- Department of Bone and Joint Sports Medicine, Longyan First Affiliated Hospital of Fujian Medical University, Longyan, China
Objective: This study was performed to compare the clinical effect of arthroscopic debridement vs. arthroscopic microfracture in the treatment of osteochondral lesions of the talus.
Methods: We retrospectively reviewed patients with osteochondral lesion of talus who were admitted to our hospital from April 2020 to April 2021. The patients were divided into Group A (arthroscopic debridement group, n = 39) and Group B (arthroscopic microfracture group, n = 42), and the intraoperative details in the two groups were analyzed. The American Orthopaedic Foot and Ankle Society (AOFAS) score and visual analogue scale (VAS) score were compared between the two groups before surgery and at the last follow-up.
Results: The postoperative AOFAS score (Group A, 40.9–82.26; Group B, 38.12–87.38), VAS score (Group A, 6.44–3.92; Group B, 6.38–2.05) significantly improved in both groups, but the improvement was significantly greater in Group B than in Group A (P < 0.05). Among all patients, the AOFAS and VAS scores of men aged ≤30 years and patients with a low body mass index (BMI) improved more significantly (P < 0.05).
Conclusion: The arthroscopic microfracture for the treatment of osteochondral lesion of talus is superior to joint debridement in terms of improving ankle function, especially in relatively young men with a relatively low BMI.
Introduction
Ankle sprain is the most common injury in sports (1). Osteochondral lesions of the talus (OLTs) are particularly common; such lesions involve any impairment of the articular surface of the talus or subchondral bone (2). These defects often cause symptoms such as deep ankle pain, swelling, weakness, a locking sensation, and instability at the ankle (3–5). The risk factors and etiology of OLTs include acute and severe ankle sprain, fracture, and recurrent ankle sprain. Nontraumatic causes include local osteonecrosis, systemic vascular disease, and congenital or endocrine abnormalities (3). However, because the articular cartilage shows limited healing and regeneration abilities (6), spontaneous healing of OLTs to normal cartilage rarely occurs (7, 8). Recent studies have shown that conservative management of OLTs has poorer outcomes than operative treatment (9, 10).
Various operative treatments of symptomatic OLTs have been reported, such as microfracture, debridement, osteochondral autograft transplantation, subchondral drilling, and autologous chondrocyte implantation (4). Debridement, microfracture, and subchondral drilling are performed for primary lesions of <1.5 cm in diameter (11, 12). These techniques are commonly performed arthroscopically using curettes and an arthroscopic shaver to remove surrounding unstable cartilage. For lesions of <10 mm in diameter, better results may be obtained by debridement combined with microfracture (13, 14). Osteochondral autograft transplantation is suitable for the treatment of total articular cartilage damage with or without subchondral bone cysts (15). Autologous chondrocyte implantation is performed in patients with large cartilage surface lesions in the talus (16). Among these techniques, arthroscopic microfracture and debridement are effective treatments for OLTs. Debridement creates a stable bleeding base through cleanup and curettage (17), and microfracture facilitates cartilage regeneration through bone marrow stimulation. These treatments have the advantages of a simple operation, minimal trauma, high safety and specificity, low cost, and mild postoperative pain (18, 19). However, whether articular debridement or microfracture is the best treatment for osteochondral lesion of talus remains controversial (5, 20). The effect of body mass index (BMI) on the outcome of arthroscopic treatment of OLTs remains unexplored. The present study was performed to compare the effect of ankle arthroscopic joint debridement vs. microfracture in the treatment of osteochondral lesion of talus, and differences in BMI were analyzed for clinical reference.
