- 1Department of Breast Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Ministry of Health of the Russian Federation, Sechenov University, Moscow, Russia
- 3Department of Radiation Oncology, Cancer Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 4Department of Oncology Plastic Surgery, Hunan Province Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
- 5L.L. Levshin Institute of Cluster Oncology, Moscow, Russia
- 6Academy of Postgraduate Education, The Federal State Budgetary Unit FSCC, Federal Medical Biological Agency, Moscow, Russia
- 7Cancer Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background and Objective: Deep Inferior Epigastric Perforator (DIEP) flap is a tissue isolated from the skin and subcutaneous tissue of the lower abdomen or rectus muscle to foster breast reconstruction. There is limited information about DIEP-flap induced complications associated with breast reconstruction surgery.
Evidence: We conducted a systematic review of the published literature in the field of breast cancer reconstruction surgery. Information was gathered through internet resources such as PubMed, Medline, eMedicine, NLM, and ReleMed etc. The following key phrases were used for effective literature collection: “DIEP flap”, “Breast reconstruction”, “Patient management”, “Postoperative DIEP”, “Intraoperative anticoagulant therapy”, “Clinical recommendations”. A total of 106 research papers were retrieved pertaining to this systematic review.
Conclusion: A successful breast reconstruction with DIEP-flap without complications is the priority achievement for this surgical procedure. This study provides various evidence-based recommendations on patient management in the perioperative, intraoperative, and postoperative periods. The clinical recommendations provided in this review can benefit surgeons to execute breast reconstruction surgery with minimal postoperative complications. These recommendations are beneficial to improve clinical outcomes when performing surgery by minimizing complications in perioperative, intraoperative, and postoperative period.
Highlights
- Breast reconstruction with the DIEP-flap can be surgical choice in the cases of structural restoration of the anterior chest wall anatomy after a mastectomy.
- This is a systematic review with evidence-based recommendations on patient management in the perioperative period after DIEP flap breast reconstruction.
- This study represents surgery an important stage in complete functional, emotional, psycho-social, and aesthetic patient rehabilitation with improved quality of life after radical mastectomy.
- The study recommendations have a strong potential to improve clinical results when performing breast reconstruction with the DIEP-flap, and will serve as a basis for new prospective studies on topics covered in this study.
Introduction
Breast reconstruction is a significant approach in the patients who received radical mastectomy to improve their quality of life by minimizing psychosocial stress. This kind of strategy is a crucial element in the successful therapy and management of breast cancer (1, 2). For instance, the autologous microvascular breast reconstruction reported to produce typically significant clinical outcomes; and this kind of reconstruction can be performed using DIEP flap or a muscle-sparing (MS) free “transverse rectus abdominis musculocutaneous” (TRAM) flap (3, 4). However, there are several perioperative, intraoperative, and postoperative complications reported during DIEP-flap breast reconstruction surgery but the clinical information pertaining to the management of these complications in surgical oncology are minimal. For instance, DIEP-flap complications can be practically separated into two groups: first group- complications associated with technical difficulties in flap mobilization such as anastomosis formation, perforate vessel traumatization, surgical mistakes in vascular anastomosis completion (5, 6); a second group - complications due to the mistakes in the patient preparation and management (7). Based on the classification of complications, we have performed a systematic review of literature in regard to the most important aspects of preoperative, intraoperative, and postoperative patient management when performing a DIEP-flap breast reconstruction.
The current review outlines articles, clinical cases, different publications pertinent to the quality of care and clinical recommendations of patient management when performing autologous breast reconstruction using a DIEP-flap. Furthermore, this review delineates the proper patient management in the preoperative period forms significant clinical recommendations on intraoperative management, and defines an appropriate postoperative management protocol suggesting a viable strategy for the breast reconstruction to foster patient management effectively in the preoperative, intraoperative, and early postoperative periods.
Overview
The usage of autologous tissue isolated from the lower abdominal wall is a reliable and popular method of breast reconstruction. The technique was first described by Holmstrom in 1979 and popularized by Hartrampf et al. in 1982 (8). The history and development of modern reconstructive surgery reported as a justified strategy to improve operative technique, consequently patient management in the perioperative period in order to minimize the postoperative complications. Substantial breakthrough in reconstructive and plastic surgery was seen in 1989, when a prospective surgeon G. Ian Taylor formulated the angiosome theory of vascularization (9).
The constant commitment of surgeons to refrain from executing the traumatizing surgical operations began due to the new era of developments in the perforator flap-based reconstruction surgery (4). A new stage in the development of breast reconstructive surgery was the DIEP-flap based reconstruction technique, which could allow minimal anterior abdominal wall traumatization, and sparing the rectus abdominis muscle and minimal injury of the abdominal aponeurosis (3). This new era of breast reconstructive surgery was dominated by the DIEP-flap. Blondeel P. N. et al. showed that compared to the TRAM-flap, mobilization of the DIEP-flap significantly can decrease surgical traumatization of the rectus and oblique abdominis muscles, which minimize the incidence of weakness in the anterior abdominal wall during the postoperative period (3). Despite the widely accepted advantages of minimally traumatizing techniques over other intervention methods, it does not prevent complications completely (9). In addition, the attributes of new surgical methods in breast reconstruction can increase the specter of possible complications (3, 7). DIEP-flap is currently referred to as the gold standard in reconstructive mammoplasty. The usage of DIEP-flap allows for minimization of donor site morbidity (3, 10–13), but the reconstructive procedure still remains a long and strenuous surgery, with a median operative time of 6–7 h.
Methods
Data Sources
The literature for this systematic review was gathered through internet resources, such as PubMed, Medline and others (eMedicine, NLM, ReleMed). All the data acquired for this study were completely in accordance with the guidelines outlined in the PRISMA statement. An automatic search with manual sorting of the selected articles was performed. The following key phrases were used: “DIEP-flap”, “breast reconstruction”, “patient management”, “postoperative DIEP”, “intraoperative anticoagulant therapy”, “clinical recommendations”. A total of 106 papers were retrieved relevant to our research and subjected for primary evaluation. Total 56 significantly informative papers were selected after primary screening procedures, and were categorized according to sources including pubmed, medline, others.
Study Selection
Potentially acceptable articles for systematic review were considered, which deciphered the topic for breast reconstruction with free flaps, including DIEP-flaps, and the analysis of this article information gave specific recommendations on patient management. Total 106 papers were primarily screened with respect to actuality, publication date, access to article text, content, number of patients, and complete flap-loss rate, whereas 56 research papers were chosen for secondary screening. Following a two-stage screening process, 21 research papers were selected for further study (Figure 1).
