- 1Department of Urology, Peking University Third Hospital, Beijing, China
- 2Department of Pathology, Peking University Third Hospital, Beijing, China
Background: Leiomyosarcoma (LMS) is a malignant spindle-cell mesenchymal tumor originating from the smooth muscle cells, which mostly affects soft tissues and abdominopelvic organs over extremities. Primary LMS of the penis is a relatively uncommon mesenchymal tissue disease and a poorly understood condition.
Case Report: A 69-year-old man presented with a growing, painless mass protruding from the penis. The irregularly lobulated lump was roughly 3 cm × 2.5 cm, with a smooth surface, tough texture, distinct boundary, and no tenderness. It was determined to be a penile tumor during the preoperative radiological evaluation. The patient underwent resection of the penile mass, followed by extended resection in the second operation. The diagnosis of LMS was verified by pathological examination. During a 20-month follow-up, the patient made a smooth recovery and remained disease-free.
Conclusion: An immunohistochemical examination is essential for rendering this rare diagnosis. Radical excision of tumor lesions with negative cut margins is guaranteed to be the best treatment for primary penile LMS. Close follow-up should be provided due to the high rate of local recurrence.
Introduction
Malignant tumors of the penis are relatively rare, with an incidence of about 1 per 100,000 in developed countries such as North America and Europe; however, the incidence in less economically developed regions such as Asia, Africa, and South America is slightly higher than that in the aforementioned developed regions. The majority of penile malignant tumors (95%) are squamous cell carcinoma, while adenocarcinoma, malignant melanoma, and sarcoma are sporadic (1). Malignant mesenchymal tissue tumors (including Kaposi’s sarcoma, smooth muscle sarcoma, rhabdomyosarcoma, and malignant fibrous histiocytoma) account for less than 5%, among which primary leiomyosarcoma (LMS) of the penis is incredibly uncommon, mostly affecting middle-aged and older males (2). In 1969, Pratt and Ross (3) first classified LMS of the penis into deep and superficial types according to the tumor site. Since deep LMS has early metastasis and poor prognosis, early diagnosis and correct identification of the type are crucial.
A 69-year-old patient with primary penile LMS was admitted to our hospital in March 2021. This article, which is based on the CARE Guideline (4) and includes a reporting checklist in the supplementary material, analyzes the case data of this patient and reviews the pertinent literature to discuss the clinical manifestations, diagnosis, and treatment of primary penile LMS in order to better understand and diagnose it.
Case presentation
A 69-year-old male patient was admitted to the hospital with a 3-year painless mass in the penis as his main complaint. Three years ago, the patient had a soy bean-sized, painless mass in the middle shaft of the penis with no obvious cause. No ulceration or effusion, urinary frequency, urgency, or urinary pain was present nor was there a fever or any other discomfort. The lump rapidly enlarged to a diameter of 3 cm 6 months ago, without any swelling, heat, pain, or any other discomfort. The patient had a history of hypertension and type 2 diabetes mellitus, both of which responded effectively to oral treatment.
Physical examination revealed an irregular lobulated mass measuring about 3 cm × 2.5 cm in the middle shaft part of the penis on the ventral side, with a smooth surface, tough texture, clear border, a moderate range of motion, and no tenderness. There was no visible skin ulceration on the surface, no redness or swelling of urethral orifice, and no aberrant secretion. The epididymis and bilateral testis were both normal, and there were no swollen lymph nodes in the inguinal region. The skin color of the scrotum was also normal.
Auxiliary examination revealed that the usual tests for blood, urine, stool, liver and kidney function, electrolytes, myocardial enzymes, coagulation function, and prostate-specific antigen were all normal. Preoperative pelvic magnetic resonance imaging (MRI) (male genital system) showed a nodular mixed signal shadow in the distal penis, with a size of about 31 mm × 27 mm × 22 mm, and a high signal on diffusion-weighted imaging (DWI). No noticeable large lymph nodes were seen in the pelvis (Figure 1). Preoperative ultrasonography showed that no enlarged lymph nodes were detected in the bilateral inguinal region.