Materials and methods
General information
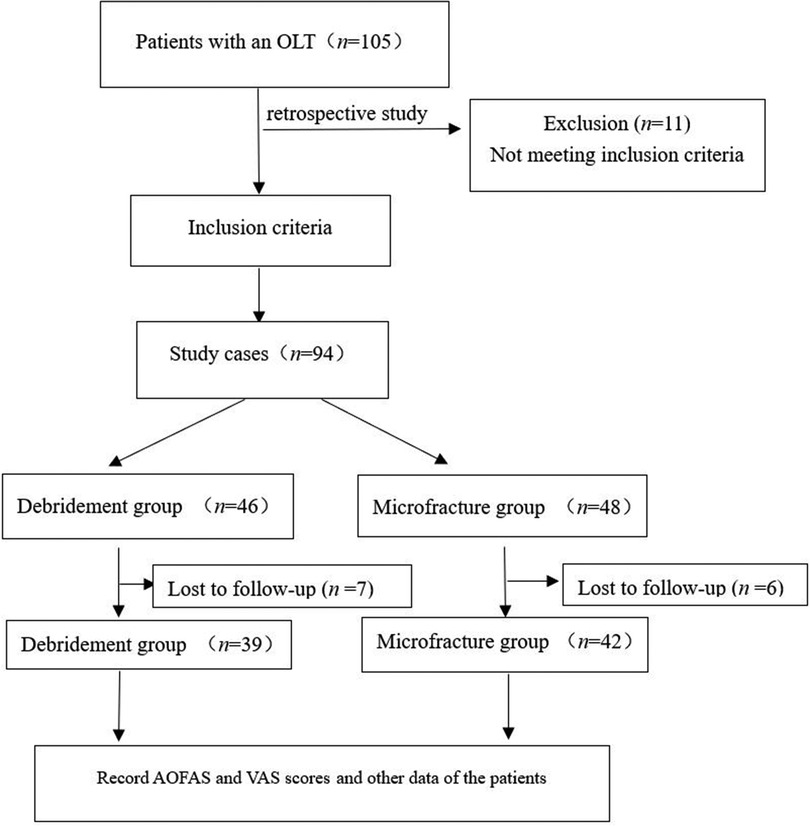
This study involved 105 patients with OLTs admitted to our hospital from April 2020 to April 2021. The inclusion criteria were an age of 18–<70 years; clinical manifestations such as ankle pain, swelling, stiffness, ankle instability, and tenderness in the injured area (2, 6, 13); imaging results that met the clinical diagnostic criteria for stage I and II OLTs; performance of ankle arthroscopic joint debridement or microfracture during hospitalization; and provision of written informed consent for participation in the study. There was no restriction on sex. The exclusion criteria were severe fractures or stiffness in other parts of the body and an osteochondral lesion area of >1.5 cm2. The discontinuation criteria were not following the doctor's advice during rehabilitation and the development of postoperative limb trauma or serious comorbidities.
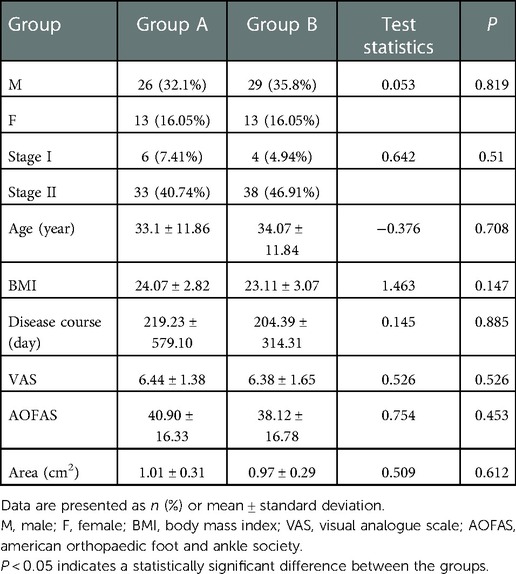
Finally, after applying these criteria, 81 patients were included in the study. The patients were divided into Group A [arthroscopic debridement group, n = 39 (48.15%)] and Group B [arthroscopic microfracture group, n = 42 (51.85%)]. All patients had a single lesion. Group A comprised 26 men and 13 women with a mean age of 33.1 ± 11.86 years and cartilage injury area of 0.5–1.5 cm2 (mean, 1.01 ± 0.31 cm2). According to the magnetic resonance imaging (MRI) staging criteria (21), 6 patients had stage I OLTs and 33 had stage II OLTs. Group B comprised 29 men and 13 women with a mean age of 34.07 ± 11.84 years and cartilage damage area of 0.4–1.44 cm2 (mean, 0.97 ± 0.29 cm2). Four patients had MRI stage I OLTs and 38 patients had stage II OLTs. The BMI was calculated according to the World Health Organization classification of BMI (22).
This study was performed in compliance with the requirements of the World Medical Association Helsinki Declaration (2013) and was approved by the Medical Ethics Committee of Longyan First Affiliated Hospital of Fujian Medical University (No. 201929). All patients provided written informed consent (Figure 1).
Preoperative examination
All patients underwent 3.0 T MRI examination before surgery. The maximum length and width of the damaged area were scanned at different levels to calculate the area of osteochondral lesion of the talus. Sequences were obtained in three planes (coronal, sagittal, and axial) by proton density-weighted and fat suppression imaging. Preoperative MRI scans were evaluated by three musculoskeletal radiologists and two orthopedic surgeons, all with more than 10 years of experience (all were experts who independently evaluated the scans). These three musculoskeletal radiologists and two orthopedic surgeons reached a consensus. The scans were evaluated at an image archiving and communication system workstation. All OLTs were consistent with stages I and II. All patients were treated conservatively for 3 months with poor results.