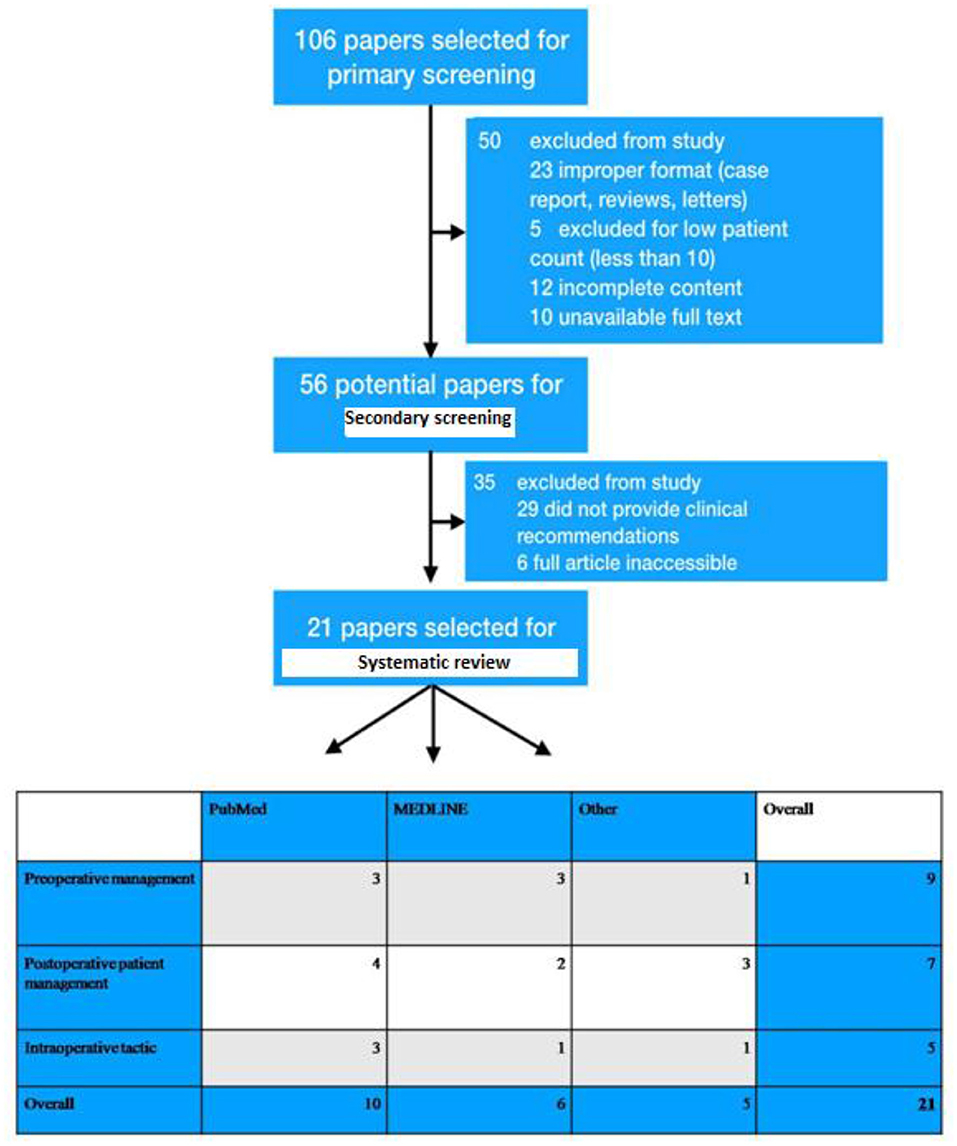
Figure 1. Depiction of exclusion and inclusion criteria of patients who were undergone breast reconstruction and total 106 papers were primarily screened with respect to actuality, publication date, access to article text, content, number of patients, and complete flap-loss rate. Primary screening was executed and selected 56 research papers for secondary screening. Consequently, a two-stage screening process was performed and selected 21 research papers for this study.
Data Extraction
According to international standards provided by the “systematic review of observational Studies in epidemiology”, the selection and categorization of data was performed according to the following criteria: number of patients included in the study, amount of flap transfers performed, incidence of complications, type of surgical intervention, patient age, year and type of publication, recommendations on patient management. Three main groups for proper categorization were derived as per the preoperative preparation, postoperative patient management, and choice of intraoperative therapy (Figure 2). The results of these three separate categories were reviewed and analyzed separately. Systematically, the crucial inferences were addressed in this review which might be beneficial to surgeons during the patient management during or after the breast reconstruction with DIEP flaps.
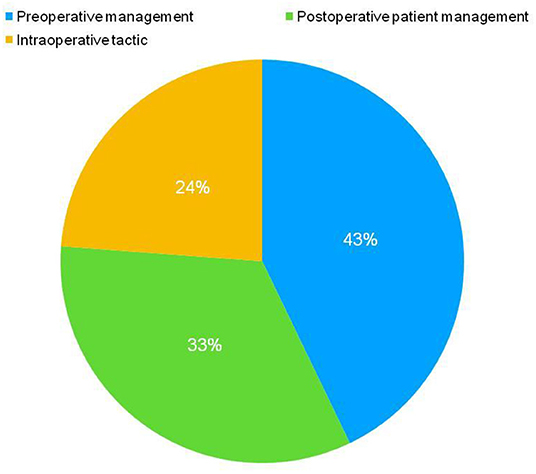
Figure 2. Three main groups were chosen for proper categorization for further inferences to delineate the efficient patient management in preoperative period during DIEP flap-based breast reconstruction. This categorization was performed according to the preoperative preparation, postoperative patient management, and choice of the intraoperative therapy.
Results
Preoperative Period
The preoperative period was overviewed by ascertaining the total 9 clinical studies in order to describe the effective patient management. A literature review of these studies outlining clinical recommendations on patient management was delineated in the preoperative period mainly in the patients undergoing breast reconstruction with a DIEP-flap.
According to previous studies, patient preparation is one of the most important factors, which could influence postoperative rehabilitation and incidence of complications (14–16). Clinical manifestations of postoperative complications are dependent on the quality of preoperative patient preparation. A plethora of clincial reports deciphered the correlation between low perioperative morbidity rates and proper “preoperative patient preparation” for the surgical treatment and postoperative rehabilitation. Hence, preoperative preparation should ascertain the “anatomical and morphological aspects of the donor and recipient sites” (7, 17–19). The success of a DIEP flap-based breast reconstruction depends on several factors; For instance, the perfusion of transferred flap is the most important factor that determines postoperative complications. Standards of perfusion monitoring of DIEP-flap should be carried out by observing temperature, vascular pulse, vessel diameter correlation, capillary response prior to surgical intervention (7, 14, 20–22). To decrease the overall complication rate, it is necessary to understand the “dynamics and anatomy of blood flow” through the deep and superior inferior epigastric systems (7). In case of dominant flap perfusion through the superficial inferior epigastric system (SIEA system), the dissecting SIEA artery during flap mobilization can increase the risk of partial DIEP-flap necrosis (23). The number of perforators available for mobilization plays a crucial role in flap viability. Comparatively, decline in the incidence of complications was observed in breast reconstructions with the DIEP-flaps based on two or more perforators used for anastomosis, as well as two and more veins (22).
Minqiang et al. have reported 22 cases of breast reconstruction with DIEP-flap. The preoperative visualization in these patients was conducted via a multidetector spiral computed tomographic angiography (MSCT-angiography). The study showed minimal complication rates including 0% of severe complications, and less than 5% postoperative complications with light and medium severity (24). Innovations in the preoperative patient visualization techniques and protocols prior to breast reconstruction with the DIEP-flap are associated with a lower overall operative risk, as well as a lower rate of perioperative complications. Preoperative CT-angiography can minimize operative time, less anterior abdominal wall morbidity subsequently enhance the conditions for a surgical intervention. Recently, CT-visualization is used not only to assess the quality, quantity, and functionality of the perforator vessels, but also to assess the suitability of flap tissue, which is available for mobilization (25). The algorithm “Volumetric Planning” reported by Chang & Ooi showed significantly impressive results in the flap preparation and preoperative availability for assessing mobilization flap volume prior to surgical procedure. The incidence of postoperative complications is statistically less in case of proper preoperative flap perfusion zone analysis (26, 27). We recommend performing a thorough preoperative visualization, mapping, and volume assessment to achieve the best postoperative results during DIEP flap-based breast reconstruction.
A significant amount of observational studies performed by reconstructive and plastic surgeons have concluded that adequate preoperative planning is an inseparable part of a successful surgical intervention to promote the complication-free postoperative period (25, 28). Different examination methods are recommended by different authors including CT-visualization, MRI-angiography, pPCF, and ultrasound-dopplerography (29–31).
