes the tumor while exerting minimal traction on the spinal cord and nerve roots (11). However, patients need to stay in bed for a long time after surgery, which may lead to serious complications. Furthermore, severe loss of posterior column structures such as the laminar ligament, loss of bony structural support for diseased segments, and loss of paravertebral muscle attachment points may result in long-term spinal deformity. According to Onyia CU et al., there is a 60% chance that a second operation will be required due to spinal instability following total laminectomy (12). As a result, some studies recommend performing internal fixation with a screw and rod fixation system when using the total laminectomy approach. While this procedure effectively prevents postoperative spinal instability, it severely restricts the range of motion of the corresponding segment and may result in accelerated degeneration of adjacent segments. Additionally, screw implants carry risks, including injury to the spinal cord and nerve roots during implantation and long-term risks, such as neurological symptoms associated with screw loosening and fracture. Because of the direct contact between the spinal canal structure and surrounding soft tissues, spinal dura mater and nerve root adhesions are quite common. According to Raffaeli et al., scar tissues surrounding the nerve root may impair the nerve root's blood supply and nutrition, resulting in chronic nerve root pain following surgery (13).
Hemilaminectomy
In 1908, Taylor AS et al. came up with the hemilaminectomy technique, thereby launching the era of minimally invasive spine surgery (14). This technique required only one side of the lamina to be removed, thereby preserving the midline spinal ligament complex and contralateral muscles necessary for spinal stability. The posterior ligament complex (such as the interspinous ligament) serves as a point of attachment for the muscle, and its preservation allows for paravertebral muscle reconstruction along the natural plane, increasing the likelihood of muscle recovery and minimizing the risk of muscle atrophy and subsequent kyphosis (15). Perez-Cruet et al. (16) previously found that an increasing number of experimental and clinical studies had established a strong correlation between vertebral column stability and the vertebral lamina's decompression degree. As a result, in clinical settings and evidence-based studies, the UHMV procedure has largely superseded total laminectomy (5, 17–20). The disadvantage of hemilaminectomy is that the surgical area between the spinous process and the ipsilateral facet joint is small, and microscope-based visualization is limited by a small depth of field, tubular vision, and inadequate illumination of deeper sites. As a result, UHMV is prone to insufficient tumor resection and unintentional spinal cord and nerve root injury. The advent of neuroendoscopic hemilaminectomy by Endo T et al. and Burkhardt BW et al. has revolutionized how hemilaminectomy is performed, with an increasing number of people believing that microscopic visualization will gradually be replaced (21, 22).
Split Laminotomy
Bognár et al. (23) reported the first successful laminectomy in six pediatric patients. The benefits of this procedure include symmetrical reconstruction of the posterior column of the spine and a low risk of bleeding due to the sparse veins in the dorsal midline epidural space. However, its disadvantages are also quite apparent. (1) After laminectomy, the horizontal width exposed is only about 1.5 cm, the operative field is constrained, and duraplasty is very challenging. (2) Occasionally, the spinous process can fracture during the separation process and could also fracture under traction (24).
Other (Neuroendoscopic Transforaminal and Interlaminar Surgeries)
At present, endoscopy is widely used to treat degenerative spinal diseases, particularly lumbar disc herniation and lumbar spinal stenosis. Due to technological advancements and increased surgical experience, endoscopic spine surgery indications have expanded considerably. Senturk et al. (25) previously described a translaminar approach for complete endoscopic removal of a lumbar intradural extramedullary tumor. Moreover, Tsai et al., Ying et al., and Zhu et al. (26–28) also supported the use of the interlaminar approach to remove intraspinal tumors. The main benefit of neuroendoscopic transforaminal and interlaminar resection is the ability to achieve complete tumor resection with minimal bone removal with improved spinal mobility and stability preservation. Among the other advantages are decreased intraoperative blood loss and postoperative pain, as well as a shorter hospital stay. Although there are few studies and controversy surrounding these surgical procedures, with the development of endoscopic instruments in recent years, endoscopic technology will become an effective alternative for treating spinal tumors surgically.