Surgical technique
All patients underwent spinal anesthesia via the lumbar canal and were placed in the supine position. A tourniquet was applied to the affected leg and thigh for hemostasis. A 30-degree, 4-mm-diameter ankle arthroscope (Arthrex, Naples, FL, United States) was used, and either an anterolateral or anteromedial approach was employed. The anterolateral approach was located at the intersection of the external end of the ankle joint line with the third fibular tendon, and the anteromedial approach was located at the intersection of the medial side of the tibialis anterior tendon with the articular line and the lateral side of the saphenous vein and nerves. Arthroscopic examination revealed varying degrees of synovial hyperplasia.
In Group A, the hyperplastic synovium was removed with curettes and shaver, and the unstable or necrotic cartilage and granulation tissue at the edge of the lesion were cleaned. The subchondral surface was then freshened with shaver and allowed to bleed slightly (23) (Figure 2A).
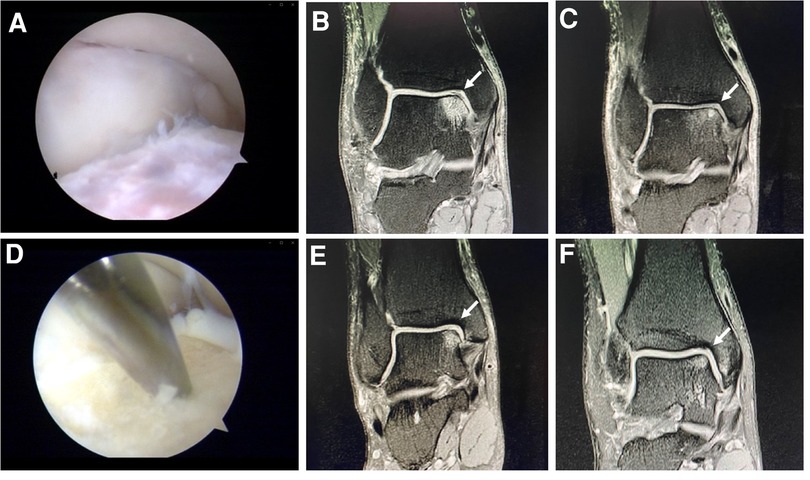
Figure 2. Arthroscopic debridement (A). Preoperative (B) and postoperative (C) MRI of a case in the group A. Microfracture (D). Preoperative (E) and postoperative (F) MRI of a case in the group B.
In Group B, the hyperplastic synovium was removed with curettes and shaver, and the unstable or necrotic cartilage and granulation tissue at the edge of the lesion were cleaned. The microfracture instrument (Arthrex) was used to perform the microfracture operation at the cartilage defect position and the talus subchondral bone plate (depth, 3 mm; spacing, 3–4 mm). If subchondral cysts were present, debridement was performed on the inside of the cysts. No other operations such as bone grafting were performed after microfracture of the capsule wall. The tourniquet was then relaxed; if blood or fat tissue leaked through the hole, the hole was properly perforated. If no blood leakage was observed, the hole was further deepened (24) (Figure 2D). Ligament repair was performed for lateral malleolar ligament injury, and the patients were then treated with a thick cotton pad dressing and posterior plaster splint.
Postoperative treatment: elevation of affected limb, administration of prophylactic anti-infection treatment, and intermittent dressing changes
In the first 6 weeks after surgery, the patients walked using a crutch, placing no weight on the affected limb. Passive ankle activity was carried out twice a day for 15–20 min each time. At 6–8 weeks after the operation, the patients began partial weight-bearing on the affected limb under the crutch. Passive ankle activity was carried out twice a day for 15–20 min each time. From 8 to 12 weeks after the operation, the patients walked with a full load on the affected limb, and the walking time was gradually extended according to their condition. Two to three times a day, the patients performed 5–10 consecutive 2- to 5-minute static squatting exercises with 30-second rest intervals. Jogging, climbing, and other sports were carried out according to the patient's proprioception and balance training (20, 25). The range of motion and wound recovery were reviewed after 12 months of follow-up.
Evaluation criteria and observation indicators of curative effect
The patients' degree of pain was scored according to a visual analogue scale (VAS) (26). Joint function was graded using the American Orthopaedic Foot and Ankle Society (AOFAS) score (27). A score of ≥90 was considered excellent, 80–89 was considered good, 70–79 was considered fair, and ≤69 was considered poor. The good/excellent rate (28) was calculated as follows: (number of excellent cases + number of good cases)/total cases × 100%. VAS scores and AOFAS scores were obtained before surgery and at the last follow-up.
Statistical analysis
SPSS ver. 23.0 statistical software (IBM Corporation, Armonk, NY, United States) was used for the statistical analysis. Measurement data are expressed as mean ± standard deviation; repeated-measures analysis of variance was used for comparison at different time points within a group, and the independent-samples t test was used for comparison between groups at the same time points. Count data are expressed as percentage and were analyzed using the χ2 test. A P-value of <0.05 was considered statistically significant.