Adequate preoperative patient preparation includes the evaluation and modification of risk factors. Previous observational studies have examined a total 758 cases of breast reconstruction with the DIEP-flaps, and underlined the importance of the evaluation of potential risk factors prior to the surgical intervention. The main etiopathogenetic factor in the manifestation of postoperative complications after a successful surgical reconstruction is “obesity”. A high BMI is one of the significant factors which can confer to a higher incidence of complication rate in both the donor and recipient sites (14). Despite this fact, the DIEP-flap is still the method of choice, because this kind of procedure has been associated with a statistically low incidence of complications in the patients undergoing breast reconstruction, when compared to other reconstruction methods (21). Nahabedian et al. showed that smoking and patient age are not the major risk factors in the pathogenesis of partial necrosis and venous thrombosis after the breast reconstruction; the main cause of these complications is overweight in these patients (32). Even though there are several International Society of Aesthetic Plastic Surgery (ISAPS) recommendations pertinent to the importance of smoking refusal at least two weeks prior to surgery, there are no statistically significant reports describing the “loss of DIEP-flap, microvascular complications or partial fat necrosis” in the patients with smoking and underwent breast reconstruction. On the other hand, the smoking patients exhibit a higher incidence of postoperative abdominal weakness and infection. Patients with a “pack/year index of 10 and more” are considered to be at the high risk of gaining postoperative complications. Surgery is not recommended for these patients (33). In their observational study, Selber et al. showed a correlation between certain risk factors and complication occurrence. For instance, the prior radiation therapy was consistent with seroma formation in the postoperative period in DIEP-flap based breast reconstruction (34).
Hormonal and chemotherapy prior to the surgical intervention of breast reconstruction with the DIEP-flap could invoke a higher incidence of vascular complications in the patient groups (35). It is a proven fact that the administration of tamoxifen prior to the surgery typically has a significant impact in inducing microvascular complications. Therefore, it is recommended to avoid administration of tamoxifen 14 days prior to the surgical intervention, and resume the administration of this drug after surgery (36).
Intraoperative Tactic
There were a total of 5 studies that overviewed 937 flaps and these reports can ascertain the intraoperative breast reconstruction and patient management. The mean complication rate was 2.9%, and mean complete flap loss rate was 1.2%. A literature review has been performed as a secondary procedure to ascertain the patient management strategies in the intraoperative period during breast reconstruction with DIEP-flap based surgical intervention.
Temperature control is another significant factor in the patient management during DIEP flap-based surgery. Previous studies reported a statistically significant correlation between “hypothermia” with a higher risk of incidence of postoperative complications (37, 38). Intraoperatively, the patients are subdued to longer periods of exposition, which predetermines hypothermia. Moreover, the anesthesia-related interventions can influence the thermoregulatory mechanisms of the patient (37, 38). For instance, the increased risk of intraoperative hypothermia could cause a higher risk of intraoperative complications; therefore it is another significant strategy to control the intraoperative patient temperature during the surgical intervention of breast reconstruction and the patient temperature should not be less than 35°C. During flap mobilization, it is recommended to monitor the patient's temperature and persistently maintained 37°C (37).
A previous study analyzed the adequacy of different intraoperative anticoagulation regimen during surgical intervention of breast reconstruction (39). This study reported that adequate heparin therapy with low-molecular-weight heparin (LMWH) is necessary as a precaution to prevent vascular thrombosis. However, the intraoperative heparin administration did not show any statistically significant influence on the microvascular anastomosis competence (level of evidence 2b). The significance of LMWH administration at the time of postoperative period is to minimize the vascular complications after breast reconstruction. Furthermore, the administration of dextran is strictly prohibited due to the complete flap loss as observed in these studies (40).
Fibrin glue usage during the surgical intervention has significant implications as a stabilizing agent to minimize the incidence of perioperative complications, and also reduce the rate of complete flap loss. Only 0.9% of complete flap loss was observed out of the total 301 transferred flaps. Fibrin glue is useful as a microvascular anastomosis stabilizing agent and it allows a significant decrease in the perfusion and microvascular-related complications, consequently improving the overall flap viability (41).
Enajat et al. delineated the Swedish-Australian microsurgical intervention of 564 cases of breast reconstruction with the DIEP flaps and suggested several useful conclusions for executing the proper intraoperative technique for patient management (42). This study recommended the completion of two venous anastomoses per flap instead of just one, because this kind of approach can minimize the risk of acquiring venous complications. In order to reduce the incidence of venous complications during breast reconstruction with the DIEP-flap, it has been recommended performing “venous superdrainage” during surgery. The significant goal of this method is to provide a back-up drainage system via a secondary venous anastomosis between the “superficial flap venous system” and a “recipient vein” (thoracodorsal vein, lateral pectoralis vein, intercostal vein, medial subcutaneous vein). The rate of venous congestion in cases of performing a venous superdrainage is significantly lower when compared to the patient cases without a secondary venous outflow substrate (43).
The prevalence of fat necrosis in the recipient zone is another postoperative complication elicited due to the variability in the diameter of the anastomosed vessels after breast reconstruction with DIEP flap (43–46). Thus, the importance of vessel diameter conformity in the reduction of postoperative complications has been observed by several authors (41, 44–47). Furthermore, the donor site complications can play a significant role in the postoperative morbidity after the breast reconstruction with DIEP-flap (48). In order to avoid donor site complications, it is recommended to follow certain sequential actions during surgical intervention. For instance, the intraoperative direction of muscular nerve branches innervating the rectus abdominis muscle can facilitate their preservation, which further helps to minimize postoperative morbidity of the donor site. A significant risk factor of anterior abdominal wall weakness in the postoperative period is intraoperative bilateral mobilization of the DIEP-flap (32, 49).
A few prospective randomized clinical studies reported a correlation between quality and quantity of infusion and the risk of developing postoperative complications (50–52). They analyzed 354 cases of microsurgical breast reconstruction with strictly regulated infusion tactics. Only 0.8% of complete flap loss was observed and minimal complication rate also observed which was due to the implementation of an optimal infusion tactic i.e. crystalloid infusion rate between 3.5 ml−6 ml/kg per hour in the first 24-h postoperative period (level of evidence 2b) (41, 53). Crystalloid infusion should not exceed 130 ml/kg in the first 24-h postoperative period. Infusion volume in the intraoperative period is derived from overall biological fluid loss, a parameter which should be counted for the levels of intravenous infusion (54).
Previous studies deciphered the intraoperative evaluation of perfusion dynamics in the revascularized flap (55, 56). The instrumental intraoperative visualization of flap perfusion, as a technique could help to evaluate flap viability as well as to predict the negative impact of zones with reduced tissue perfusion. Application of the intraoperative dynamic infrared tomography (DIRT) reported typically fruitful results during the assessment of quality of flap perfusion (57). The quality of flap perfusion defines the consequent surgical tactic, including, but not limited to flap volume correction. Intraoperative thermography can significantly minimize the risk of postoperative perfusion-related complications (58, 59). In addition, laser-assisted angiography with indocyanine green is another method applied to evaluate the intraoperative flap perfusion. This method is used to visualize vessel anatomy in the preoperative period, and also to evaluate perfusion dynamics of the transferred free flap, as well as quality and stability of the microvascular anastomosis (56, 60).
Furthermore, it is crucial to evaluate the risk of developing postoperative complications in patients, who received a blood transfusion during surgery. The intraoperative transfusion is required directly based on the length of surgery and volume of reconstructive intervention. Appleton et al. recorded a higher complication risk in the patients with bilateral breast reconstruction with DIEP-flap, as well as in patients with a prolonged operative time, who received hemotransfusion (61).
Postoperative Period
In order to adequately analyze the proper patient management tactic in the postoperative period, a total of 3,335 cases of breast reconstruction with the DIEP-flap were included in the study. The mean complication rate in the selected cases was 4.44% whereas the “severe complications rate”, and “complete flap loss rate” were 3.73, and 2.48% respectively.