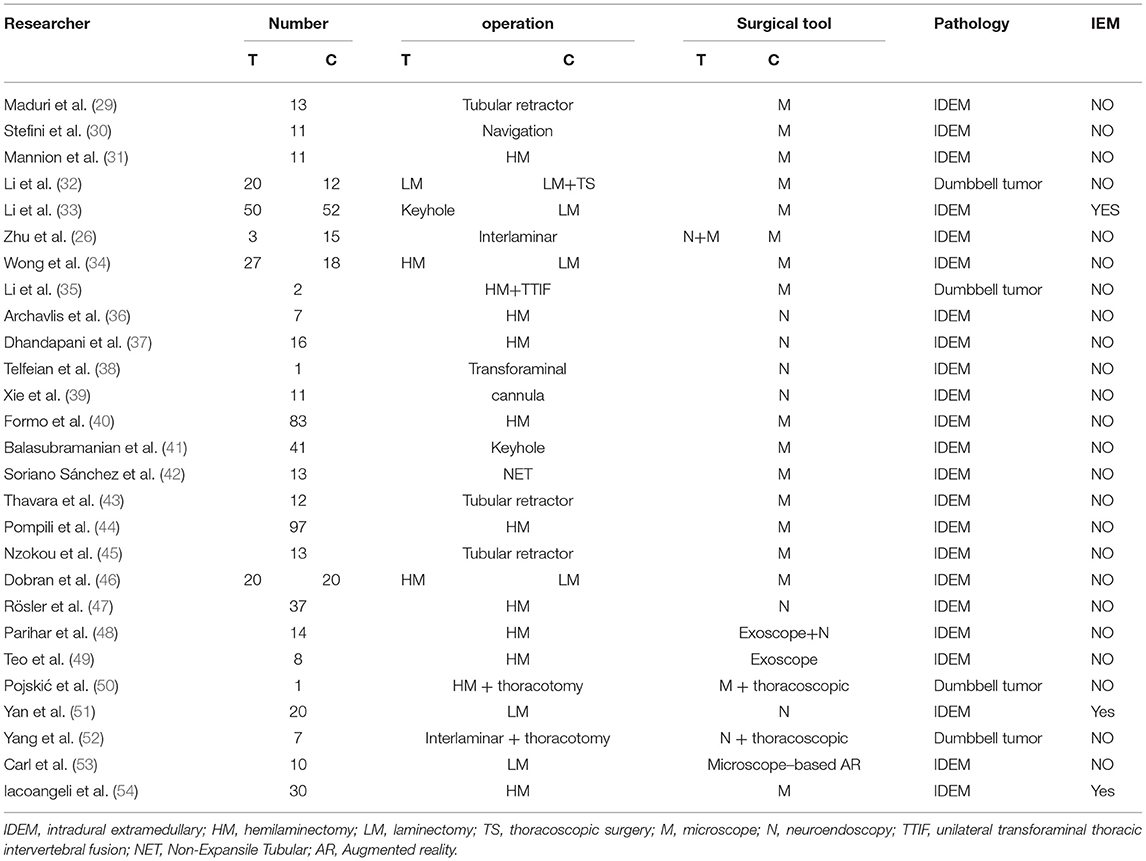
Herein, we collected important surgical information on spinal cord meningiomas and schwannomas in the past 10 years and summarized the representative articles (Table 5).
In this study, most patients undergoing UHMV exhibited satisfactory tumor resection and postoperative improvements in clinical symptoms, in line with prior reports (8). Meanwhile, the UHNV group had favorable outcomes, and this approach was associated with benefits such as decreased blood loss, tissue injury, hospitalization duration, length of stay (LOS), and postoperative complication rates. Parihar et al. previously evaluated the outcomes of 18 patients who underwent UHNV and determined that this technique was a safe and effective alternative to the UHMV approach (55). Di et al. similarly observed reductions in soft tissue injury, blood loss, incision length, postoperative pain, and hospitalization duration in patients who underwent the UHNV procedure (56). Besides, Ren et al. reported that UHNV was an effective minimally invasive procedure that reduced the presence of residual tumors in treated patients (57). Overall, small bony fenestrations or enlarged interlaminar corridors offer a similar effect to tubular retractors, and using endoscopy with “tubular retractors” provides a better view than “tubular” microsurgery.
