
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 11 January 2023
Sec. Cardiovascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1102742
This article is part of the Research Topic Case Reports in Heart Surgery: 2022 View all 21 articles
Primary cardiac tumors are extremely uncommon in young children and infants. Cardiac myxoma are typically found in the atria, predominately in the left atrium, with relatively few found on the right side, such as in the right ventricle or pulmonary artery. Numerous significant complications, including sudden death, can result from obstruction of the main pulmonary artery trunk and right ventricular outflow tract. Here, we describe the case of a 14-year-old Chinese girl diagnosed with a right ventricular myxoma located in the right ventricle and extended into the main pulmonary trunk. Complete resection of the myxoma and histological confirmation were performed.
Myxomas occur in all age groups, most commonly between the ages of 30 and 60, and are predominantly found in females. Rhabdomyomas and teratomas are the most common tumors found in children, while myxomas and fibroids are less common. They are most commonly found in the left atrium. The right atrium (RA) is the second most frequent place where myxomas arise, constituting about 7%–12% of cases. Only a few cases of myxoma found in the right ventricle have been documented (1–4). The majority of medical professionals advise early surgical resection to lower mortality brought on by complications, such obstruction of the heart's inflow or outflow system (5). Here, we present a case of a primary cardiac myxoma in a child that had protruded into the pulmonary trunk from the right ventricle (RV).
This study obtained the informed consent of the patients and their families for publication. Due to chest congestion and edema, a 14-year-old girl with nephrotic syndrome who had been identified in a smaller district hospital in China was sent to our division. Her blood pressure was 109/80 mmHg at the time of admission, and her pulse rate was 107 beats per minute. Physical examination revealed face and ankle edema as well as a grade 4/6 systolic ejection murmur at the left upper sternal border.
Transthoracic echocardiography revealed a significant 70 × 30 mm RV mass which protruded into the right ventricular outflow tract (RVOT) and the pulmonary trunk. The pressure gradient from the pulmonary artery to the RV was 64 mmHg (Figure 1A). There was also enlargement of the RV and right axis deviation along with sinus tachycardia. A filling deficit in the RV, pulmonary trunk, and right pulmonary artery was discovered by computed tomography (CT) (Figures 1C,D).
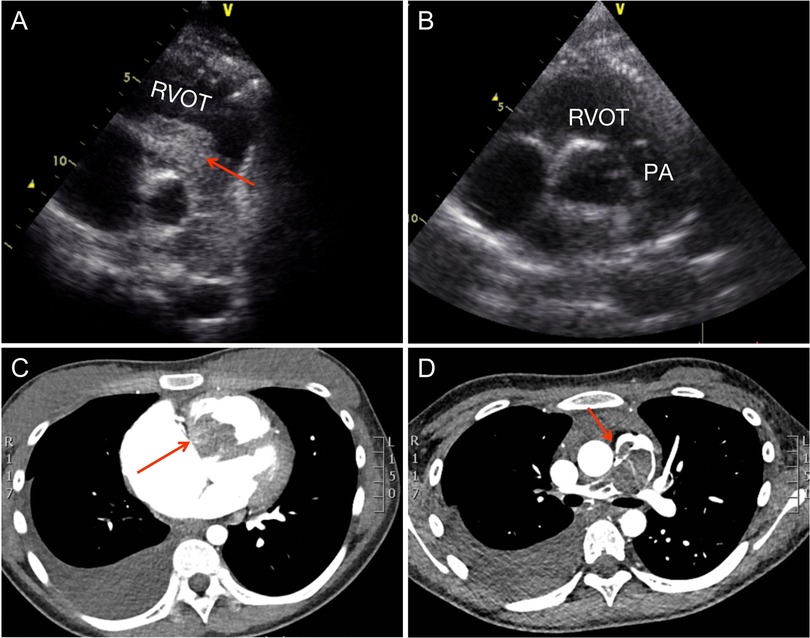
Figure 1. Preoperative and postoperative examination. (A) Preoperative parasternal short-axis view at the level of the aortic valve showing the obstructed right ventricular outflow tract and pulmonary trunk. (B) Postoperative parasternal short-axis view at the level of the aortic valve showing the unobstructed right ventricular outflow tract and pulmonary trunk. (C) Computed tomography reveals the right ventricular outflow tract. (D) Computed tomography reveals the pulmonary trunk and right pulmonary artery. RVOT, right ventricular outflow tract; PA, pulmonary artery.