In addition, adequate pain management is an inseparable part of postoperative treatment. Patients undergoing the breast reconstruction using DIEP-flap can be segregated into two different categories based on the pain threshold: “patients with a normal pain threshold', and ‘patients with a low pain threshold”. The first group (70–75%) requires patient-controlled treatment (PCT) for mitigating pain in the initial two days after surgery followed by the administration of oral analgesics (62, 63). The second patient group (25–30%) consists of patients, who need a longer course of PCT- upto three days; this group is characterized by a longer hospitalization stay and a lengthier rehabilitation period. Furthermore, the patients after a simultaneous mastectomy with breast reconstruction more often fall into the second patient group (62). In order to minimize the requirement of narcotic analgesics administration, the authors recommend different pain management strategies postoperatively after breast reconstruction (63). Blockade of the transverse abdominal space with the ultrasound control can eliminate the requirement of narcotic analgesic administration. Catheterization of the donor zone for local anesthetic administration can allow quality anesthesia for up to 72 h without the need of narcotic analgesics (64). Epidural anesthesia is another significant pain control strategy during surgery, which allows adequate pain management without the intravenous administration and PCT. As per several studies, the most innovative anesthetic methods are local anesthesia applied during blockade and catheterization (65, 66).
The loss of hemoglobin is one of the most important complications in postoperative patient management. The length of hospitalization, overall blood loss, and blood transfusions considerably exacerbate the complications in postoperative period. Hemoglobin level is one of the most important indicators, which is an indicator of the hemodynamic and rheologic balance of the blood (67). A direct correlation was reported between anemia and the rate of postoperative complications during autologous breast reconstruction. This report also delineated a significant risk of developing postoperative complications observed in the patients with hemoglobin levels less than 100 g/l, which is not yet considered as an anemic state (67). Despite this, the authors conclude that hemoglobin levels less than 100 g/l, bilateral breast reconstruction, simultaneous reconstruction, and blood transfusion are statistically significant and indicate postoperative complication development (68). The length of surgical intervention defines the loss of hemoglobin. For instance, one hour of surgery is accompanied by an average loss of 0.25 g/l of hemoglobin. Intraoperative complications conferred substantial rise in the average loss of 0.45 g/l hemoglobin. Each gram of the removed tissue amounts to a corresponding average of loss of 0.001 g/l hemoglobin. For instance, tranexam (TXM) is an effective drug, which prevents significant hemoglobin loss in the postoperative period. TXM administration could mitigate the average blood loss by 18.2 ml/kg (p = 0.001); therefore this drug can significantly be reported to increase flap survival prognosis (69). The influence of TXM administration on microvascular anastomosis was emphasized by Zhang and Wieslander. As per this study, the administration of a clinical dose and double dose of tranexam (14 mg/kg and 28 mg/kg accordingly) has not exhibited a statistically significant effect on thrombus formation and bleeding in microvascular anastomosis (70).
Fluid loss compensation is primarily performed according to volumetric parameters. Total 354 cases of breast reconstruction with free abdominal flaps were examined; these reports showed the optimal rate of infusion parameters for the perioperative period (51, 52). The optimal measure of crystalloid infusion should be between 3.5 ml-6 ml/kg per hour to replenish the fluid loss. Blood transfusion should be performed according to clinical readings and if the patient's hemoglobin levels are less than 70 g/l (level of evidence 2b). Crystalloid infusion rate should not exceed 130 ml/kg a day. Existing data does not support the usage of albumin over synthetic colloid solutions (52). Proper strategies of fluid infusion performed in this study to reduce the overall complication rate to 4.1%, where the complete flap loss only 0.8%.
Maintaining flap perfusion is another significant surgical priority after breast reconstruction. Perfusion control and pharmacological support are necessary in order to maintain flap perfusion effectively. Flap perfusion control is performed using dynamic infrared thermography (DIRP). This strategy allows surgeons to detect perfusion pathology at the earliest; therefore timely management of complications can be executed. Early hypoperfusion identification could allow a conservative approach in preventing further development of serious complications, such as border necrosis, wound dehiscence, adipose necrosis, and flap loss (71). Standard strategies such as angiography and ultrasound dopplerography (USDG) could be used to evaluate the anastomosed vessels pertaining to the quality of flap perfusion, which can also be analyzed by subjective methods. The application of Doppler-catheter “Cook-Swartz” implantation has shown a significant decline in false-diagnosis of complications and allowed surgeons to detect early microvascular complications. This method is referred to as a new method, which could be useful to predict and prevent microvascular complications by ascertaining the tissue oxygen saturation (StO2, ΔStO2), and saturation change speed (ΔStO2/Δt). Rate of change of ΔStO2/Δt by −20% in 30 min preceded vascular complication manifestation (72, 73). This kind of monitoring can be executed with the aid of a “T.Ox Tissue Oximeter” and it can predict the development of vascular complications 60 min prior to their true manifestation (74).
The flap perfusion quality through the pharmacological therapies is an important aspect for the effective postoperative patient management. Previous studies deciphered the effectiveness of heparin medication in the postoperative period. Among 493 cases of free flap reconstruction, a higher risk of complication development was observed in the patient cases receiving inadequate administration of heparin therapy. Postoperative subcutaneous administration of heparin significantly can mitigate the risk of microvascular thrombosis. There is no statistically significant data in favor of systemic administration of heparin when performing free flap transfer (level of evidence 2b). The administration of dextran as a prophylaxis for venous thrombosis is contraindicated due to a higher incidence of microvascular and perfusion-related complications when using this drug (level of evidence 1b) (39, 75).
The need for radiation therapy after a mastectomy and breast reconstruction has been reported to be varied between 25–30% (76). A majority of surgeons are very concerned to use radiation therapy onto the “area of anastomosis” as the radiation could enhance the development of associated complications. Chatterjee et al. conducted a clinical study, which analyzed the rate of flap volume loss depending on dosage of radiation. The results of these studies showed that there is no statistically significant correlation between radiation therapy on the flap and flap volume loss (77). It is therefore not recommended to postpone radiation therapy. Above all the complications during DIEP flap-breast reconstruction were deciphered vividly in the following (Table 1).

Table 1. The patient management strategies in preoperative, intraoperaitve, and postoperative periods in order to minimize different complications observed in the DIEP flap-based breast reconstruction.
Discussion
In this study, we reviewed a total of 6,475 cases of reconstructive interventions, covered in 21 studies. The mean complication rate in these studies on patient management in breast reconstructive surgery was 5.08% (78).
Patient body temperature should be properly monitored at the time of surgical intervention. This physiological parameter is often disregarded in the operating room. There is a proven correlation between hypothermia and the increased risk of complications. Patients during surgery are prone to the prolonged exposition, which predisposes them to hypothermia. Anesthesia administration could have a direct effect on the patient's thermoregulatory mechanisms, and the administration of anesthesia can modulate heat emission and heat production during surgery. Therefore, persistent control of the core body temperature should be implemented. As per the analysis of all the above reports systematically, we recommend the maintenance of a patient's core body temperature more than 35°C at all times during DIEP flap-based breast reconstruction. A mean temperature of over >37°C is recommended during flap transfer and vascular anastomosis. The mean recommended core body temperature in the operating room to prevent hypothermia should be 24°C. In all cases, it is recommended to warm patients prior to surgical intervention and 24–48 h postoperatively, preventing difference between peripheral and core body temperature of more than >2°C (54) (Table 2).