Results
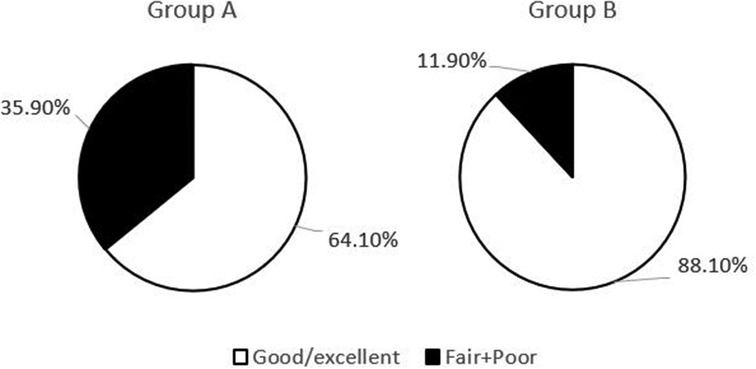
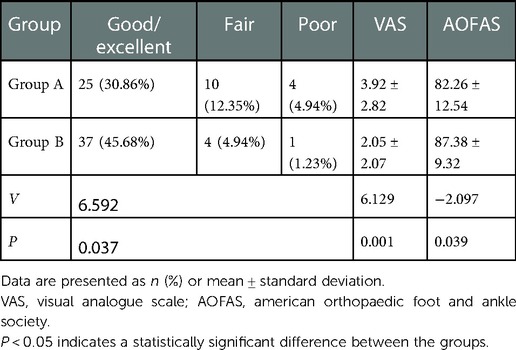
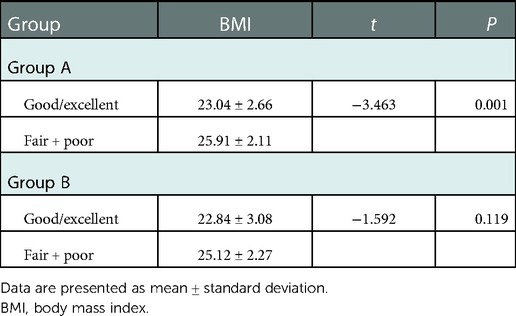
The postoperative follow-up duration was 1 year. There was no statistically significant difference in sex, age, disease course, BMI, VAS score, AOFAS score, or cartilage injury area between the two groups (Table 1). The VAS score in Group A decreased from 6.44 to 3.92, and that in Group B decreased from 6.38 to 2.05. The AOFAS score in Group A improved from 40.9 to 82.26, and that in Group B increased from 38.12 to 87.38 (P < 0.05) (Table 2). At the last follow-up, the AOFAS score in Group A indicated good/excellent joint function in 25 (30.86%) patients, fair in 10 (12.35%), and poor in 4 (4.94%). The AOFAS score in Group B indicated good/excellent joint function in 37 (45.68%) patients, fair in 4 (4.94%), and poor in 1 (1.23%). There was a statistically significant difference between the two groups (P < 0.05) (Table 2). The good/excellent rate in Group B was significantly higher than that in Group A (P < 0.05) (Figure 3). MRI at final follow-up showed more obvious cartilage regeneration in Group B than in Group A (Figures 2B,C,E,F). The patients with an overall excellent AOFAS score at the last follow-up comprised 32 (39.51%) men and 9 (11.11%) women aged ≤30 years and 11 (13.58%) men and 10 (12.35%) women aged >30 years, and most of the patients with excellent joint function were young men (P < 0.05) (Table 3). There was no statistically significant difference in area between patients with excellent (1.09 ± 0.29) and poor (1.07 ± 0.32) scores (P > 0.05). There was also no significant difference in disease course between patients with excellent (225.48 ± 516.13) and fair or poor (168.11 ± 165.83) scores (P > 0.05). The BMI of patients with excellent joint function (22.92 ± 2.90 kg/m2) was significantly lower than that of patients with fair and poor joint function (25.71 ± 2.12 kg/m2) (P < 0.05) (Table 4). In Group A, there was a statistically significant difference in the BMI between patients with a good/excellent outcome (23.04 ± 2.66) and those with a fair or poor outcome (25.91 ± 2.11) (P < 0.05). In Group B, the BMI was not significantly different between patients with a good/excellent outcome (22.84 ± 3.08) and those with a fair or poor outcome (25.12 ± 2.27) (P > 0.05) (Table 5).