In light of our findings and prior studies, we believe that the UHNV procedure has several significant advantages over the UHMV procedure. Among them are the following:
(1) For tumors of comparable size (diameter, length, and location), surgical incisions were typically 3–4 cm longer in the UHMV group than in the UHNV group. Due to the larger incision and associated scar tissue development, these larger surgical incisions are likely to increase the incidence of postoperative complications such as pain, infection, myasthenia, and cerebrospinal fluid leakage (58).
(2) The UHNV procedure can minimize soft tissue separation and excessive bone removal, thereby avoiding extensive muscle stripping and lowering postoperative pain, bleeding, and spinal instability rates (59).
(3) Neuroendoscopic visualization enables a more reliable panoramic view of the operating field, allowing for more detailed examination and illumination in deep tissue sites (60). As the operation progresses, the neuroendoscope can be continuously adjusted to meet changing operative requirements, allowing for more precise tumor separation and resection. For dumbbell-shaped intraspinal tumors primarily located outside the spinal canal or for other large tumors, an initial tumor portion can be removed to create a channel, followed by careful separation of the upper and lower tumor edges and segmental tumor resection (Figure 7). Overall, our findings corroborate with those of Barrenechea et al. (61) and offer further insight into this field.
(4) Using an angled lens eliminates blind spots within the visual field during surgery. The use of a flexible neuroendoscope that enables “round-angle observation” can ensure complete tumor resection and visualization of the spinal canal from multiple angles and directions while minimizing iatrogenic damage to the spinal cord, nerve roots, or blood vessels, thereby lowering the incidence of postoperative complications. This advantage is especially advantageous when tumors are located primarily in the midline and have a large vertical diameter, when tumors are closely adherent to the spinal cord or nerve roots, or when tumors are located ventrally or laterally.
(5) The UHNV approach is minimally invasive, allowing for a more rapid postoperative recovery, a shorter length of stay, lower hospitalization costs, and more efficient use of medical resources. Singh et al. (62) also confirmed that the UHNV approach is superior due to its accessibility, mobility, illumination, and visualization advantages.
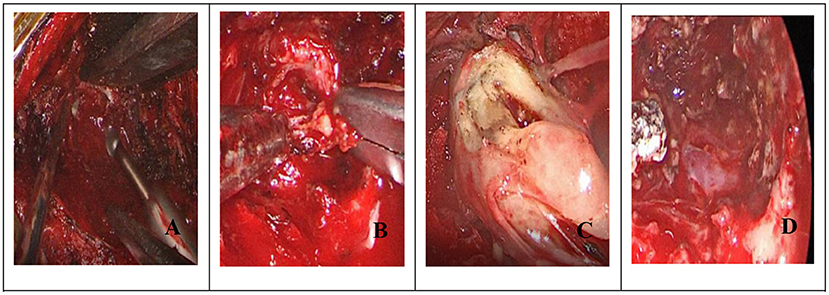
Figure 7. (Dumbbell): (A) Endoscopic removal of hemilamellae; (B) Endoscopic intratumoral resection; (C) After shrinking the tumor to create a channel, carefully separate the tumor from upper and lower levels; (D) Tumor removal following endoscopic electrocoagulation.
There are certain limitations associated with the UHNV approach. First, the neuroendoscopic approach imposes a high burden on the operator, who must be familiar with correct neuroendoscopic surgical protocols. Additionally, this approach necessitates improved hand-eye coordination, spatial positioning, and additional training on cadavers before clinical translation (63).