She underwent a sternotomy and cardiopulmonary bypass creation by aortic and bicaval cannulation under general anesthesia. She had her RA and RVOT opened. The myxoma was attached to the membranous portion of interventricular septum and had grown into the main pulmonary artery and the right pulmonary artery. The mass was entirely eliminated (Figure 2). According to pathological findings, there were two pieces of tumor tissue totaling 5 × 4 × 2 cm, the majority of which was mucus. It appeared to be a myxoma under a microscope, with slightly larger individual nuclei. CD34(vascular+), NSE(−), SMA(+), CK(−), CD68(+), MC(−), CR(+), Desmin(−), CEA(−), EMA (1), and F8(+) were immunohistochemically positive. She was discharged 7 days after surgery and the recovery time was uncomplicated. Following surgery, echocardiography showed an unobstructed RVOT (Figure 1B). Seven-year postoperative follow-up showed no recurrence of the patient's tumor. The entire process of disease development is described in Table 1.
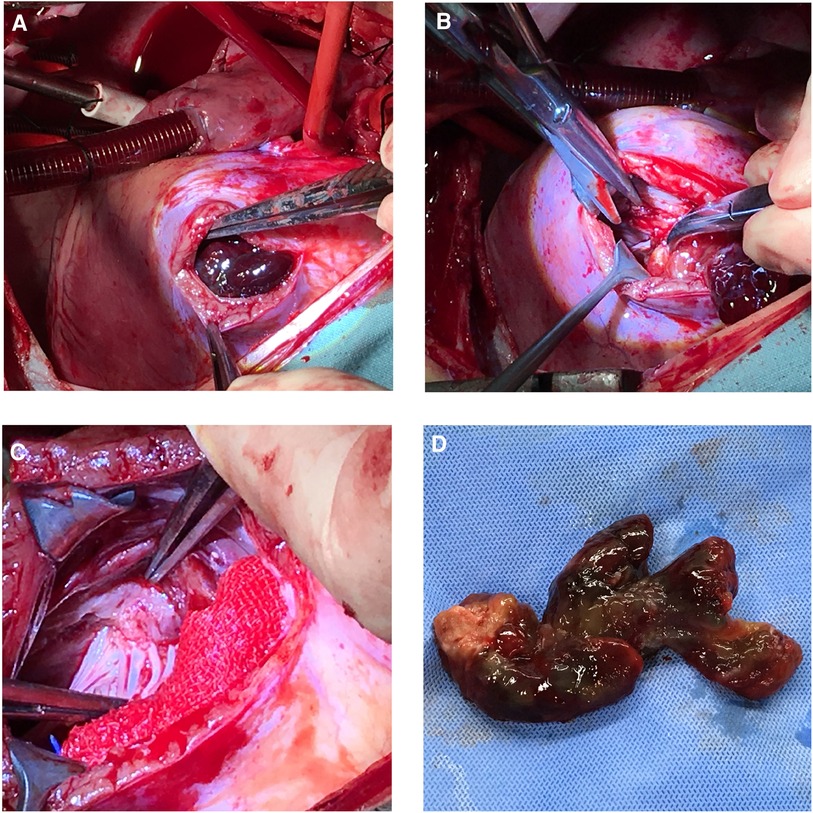
Figure 2. Surgery pictures. Operative procedures. (A) Right ventricular outflow tract incision was made to expose the tumor. (B) and (C) The tumor root which attached to the membranous portion of interventricular septum was surgically removed. (D) The entire tumor.
Up to 75% of myxomas are found in the left atrium. Right-sided myxomas are uncommon (15%–20%), and RV (3%–4%) or pulmonary artery myxomas are exceedingly uncommon (6). Syncope, pulmonary embolism, chest congestion, and sudden death are complications that can result from obstruction of the outflow of blood from the RV (2, 7).