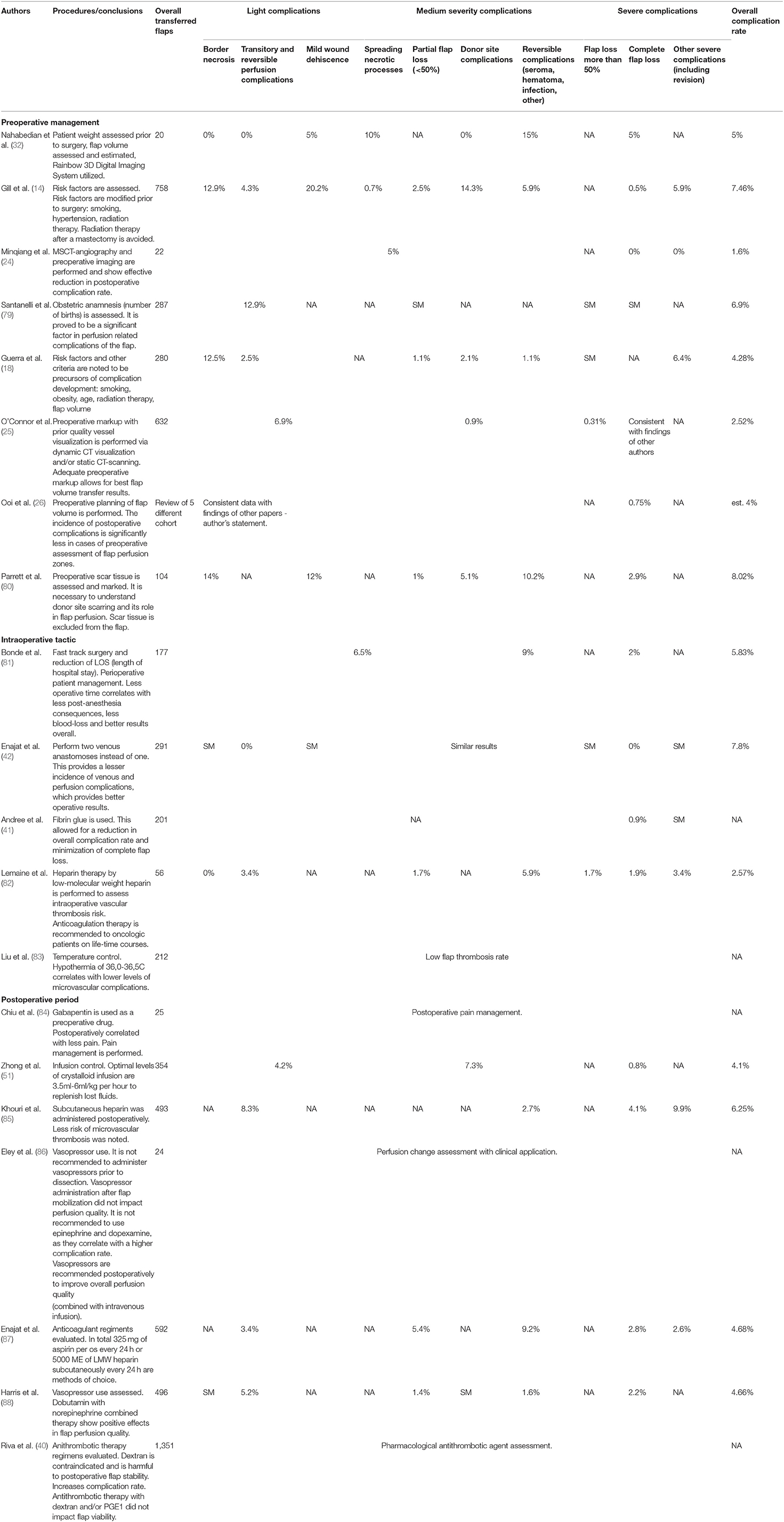
Table 2. Different clinical reports of overall complication rate (light/medium/severe) in the patient management in the DIEP flap-based breast reconstruction.
Conclusion
Breast reconstruction using DIEP-flap based surgical intervention is a specific choice in order to foster the structural restoration of anterior chest wall anatomy after a mastectomy. This surgery represents an important stage in complete functional, emotional, psycho-social, and aesthetic patient rehabilitation. Implant-based reconstruction of breast results in the associated risks in perioperative period and complications such as infection, implant loss due to capsular contractures during postoperative periods. This can enhance the rate of failure of breast reconstruction by 30% and implant loss up to 4 to 18% among all the prosthetic breast reconstructions making the patients, surgeons hard to take appropriate decisions in order to eliminate these complications. Hence, a successful surgical reconstruction without complications is the priority of any surgeon. This study provides evidence-based recommendations on patient management in the perioperative period, and postoperative periods. These recommendations have a strong potential to improve clinical results when performing breast reconstruction with the DIEP-flap, and will serve as a basis for new prospective studies on topics covered in this study. Completing a series of patient management strategies may facilitate a high-quality surgery with minimal blood loss and minimal postoperative complications, which further correlates with a better patient rehabilitation and improved overall quality of life.
Limitations and Strengths
This study described an in-depth review of existing associations between patient management and clinical characteristics, and associated postsurgical complications pertinent to the DIEP flap-based breast reconstruction. For the first time a large stratification and systematization of data was deciphered pertinent to the DIEP flap-based surgical intervention. The main limitation of this study is the lack of direct statistical comparison, mainly due to the largely differing reporting styles of the selected publications. Nonetheless, our review offers a valuable and important insight for the application of DIEP flap-based surgery to minimize the postsurgical complications in clinical practice.
Author's Note
Breast reconstruction using DIEP-flap can be a significant surgical choice for the tissue restoration of anterior chest wall anatomy after a mastectomy. This systematic review deciphers the evidence-based recommendations on patient management in the perioperative period after DIEP flap-breast reconstruction.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
KC, MS, NB, DS, YG, JLi, JZ, IR, OS, RF, JLiu, and PL: conceptualized the study. NB, KC, DS, YG, MS, and PL: performed the literature analysis and wrote the original manuscript draft. NB, PL, RF, and KC: revised, edited, and extended the final draft. All authors have reviewed and approved the manuscript before submission.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81703158).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hofer SO, Damen TH, Mureau MA, Rakhorst HA, Roche NA. A critical review of perioperative complications in 175 free deep inferior epigastric perforator flap breast reconstructions. Ann Plast Surg. (2007) 59:137–42. doi: 10.1097/01.sap.0000253326.85829.45
2. Chen K, Lu P, Beeraka NM, Sukocheva OA, Madhunapantula SV, Liu J, et al. Mitochondrial mutations and mitoepigenetics: focus on regulation of oxidative stress-induced responses in breast cancers. In: Seminars in Cancer Biology. (2020). doi: 10.1016/j.semcancer.2020.09.012
3. Blondeel PN, Vanderstraeten G, Monstrey S, Van Landuyt K, Tonnard P, Lysens R, et al. The donor site morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br J Plast Surg. (1997) 50:322–30. doi: 10.1016/S0007-1226(97)90540-3
4. Geddes CR, Morris SF, Neligan PC. Perforator flaps: evolution, classification, and applications. Ann Plast Surg. (2003) 50:90–9. doi: 10.1097/00000637-200301000-00016
5. Dancey A, Blondeel PN. Technical tips for safe perforator vessel dissection applicable to all perforator flaps. Clin Plast Surg. (2010) 37:593–606. doi: 10.1016/j.cps.2010.06.008
6. Martinez CA, Reis SM, Rednam R, Boutros Boutros SG, The outpatient DIEP: safety and viability following a modified recovery protocol. Plastic Reconstr Surg Global Open. (2018) 6:e1898. doi: 10.1097/GOX.0000000000001898
7. Tran NV, Buchel EW, Convery PA. Microvascular complications of DIEP flaps. Plast Reconstr Surg. (2007) 119:1397–405. doi: 10.1097/01.prs.0000256045.71765.96
8. Moradi P, Durrant C, Glass GE, Askouni E, Wood S, Rose V. SIEA flap leads to an increase in abdominal seroma rates compared to DIEP flap for breast reconstruction. Eur J Plast Surg. (2011) 34:87–91. doi: 10.1007/s00238-010-0470-0
