
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 24 January 2023
Sec. Neurosurgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1092140
This article is part of the Research TopicRare and Misdiagnosed Neurosurgical ConditionsView all 8 articles
 Long Chen1,2,3
Long Chen1,2,3 Zujian Xiong1,2,3
Zujian Xiong1,2,3 Yian Zhou4
Yian Zhou4 Yanwen Li1,2,3
Yanwen Li1,2,3 Yuanyang Xie1,3
Yuanyang Xie1,3 Yi Xiong1,2,3
Yi Xiong1,2,3 Siyi Wanggou1,3*
Siyi Wanggou1,3* Xuejun Li1,3*
Xuejun Li1,3*
Background: Supratentorial hemangioblastoma is an extremely rare neoplasm. The aim of this study is to delineate the clinical features among cystic and solid supratentorial hemangioblastoma patients and evaluate the risk factors for progression-free survival (PFS).
Methods: We conducted a literature search in PubMed for histopathologically identified supratentorial hemangioblastoma between 1947 and 2021 and extracted and collected the clinical features of patients treated at our own institute. The rate of PFS was determined using Kaplan–Meier analysis. Differences in categorical factors, such as the location of tumor and diagnosis of von Hippel–Lindau disease, were analyzed using the Pearson χ2 test. A Cox regression analysis was performed to evaluate the association between various variates and survival outcomes.
Results: A total of 237 cases of supratentorial hemangioblastoma were identified from 169 studies. A survival analysis found that patients with cystic tumors had a significantly better prognosis than those with solid tumors (log-rank, p = 0.0122). Cox regression analysis suggested that cystic hemangioblastoma (hazard ratio (HR): 0.186, 95% CI: 0.043–0.803, p < 0.05) and gross total resection (GTR) (HR: 0.126, 95% CI: 0.049–0.323, p < 0.001) were significant predictors of longer survival (PFS) for supratentorial hemangioblastoma. Following an analysis of 13 supratentorial hemangioblastoma cases from our institute, we validated that cystic tumor had improved prognosis than solid tumor (log-rank, p = 0.0096) and GTR was superior to subtotal resection (log-rank, p = 0.0029).
Conclusions: Cystic hemangioblastoma vs. solid hemangioblastoma may be two tumoral statuses with different clinical features, and a specific treatment strategy should be considered.
Hemangioblastoma, a rare tumor of the central nervous system (CNS), was first reported by von Hippel–Lindau in 1895 (1). It is a benign, vascular tumor that accounts for 1%–3% of intracranial space-occupying lesions (1, 2), and recent studies have demonstrated that hemangioblastoma arises from arrested mesoderm-derived hemangioblasts during embryonic development (3–5). Hemangioblastoma occurs frequently in the cerebellum, brainstem, and spinal cord, but rarely does supratentorial hemangioblastoma occur either sporadically or in conjunction with von Hippel–Lindau (VHL) disease, which accounts for approximately one-third of cases (6–8).
VHL disease is an autosomal-dominant inherited disorder, mainly caused by germline mutations of the VHL gene located on the short arm of chromosome 3. The prevalence of VHL is 1/(91,000–36,000) (9) and penetrance can reach 87%–97% (10, 11). A VHL patient often presents with a multiorgan tumor syndrome, including CNS hemangioblastoma, retinal hemangioblastoma, renal carcinoma or renal cyst, pancreatic neuroendocrine tumor or cysts, and adrenal pheochromocytoma (12, 13). The development and progression of CNS-associated hemangioblastoma is the most common cause of morbidity and mortality in VHL disease, among which supratentorial hemangioblastoma accounts for only 1%–6% of VHL-related hemangioblastoma (7, 14). However, studies focusing on supratentorial hemangioblastoma have been rarely reported, which makes it difficult to provide valuable information on clinical diagnosis and treatment.
This study was performed mainly to summarize the clinical characteristics of sporadic and VHL-related supratentorial hemangioblastoma and explore the differences in clinical characteristics between cystic and solid tumors in supratentorial hemangioblastoma in order to enrich the current evidence of biological and clinical features and further optimize the management of intracranial hemangioblastoma, especially supratentorial hemangioblastoma.
The first author (LC) conducted a literature search in the US National Library of Medicine (PubMed) in 2021 to identify all reported cases of supratentorial hemangioblastoma (between October 1947 and March 2021). The following specific search terms were used: “hemangioblastoma,” “hemangioblastomas,” “haemangioblastoma,” “supratentorial hemangioblastoma,” “angioblastoma,” and “angioreticuloma.” A total of 2,964 articles were identified, of which 169 contained patient data regarding supratentorial hemangioblastomas.
Publications were eligible if they met the following criteria: acquisition of full-text articles; studies or case reports containing individual patient clinical data or purely supratentorial hemangioblastoma–aggregated datasets of either histopathologically confirmed or presented in the context of a confirmed diagnosis of VHL disease. The standard Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Individual Participant Data (PRISMA-IPD) methodology was used in this study.
Diagnosis of VHL was confirmed in study patients using genetic testing and/or diagnostic clinical criteria (14, 15): a family history of VHL disease with CNS hemangioblastoma, pheochromocytoma, or renal clear cell carcinoma; two or more CNS hemangioblastomas or one CNS hemangioblastoma with a visceral tumor, including pancreatic cysts, neuroendocrine tumors, and endolymphatic sac tumors, but not renal and epididymal cysts.
For each case, detailed clinical information [sex, age, symptom, VHL, retinal hemangioblastoma, visceral lesion, preoperation Karnofsky Performance Scale (KPS), postoperation KPS, progression-free survival (PFS), etc.] was extracted from the original article. The KPS score was mainly obtained directly through the content of the article. The postoperative KPS score is an assessment of the status of surgical patients 1 week after surgery. If it is not mentioned in the article, we will score it according to the preoperative and postoperative conditions by using the KPS. All data were entered into a dedicated form and validated by two reviewers (ZX and YX).