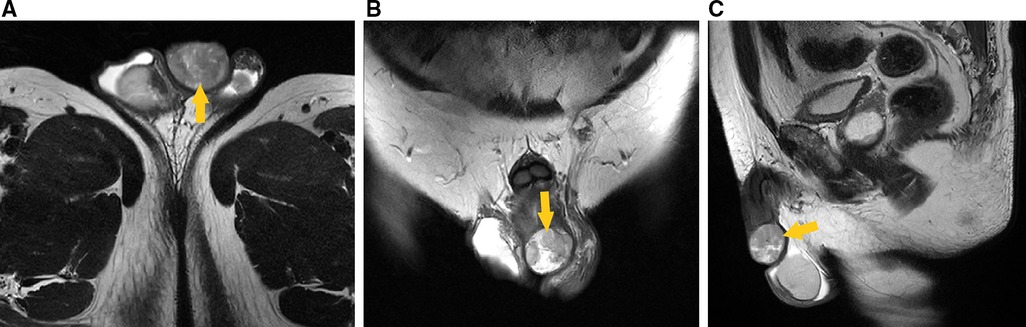
Figure 1. Preoperative pelvic MRI (male genital system) showed nodular mixed signal shadow in the distal penis (arrow head), with a size of about 31 mm × 27 mm × 22 mm, and a high signal on DWI. (A) Transverse section, T2-weighted imaging. (B) Coronal section, T2-weighted imaging. (C) Median sagittal section, T2-weighted imaging.
Under intralesional anesthetic, the patient had the penile lump removed. The mass was in the lower fascia layer (Eberth fascia), totally excised, and there was no visible adhesion between it and the surrounding tissues.
Gross examination revealed a grey-yellow necrotic tumor, measuring 3 cm × 3 cm × 2.5 cm. Microscopic examination showed severe atypia and high mitoses. Immunohistochemical results showed that S-100 (−), CD34 (−), Ki-67 (about 70% +), P53 (consistent with wild type), smooth muscle actin (SMA) (+), Desmin (+), and Caldesmon (+). Results from immunohistochemistry and morphology were consistent with LMS (Figure 2).
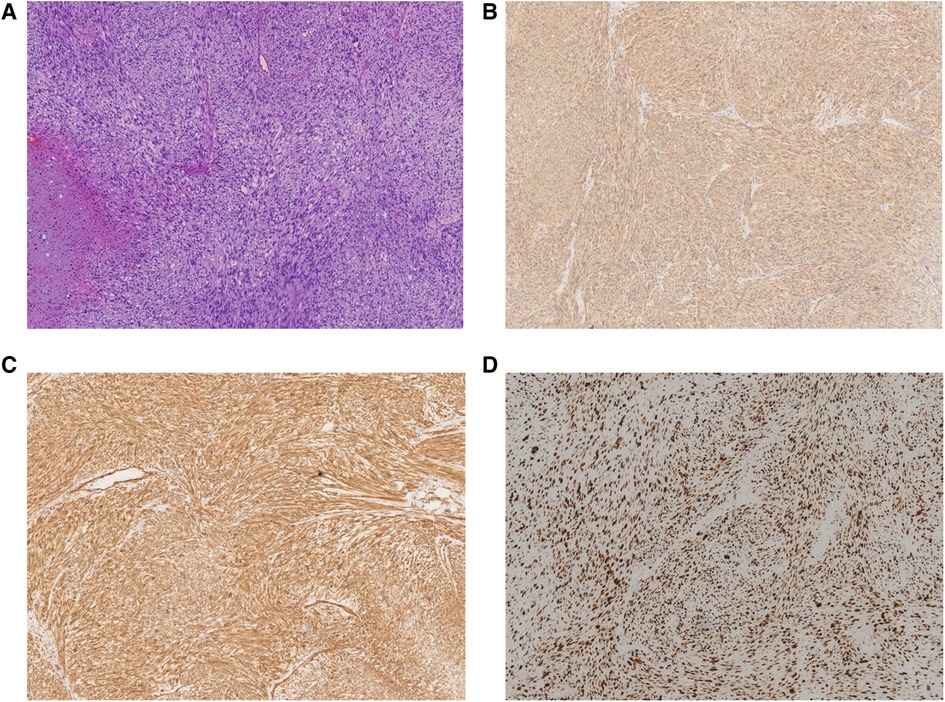
Figure 2. Pathological examination showing that the mass was composed of atypical spindle-shaped cells tumor with focal necrosis. (A) Hematoxylin and eosin (HE) staining with high mitoses and atypical mitoses (×40). (B) Immunohistochemistry staining of SMA (×40). (C) Immunohistochemistry staining of Caldesmon (×40). (D) Immunohistochemistry staining of Ki-67 (×40).