Nevertheless, this study has some limitations. Firstly, this was a non-randomized cohort study which might cause selection bias. Secondly, due to the rarity of intramedullary and spinal cord malignancies, those patients were excluded from this study. As a result, additional research is warranted to determine the safety and efficacy of UHNV in treating intramedullary and spinal cord malignancies. Furthermore, this was a relatively small study with a short-term follow-up period, and additional large-scale studies with a longer follow-up period will be needed to confirm and expand upon the results of this study.
Conclusions
The resection of intradural extramedullary tumors using the UHNV technique is a safe and effective alternative to the UHMV approach while also minimizing surgical injury.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
WZ: conception and design and manuscript writing. CW: administrative support. WZ, HJ, and SH: provision of study materials or patients. WZ, YZ, and HJ: collection and assembly of data. WZ, BY, and HW: data analysis and interpretation. All authors approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.823770/full#supplementary-material
Supplementary Video 1. Endoscopically, we looked for the location of the tumor and carefully isolated the peripheral nerves.
Supplementary Video 2. Under endoscopy, we isolated the tumor and removed part of it to expand the surgical space.
Supplementary Video 3. Endoscopically, we isolated the parent nerve while protecting the nerve.
Supplementary Video 4. An initial tumor portion can be removed to create a channel, and remaining tumor segmental resection.
Supplementary Video 5. Complete tumor resection, tumor cavity hemostasis.
Supplementary Video 6. The corresponding vertebral laminae were partially removed under neuroendoscope according to location and size, and basal range of tumor, and the window size is suitable for the operation of two instruments such as an aspirator and a detacher under endoscopic visualization.
Supplementary Video 7. After careful hemostasis, the endorhachis was cut longitudinally.
Supplementary Video 8. The endorhachis was cut longitudinally, and parts of the tumor can be removed to create a channel.
References
1. Azad TD, Jiang B, Bettegowda C. Molecular foundations of primary spinal tumors-implications for surgical management. Ann Transl Med. (2019) 7:222. doi: 10.21037/atm.2019.04.46
2. Weber C, Gulati S, Jakola AS, Habiba S, Nygaard ØP, Johannesen TB, Solheim O. Incidence rates and surgery of primary intraspinal tumors in the era of modern neuroimaging: a national population-based study. Spine (Phila Pa 1976). (2014) 39:E967–73. doi: 10.1097/BRS.0000000000000412
3. Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. (2008) 87:173–9. doi: 10.1007/s11060-007-9507-z
4. Arnautovic K, Arnautovic A. Extramedullary intradural spinal tumors: a review of modern diagnostic and treatment options and a report of a series. Bosn J Basic Med Sci. (2009) 9(Suppl 1):40–5. doi: 10.17305/bjbms.2009.2755
5. Lei D, Zhou Y, Yao D, Zhang F, Wang X, Jiang X, et al. Efficacy of unilateral hemilaminectomy for intraspinal tumor resection: a systematic review and meta-analysis. Ann Palliat Med. (2021) 10:984–99. doi: 10.21037/apm-20-499
6. Mobbs RJ, Maharaj MM, Phan K, Rao PJ. Unilateral hemilaminectomy for intradural lesions. Orthop Surg. (2015) 7:244–9. doi: 10.1111/os.12184
7. Sun CX, Meng XL, Xie SN, Yu Y, Yang HJ, Wu B. Unilateral hemilaminectomy for patients with intradural extramedullary tumors. J Zhejiang Univ Sci B. (2011) 12:575–81. doi: 10.1631/jzus.B1000402
8. Chen HF, Yang KY, Ju Y, Li GP, Huang SQ. Clinical analysis of 542 intraspinal tumors microsurgically resected by hemilaminectomy. Zhonghua Yi Xue Za Zhi. (2010) 90:874–7.