Myxomas are uncommon in children despite being the most frequent primary heart tumor. A study reported that 8 children with a clinical diagnosis of cardiac tumor underwent surgery between 1986 and 2003. Surgical pathology only revealed myxomas in 2 patients (25%) (8). In another study, 56 children had primary heart tumors. Of those, 6 children had fibrosarcoma and 44 children had rhabdomyosarcoma. No myxomas were discovered (9). Thus, pediatric cardiac myxomas are quite uncommon. Although right ventricular myxomas in children have been documented in the past, pediatric cases of enormous right ventricular mucinous tumors that extend into the pulmonary artery are extremely rare. Patients of this type are at a significant risk for sudden death and pulmonary embolism.
It is especially useful for the diagnosis and treatment of patients to properly represent the size, quantity, and attachments of tumors. Consequently, it is crucial to appropriately describe cardiac tumor using imaging. CT, MRI and echocardiography are frequently used to assess cardiac tumor, according to the guidelines. The location, size, form, attachment points, and motion features of mucinous tumors can be determined via transthoracic echocardiography and, if required, transesophageal access. CT provides a better assessment of tumor extent, including invasion of adjacent vessels and pulmonary metastases, than echocardiography. In addition, CT is capable of directly imaging tumorigenic pulmonary emboli and can also be used to assess calcification (10). MRI can assess myocardial infiltration, pericardial involvement, and/or extracardiac extension. MRI overcomes the usual limitations of echocardiography and can more accurately assess changes in cardiac function. The use of intravenous contrast agents has improved tumor characterization and delineation of tumor boundaries. MRI can also differentiate tumors from other non-tumor masses (11–13). In our case, the patient did not undergo the MRI, because MRI is still a relatively expensive examination. The financial burden on the patient is why this examination was not performed. Although not used in our report, MRI is still important as an adjunct to diagnose tumors and determine the type of tumor.
In our case, the symptoms of chest tightness, facial edema, and lower extremity edema were so first diagnosed as nephrotic syndrome. This may be due to a possible lack of cardiac auscultation. Therefore, in suspicious cases, a brief auscultation and routine imaging should be performed at the regional hospital before the patient is referred to a higher level hospital. This is important because delays in diagnosis and treatment can lead to complications and, in severe cases, death.
Since no medications have been developed to diminish or stop the growth of myxomas, surgical excision is the only effective treatment for them in any heart cavity. The surgical plan includes complete removal of the tumor while restoring any tumor-affected valves that may be present. Vigorous palpation and other cardiac manipulation should be avoided until extracorporeal circulation is started since the threat of tumor fragmentation and embolization remains high. Asymptomatic myxoma patients have also undergone surgery with positive outcomes and no postoperative fatalities (14, 15). Recurrence has been observed in approximately 5% of patients months or years after surgery. On the surgical approach, some surgeons choose either the RA or RVOT approach. We chose both the RA and RVOT approach because the root of the patient's tumor was located in the membranous portion of interventricular septum and entered the pulmonary artery along the RVOT. Therefore, we believe that a simple right atrial incision is not sufficient to remove the tumor cleanly, so we choose a dual approach for an incision. After the operation, the child did very well and was almost back to normal upon discharge from the hospital. Rarely, a RV myxoma will block the RVOT in children (16). However, immediate and thorough surgical resection should be performed to prevent outflow tract occlusion, which could cause rapid mortality.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethics Committee of the Second Xiangya Hospital of Central South University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