The specific imaging data of each patient were extracted, such as CT scan images, fluid attenuated inversion recovery (FLAIR), and T1- and T2-weighted MRI. The cystic hemangioblastoma in this study included peritumoral and intratumoral cysts. Peritumoral cysts were judged by using T2-weighted MRI, and intratumoral cysts were determined by using T1-weighted postcontrast MRI, which manifested as hypo-intensity within enhancing tumor. In supratentorial multiple tumors, the tumor that was the largest in terms of volume or that caused symptoms was used to define whether the patient had a cystic or solid tumor. The definition of deep and superficial anatomical locations is as follows: superficial refers to the frontal, temporal, parietal, and occipital lobes and deep denotes the sellar region, basal ganglia, ventricles, and hippocampal regions. Peritumoral edema was obtained as described from the content of the study or judged by using CT and T2 FLAIR images. Information about tumor volume was obtained from the description in the article, which refers to the total volume of cystic and solid components. If tumor volume was not mentioned in the paper, NA (not available) was entered instead. In accordance with the cases reported in our institute, tumor size evaluation was performed as previously described (16) and the extent of resection was determined, which was recorded as gross total resection (GTR) or subtotal resection (STR) on postoperative contrast MRI scans before discharge. Detailed anatomical location of the tumor and cyst was obtained from the article content; if not, we judged the specific location by image data. All information was entered into the form and reviewed by two people (SW and YX). All disagreements were resolved through consultation with a third reviewer when needed (XL).
For categorical data, Pearson's χ2 test, Fisher's exact test, Wilcoxon rank-sum test, or Fisher's exact test were used to analyze the differences among preoperation variates. Spearman rank correlation analysis was used in different categorical data. The data were analyzed statistically using descriptive analysis by Cramer's V to determine the correlation degree between the groups. The Kaplan–Meier (K–M) survival analysis method was employed to generate time–to-progression curves and these were tested by using log-rank. Cox regression analysis was performed to evaluate the association between various variates and survival outcomes. A p-value of <0.05 was considered statistically significant. All statistical analyses and graphics were prepared using Statistical Package for the Social Sciences (IBM SPSS Statistics 23.0) and Graphpad Prism 8.0 software.
Following the selection criteria (Figure 1), a total of 169 articles were retrieved, from which 237 supratentorial hemangioblastoma patients were identified, with a less female predominance (48.5%), and the mean age of onset was 38.51 ± 19.11 years (range 0.06–85.00 years) (Supplementary Figure S1A). The most common symptoms included vision deficit (29.1%), followed by headache/dizziness (24.5%), and increased intracranial pressure (ICP) (12.7%). A total of 45.1% of patients were genetically or clinically diagnosed with VHL disease (Table 1).
Of the 237 patients, 222 (93.7%) had supratentorial solitary hemangioblastoma, the remaining 15 (6.3%) had supratentorial hemangioblastomas with multiple foci, and the total number of patients with tumors was 260. Of these patients, 147 (62.0%) had solid tumors, and 64 (27.0%) were classified as having cystic tumors, which accounted for approximately one-third of the patients (Table 1). Among patients with VHL, the presence of extracranial tumors was also reported, of which renal cell carcinoma is the most common, followed by adrenal pheochromocytoma (Supplementary Table S1). In supratentorial hemangioblastoma, the cerebrum (36.3%) is the most common location, followed by the sellar region (24.5%) (Supplementary Table S2). Notably, dual attachment (10.5%) and calcification (2.5%) can also be seen in CT imaging and MRI for supratentorial hemangioblastoma (Supplementary Table 2). The total follow-up of these cases ranged from 0 to 276 months (Supplementary Table S3).
Of these cases with available treatment modality, 187 (78.9%) patients underwent surgery alone, and 12 (5.1%) and 31 (13.1%) received radiotherapy and conservative treatment, respectively (Table 1). Among the 187 surgically treated patients, 27 (11.4%) underwent STR.
An analysis of the distribution of supratentorial hemangioblastoma stratified by age subgroup showed that, at the age subgroup range of 0–20 years, the optic nerve was the least frequently involved, whereas the ventricular nerve was rare in the age subgroup of more than 20 years (Supplementary Table S4). A further exploration of the tumor distribution in different locations suggested that the frontal (32.6%) and temporal (31.4%) lobes were the most frequently involved in the cerebrum, least in the occipital lobe (7.0%), whereas the sellar region was mainly suprasellar (44.8%) and pituitary stalk (39.7%) (Supplementary Table S5). A differential analysis of hemangioblastoma at each site showed that tumor size located in the sellar region and the optic nerve were generally smaller, which reached statistical significance compared with those located in the cerebrum (Supplementary Figure S1B). A correlation analysis was performed between tumor anatomical regions and symptoms. We found that patients with sellar lesions mainly experienced endocrine-related symptoms (Cramer's V = 0.33), and the optic nerve was strongly correlated with visual defect (Cramer's V = 0.53) (Supplementary Figure S1C).
Compared with solid tumors, cystic tumors are more prone to superficial location (p = 2.08 × 10−3) (Supplementary Table S6). A comparison of the incidence of edema by tumor sites revealed that edema was less common in sellar region lesions than in the cerebrum and optic nerve (p = 0.0003 and p = 0.012, respectively) (Supplementary Table S7). We also found a significant correlation between cysts and edema (r = 0.307, p < 0.05). More importantly, an analysis of tumor anatomical distribution of supratentorial cystic and solid tumors revealed that cystic tumors were mainly located in the cerebrum (62.5%) and the highest proportion of solid tumors was found in the sellar region (29.3%), and the proportion of cystic tumors in the cerebrum was significantly higher than that in the optic nerve (p = 4.78 × 10−4) and sellar region (p = 5.50 × 10−5) (Supplementary Table S8).