9. Rodriguez JH, Ponsky JL. Operating with the endoscope. Surg Clin North Am. (2020) 100:xvii–xviii. doi: 10.1016/j.suc.2020.09.012
10. Gowers WR, Horsley V. A case of tumour of the spinal cord. removal; recovery. Med Chir Trans (Report missing IFs). (1888) 71:377–430. doi: 10.1177/095952878807100125
11. Ottenhausen M, Ntoulias G, Bodhinayake I, Ruppert FH, Schreiber S, Förschler A, et al. Intradural spinal tumors in adults-update on management and outcome. Neurosurg Rev. (2019) 42:371–88. doi: 10.1007/s10143-018-0957-x
12. Onyia CU, Menon SK. Laminectomy vs. laminoplasty in the surgical management of long-segment intradural spinal tumors: any difference in neurological outcomes? Asian J Neurosurg (Report missing IFs). (2018) 13:1128–33. doi: 10.4103/ajns.AJNS_67_18
13. Raffaeli W, Righetti D, Andruccioli J, Sarti D. Periduroscopy: general review of clinical features and development of operative models. Acta Neurochir Suppl. (2011) 108:55–65. doi: 10.1007/978-3-211-99370-5_10
14. Villalonga JF, Cervio A. Surgical treatment of intradural extramedullary lesions by hemilaminectomy. Surg Neurol Int. (2017) 8(Suppl 2):S11–7. doi: 10.4103/sni.sni_253_17
15. KrishnanKutty R, Sreemathyamma SB, Sivanandapanicker JL, Asher P, Prabhakar RB, Peethambaran A. Hemilaminectomy for spinal cord intradural tumors: an institutional experience. Asian J Neurosurg (Report missing IFs). (2018) 13:760–5. doi: 10.4103/ajns.AJNS_106_18
16. Perez-Cruet MJ, Fessler RG, Perin NI. Review: complications of minimally invasive spinal surgery. Neurosurgery. (2002) 51(5 Suppl):S26–36. doi: 10.1097/00006123-200211002-00005
17. Yüce I, Kahyaoglu O, Çavuşoglu HA, Ataseven M, Çavuşoglu H, Aydin Y. Surgical treatment and outcomes of intramedullary tumors by minimally invasive approach. J Clin Neurosci. (2021) 86:26–31. doi: 10.1016/j.jocn.2021.01.001
18. Aydin Y, Yüce I, Çavuşoglu H. Surgical treatment and outcomes of intramedullary tumors by minimally invasive approach: answer. J Clin Neurosci. (2021) 93:260–1. doi: 10.1016/j.jocn.2021.05.066
19. Turel MK?D'Souza WP?Rajshekhar V. Hemilaminectomy approach for intradural extramedullary spinal tumors: an analysis of 164 patients. Neurosurg Focus. (2015) 39:E9. doi: 10.3171/2015.5.FOCUS15170
20. Naganawa T, Miyamoto K, Hosoe H, Suzuki N, Shimizu K. Hemilaminectomy for removal of extramedullary or extradural spinal cord tumors: medium to long-term clinical outcomes. Yonsei Med J. (2011) 52:121–9. doi: 10.3349/ymj.2011.52.1.121
21. Endo T, Tominaga T. Use of an endoscope for spinal intradural pathology. J Spine Surg. (2020) 6:495–501. doi: 10.21037/jss.2020.01.06
22. Burkhardt BW, Oertel JM. Endoscopic posterior cervical foraminotomy: 2-dimensional operative video. Oper Neurosurg. (2020) 18:E41. doi: 10.1093/ons/opz131
23. Bognár L, Madarassy G, Vajda J. Split laminotomy in pediatric neurosurgery. Childs Nerv Syst. (2004) 20:110–3. doi: 10.1007/s00381-003-0848-4
24. Arocho-Quinones EV, Kolimas A, LaViolette PS, Kaufman BA, Foy AB, Zwienenberg M, et al. Split laminotomy vs. conventional laminotomy: postoperative outcomes in pediatric patients. J Neurosurg Pediatr. (2018) 21:615–25. doi: 10.3171/2017.12.PEDS17368