KG, YDS, HDL, LX and JJL: contributed to conception and design of the study. KG: wrote the first draft of the manuscript. KG, YFY and JJL: wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Shah IK, Dearani JA, Daly RC, Suri RM, Park SJ, Joyce LD, et al. Cardiac myxomas: a 50-year experience with resection and analysis of risk factors for recurrence. Ann Thorac Surg. (2015) 100(2):495–500. doi: 10.1016/j.athoracsur.2015.03.007
2. Karagoz A, Keskin B, Karaduman A, Tanyeri S, Adademir T. Multidisciplinary approach to right ventricular myxoma. Braz J Cardiovasc Surg. (2021) 36(2):257–60. doi: 10.21470/1678-9741-2020-0177
3. Kaulitz R, Haen S, Sieverding L. Neonatal aortic arch obstruction due to pedunculated left ventricular foetal myxoma. Cardiol Young. (2015) 25(7):1393–5. doi: 10.1017/S1047951114001929
4. Li Y, Yang W, Liao S, Zuo H, Liu M. Cardiac myxomas as great imitators: a rare case series and review of the literature. Heart Lung. (2022) 52:182–9. doi: 10.1016/j.hrtlng.2022.01.010
5. Samanidis G, Khoury M, Balanika M, Perrea DN. Current challenges in the diagnosis and treatment of cardiac myxoma. Kardiol Pol. (2020) 78(4):269–77. doi: 10.33963/KP.15254
6. Mittle S, Makaryus AN, Boutis L, Hartman A, Rosman D, Kort S. Right-sided myxomas. J Am Soc Echocardiogr. (2005) 18(6):695. doi: 10.1016/j.echo.2005.03.007
7. Lu C, Yang P, Hu J. Giant right ventricular myxoma presenting as right heart failure with systemic congestion: a rare case report. BMC Surg. (2021) 21(1):64. doi: 10.1186/s12893-020-00977-4
8. Padalino MA, Basso C, Milanesi O, Vida VL, Moreolo GS, Thiene G, et al. Surgically treated primary cardiac tumors in early infancy and childhood. J Thorac Cardiovasc Surg. (2005) 129(6):1358–63. doi: 10.1016/j.jtcvs.2004.10.020
9. Beghetti M, Gow RM, Haney I, Mawson J, Williams WG, Freedom RM. Pediatric primary benign cardiac tumors: a 15-year review. Am Heart J. (1997) 134(6):1107–14. doi: 10.1016/S0002-8703(97)70032-2
10. Ekmektzoglou KA, Samelis GF, Xanthos T. Heart and tumors: location, metastasis, clinical manifestations, diagnostic approaches and therapeutic considerations. J Cardiovasc Med. (2008) 9(8):769–77. doi: 10.2459/JCM.0b013e3282f88e49
11. Lyon AR, Lopez-Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur Heart J. (2022) 43(41):4229–361. doi: 10.1093/eurheartj/ehac244
12. Poterucha TJ, Kochav J, O'Connor DS, Rosner GF. Cardiac tumors: clinical presentation, diagnosis, and management. Curr Treat Options Oncol. (2019) 20(8):66. doi: 10.1007/s11864-019-0662-1
13. Luna A, Ribes R, Caro P, Vida J, Erasmus JJ. Evaluation of cardiac tumors with magnetic resonance imaging. Eur Radiol. (2005) 15(7):1446–55. doi: 10.1007/s00330-004-2603-y
14. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine. (2001) 80(3):159–72. doi: 10.1097/00005792-200105000-00002
15. Tetera W, Wilk A, Krol W, Braksator W. Asymptomatic left atrial myxoma. J Cardiovasc Echogr. (2022) 32(2):116–8. doi: 10.4103/jcecho.jcecho_70_21
Keywords: primary cardiac tumors, right ventricle, diagnosis, treatment, surgery
Citation: Gong K, Yang Y, Shen Y, Liu H, Xie L and Liu J (2023) Successful management of a rare case of juvenile giant right ventricular myxoma. Front. Surg. 9:1102742. doi: 10.3389/fsurg.2022.1102742
Received: 19 November 2022; Accepted: 19 December 2022;
Published: 11 January 2023.
Edited by:
Hendrik Tevaearai Stahel, Bern University Hospital, SwitzerlandReviewed by:
Anshuman Darbari, All India Institute of Medical Sciences, India© 2023 Gong, Yang, Shen, Liu, Xie and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jijia Liu bWNsaXVqaWppYUBjc3UuZWR1LmNu
Specialty Section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.