Three weeks later, the patient was readmitted and an extended resection was performed under endotracheal anesthesia based on sufficient dialog with the patient and his families. The skin was excised from the surgical site within 3 cm, and subcutaneous tissue was dissected all the way to Buck’s fascia. Postoperative pathology suggested no residual tumor cells.
There was no local discomfort after surgery and slight scarring at the wound; postoperative erectile function was basically the same as before. No local tumor recurrence, inguinal lymph node enlargement, and metastasis were noticed over the 20-month follow-up.
Discussion
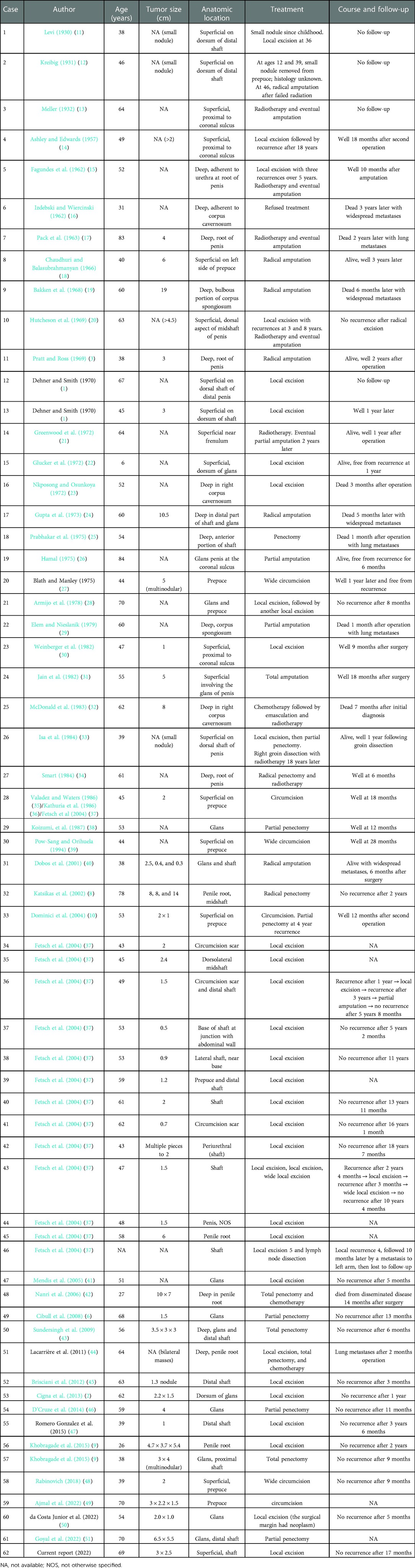
LMS is one of the most common subtypes of malignant mesenchymal tissue tumors, accounting for approximately 10%–20% of soft tissue sarcomas, which often affects the abdomen, retroperitoneum, large vessel wall, and uterus (5), and rarely involves the penis. Primary sarcomas of the penis also include Kaposi’s sarcoma, epithelioid hemangioendothelioma, hemangiosarcoma, and rhabdomyosarcoma. Since Levi’s initial report in 1930, 61 cases of primary LMS of the penis have been reported in English (Table 1), ranging in age from 6 to 84 years, with cases most frequently occurring in people in their forties and fifties (6–9). Penile LMS often originates from the following structures: (1) the dermal layer of the erector spinae; (2) the superficial fascial muscular layer of the penis; (3) the muscular layer of the superficial vessels outside the tunica albuginea; and (4) the muscular layer of the deep vascular complex that make up the corpus cavernosum and corpus spongiosum. With the tunica albuginea acting as the boundary, it can be divided into deep LMS and superficial LMS. Superficial LMS mostly appears on the surface of the distal penis or glans and is characterized by painless nodules, sluggish development, and a few deep infiltrations. Deep LMS, however, can involve the smooth muscle of the corpus cavernosum and invade the urethra and other surrounding structures (10).