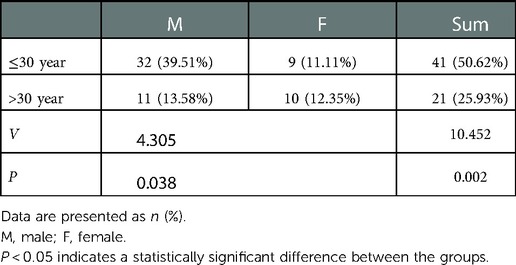
Table 3. Male-to-female ratio of patients with good/excellent joint function aged ≤30 and >30 years.

Table 4. Comparison of cartilage lesion area, BMI, and disease course between patients with good/excellent joint function and those with fair and poor joint function.
Discussion
Among the available surgical management techniques, arthroscopic debridement and microfracture yields a success rate of approximately 85%. Because arthroscopic debridement and microfracture provides rapid recovery with high cost-effectiveness, technical feasibility, a high success rate, and a low incidence rate of complications, this treatment has been widely accepted as a major therapeutic strategy with good functional prognosis (29, 30). Debridement results in freshening of the cartilage surface and enhanced cartilage growth and healing, but the irregularity and depression of the subchondral bone plate after cartilage debridement may create the risk of hyaline cartilage detachment (31). The basic principle of microfracture is penetration of the subchondral bone to induce a repair reaction. Subchondral bone penetration induces the release of serum factors, resulting in the formation of fibrocartilage that covers the wound. After its initial formation, the fibrocartilage must be stabilized to reach a certain mechanical strength (32). The biomechanical properties of fibrocartilage are inferior to those of hyaline cartilage; over time, fibrocartilage decreases in quality and shows inferior wear characteristics. Microfracture is an effective method to relieve clinical symptoms through subchondral decompression (33). Chuckpaiwong et al. (11) reported good functional results of OLTs treated by arthroscopic microfracture in 105 patients with a mean follow-up of 31.6 months. In another study, the patients' ankle function and quality of life were satisfactorily improved after 6.7 years of follow-up after microfracture (34). Therefore, according to these studies, the long-term clinical outcomes after microfracture are just as good as the short- to medium-term outcomes (30). The present study suggests that existing degenerative changes and persistence of fibrochondral deficiency may be related to poor outcomes. Shimozono et al. (35) studied the morphologic changes in the upper subchondral bone on MRI scans 2 years after microfracture and reported worsening clinical outcomes and poor radiographic results over time.
The VAS and AOFAS scores in both groups were significantly improved at the last follow-up compared with those before surgery, indicating that both debridement and microfracture were effective. The good/excellent rate in Group B was higher than that in Group A (P < 0.05), indicating that the effect of microfracture was better than that of debridement. These findings suggest that broken cartilage itself can form cartilage, and microfracture can induce an overflow of growth factors into the bone marrow and promote cartilage growth. Microfracture was also more effective in men aged ≤30 than >30 years. Studies have shown that broken cartilage itself has a high potential to form cartilage (36). Therefore, microfractures promote growth factor exudation and promote cartilage repair. Considering that young chondrocytes have superior chondrogenesis potential, that joint surface fibrillary formation and cartilage degeneration are age-related processes, and that tensile stiffness and strength gradually decline (37), patients are not advised to continue to engage in competitive sports activities or heavy physical labor after surgical rehabilitation. In the present study, the treatment effect in both groups was better in young patients. Various changes in the synthetic properties of articular cartilage as well as increased apoptosis have been shown to weaken the ability of chondrocytes to repair damaged tissues over time (38), and young cartilage produces more proteoglycan C, type II collagen, and IX mRNA than old cartilage. Additionally, the growth of young cartilage cells in monolayer culture is significantly faster than that of old cartilage cells (39). This is considered to be related to the strong ability of chondrocytes to repair damaged tissues, which is consistent with the results of this study.
The reported sensitivity and specificity of MRI for osteochondral lesion of the talus is 96% (40). Surgical treatment is recommended for patients with stage I and II MRI manifestations who undergo failed standard conservative treatment (41). In this study, the stripped talus cartilage and surrounding proliferative tissues were completely debrided by a shaver under arthroscopy (42). Patients in Group B underwent microfracture of cartilage on the basis of complete dissection of proliferating cartilage and peripheral tissue, uneven fibular cartilage in the postoperative cartilage-injured area, excellent midterm follow-up results, and reliable results. Effective repair of talus cartilage lesions and restoration of normal joint function were achieved (12). There was no statistically significant difference in the cartilage damage area or disease course between patients with good/excellent joint function and those with fair and poor joint function. Additionally, there was no significant difference in the cartilage damage area of the patients included in this study (all areas were within 1.5 cm2). The patients did not exercise aggressively after surgery, and pain and swelling did not aggravate their condition; thus, there was no difference in the disease course. The BMI of patients with good/excellent joints was significantly lower than that of patients with fair and poor joints, and our analysis was based on the following two reasons: Firstly, obesity has negative physiologic and psychological impacts on patients' postoperative quality of life and affects their postoperative recovery. Secondly, obese patients have a poor prognosis for the development of degenerative joint disease, consistent with previous studies (43, 44). Finally, obesity-associated behavior such as functional limitations, constant dieting, and mental stress from their body image and poor self-esteem, coupled with social stigma, all play a strong role linking obesity with depression (45). In Group B, we concluded that an elevated BMI did not adversely affect pain and function, and a high proportion of patients reported greater postoperative satisfaction and achievement, similar to other studies (44). Instead, patients should be encouraged to normalize their BMI in view of the negative impact on their quality of life and the physical limitations associated with a raised BMI.