Patients were divided into cystic and solid subgroups and then stratified by age subgroups; the mean age at initial diagnosis was found to be younger in cystic (33.35 ± 20.24 years) than in solid tumor (41.16 ± 18.58 years) (Figure 2A). A further analysis of each age subgroup revealed statistical significance between ≤10 years and other subgroups for cystic and solid tumors; that is, younger patients (≤10 years) were more likely to have cystic tumor (Supplementary Table S9). In addition, preoperative KPS (Figure 2B) and postoperative complications (Table 2) were analyzed in patients with cystic and solid tumors. We found that patients with cystic tumors had significantly lower preoperative KPSs than those with solid tumors (p = 0.001), but patients with cystic tumors had a significantly lower risk of developing complications than those with solid tumors (p = 0.012). There was an obvious trend that the volume of the cystic tumor was larger than that of solid tumor but with no significant difference (Figure 2C). Survival analysis was performed using the Kaplan–Meir survival curve, which was examined by employing the log-rank test. We found a clear benefit for GTR patients than for STR (log-rank, p = 0.0005) or radiotherapy (RT) patients with statistically significant values (log-rank, p = 0.003) (Figure 2D). Further grouped by cystic and solid status, Kaplan–Meir survival curves revealed that patients with cystic tumors had a significantly better prognosis than those with solid status (log-rank, p = 0.0122) (Figure 2E).
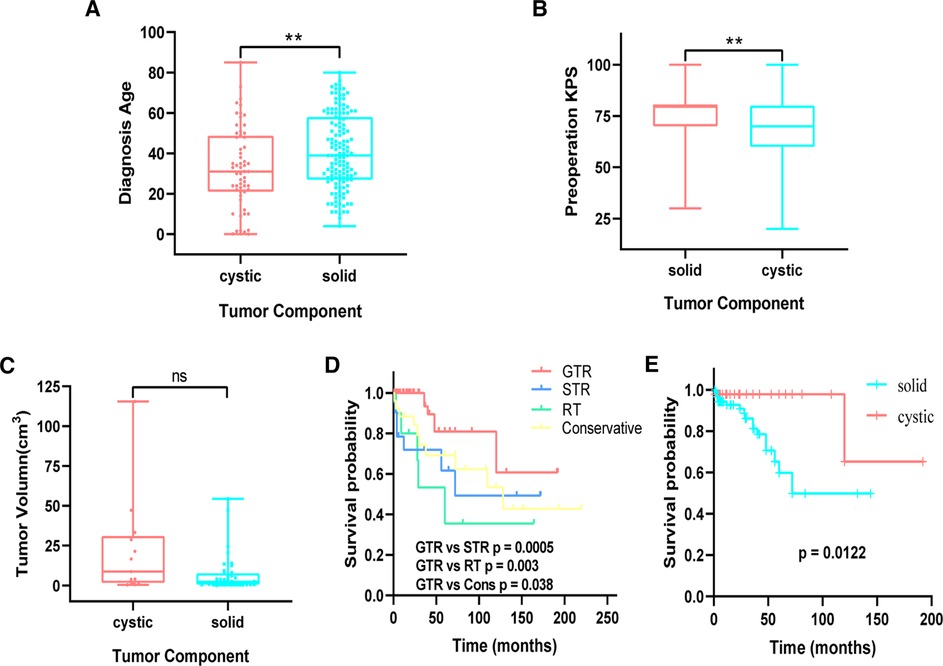
Figure 2. (A) comparison of age between cystic and solid patients. (B) Comparison of preoperation KPS between cystic and solid patients. (C) Comparison of cystic and solid tumor volume in purely supratentorial solitary hemangioblastoma. (D) Kaplan–Meier analysis of progression-free survival of supratentorial hemangioblastoma stratified by treatment modalities. GTR vs. STR (p = 0.0005), GTR vs. RT (p = 0.003). (E) Cystic vs. solid (p = 0.0122). KPS, Karnofsky Performance Scale; STR, subtotal resection.
The patients were divided into two groups: VHL and sporadic. We compared the median onset age between both groups (Supplementary Table 10), which suggested that there was a significant difference between sporadic (median age: 42.50 years) and VHL-related hemangioblastoma (median age 33.00 years) (p = 0.010). A further analysis stratified by gender revealed that the median age of VHL patients (32.70 ± 12.82 years) was significantly lower than that of sporadic patients (42.15 ± 20.93 years) in the female category (p = 0.010) (Supplementary Table 10). In addition, those with supratentorial solitary lesion mainly belonged to the sporadic group (91.82%) (Supplementary Figure S2A). A comparison of tumor volumes between sporadic and VHL-related supratentorial hemangioblastomas revealed that the volume in the former was significantly larger than that of the latter (p < 0.0001) (Supplementary Figure S2B). Meanwhile, those with VHL differ from their counterparts in terms of symptomatic occurrence, ICP, infratentorial lesions, preoperative KPS, visceral lesion, retinal hemangioblastoma, and intratumoral hemorrhage (Supplementary Table 10). Furthermore, the difference in tumor location between the two groups was mainly reflected in the cerebrum and optic nerve (Supplementary Table 11). A correlation analysis revealed that VHL disease was negatively correlated with the cerebrum (Spearman r = −0.305, p < 0.05) and positively correlated with the optic nerve (Spearman r = 0.322, p < 0.01) but not with the sellar region (p = 0.439) and the ventricle (p = 0.713).
We performed a univariate analysis for determining the potential risk factors associated with progression-free survival in patients with a supratentorial hemangioblastoma, such as gender, diagnosis age, symptom, VHL disease, infratentorial lesion, tumor component (solid and cystic), preoperation and postoperation KPS (<70 and ≥70), surgery strategy (GTR and non-GTR), and others. Of these factors, cystic tumor and GTR were significant predictors of longer PFS for supratentorial hemangioblastoma. Factors associated with longer control were symptoms (hazard ratio (HR): 4.26, 95% CI: 1.281–14.167, p = 0.018), cystic lesion (HR: 0.277, 95% CI: 0.095–0.803, p = 0.018), and GTR (HR: 0.243, 95% CI: 0.104–0.568, p = 0.001) in univariate analysis. These factors were then subjected to multivariate Cox regression analysis, and the result showed that cystic tumor (HR: 0.186, 95% CI: 0.043–0.803, p = 0.024) and GTR (HR: 0.126, 95% CI: 0.049–0.323, p < 0.001) was a significant independent predictor for improved PFS when compared with solid tumor and non-GTR (Table 3).