LMS of the penis can present with a variety of clinical manifestations, such as painless nodules or masses, localized pain and swelling, superficial skin ulceration, hematuria, urethral obstruction, and enlargement of inguinal lymph node. In comparison to superficial LMS, deep LMS located below the deep fascia of the penis are more likely to manifest with hematuria and urethral obstruction (10). The most frequent location of tumor was the shaft, followed by the prepuce, whereas the coronal sulcus, the frenulum, and circumcision scars were additional uncommon locations (2).
Due to the rarity of penile LMS and the lack of distinct clinical symptoms, tumor excision and biopsy are typically required for diagnosis. A careful inspection of the lesion and palpation of the inguinal lymph nodes are required if a clinical suspicion of soft tissue sarcoma is strong. It is preferable to use MRI to demonstrate the depth of tumor invasion and evaluate both inguinal and pelvic lymph nodes. Deep LMS is prone to blood metastases, with the lung and liver being the most common sites of metastasis; therefore, a simultaneous CT scan of the chest and abdomen is recommended for high-risk tumors.
The pathologic diagnosis often includes a pretreatment biopsy and further pathologic evaluation after the tumor has been surgically removed to differentiate it from other sarcomas (52). It is important to note that fine needle aspiration frequently yields inadequate tissue to make a diagnosis. LMS consists of cells with a smooth muscle spectrum; both superficial and deep LMS have the same characteristic histologic features: spindle-shaped cells, with eosinophilic cytoplasm, long rod-shaped and darkly stained nuclei (53). The mitotic rate and other mitotic variables could predict the tendency of tumor invasion to adjacent structures or metastasis (8). In immunohistochemistry, SMA, Desmin, and Caldesmon are typically positive, but none of these markers are specific for smooth muscle differentiation (53). Cytokeratin and S100 were negative and could be differentiated from epithelial tissue (39). Negative CD34 could be identified from Kaposi’s sarcoma (46). Immunopositive results for P16 and P53 with high Ki-67 proliferation index are highly sensitive and specific for the distinction of LMS and leiomyoma (52).
The principle of treatment for primary penile LMS is currently considered to be radical resection of the tumor lesion (R0 surgery) with maximum local organs preservation. Whether local lesion excision, partial penectomy, or total penectomy is performed depends on tumor type, size, and presence of metastasis. Tumor size is one of the best predictors of outcome for primary LMS of penis, when stratified as follows: ≤2 vs. >2 cm and ≤5 vs. >5 cm (37). First, local lesion and extensive resection is the best choice for superficial LMS and ≤2 cm in diameter, and the prognosis is better because distant metastasis of superficial LMS is rare. Due to the risk of recurrence, it is important to ensure a safe margin. The most critical factor for recurrence-free survival is the microscope-negative tumor margin. The majority of studies recommend a margin of at least 1 cm, while some have found that a margin of 2–5 cm is associated with a decreased rate of recurrence after resection (54). For subcutaneous LMS of the skin, it is recommended and desirable for complete excision of the subcutaneous tissue with at least 2–3 cm of the skin margin and subcutaneous tissue (55). A second surgery was performed in our patient that included a deep subcutaneous tissue excision that reached the penile fascia (Buck’s fascia) and an expanded excision of the skin that was removed within 3 cm of the surgical site margin. However, partial penile resection or radical excision is typically the mainstay of treatment for profound LMS (9). When distant metastasis has occurred, the aim of treatment includes symptom relief, tumor volume reduction, and prolonging survival. Because of rare local lymph node metastasis in LMS and the distant metastasis is often present when the peripheral lymph nodes are involved, regional lymph node dissection is not advised in the absence of clearly clinical or imaging evidence of lymph node metastasis (52).
Adjuvant radiation treatment (RT) and chemotherapy may help in the treatment of LMS in order to preserve organ function and reduce local recurrence, although with a limited impact on survival rates (52). In a retrospective study of 14 patients with primary penile LMS in 1994 (39), local tumor recurrence was found in all patients treated with chemotherapy or radiotherapy only, and distant metastasis was found in 2 of them. Hensley et al. (56) reported that gemcitabine combined with docetaxel chemotherapy was significantly effective as first- and second-line treatment for primary penile LMS. The effectiveness of adjuvant radiation and chemotherapy in treating primary penile LMS has not been verified because of the small number of cases.