At the 2018 International Consensus Meeting on Cartilage Repair of the Ankle (46), complete weight bearing was not recommended within 2–3 months after ankle cartilage surgery. In this study, the patients performed partial weight bearing under a crutch within 2 months after surgery. As their rehabilitation training progressed, the patients could carry out complete weight bearing 3 months after surgery depending on their condition, and jogging, climbing, and other sports activities could be carried out according to their proprioception and balance training. Thus, we believe that the herein-described rehabilitation program is reasonable.
There were still some limitations for the present study. Firstly, the follow-up time was relatively short. Secondly, there was a selection bias in the retrospective study in spite of no significant difference of the basic parameters between the two groups. Finally, not all the patients underwent MRI at final follow-up and most of the results were the subjective evaluation. Further prospective study with more cases, long-term follow-up and objective evaluation was needed in the future.
In conclusion, the microfracture for the treatment of osteochondral lesion of talus is more effective than debridement in improving ankle function, especially in relatively young men with a relatively low BMI.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by this study was performed in compliance with the requirements of the World Medical Association Helsinki Declaration (2013) and was approved by the Medical Ethics Committee of Longyan First Affiliated Hospital of Fujian Medical University (No. 201929). The patients/participants provided their written informed consent to participate in this study.
Author contributions
MZ, DC, DC, JL, PZ, QW, SH, YL and JJ: collected the clinical data and instructed the patients in the rehabilitation training. DC: was in charge of the study design, conducted the literature research and was the primary surgeon. MZ: majorly contributed to the completion of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a startup fund for scientific research from Fujian Medical University (grant no. 2019QH1209).
Acknowledgments
We thank Angela Morben, DVM, ELS, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. (2007) 42(2):311–9. PMID: 1771018117710181
2. Looze CA, Capo J, Ryan MK, Begly JP, Chapman C, Swanson D, et al. Evaluation and management of osteochondral lesions of the talus. Cartilage. (2017) 8(1):19–30. doi: 10.1177/1947603516670708
3. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. (2014) 7(5):414–22. doi: 10.1177/1938640014543362
4. Rikken Q, Kerkhoffs G. Osteochondral lesions of the talus: an individualized treatment paradigm from the amsterdam perspective. Foot Ankle Clin. (2021) 26(1):121–36. doi: 10.1016/j.fcl.2020.10.002
5. O'Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. (2010) 38(2):392–404. doi: 10.1177/0363546509336336
6. Fermín T, Hovsepian JM, D'Hooghe P, Papakostas ET. Arthroscopic debridement of osteochondral lesions of the talus: a systematic review. Foot. (2021) 49:101852. doi: 10.1016/j.foot.2021.101852
7. Wang CC, Yang KC, Chen IH. Current treatment concepts for osteochondral lesions of the talus. Tzu Chi Med J. (2021) 33(3):243–9. doi: 10.4103/tcmj.tcmj_106_20
8. Giannini S, Vannini F. Operative treatment of osteochondral lesions of the talar dome: current concepts review. Foot Ankle Int. (2004) 25(3):168–75. doi: 10.1177/107110070402500311
9. Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. (2010) 18(2):238–46. doi: 10.1007/s00167-009-0942-6
10. Tol JL, Struijs PA, Bossuyt PM, Verhagen RA, van Dijk CN. Treatment strategies in osteochondral defects of the talar dome: a systematic review. Foot Ankle Int. (2000) 21(2):119–26. doi: 10.1177/107110070002100205
11. Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. (2008) 24(1):106–12. doi: 10.1016/j.arthro.2007.07.022
12. Duramaz A, Baca E. Microfracture provides better clinical results than debridement in the treatment of acute talar osteochondral lesions using arthroscopic assisted fixation of acute ankle fractures. Knee Surg Sports Traumatol Arthrosc. (2018) 26(10):3089–95. doi: 10.1007/s00167-018-4963-x