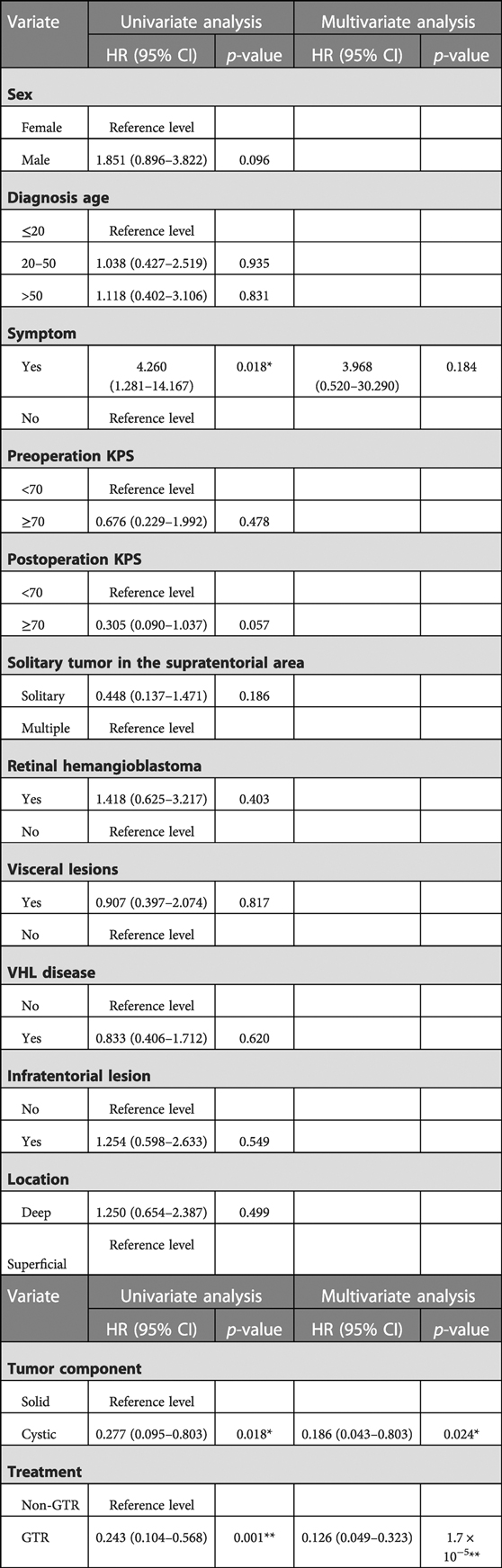
Table 3. Univariate and multivariate Cox regression analyses of potential risk factors influencing the PFS of patients with supratentorial hemangioblastoma.
We collected a cohort of 13 supratentorial hemangioblastoma patients diagnosed at the Xiangya Hospital, Central South University, between the years 2010 and 2021, with the breakup being four VHL-related and nine sporadic supratentorial hemangioblastomas. Patient clinical characteristics are given in Supplementary Table 12, and representative patients with hemangioblastomas are described in Figure 3 and Supplementary Figure S3.
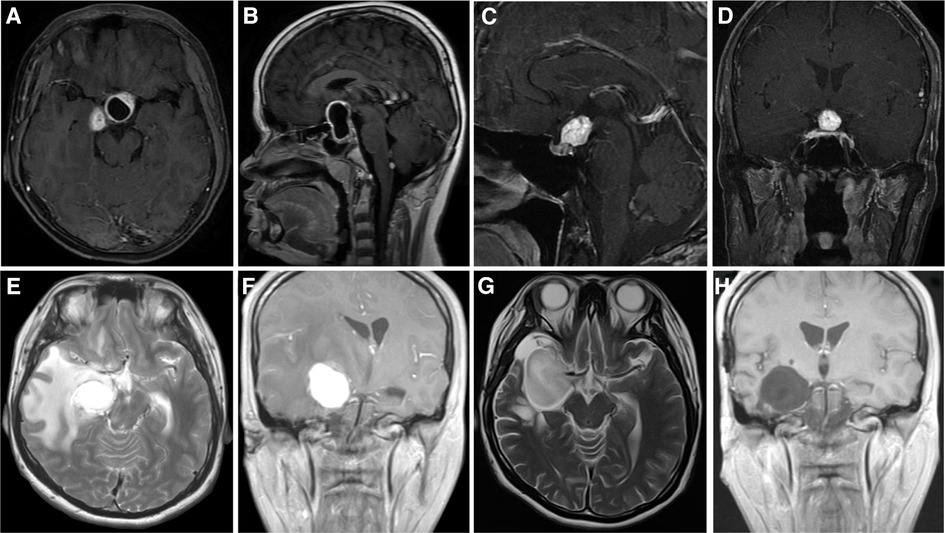
Figure 3. Imaging data of Xiangya cases. (A,B) Case 1: Cystic hemangioblastoma in the sellar region. (C,D) Case 3: Solid hemangioblastoma in pituitary stalk. Case 7: Solid hemangioblastoma in the basal ganglia region accompanied by massive edema (E,F), the edema subsided completely 3 weeks after operation (G), and there was no recurrence 26 months after operation (H).
The median age at initial diagnosis was 36.38 ± 15.49 years in cystic and 37.40 ± 14.94 years in solid tumors, respectively (p = 0.909), and the mean preoperative KPS scores of cystic and solid tumors were 70.00 ± 9.26 and 82.00 ± 4.47, respectively (p = 0.022) (Supplementary Table 13). Six patients were diagnosed with peritumoral edema around the tumor (five cerebrum and one hippocampal), and all give cerebrum patients who underwent surgical resection achieved GTR. Survival analysis was performed (K–M curves), which revealed that the PFS of patients with cystic supratentorial hemangioblastoma was significantly longer than that of patients with solid tumors, and the result was statistically significant (log-rank, p = 0.0096) (Figure 4A). Patients who underwent GTR had a better PFS than those who underwent STR, and the result was statistically significant (Figure 4B) (log-rank, p = 0.0029).