9. Taylor GI, Corlett RJ, Dhar SC, Ashton MW. The anatomical (angiosome) and clinical territories of cutaneous perforating arteries: development of the concept and designing safe flaps. Plast Reconstr Surg. (2011) 127:1447–59. doi: 10.1097/PRS.0b013e318208d21b
10. Man L-X, Selber JC, Serletti JM. Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast Reconstr Surg. (2009) 124:752–64. doi: 10.1097/PRS.0b013e31818b7533
11. Wan DC, Tseng CY, Anderson-Dam J, Dalio AL, Crisera CA, Festekjian JH. Inclusion of mesh in donor-site repair of free TRAM and muscle-sparing free TRAM flaps yields rates of abdominal complications comparable to those of DIEP flap reconstruction. Plast Reconstr Surg. (2010) 126:367–74. doi: 10.1097/PRS.0b013e3181de1b7e
12. Selber JC, Nelson J, Fosnot J, Goldstein J, Bergey M, Sonnad SS, et al. A prospective study comparing the functional impact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: Part I. Unilateral reconstruction. Plast Reconstr Surg. (2010) 126:1142–53. doi: 10.1097/PRS.0b013e3181f02520
13. Selber JC, Fosnot J, Nelson J, Goldstein J, Bergey M, Sonnad S, et al. A prospective study comparing the functional impact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: Part II. Bilateral reconstruction. Plast Reconstr Surg. (2010) 126:1438–53. doi: 10.1097/PRS.0b013e3181ea42ed
14. Gill PS, Hunt JP, Guerra AB, Dellacroce FJ, Sullivan SK, Boraski J, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg. (2004) 113:1153–60. doi: 10.1097/01.PRS.0000110328.47206.50
15. Bajaj AK, Chevray PM, Chang DW. Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg. (2006) 117:737–46. doi: 10.1097/01.prs.0000200062.97265.fb
16. KRIZEK TJ TANI T, DESPREZ JD, KIEHN CL. Experimental transplantation of composite grafts by microsurgical vascular anastomoses. Plast Reconstr Surg. (1965) 36:538–46. doi: 10.1097/00006534-196511000-00005
17. Blondeel P. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg. (1999) 52:104–11. doi: 10.1054/bjps.1998.3033
18. Guerra AB, Metzinger SE, Bidros RS, Rizzuto RP, Gill PS, Nguyen AH, et al. Bilateral breast reconstruction with the deep inferior epigastric perforator (DIEP) flap: an experience with 280 flaps. Ann Plast Surg. (2004) 52:246–52. doi: 10.1097/01.sap.0000110529.37143.96
19. Granzow JW, Levine JL, Chiu ES, Allen RJ. Breast reconstruction with the deep inferior epigastric perforator flap: history and an update on current technique. J Plast Reconstr Aesthet Surg. (2006) 59:571–9. doi: 10.1016/j.bjps.2006.01.004
20. Healy C, Allen Sr RJ. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg. (2014) 30:121–6. doi: 10.1055/s-0033-1357272
21. Ochoa O, Chrysopoulo M, Nastala C, Ledoux P, Pisano S. Abdominal wall stability and flap complications after deep inferior epigastric perforator flap breast reconstruction: does body mass index make a difference? Analysis of 418 patients and 639 flaps. Plast Reconstr Surg. (2012) 130:21e−33e. doi: 10.1097/PRS.0b013e3182547d09
22. Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. (1994) 32:32–8. doi: 10.1097/00000637-199401000-00007
23. Heitmann C, Felmerer G, Durmus C, Matejic B, Ingianni G. Anatomical features of perforator blood vessels in the deep inferior epigastric perforator flap. Br J Plast Surg. (2000) 53:205–8. doi: 10.1054/bjps.1999.3257
24. Minqiang X, Lanhua M, Jie L, Dali M, Jinguo L. The value of multidetector-row CT angiography for pre-operative planning of breast reconstruction with deep inferior epigastric arterial perforator flaps. Br J Radiol. (2010) 83:40–3. doi: 10.1259/bjr/29140440
25. O'Connor EF, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland surgery. (2016) 5:93. doi: 10.3978/j.issn.2227-684X.2015.05.17
26. Ooi AS, Chang DW. Discussion: volumetric planning using computed tomographic angiography improves clinical outcomes in DIEP flap breast reconstruction. Plast Reconstr Surg. (2016) 137:781e−2e. doi: 10.1097/PRS.0000000000002084
27. Lee K-T, Mun G-H. Volumetric planning using computed tomographic angiography improves clinical outcomes in DIEP flap breast reconstruction. Plast Reconstr Surg. (2016) 137:771e−80e. doi: 10.1097/PRS.0000000000002045
28. Schaverien MV, McCulley SJ. Contrast-enhanced magnetic resonance angiography for preoperative imaging in DIEP flap breast reconstruction. In: Breast Reconstruction. edn.: Springer; (2016). p. 163–70. doi: 10.1007/978-3-319-18726-6_14
29. Yang X, Miller MJ, Friel HT, Slijepcevic A, Knopp MV. Perforator phase contrast angiography of deep inferior epigastric perforators: a better preoperative imaging tool for flap surgery than computed tomographic angiography? Invest Radiol. (2017) 52:334–42. doi: 10.1097/RLI.0000000000000348
30. Dražan L, Lombardo G. Tridimensional doppler assessment: a reliable, non-invasive and cost-effective method for preoperative perforator assessment in DIEP flap. Acta Chir Plast. (2016) 58:60–3.