25. Sentürk S, Ünsal ÜÜ. Percutaneous full-endoscopic removal of lumbar intradural extramedullary tumor via translaminar approach. World Neurosurg. (2019) 125:146–9. doi: 10.1016/j.wneu.2019.01.206
26. Zhu YJ, Ying GY, Chen AQ, Wang LL, Yu DF, Zhu LL, Ren YC, et al. Minimally invasive removal of lumbar intradural extramedullary lesions using the interlaminar approach. Neurosurg Focus. (2015) 39:E10. doi: 10.3171/2015.5.FOCUS15182
27. Ying GY, Yao Y, Shen F, Wu ZY, Chen CM, Zhu YJ. Percutaneous endoscopic removal of cervical foraminal schwannoma via interlaminar approach: a case report. Oper Neurosurg. (2018) 14:1–5. doi: 10.1093/ons/opx088
28. Tsai SH, Wu HH, Cheng CY, Chen CM. Full endoscopic interlaminar approach for nerve root decompression of sacral metastatic tumor. World Neurosurg. (2018) 112:57–63. doi: 10.1016/j.wneu.2018.01.075
29. Maduri R, Bobinski L, Duff JM. Image merge tailored access resection (IMTAR) of spinal intradural tumors. Technical Report of 13 Cases World Neurosurg. (2017) 98:594–602. doi: 10.1016/j.wneu.2016.05.092
30. Stefini R, Peron S, Mandelli J, Bianchini E, Roccucci P. Intraoperative spinal navigation for the removal of intradural tumors: technical notes. Oper Neurosurg. (2018) 15:54–9. doi: 10.1093/ons/opx179
31. Mannion RJ, Nowitzke AM, Efendy J, Wood MJ. Safety and efficacy of intradural extramedullary spinal tumor removal using a minimally invasive approach. Neurosurgery. (2011) 68(1 Suppl Operative):208–16. doi: 10.1227/NEU.0b013e318207b3c7
32. Li Y, Wang B, Li L, Lü G. Posterior surgery vs. combined laminectomy and thoracoscopic surgery for treatment of dumbbell-type thoracic cord tumor: a long-term follow-up. Clin Neurol Neurosurg. (2018) 166:31–5. doi: 10.1016/j.clineuro.2018.01.019
33. Li Y, Xu J, Zheng W. Comparison of the safety and outcomes between keyhole surgery and total laminectomy for resection of intradural extramedullary spinal cord tumors. Zhonghua Yi Xue Za Zhi. (2020) 100:3093–8. doi: 10.3760/cma.j.cn112137-20200305-00605
34. Wong AP, Lall RR, Dahdaleh NS, Lawton CD, Smith ZA, Wong RH, et al. Comparison of open and minimally invasive surgery for intradural-extramedullary spine tumors. Neurosurg Focus. (2015) 39:E11. doi: 10.3171/2015.5.FOCUS15129
35. Li C, Ye Y, Gu Y, Dong J. Minimally invasive resection of extradural dumbbell tumors of thoracic spine: surgical techniques and literature review. Eur Spine J. (2016) 25:4108–15. doi: 10.1007/s00586-016-4677-z
36. Archavlis E, Schwandt E, Kosterhon M, et al. A modified microsurgical endoscopic-assisted transpedicular corpectomy of the thoracic spine based on virtual 3-dimensional planning. World Neurosurg. (2016) 91:424–33. doi: 10.1016/j.wneu.2016.04.043
37. Dhandapani S, Karthigeyan M. “Microendoscopic” versus “pure endoscopic” surgery for spinal intradural mass lesions: a comparative study and review. Spine J. (2018) 18:1592–602. doi: 10.1016/j.spinee.2018.02.002
38. Telfeian AE, Choi DB, Aghion DM. Transforaminal endoscopic surgery under local analgesia for ventral epidural thoracic spinal tumor: case report. Clin Neurol Neurosurg. (2015) 134:1–3. doi: 10.1016/j.clineuro.2015.03.022
39. Xie T, Xiu P, Song Y. Zeng JC,Huang SS. Percutaneous endoscopic excision and ablation of osteoid osteoma of the lumbar spine and sacrum: a technical note and outcomes. World Neurosurg. (2019) 133:121–6. doi: 10.1016/j.wneu.2019.09.039