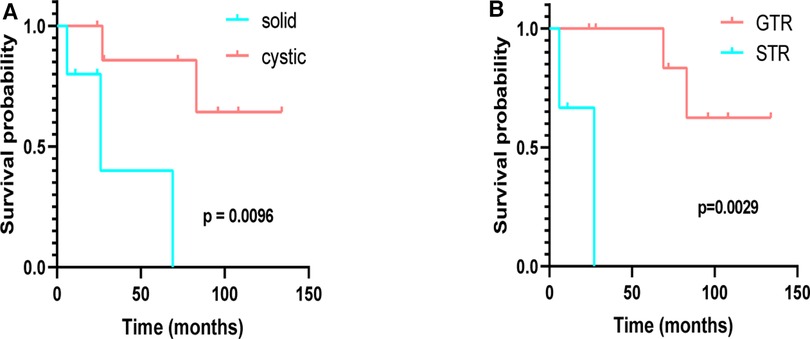
Figure 4. Kaplan–Meier analysis of progression-free survival of supratentorial hemangioblastoma stratified by treatment modalities. (A) Cystic vs. solid (p = 0.0096); (B) GTR vs. STR (p = 0.0029).
CNS hemangioblastomas are the most common clinical manifestations of VHL disease. Previous studies have indicated that the growth rate of intracranial hemangioblastomas is substantially greater than that of other benign intracranial tumors (17, 18). However, CNS-associated hemangioblastomas are highly variable in supratentorial and infratentorial anatomical distribution. Lonser et al. (19) analyzed 225 VHL patients with CNS hemangioblastomas, and supratentorial patients accounted for 10.2% (23 cases in total). In terms of the number of tumors, there were 1921 tumors in 225 patients, of which 1% (21 supratentorial hemangioblastomas) were supratentorial locations. By analyzing 152 CNS-associated hemangioblastomas, Peyre et al. (7) showed that VHL accounted for 54% and supratentorial hemangioblastoma occurred in 3.2%, which is in agreement with reports on VHL patients, with supratentorial hemangioblastoma occurring in 1%–6% (14, 15). In addition, they found that only 13 (3.2%) of 409 VHL patients had supratentorial tumors, of which 4 were supratentorial multiple hemangioblastomas. A further analysis of the imaging data of 14 tumors revealed that 13 tumors had progressed (92.9%); in contrast, of 373 infratentorial cases reported by Wanebo et al. (20), only 44% reported tumor growth. Thus, based on the progression of supratentorial hemangioblastoma reported by Peyre et al. the possibility of supratentorial hemangioblastoma developing further seems to be higher (92.9%) than infratentorial and spinal hemangioblastoma (44%).
It is of great practical significance to clarify the clinical characteristics between cystic and solid components in hemangioblastoma. Our data showed that among supratentorial tumors, cystic tumors accounted for 27%, compared with the rate of 33% reported by Mills et al. (21). We further analyzed the location distribution of supratentorial cystic tumors and found that cystic tumors were mainly located in the cerebrum. Similar to cerebellar hemangioblastoma, which is mainly located in the posterior (74%) and superficial (68%) regions of the cerebellar cortex rather than in the anterior or deep region (6), supratentorial cystic hemangioblastomas are more frequently located in the superficial (58.2%) than in the deep cerebral cortex. This may be due to differences in the hydraulic conductivity of the cerebrum compared with other supratentorial locations. These intrinsic anatomic differences in fluid transport may make the cerebrum more prone to the generation of peritumoral cysts (22). In addition, peritumoral edema precedes and underlies the propagation of peritumoral and intratumoral cysts. Our data showed that there is a significant correlation between the presence of edema and the presence of cystic components in supratentorial tumors (r = 0.307, p < 0.01). Previous research has provided insights into the potential mechanisms leading to the formation and progression of tumor-related peritumoral cysts, which has an association with high intratumoral pressure, increased vascular permeability, and the levels of vascular endothelial growth factor (VEGF) (23, 24).
The formation of peritumoral edema or cyst is an important reason for the occurrence of symptoms of patients, but this edema will subside with tumor resection. For example, in patient 7 from our institute, the preoperative massive peritumoral edema disappeared after total tumor resection. Moreover, Peyre et al. (7) found no significant correlation between the formation of cysts and tumor size in a small series of cases. Similarly, consistent with their results, our data showed no correlation between supratentorial cyst formation and tumor size (p = 0.053). In view of its highly vascular characteristics, hemangioblastoma often shows obvious contrast enhancement on T1-MRI (25, 26). The signal of flow void within the tumor on the T2-weighted images is occasionally observed and regarded as a characteristic feature of the tumor (27, 28). These regions are considered to be intratumoral vascular structures. In our data, the hemangioblastoma with the flow void effect accounted for 13.5%. The typical vascular flow void signal was shown in patient 2, which is a low signal on T2 MRI and postcontrast enhancement. In addition, by analyzing the surgical resection degree of supratentorial tumors, we found that the total resection of cystic tumors located in the cerebral lobe could be easily performed, while for sellar tumors, whether cystic or solid type, the total resection of GTR was more difficult to perform compared with that of the other parts (29). Therefore, it is suggested that both cystic and solid tumors in the sellar region should be inspected before surgery to evaluate whether they can be completely resected.
Previous studies have confirmed that, during the assessment of the progression from the asymptomatic to the symptomatic state, the tumor size gradually increases, and the volume of the cyst is significantly larger than that of the tumor (20, 22). We found that in patients with supratentorial solitary tumor, the size of the cystic tumor was significantly larger than that of solid tumors; however, this result had no statistical difference. A further analysis of the difference in tumor size between symptomatic and asymptomatic hemangioblastoma showed that the mean size of symptomatic hemangioblastoma was larger than that of asymptomatic hemangioblastoma.