13. Powers RT, Dowd TC, Giza E. Surgical treatment for osteochondral lesions of the talus. Arthroscopy. (2021) 37(12):3393–6. doi: 10.1016/j.arthro.2021.10.002
14. Singh N, Pandey CR, Tamang B, Singh R. Scranton type V osteochondral defects of talus: does one-stage arthroscopic debridement, microfracture and plasma rich in growth factor cause the healing of cyst and cessation of progression to osteoarthritis. Malays Orthop J. (2020) 14(2):64–71. doi: 10.5704/MOJ.2007.014
15. Redondo ML, Beer AJ, Yanke AB. Cartilage restoration: microfracture and osteochondral autograft transplantation. J Knee Surg. (2018) 31(3):231–8. doi: 10.1055/s-0037-1618592
16. Aurich M, Albrecht D, Angele P, Becher C, Fickert S, Fritz J, et al. Treatment of osteochondral lesions in the ankle: a guideline from the group “clinical tissue regeneration” of the German society of orthopaedics and traumatology (DGOU). Z Orthop Unfall. (2017) 155(1):92–9. doi: 10.1055/s-0042-116330
17. Ventura A, Terzaghi C, Legnani C, Borgo E. Treatment of post-traumatic osteochondral lesions of the talus: a four-step approach. Knee Surg Sports Traumatol Arthrosc. (2013) 21(6):1245–50. doi: 10.1007/s00167-012-2028-0
18. Corr D, Raikin J, O’Neil J, Raikin S. Long-term outcomes of microfracture for treatment of osteochondral lesions of the talus. Foot Ankle Int. (2021) 42(7):833–40. doi: 10.1177/1071100721995427
19. Qulaghassi M, Cho YS, Khwaja M, Dhinsa B. Treatment strategies for osteochondral lesions of the talus: a review of the recent evidence. Foot. (2021) 47:101805. doi: 10.1016/j.foot.2021.101805
20. Lee KB, Park HW, Cho HJ, Seon JK. Comparison of arthroscopic microfracture for osteochondral lesions of the talus with and without subchondral cyst. Am J Sports Med. (2015) 43(8):1951–6. doi: 10.1177/0363546515584755
21. Hepple S, Winson IG, Glew D. Osteochondral lesions of the talus: a revised classification. Foot Ankle Int. (1999) 20(12):789–93. doi: 10.1177/107110079902001206
22. Caballero B. Humans against obesity: who will win. Adv Nutr. (2019) 10(Suppl_1):S4–S9. doi: 10.1093/advances/nmy055
23. Yang HY, Lee KB. Arthroscopic microfracture for osteochondral lesions of the talus: second-Look arthroscopic and magnetic resonance analysis of cartilage repair tissue outcomes. J Bone Joint Surg Am. (2020) 102(1):10–20. doi: 10.2106/JBJS.19.00208
24. Kim TY, Song SH, Baek JH, Hwang YG, Jeong BO. Analysis of the changes in the clinical outcomes according to time after arthroscopic microfracture of osteochondral lesions of the talus. Foot Ankle Int. (2019) 40(1):74–9. doi: 10.1177/1071100718794944
25. Lee DH, Lee KB, Jung ST, Seon JK, Kim MS, Sung IH. Comparison of early versus delayed weightbearing outcomes after microfracture for small to midsized osteochondral lesions of the talus. Am J Sports Med. (2012) 40(9):2023–8. doi: 10.1177/0363546512455316
26. McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. (1988) 18(4):1007–19. doi: 10.1017/S0033291700009934
27. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. (1994) 15(7):349–53. doi: 10.1177/107110079401500701
28. Friedrich KM, Mamisch TC, Plank C, Langs G, Marlovits S, Salomonowitz E, et al. Diffusion-weighted imaging for the follow-up of patients after matrix-associated autologous chondrocyte transplantation. Eur J Radiol. (2010) 73(3):622–8. doi: 10.1016/j.ejrad.2008.12.017
29. Park HW, Lee KB. Comparison of chondral versus osteochondral lesions of the talus after arthroscopic microfracture. Knee Surg Sports Traumatol Arthrosc. (2015) 23(3):860–7. doi: 10.1007/s00167-014-3061-y
30. Hannon CP, Bayer S, Murawski CD, Canta GL, Clanton TO, Haverkamp D, et al. Debridement, curettage, and bone marrow stimulation: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. (2018) 39(1_Suppl):16S–22S. doi: 10.1177/1071100718779392
31. Reilingh ML, Lambers K, Dahmen J, Opdam K, Kerkhoffs G. The subchondral bone healing after fixation of an osteochondral talar defect is superior in comparison with microfracture. Knee Surg Sports Traumatol Arthrosc. (2018) 26(7):2177–82. doi: 10.1007/s00167-017-4654-z