40. Formo M, Halvorsen CM, Dahlberg D, Brommeland T, Fredø H, Hald J, et al. Minimally invasive microsurgical resection of primary, intradural spinal tumors is feasible and safe: a consecutive series of 83 patients. Neurosurgery. (2018) 82:365–71. doi: 10.1093/neuros/nyx253
41. Balasubramanian SC, Nair AR, Saphiya NN, Madan A, Mathews SS. Minimally invasive resection of spinal tumors with tubular retractor: case series, surgical technique, and outcome. World Neurosurg. (2021) 149:e612–21. doi: 10.1016/j.wneu.2021.01.124
42. Soriano Sánchez JA, Soto García ME, Soriano Solís S, Rodríguez García M, Trejo Huerta P, Sánchez Escandón O, et al. Microsurgical resection of intraspinal benign tumors using non-expansile tubular access. World Neurosurg. (2020) 133:e97–e104. doi: 10.1016/j.wneu.2019.08.170
43. Thavara BD, Kidangan GS, Rajagopalawarrier B. Analysis of the surgical technique and outcome of the thoracic and lumbar intradural spinal tumor excision using minimally invasive tubular retractor system. Asian J Neurosurg Apr-Jun. (2019) 14:453–60. doi: 10.4103/ajns.AJNS_254_18
44. Pompili A, Caroli F, Crispo F, Giovannetti M, Raus L, Vidiri A, et al. Unilateral laminectomy approach for the removal of spinal meningiomas and schwannomas: impact on pain, spinal stability, and neurologic results. World Neurosurg. (2016) 85:282–91. doi: 10.1016/j.wneu.2015.09.099
45. Nzokou A, Weil AG, Shedid D. Minimally invasive removal of thoracic and lumbar spinal tumors using a non-expandable tubular retractor. J Neurosurg Spine. (2013) 19:708–15. doi: 10.3171/2013.9.SPINE121061
46. Dobran M, Paracino R, Nasi D, Aiudi D, Capece M, Carrassi E, et al. Laminectomy vs. unilateral hemilaminectomy for the removal of intraspinal schwannoma: experience of a single institution and review of literature. J Neurol Surg A Cent Eur Neurosurg. (2021) 82:552–5. doi: 10.1055/s-0041-1722968
47. Rösler J, Georgiev S, Roethe AL, Chakkalakal D, Acker G, Dengler NF, et al. Clinical implementation of a 3D4K-exoscope (Orbeye) in microneurosurgery. Neurosurg Rev. (2021) 45:627–35. doi: 10.21203/rs.3.rs-425632/v1
48. Parihar V, Yadav YR, Kher Y, Ratre S, Sethi A, Sharma D. Learning neuroendoscopy with an exoscope system (video telescopic operating monitor): early clinical results. Asian J Neurosurg Oct-Dec. (2016) 11:421–6. doi: 10.4103/1793-5482.145551
49. Teo THL, Tan BJ, Loo WL, Yeo AKS, Dinesh SK. Utility of a high-definition 3D digital exoscope for spinal surgery during the COVID-19 pandemic. Bone Jt Open. (2020) 1:359–63. doi: 10.1302/2633-1462.17.BJO-2020-0079.R1
50. Pojskić M, Zbytek B, Mutrie CJ, Arnautović KI. Spinal dumbbell epidural. Hemangioma: two stage/same sitting/same position posterior microsurgical and transthoracic endoscopic resection - case report and review of the literature. Acta Clin Croat. (2018) 57:797–808. doi: 10.20471/acc.2018.57.04.27
51. Yan X, Wang H, Li C, Lin Y, Lin L, Zhu S, et al. Endoscopically controlled surgery with open hemilaminectomy for the treatment of intradural extramedullary tumors: an operative technique and short-term outcomes of 20 consecutive cases. Chin Neurosurg J. (202) 7:1. doi: 10.1186/s41016-020-00222-0