31. Teunis T, van Voss MH, Kon M, van Maurik JM. CT-angiography prior to DIEP flap breast reconstruction: a systematic review and meta-analysis. Microsurgery. (2013) 33:496–502. doi: 10.1002/micr.22119
32. Nahabedian MY, Momen B, Galdino G, Manson PN, Namnoum JD. Breast reconstruction with the free TRAM or DIEP flap: Patient selection, choice of flap, and outcome. Plast Reconstr Surg. (2002) 110:466–75. doi: 10.1097/00006534-200208000-00015
33. Chang DW, Reece GP, Wang B, Robb GL, Miller MJ, Evans GR, et al. Effect of smoking on complications in patients undergoing free TRAM flap breast reconstruction. Plast Reconstr Surg. (2000) 105:2374–80. doi: 10.1097/00006534-200006000-00010
34. Selber JC, Kurichi JE, Vega SJ, Sonnad SS, Serletti JM. Risk factors and complications in free TRAM flap breast reconstruction. Ann Plast Surg. (2006) 56:492–7. doi: 10.1097/01.sap.0000210180.72721.4a
35. Disa JJ. Discussion: Tamoxifen increases the risk of microvascular flap complications in patients undergoing microvascular breast reconstruction. Plast Reconstr Surg. (2012) 129:315–6. doi: 10.1097/PRS.0b013e31823aeccd
36. Kelley BP, Valero V, Yi M, Kronowitz SJ. Tamoxifen increases the risk of microvascular flap complications in patients undergoing microvascular breast reconstruction. Plast Reconstr Surg. (2012) 129:305. doi: 10.1097/PRS.0b013e31823ae86c
37. Gardiner M, Nanchahal J. Strategies to ensure success of microvascular free tissue transfer. J Plast Reconstr Aesthet Surg. (2010) 63:e665–73. doi: 10.1016/j.bjps.2010.06.011
38. Young VL, Watson ME. Prevention of perioperative hypothermia in plastic surgery. Aesthetic Surg J. (2006) 26:551–71. doi: 10.1016/j.asj.2006.08.009
39. Disa JJ, Polvora VP, Pusic AL, Singh B, Cordeiro PG. Dextran-related complications in head and neck microsurgery: do the benefits outweigh the risks? A prospective randomized analysis. Plast Reconstr Surg. (2003) 112:1534–9. doi: 10.1097/01.PRS.0000083378.58757.54
40. Riva FM, Chen YC, Tan NC, Lin PY, Tsai YT, Chang HW, et al. The outcome of prostaglandin-E1 and dextran-40 compared to no antithrombotic therapy in head and neck free tissue transfer: analysis of 1,351 cases in a single center. Microsurgery. (2012) 32:339–43. doi: 10.1002/micr.21958
41. Andree C, Munder BI, Behrendt P, Hellmann S, Audretsch W, Voigt M, et al. Improved safety of autologous breast reconstruction surgery by stabilisation of microsurgical vessel anastomoses using fibrin sealant in 349 free DIEP or fascia-muscle-sparing (fms)-TRAM flaps: a two-centre study. Breast. (2008) 17:492–8. doi: 10.1016/j.breast.2008.03.010
42. Enajat M, Rozen WM, Whitaker IS, Smit JM, Acosta R. A single center comparison of one versus two venous anastomoses in 564 consecutive DIEP flaps: investigating the effect on venous congestion and flap survival. Microsurgery. (2010) 30:185–91. doi: 10.1002/micr.20712
43. Tutor EG, Auba C, Benito A, Rábago G, Kreutler W. Easy venous superdrainage in DIEP flap breast reconstruction through the intercostal branch. J Reconstr Microsurg. (2002) 18:595–8. doi: 10.1055/s-2002-35098
44. Sailon AM, Schachar JS, Levine JP. Free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps for breast reconstruction: a systematic review of flap complication rates and donor-site morbidity. Ann Plast Surg. (2009) 62:560–3. doi: 10.1097/SAP.0b013e31819faf0d
45. Kroll SS. Fat necrosis in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. (2000) 106:576–83. doi: 10.1097/00006534-200009010-00008
46. Hamdi M, Blondeel P, Van Landuyt K, Monstrey S. Algorithm in choosing recipient vessels for perforator free flap in breast reconstruction: the role of the internal mammary perforators. Br J Plast Surg. (2004) 57:258–65. doi: 10.1016/j.bjps.2003.12.004
47. Cina A, Salgarello M, Barone-Adesi L, Rinaldi P, Bonomo L. Planning breast reconstruction with deep inferior epigastric artery perforating vessels: multidetector CT angiography versus color Doppler US. Radiology. (2010) 255:979–87. doi: 10.1148/radiol.10091166
48. Rozen WM, Ashton MW, Murray AC, Taylor GI. Avoiding denervation of rectus abdominis in DIEP flap harvest: the importance of medial row perforators. Plast Reconstr Surg. (2008) 122:710–6. doi: 10.1097/PRS.0b013e318180ed8b
49. Futter CM, Webster MHC, Hagen S, Mitchell SL. A retrospective comparison of abdominal muscle strength following breast reconstruction with a free TRAM or DIEP flap. Br J Plast Surg. (2000) 53:578–83. doi: 10.1054/bjps.2000.3427
50. Clark JR, McCluskey SA, Hall F, Lipa J, Neligan P, Brown D, et al. Predictors of morbidity following free flap reconstruction for cancer of the head and neck. Head Neck. (2007) 29:1090–101. doi: 10.1002/hed.20639
51. Zhong T, Neinstein R, Massey C, McCluskey SA, Lipa J, Neligan P, et al. Intravenous fluid infusion rate in microsurgical breast reconstruction: important lessons learned from 354 free flaps. Plast Reconstr Surg. (2011) 128:1153–60. doi: 10.1097/PRS.0b013e318221da56
52. Boldt J. New Light on Intravascular Volume Replacement Regimens: What Did We Learn from the Past Three Years?[RETRACTED]. Anesthesia Analgesia. (2003) 97:1595–604. doi: 10.1213/01.ANE.0000089961.15975.78
53. Rubino C, Coscia V, Cavazzuti A, Canu V. Haemodynamic enhancement in perforator flaps: The inversion phenomenon and its clinical significance. A study of the relation of blood velocity and flow between pedicle and perforator vessels in perforator flaps. J Plast Reconstr Aesthet Surg. (2006) 59:636–43. doi: 10.1016/j.bjps.2005.07.010
54. Hagau N, Longrois D. Anesthesia for free vascularized tissue transfer. Microsurgery. (2009) 29:161–7. doi: 10.1002/micr.20584
55. Tindholdt TT, Saidian S, Pripp AH, Tønseth KA. Monitoring microcirculatory changes in the deep inferior epigastric artery perforator flap with laser Doppler perfusion imaging. Ann Plast Surg. (2011) 67:139–42. doi: 10.1097/SAP.0b013e3181f3e39b
56. Newman MI, Samson MC. The application of laser-assisted indocyanine green fluorescent dye angiography in microsurgical breast reconstruction. J Reconstr Microsurg. (2009) 25:021–6. doi: 10.1055/s-0028-1090617
57. Thiessen FE, Tondu T, Cloostermans B, Dirkx YA, Auman D, Cox S, et al. Dynamic InfraRed Thermography (DIRT) in DIEP-flap breast reconstruction: A review of the literature. Eur J Obstetr Gynecol Reproduc Biol. (2019) 242:47–55. doi: 10.1016/j.ejogrb.2019.08.008
58. de Weerd L, Weum S, Mercer JB. The value of dynamic infrared thermography (DIRT) in perforator selection and planning of free DIEP flaps. Ann Plast Surg. (2009) 63:274–9. doi: 10.1097/SAP.0b013e318190321e
59. de Weerd L, Mercer JB, Setså LB. Intraoperative dynamic infrared thermography and free-flap surgery. Ann Plast Surg. (2006) 57:279–84. doi: 10.1097/01.sap.0000218579.17185.c9
60. Francisco BS, Kerr-Valentic MA, Agarwal JP, Laser-assisted indocyanine green angiography and DIEP breast reconstruction. Plast Reconstr Surg. (2010) 125:116e−8e. doi: 10.1097/PRS.0b013e3181cb67a9
61. Appleton SE, Ngan A, Kent B, Morris SF. Risk factors influencing transfusion rates in DIEP flap breast reconstruction. Plast Reconstr Surg. (2011) 127:1773–82. doi: 10.1097/PRS.0b013e31820cf1dd
62. Bar-Meir ED, Yueh JH, Hess PE, Hartmann CE, Maia M, Tobias AM, et al. Postoperative pain management in DIEP flap breast reconstruction: identification of patients with poor pain control. Eplasty. (2010) 10:e59.