In our analysis, we combined STR and RT as an independent treatment modality to compare with GTR in extending PFS. The results showed that STR and RT were significantly inferior to GTR. In previous studies, high-dose fractioned external beam radiotherapy (EBRT) has been demonstrated to improve overall survival (OS) and disease-free survival (DFS) for CNS-related hemangioblastoma (30, 31). Recently, numerous studies of stereotactic radiosurgery (SRS) for CNS hemangioblastoma showed that local tumor control rates at 5 and 10 years ranged from 83% to 94% and 61% to 80%, respectively (32–34), with a 0%–7% risk for adverse radiation effects. Of these, Gamma Knife Radiosurgery (GKRS) is considered an acceptable treatment modality for CNS hemangioblastoma, the 5-year tumor control rate was 74%–85% (35, 36), and compared with patients with a solitary hemangioblastoma, those with multiple lesions in CNS were more likely to show progress after GKRS treatment (35). Liebenow et al. (37) held the idea that GKRS altered the clinical course from a saltatory growth pattern to a reduction of tumor size. They reported that the formation rates of new hemangioblastomas at 1, 3, and 5 years were 97%, 80%, and 46%, respectively. In addition, GKRS is not an ideal treatment for cystic hemangioblastoma (32, 34, 37), especially for small mural nodules with large cysts and for those who need urgent relief from symptoms (38). As shown in patient 11 from our institution (Supplementary Figure S3), changes occurring in tumors were highly uncertain during gamma knife treatment, and it was shown during dynamic observation that the tumor could shrink, stabilize, or increase in size, and even cystic changes occurred after this treatment. Gamma knife treatment did not prevent the development of new lesions. If the tumor appears to undergo a cystic change, surgery should be the only curative treatment modality.
However, it is worth noting that GKRS may be an effective treatment for cystic lesions, when they are located in an unfavorable surgical location, or for multiple intracranial lesions in an individual patient (39–41). Despite few studies having reported the efficacy of RT for supratentorial hemangioblastoma, studies on CNS hemangioblastoma have shown that the 5-year OS of the VHL subgroup was better than that of the non-VHL when patients were stratified by VHL status (32, 34). Evidence suggests that surgical resection can be the first line of treatment of supratentorial hemangioblastoma, and GTR should be the first priority. However, in those in whom lesions occur in the eloquent area or in whom only STR can be achieved, EBRT, SRS, or GKRS could be recommended as an alternative treatment modality for small, solid, or noncystic and VHL-associated lesions.
We explored the relationship between different age subgroups and cystic or solid tumors. The results showed that younger symptomatic patients tended to have cystic hemangioblastoma, while older symptomatic patients tended to have solid hemangioblastoma. Furthermore, survival analysis showed that the PFS of cystic patients was better than that of solid patients (p < 0.05). Combined with the Cox regression analysis, it was found that cystic tumor and GTR were two factors of PFS. Based on these results, we recommend that, for young patients with supratentorial cystic hemangioblastoma accompanied by edema, if the tumor is not located in the sellar region, for which the total resection rate was 65% in our study, which was lesser than that for cerebrum (89%), ventricle (69%), and optic nerve (80%), GTR could be the first priority for surgery for achieving a better prognosis.
Craniospinal hemangioblastoma occurs in over 80% of patients with VHL-associated hemangioblastoma, and multiple lesions occur in over 90% of patients (25). The results of our data analysis showed that sporadic patient cases (91.82%) were found for supratentorial single lesions. While sporadic and VHL-related hemangioblastomas share histological characteristics in the CNS, the clinical course of these tumors may differ significantly (42). Compared with sporadic patient cases, VHL patients, whose lesions were detected at a younger age, are more likely to develop multiple tumors. Lonser et al. (19) also found that in patients with VHL, new tumorigenesis seemed to correlate with younger age. Patients younger than 20 years were more likely to develop new tumors than those older than 40 years. In our study, we found that the average age of VHL patients developing supratentorial hemangioblastoma was younger than that of their sporadic counterparts, indicating that, during the lifetime of a VHL patient, the risk of new tumors decreases with age. This age-related tumorigenesis may be due to VHL gene missense mutated pVHL (VHL protein) (8), which still potently participates in the course of hypoxia inducible factor (HIF) degradation. However, due to the instability of the mutant protein, it is often degraded by chaperone proteins (e.g. Hsp70 and Hsp90) shortly after transcription (43). Previous studies have confirmed a progressive decline in the function of these chaperones with aging, which may contribute to the prolongation of missense VHL protein activity and attenuation of the new tumor growth (44). This age-related decline in proteasome function may partially account for the difference in the onset age of supratentorial hemangioblastoma between the VHL and the sporadic patient cases.
In conclusion, in this study, we mainly performed a detailed and in-depth analysis of the clinically relevant features of supratentorial hemangioblastoma by summarizing previous case reports from the literature and case series from our institute. We found that, in supratentorial hemangioblastoma, GTR significantly prolonged patients’ PFS compared with STR and RT. Meanwhile, we also found that cystic hemangioblastoma existed as a separate entity and had significant differences with solid tumors in terms of preoperative KPS, treatment modality, and PFS. However, due to the limited number of cases and insufficient clinical data in this study, further multicentric prospective studies and in-depth exploration are needed to refine the management of supratentorial hemangioblastoma.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the medical ethics committee of Xiangya Hospital of Central South University. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with national legislation and institutional requirements.
Study design and data acquisition were done by LC and XL; funding acquisition was done by SW and XL; all data were reviewed and validated by YX and YX; radiological information was reviewed and validated by SW and YL; clinical interpretation was done by LC and YL; statistical guidance and data analysis were provided by ZX and YZ; result interpretation and manuscript writing were done by LC and XL. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grant no. 81770781, 81472594) and the Natural Science Foundation of Hunan Province, China (grant no. 2019JJ50978).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1092140/full#supplementary-material.