52. Yang K, Ji C, Luo D, Li K, Pang L, Xu D, et al. percutaneous spinal endoscopic combined with thoracoscopic surgery for treatment of thoracic eden IV dumbbell tumors. World Neurosurg. (2022) 157:e492–6. doi: 10.1016/j.wneu.2021.10.134
53. Carl B, Bopp M, Saß B, Pojskic M, Nimsky C. Augmented reality in intradural spinal tumor surgery. Acta Neurochir (Wien). (2019) 161:2181–93. doi: 10.1007/s00701-019-04005-0
54. Iacoangeli M, Gladi M, Di Rienzo A, Dobran M, Alvaro L, Nocchi N, et al. Minimally invasive surgery for benign intradural extramedullary spinal meningiomas: experience of a single institution in a cohort of elderly patients and review of the literature. Clin Interv Aging. (2012) 7:557–64. doi: 10.2147/CIA.S38923
55. Parihar VS, Yadav N, Yadav YR, Ratre S, Bajaj J, Kher Y. Endoscopic management of spinal intradural extramedullary tumors. J Neurol Surg A Cent Eur Neurosurg. (2017) 78:219–26. doi: 10.1055/s-0036-1594014
56. Di X. Endoscopic spinal tethered cord release: operative technique. Childs Nerv Syst. (2009) 25:577–81. doi: 10.1007/s00381-008-0800-8
57. Ren YC, Zhao BJ, Xie ZY, Ying GY, Shen F, Zhu YJ. Flexible endoscope visualization to assist in the removal of a string of 10 schwannomas at the cauda equina: technical case report. J Neurosurg Spine. (2020) 3:1–4. doi: 10.3171/2020.4.SPINE191390
58. Barber SM, Fridley JS, Konakondla S, Nakhla J, Oyelese AA, Telfeian AE, et al. Cerebrospinal fluid leaks after spine tumor resection: avoidance, recognition and management. Ann Transl Med. (2019) 7:217. doi: 10.21037/atm.2019.01.04
59. Ogihara S, Seichi A, Iwasaki M, Kawaguchi H, Kitagawa T, Tajiri Y, et al. Concurrent spinal schwannomas and meningiomas. Case illustration. J Neurosurg. (2003) 98(3 Suppl):300. doi: 10.3171/spi.2003.98.3.0300
60. Vasudevan K, Saad H, Oyesiku NM. The role of three-dimensional endoscopy in pituitary adenoma surgery. Neurosurg Clin N Am. (2019) 30:421–32. doi: 10.1016/j.nec.2019.05.012
61. Barrenechea IJ, Fukumoto R, Lesser JB, Ewing DR, Connery CP, Perin NI. Endoscopic resection of thoracic paravertebral and dumbbell tumors. Neurosurgery. (2006) 59:1195–201. doi: 10.1227/01.NEU.0000245617.39850.C9
62. Singh H, Essayed WI, Cohen-Gadol A, Zada G, Schwartz TH. Resection of pituitary tumors: endoscopic vs. microscopic. J Neurooncol. (2016) 130:309–17. doi: 10.1007/s11060-016-2124-y
Keywords: endoscope, unilateral hemilaminectomy, intraspinal tumor, microscope, retrospective single center analysis
Citation: Zeng W, Jiang H, He S, Zhang Y, Yu B, Wang H and Wang C (2022) Comparison of Neuroendoscopic and Microscopic Surgery for Unilateral Hemilaminectomy: Experience of a Single Institution. Front. Surg. 9:823770. doi: 10.3389/fsurg.2022.823770
Received: 28 November 2021; Accepted: 04 March 2022;
Published: 29 March 2022.
Edited by:
Roberto Colasanti, University Hospital of Padua, ItalyReviewed by:
Carlos A. Bagley, University of Texas Southwestern Medical Center, United StatesMirza Pojskic, University Hospital of Giessen and Marburg, Germany
Copyright © 2022 Zeng, Jiang, He, Zhang, Yu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cunzu Wang, bmV1cm9zdXJnZW9ud2FuZ0AxMjYuY29t
 Wei Zeng
Wei Zeng Haixiao Jiang1
Haixiao Jiang1 Cunzu Wang
Cunzu Wang