63. Kroll SS, Sharma S, Koutz C, Langstein HN, Evans GR, Robb GL, et al. Postoperative morphine requirements of free TRAM and DIEP flaps. Plast Reconstr Surg. (2001) 107:338–41. doi: 10.1097/00006534-200102000-00006
64. Hivelin M, Wyniecki A, Plaud B, Marty J, Lantieri L. Ultrasound-guided bilateral transversus abdominis plane block for postoperative analgesia after breast reconstruction by DIEP flap. Plast Reconstr Surg. (2011) 128:44–55. doi: 10.1097/PRS.0b013e3182174090
65. Zhong T, Wong KW, Cheng H, Ojha M, Srinivas C, McCluskey SA, et al. Transversus abdominis plane (TAP) catheters inserted under direct vision in the donor site following free DIEP and MS-TRAM breast reconstruction: a prospective cohort study of 45 patients. J Plast Reconstr Aesthet Surg. (2013) 66:329–36. doi: 10.1016/j.bjps.2012.09.034
66. Zhong T, Ojha M, Bagher S, Butler K, Srinivas C, McCluskey SA, et al. Transversus abdominis plane block reduces morphine consumption in the early postoperative period following microsurgical abdominal tissue breast reconstruction: a double-blind, placebo-controlled, randomized trial. Plast Reconstr Surg. (2014) 134:870–8. doi: 10.1097/PRS.0000000000000613
67. Nelson JA, Fischer JP, Grover R, Cleveland E, Erdmann-Sager J, Serletti JM, et al. The impact of anemia on microsurgical breast reconstruction complications and outcomes. Microsurgery. (2014) 34:261–70. doi: 10.1002/micr.22202
68. Lymperopoulos NS, Sofos S, Constantinides J, Koshy O, Graham K. Blood loss and transfusion rates in DIEP flap breast reconstruction. Introducing a new predictor. J Plast Reconstr Aesthet Surg. (2013) 66:1659–64. doi: 10.1016/j.bjps.2013.07.013
69. Murphy GRF, Glass GE, Jain A. The efficacy and safety of tranexamic acid in cranio-maxillofacial and plastic surgery. J Craniofac Surg. (2016) 27:374–9. doi: 10.1097/SCS.0000000000002250
70. Zhang B, Wieslander JB. Influence of early fibrinolysis inhibition on thrombus formation following microvascular trauma. Microsurgery. (1996) 17:278–85. doi: 10.1002/(SICI)1098-2752(1996)17:5<278::AID-MICR8>3.0.CO;2
71. de Weerd L, Miland ÅO, Mercer JB. Perfusion dynamics of free DIEP and SIEA flaps during the first postoperative week monitored with dynamic infrared thermography. Ann Plast Surg. (2009) 62:42–7. doi: 10.1097/SAP.0b013e3181776374
72. Rozen WM, Chubb D, Whitaker IS, Acosta R. The efficacy of postoperative monitoring: a single surgeon comparison of clinical monitoring and the implantable Doppler probe in 547 consecutive free flaps. Microsurgery. (2010) 30:105–10. doi: 10.1002/micr.20706
73. Smit J, Whitaker IS, Liss A, Audolfsson T, Kildal M, Acosta R. Post operative monitoring of microvascular breast reconstructions using the implantable Cook–Swartz doppler system: a study of 145 probes & technical discussion. J Plast Reconstr Aesthet Surg. (2009) 62:1286–92. doi: 10.1016/j.bjps.2008.06.007
74. Keller A. A new diagnostic algorithm for early prediction of vascular compromise in 208 microsurgical flaps using tissue oxygen saturation measurements. Ann Plast Surg. (2009) 62:538–43. doi: 10.1097/SAP.0b013e3181a47ce8
75. Chien W, Varvares MA, Hadlock T, Cheney M, Deschler DG. Effects of aspirin and low-dose heparin in head and neck reconstruction using microvascular free flaps. Laryngoscope. (2005) 115:973–6. doi: 10.1097/01.MLG.0000163539.97485.F4
76. Rogers NE, Allen Allen RJ, Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plast Reconstr Surg. (2002) 109:1919–24. doi: 10.1097/00006534-200205000-00022
77. Chatterjee J, Lee A, Anderson W, Baker L, Stevenson J, Dewar J, et al. Effect of postoperative radiotherapy on autologous deep inferior epigastric perforator flap volume after immediate breast reconstruction. J Br Surg. (2009) 96:1135–40. doi: 10.1002/bjs.6693
78. Pattani KM, Byrne P, Boahene K, Richmon J. What makes a good flap go bad? A critical analysis of the literature of intraoperative factors related to free flap failure. Laryngoscope. (2010) 120:717–23. doi: 10.1002/lary.20825
79. Santanelli F, Longo B, Cagli B, Pugliese P, Sorotos M, Paolini G. Predictive and protective factors for partial necrosis in DIEP flap breast reconstruction: does nulliparity bias flap viability? Ann Plast Surg. (2015) 74:47–51. doi: 10.1097/SAP.0b013e31828d994d
80. Parrett BM, Caterson SA, Tobias AM, Lee BT. DIEP flaps in women with abdominal scars: are complication rates affected? Plast Reconstr Surg. (2008) 121:1527–31. doi: 10.1097/PRS.0b013e31816b14a5
81. Bonde C, Khorasani H, Eriksen K, Wolthers M, Kehlet H, Elberg J. Introducing the fast track surgery principles can reduce length of stay after autologous breast reconstruction using free flaps: a case control study. J Plast Surg Hand Surg. (2015) 49:367–71. doi: 10.3109/2000656X.2015.1062387
82. Lemaine V, McCarthy C, Kaplan K, Mehrara B, Pusic AL, Cordeiro PG, et al. Venous thromboembolism following microsurgical breast reconstruction: an objective analysis in 225 consecutive patients using low-molecular-weight heparin prophylaxis. Plast Reconstr Surg. (2011) 127:1399–406. doi: 10.1097/PRS.0b013e318208d025
83. Liu Y-J, Hirsch BP, Shah AA, Reid MA, Thomson JG. Mild intraoperative hypothermia reduces free tissue transfer thrombosis. J Reconstr Microsurg. (2011) 27:121–6. doi: 10.1055/s-0030-1268211
84. Chiu T, Leung C, Lau E, Burd A. Analgesic effects of preoperative gabapentin after tongue reconstruction with the anterolateral thigh flap. Hong Kong Med J. (2012) 18:30–4.
85. Khouri RK, Cooley BC, Kunselman AR, Landis JR, Yeramian P, Ingram D, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. (1998) 102:711–21. doi: 10.1097/00006534-199809010-00015
86. Eley KA, Young JD, Watt-Smith SR. Epinephrine, norepinephrine, dobutamine, and dopexamine effects on free flap skin blood flow. Plast Reconstr Surg. (2012) 130:564–70. doi: 10.1097/PRS.0b013e31825dbf73
87. Enajat M, Mohammadi MA, Debeij J, van der Hulst RR, Mureau MA. Effect of acetylsalicylic acid on microvascular thrombosis in autologous breast reconstruction. J Reconstr Microsurg. (2014) 30:065–70. doi: 10.1055/s-0033-1356553
Keywords: breast reconstruction, DIEP flap, surgery, patient management, perioperative, intraoperative, postoperative period
Citation: Chen K, Beeraka NM, Sinelnikov MY, Zhang J, Song D, Gu Y, Li J, Reshetov IV, Startseva OI, Liu J, Fan R and Lu P (2022) Patient Management Strategies in Perioperative, Intraoperative, and Postoperative Period in Breast Reconstruction With DIEP-Flap: Clinical Recommendations. Front. Surg. 9:729181. doi: 10.3389/fsurg.2022.729181
Received: 22 June 2021; Accepted: 19 January 2022;
Published: 15 February 2022.
Edited by:
Arshad R. Muzaffar, University of Missouri, United StatesReviewed by:
Olga A. Sukocheva, Flinders University, AustraliaFatih Zor, Wake Forest School of Medicine, United States
Copyright © 2022 Chen, Beeraka, Sinelnikov, Zhang, Song, Gu, Li, Reshetov, Startseva, Liu, Fan and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengwei Lu, bHZwZW5nd2VpMTk4M0Bob3RtYWlsLmNvbQ==; Ruitai Fan, ZmFucnVpdGFpQDEyNi5jb20=
†These authors have contributed equally to this work
 Kuo Chen
Kuo Chen Narasimha M. Beeraka
Narasimha M. Beeraka Mikhail Y. Sinelnikov
Mikhail Y. Sinelnikov Jin Zhang
Jin Zhang Dajiang Song
Dajiang Song Yuanting Gu1
Yuanting Gu1 Junqi Liu
Junqi Liu Ruitai Fan
Ruitai Fan Pengwei Lu
Pengwei Lu