1. Rocha L, Noronha C, Taipa R, Reis J, Gomes M, Carvalho E. Supratentorial hemangioblastomas in von Hippel–Lindau wild-type patients—case series and literature review. Int J Neurosci. (2018) 128(3):295–303. doi: 10.1080/00207454.2017.1385613
2. Hussein MR. Central nervous system capillary haemangioblastoma: the pathologist’s viewpoint. Int J Exp Pathol. (2007) 88(5):311–24. doi: 10.1111/j.1365-2613.2007.00535.x
3. Vortmeyer AO, Frank S, Jeong SY, Yuan K, Ikejiri B, Lee YS, et al. Developmental arrest of angioblastic lineage initiates tumorigenesis in von Hippel–Lindau disease. Cancer Res. (2003) 63(21):7051–5. Available at: https://aacrjournals.org/cancerres/article/63/21/7051/510736/Developmental-Arrest-of-Angioblastic-Lineage14612494
4. Shively SB, Beltaifa S, Gehrs B, Duong H, Smith J, Edwards NA, et al. Protracted haemangioblastic proliferation and differentiation in von Hippel–Lindau disease. J Pathol. (2008) 216(4):514–20. doi: 10.1002/path.2435
5. Zhuang Z, Frerich JM, Huntoon K, Yang C, Merrill MJ, Abdullaev Z, et al. Tumor derived vasculogenesis in von Hippel–Lindau disease-associated tumors. Sci Rep. (2014) 4:4102. doi: 10.1038/srep04102
6. Jagannathan J, Lonser RR, Smith R, DeVroom HL, Oldfield EH. Surgical management of cerebellar hemangioblastomas in patients with von Hippel–Lindau disease. J Neurosurg. (2008) 108(2):210–22. doi: 10.3171/JNS/2008/108/2/0210
7. Peyre M, David P, Van Effenterre R, Francois P, Thys M, Emery E, et al. Natural history of supratentorial hemangioblastomas in von Hippel–Lindau disease. Neurosurgery. (2010) 67(3):577–87, discussion 587. doi: 10.1227/01.NEU.0000374846.86409.A7
8. Signorelli F, Piscopo G, Giraud S, Guerriero S, Laborante A, Latronico ME, et al. von Hippel–Lindau disease: when neurosurgery meets nephrology, ophthalmology and genetics. J Neurosurg Sci. (2019) 63(5):548–65. doi: 10.23736/S0390-5616.17.04153-4
9. Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. (2010) 152A(2):327–32. doi: 10.1002/ajmg.a.33139
10. Wait SD, Vortmeyer AO, Lonser RR, Chang DT, Finn MA, Bhowmick DA, et al. Somatic mutations in VHL germline deletion kindred correlate with mild phenotype. Ann Neurol. (2004) 55(2):236–40. doi: 10.1002/ana.10807
11. Wang JY, Peng SH, Li T, Ning XH, Liu SJ, Hong BA, et al. Risk factors for survival in patients with von Hippel–Lindau disease. J Med Genet. (2018) 55(5):322–8. doi: 10.1136/jmedgenet-2017-104995
12. Filling-Katz MR, Choyke PL, Oldfield E, Charnas L, Patronas NJ, Glenn GM, et al. Central nervous system involvement in von Hippel–Lindau disease. Neurology. (1991) 41(1):41–6. doi: 10.1212/WNL.41.1.41
13. Walther MM, Linehan WM. von Hippel–Lindau disease and pheochromocytoma. JAMA. (1996) 275(11):839–40. doi: 10.1001/jama.275.11.839
14. Neumann HP, Eggert HR, Scheremet R, Schumacher M, Mohadjer M, Wakhloo AK, et al. Central nervous system lesions in von Hippel–Lindau syndrome. J Neurol Neurosurg Psychiatry. (1992) 55(10):898–901. doi: 10.1136/jnnp.55.10.898
15. Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel–Lindau disease. Lancet. (2003) 361(9374):2059–67. doi: 10.1016/S0140-6736(03)13643-4
16. Weng JC, Song LR, Li D, Wang L, Wu Z, Wang JM, et al. Surgical management and prognostic factors for primary intracranial myxoma: a single-institute experience with a systematic review. J Neurosurg. (2018) 131(4):1115–25. doi: 10.3171/2018.6.JNS181132
17. Firsching RP, Fischer A, Peters R, Thun F, Klug N. Growth rate of incidental meningiomas. J Neurosurg. (1990) 73(4):545–7. doi: 10.3171/jns.1990.73.4.0545
18. Byun J, Yoo HJ, Kim JH, Kim YH, Cho YH, Hong SH, et al. Growth rate and fate of untreated hemangioblastomas: clinical assessment of the experience of a single institution. J Neurooncol. (2019) 144(1):147–54. doi: 10.1007/s11060-019-03213-z
19. Lonser RR, Butman JA, Huntoon K, Asthagiri AR, Wu T, Bakhtian KD, et al. Prospective natural history study of central nervous system hemangioblastomas in von Hippel–Lindau disease. J Neurosurg. (2014) 120(5):1055–62. doi: 10.3171/2014.1.JNS131431
20. Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel–Lindau disease. J Neurosurg. (2003) 98(1):82–94. doi: 10.3171/jns.2003.98.1.0082
21. Mills SA, Oh MC, Rutkowski MJ, Sughrue ME, Barani IJ, Parsa AT. Supratentorial hemangioblastoma: clinical features, prognosis, and predictive value of location for von Hippel–Lindau disease. Neuro Oncol. (2012) 14(8):1097–104. doi: 10.1093/neuonc/nos133
22. Huntoon K, Wu T, Elder JB, Butman JA, Chew EY, Linehan WM, et al. Biological and clinical impact of hemangioblastoma-associated peritumoral cysts in von Hippel–Lindau disease. J Neurosurg. (2016) 124(4):971–6. doi: 10.3171/2015.4.JNS1533
23. Lohle PN, van Mameren H, Zwinderman KH, Teepen HL, Go KG, Wilmink JT. On the pathogenesis of brain tumour cysts: a volumetric study of tumour, oedema and cyst. Neuroradiology. (2000) 42(9):639–42. doi: 10.1007/s002340000363
24. Berkman RA, Merrill MJ, Reinhold WC, Monacci WT, Saxena A, Clark WC, et al. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest. (1993) 91(1):153–9. doi: 10.1172/JCI116165
25. Butman JA, Linehan WM, Lonser RR. Neurologic manifestations of von Hippel–Lindau disease. JAMA. (2008) 300(11):1334–42. doi: 10.1001/jama.300.11.1334