32. Wei M, Wei Y, Liu Y. Effects of early weightbearing on microfracture treatment of osteochondral lesions of talus with subchondral bone defects. Curr Med Sci. (2019) 39(1):88–93. doi: 10.1007/s11596-019-2004-8
33. Yontar NS, Aslan L, Can A, Ogut T. One step treatment of talus osteochondral lesions with microfracture and cell free hyaluronic acid based scaffold combination. Acta Orthop Traumatol Turc. (2019) 53(5):372–5. doi: 10.1016/j.aott.2019.04.002
34. Lee YK, Young KW, Kim JS, Lee HS, Cho WJ, Kim HN. Arthroscopic microfracture with atelocollagen augmentation for osteochondral lesion of the talus: a multicenter randomized controlled trial. BMC Musculoskelet Disord. (2020) 21(1):716. doi: 10.1186/s12891-020-03730-3
35. Shimozono Y, Coale M, Yasui Y, Halloran A, Deyer TW, Kennedy JG. Subchondral bone degradation after microfracture for osteochondral lesions of the talus: an MRI analysis. Am J Sports Med. (2018) 46(3):642–8. doi: 10.1177/0363546517739606
36. Kreulen C, Giza E, Kim J, Campanelli V, Sullivan M. Viability of talus osteochondral defect cartilage for chondrocyte harvesting: results of 151 patients. Foot Ankle Int. (2014) 35(4):341–5. doi: 10.1177/1071100714523272
37. Martin JA, Buckwalter JA. Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. Iowa Orthop J. (2001) 21:1–7. PMID: 1181393911813939
38. Luria A, Chu CR. Articular cartilage changes in maturing athletes: new targets for joint rejuvenation. Sports Health. (2014) 6(1):18–30. doi: 10.1177/1941738113514369
39. Adkisson HD 4th, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, et al. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. (2010) 38(7):1324–33. doi: 10.1177/0363546510361950
40. Elias I, Zoga AC, Morrison WB, Besser MP, Schweitzer ME, Raikin SM. Osteochondral lesions of the talus: localization and morphologic data from 424 patients using a novel anatomical grid scheme. Foot Ankle Int. (2007) 28(2):154–61. doi: 10.3113/FAI.2007.0154
41. Rungprai C, Tennant JN, Gentry RD, Phisitkul P. Management of osteochondral lesions of the talar dome. Open Orthop J. (2017) 11:743–61. doi: 10.2174/1874325001711010743
42. Shimozono Y, Hurley ET, Yasui Y, Deyer TW, Kennedy JG. The presence and degree of bone marrow edema influence midterm clinical outcomes after microfracture for osteochondral lesions of the talus. Am J Sports Med. (2018) 46(10):2503–8. doi: 10.1177/0363546518782701
43. Perry KI, MacDonald SJ. The obese patient: a problem of larger consequence. Bone Joint J. (2016) 98-B(1 Suppl A):3–5. doi: 10.1302/0301-620X.98B1.36415
44. Koh D, Tan M, Zhan X, Li Z, Tay KS, Tan SM, et al. Association of elevated body mass Index and outcomes of arthroscopic treatment for osteochondral lesions of the talus. Foot Ankle Orthop. (2022) 7(2):24730114221103263. doi: 10.1177/24730114221103263
45. Braun TD, Quinn DM, Stone A, Gorin AA, Ferrand J, Puhl RM, et al. Weight bias, shame, and self-compassion: risk/protective mechanisms of depression and anxiety in prebariatic surgery patients. Obesity. (2020) 28(10):1974–83. doi: 10.1002/oby.22920
Keywords: osteochondral lesion of talus, ankle arthroscopy, debridement, microfracture, BMI
Citation: Zhang M, Chen D, Wang Q, Li Y, Huang S, Zhan P, Lai J, Jiang J and Chen D (2023) Comparison of arthroscopic debridement and microfracture in the treatment of osteochondral lesion of talus. Front. Surg. 9:1072586. doi: 10.3389/fsurg.2022.1072586
Received: 17 October 2022; Accepted: 19 December 2022;
Published: 13 January 2023.
Edited by:
Dong Jiang, Peking University Third Hospital, ChinaReviewed by:
Chen Jiao, Peking University Third Hospital, ChinaYunfeng Yang, Tongji University, China
© 2023 Zhang, Chen, Wang, Li, Huang, Zhan, Lai, Jiang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongfeng Chen Y2RmeWR5eEAxMjYuY29t
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
Abbreviations OLT, osteochondral lesion of the talus; AOFAS, american orthopaedic foot and ankle society; VAS, visual analogue scale; MRI, magnetic resonance imaging; BMI, body mass index.
 Minghua Zhang
Minghua Zhang Dongfeng Chen
Dongfeng Chen