26. Chittiboina P, Lonser RR. von Hippel–Lindau disease. Handb Clin Neurol. (2015) 132:139–56. doi: 10.1016/B978-0-444-62702-5.00010-X
27. Peker S, Kurtkaya-Yapicier O, Sun I, Sav A, Pamir MN. Suprasellar haemangioblastoma. Report of two cases and review of the literature. J Clin Neurosci. (2005) 12(1):85–9. doi: 10.1016/j.jocn.2004.02.025
28. Li Z, Feng T, Teng H, Hu Y, Yao Y, Liu Y. Suprasellar hemangioblastoma without von Hippel–Lindau disease: a case report and literature review. Int J Clin Exp Pathol. (2015) 8(6):7553–8. Available at: https://aacrjournals.org/cancerres/article/63/21/7051/510736/Developmental-Arrest-of-Angioblastic-Lineage26261668
29. Messina R, Cirrottola G, Tacconi L, Guyotat J, Signorelli F. Pituitary stalk hemangioblastoma: complete resection through orbitozygomatic approach with extradural anterior clinoidectomy. J Neurol Surg B Skull Base. (2022) 83(Suppl 3):e661–2. doi: 10.1055/s-0042-1757618
30. Smalley SR, Schomberg PJ, Earle JD, Laws ER Jr, Scheithauer BW, O’Fallon JR. Radiotherapeutic considerations in the treatment of hemangioblastomas of the central nervous system. Int J Radiat Oncol Biol Phys. (1990) 18(5):1165–71. doi: 10.1016/0360-3016(90)90454-R
31. Koh ES, Nichol A, Millar BA, Menard C, Pond G, Laperriere NJ. Role of fractionated external beam radiotherapy in hemangioblastoma of the central nervous system. Int J Radiat Oncol Biol Phys. (2007) 69(5):1521–6. doi: 10.1016/j.ijrobp.2007.05.025
32. Hanakita S, Koga T, Shin M, Takayanagi S, Mukasa A, Tago M, et al. The long-term outcomes of radiosurgery for intracranial hemangioblastomas. Neuro Oncol. (2014) 16(3):429–33. doi: 10.1093/neuonc/not201
33. Asthagiri AR, Mehta GU, Zach L, Li X, Butman JA, Camphausen KA, et al. Prospective evaluation of radiosurgery for hemangioblastomas in von Hippel–Lindau disease. Neuro Oncol. (2010) 12(1):80–6. doi: 10.1093/neuonc/nop018
34. Kano H, Shuto T, Iwai Y, Sheehan J, Yamamoto M, McBride HL, et al. Stereotactic radiosurgery for intracranial hemangioblastomas: a retrospective international outcome study. J Neurosurg. (2015) 122(6):1469–78. doi: 10.3171/2014.10.JNS131602
35. Sayer FT, Nguyen J, Starke RM, Yen CP, Sheehan JP. Gamma knife radiosurgery for intracranial hemangioblastomas—outcome at 3 years. World Neurosurg. (2011) 75(1):99–105, discussion 45–8. doi: 10.1016/j.wneu.2010.09.032
36. Silva D, Grabowski MM, Juthani R, Sharma M, Angelov L, Vogelbaum MA, et al. Gamma knife radiosurgery for intracranial hemangioblastoma. J Clin Neurosci. (2016) 31:147–51. doi: 10.1016/j.jocn.2016.03.008
37. Liebenow B, Tatter A, Dezarn WA, Isom S, Chan MD, Tatter SB. Gamma knife stereotactic radiosurgery favorably changes the clinical course of hemangioblastoma growth in von Hippel–Lindau and sporadic patients. J Neurooncol. (2019) 142(3):471–8. doi: 10.1007/s11060-019-03118-x
38. Dornbos D 3rd, Kim HJ, Butman JA, Lonser RR. Review of the neurological implications of von Hippel–Lindau disease. JAMA Neurol. (2018) 75(5):620–7. doi: 10.1001/jamaneurol.2017.4469
39. Moss JM, Choi CY, Adler JR Jr., Soltys SG, Gibbs IC, Chang SD. Stereotactic radiosurgical treatment of cranial and spinal hemangioblastomas. Neurosurgery. (2009) 65(1):79–85. doi: 10.1227/01.NEU.0000348015.51685.D2
40. Qiu J, Cai D, Yang F, Zhou J, Gong Y, Cai L, et al. Stereotactic radiosurgery for central nervous system hemangioblastoma in von Hippel–Lindau disease: a systematic review and meta-analysis. Clin Neurol Neurosurg. (2020) 195:105912. doi: 10.1016/j.clineuro.2020.105912
41. Simone CB 2nd, Lonser RR, Ondos J, Oldfield EH, Camphausen K, Simone NL. Infratentorial craniospinal irradiation for von Hippel–Lindau: a retrospective study supporting a new treatment for patients with CNS hemangioblastomas. Neuro Oncol. (2011) 13(9):1030–6. doi: 10.1093/neuonc/nor085
42. Capitanio JF, Mazza E, Motta M, Mortini P, Reni M. Mechanisms, indications and results of salvage systemic therapy for sporadic and von Hippel–Lindau related hemangioblastomas of the central nervous system. Crit Rev Oncol Hematol. (2013) 86(1):69–84. doi: 10.1016/j.critrevonc.2012.10.001
43. Yang C, Huntoon K, Ksendzovsky A, Zhuang Z, Lonser RR. Proteostasis modulators prolong missense VHL protein activity and halt tumor progression. Cell Rep. (2013) 3(1):52–9. doi: 10.1016/j.celrep.2012.12.007
Keywords: solid hemangioblastoma, supratentorial hemangioblastoma, von Hippel–Lindau disease, neuro-oncology, cystic hemangioblastoma
Citation: Chen L, Xiong Z, Zhou Y, Li Y, Xie Y, Xiong Y, Wanggou S and Li X (2023) Clinical characteristics, surgical management, and prognostic factors for supratentorial hemangioblastoma: A retrospective study. Front. Surg. 9:1092140. doi: 10.3389/fsurg.2022.1092140
Received: 7 November 2022; Accepted: 28 December 2022;
Published: 24 January 2023.
Edited by:
Jun Yang, Peking University Third Hospital, ChinaReviewed by:
Francesco Signorelli,The Catholic University of America, United States© 2023 Chen, Xiong, Zhou, Li, Xie, Xiong, Wanggou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siyi Wanggou em9reWd3b25nQGdtYWlsLmNvbQ== Xuejun Li bHhqbmV1cm9AY3N1LmVkdS5jbg==
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.