Tumor size, tumor depth, and histologic grade are the main factors affecting the risk and prognosis of primary penile LMS, as with other soft tissue sarcomas. The American Joint Committee on Cancer (AJCC) grading system has a grading system that takes into account characteristics such as tumor size, lymph node involvement, and distant metastasis; however, no research has determined if penile LMS falls within this system. In the TNM grading system, T stage is divided into T1 and T2 stages with the maximum diameter of 5 cm, but the majority of penile LMS is less than 5 cm. Thus, a lower cut-off value could be more applicable. In addition, for penile LMS, T stage should distinguish the superficial type from the deep type according to the depth of invasion, rather than just by the size of tumor. Moreover, poor differentiation (grade 3 or 4) results in upstaging to stages II or III irrespective of the tumor size (55). The Fédération Nationale des Centres de Lutte Contre Le Cancer (FNCLCC) grading system is the most widely used in the histological grading of soft tissue sarcomas. Based on the degree of differentiation, mitosis, and tumor necrosis, the FNCLCC grading can be divided into X, 1, 2, and 3 grades. The higher the grade is, the worse the prognosis will be (57). Eventually, superficial penile LMS has a better prognosis than deep LMS, especially for primary LMS with infiltration depth ≤2 cm and size ≤5 cm and being treated by extensive local excision with negative incisional margins. However, larger and deep LMS, especially located at the root of the penis, usually have a poor prognosis (33).
Local recurrence may occur after the surgery of primary penile LMS, and tumor cells often become poorly differentiated after recurrence. For superficial and deep lesions, the recurrence rates are 23% and 29%, respectively. However, the risk rate of distant metastasis was higher for deep-type LMS (50%) compared to 8% for superficial LMS. In addition, the risk of metastasis increased with tumor size, with a 29% and 50% chance of metastasis for tumors with a diameter of 5 cm and greater. Local lymph node metastasis is uncommon and occurs mostly in the advanced stages of the disease, when distant metastases are often already present and the prognosis is poor (37).
Follow-up guideline for primary penile LMS are little standardized and adapted from the soft tissue sarcomas in general (55). A complete examination, especially the operation site and inguinal lymph nodes, should be carried out every 3 months for 3 years after resection, every 6 months for the following 2 years, and then annually for up to 10 years. In high-risk cases (>5 cm tumor size, deep LMS, local relapse, high-grade LMS), chest CT should be performed every 3–6 months together with MRI of the primary tumor site and sonography of regional lymph nodes and abdomen.
Additionally, the patient underwent surgery twice. As an improvement measure, it is recommended to be alert to the possibility of malignant mass, particularly given its rapid growth during the last period of the present case, and intraoperative frozen section analysis is necessary. In addition, after local excision, expanded resection of the margins and base of the lesion for biopsy is advised.
Conclusion
In summary, though primary LMS of penis is very rare, it is not difficult to diagnose when pathology is included. Patients with deep lesions are likely to experience distant metastases at an early stage, which often has a bad prognosis. The best therapy for primary penile LMS to date is assured to be radical removal of malignant lesions with negative cut margins, and close monitoring should be administered.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Peking University Third Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1068935/full#supplementary-material.
References
1. Dehner LP, Smith BH. Soft tissue tumors of the penis. A clinicopathologic study of 46 cases. Cancer. (1970) 25(6):1431–47. doi: 10.1002/1097-0142(197006)25:6%3C1431::AID-CNCR2820250624%3E3.0.CO;2-B
2. Cigna E, Maruccia M, Parisi P, Soda G, Nasca MR, Micali G, et al. Superficial leiomyosarcoma of the glans: report of a case and literature review. Aesthetic Plast Surg. (2013) 37(5):1052–8. doi: 10.1007/s00266-013-0199-9
3. Pratt RM, Ross RT. Leiomyosarcoma of the penis. A report of a case. Br J Surg. (1969) 56(11):870–2. doi: 10.1002/bjs.1800561122
4. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
5. George S, Serrano C, Hensley ML, Ray-Coquard I. Soft tissue and uterine leiomyosarcoma. J Clin Oncol. (2018) 36(2):144–50. doi: 10.1200/JCO.2017.75.9845
6. Cibull TL, Thomas AB, Badve S, Billings SD. Leiomyosarcoma of the penis presenting as a cutaneous lesion. J Cutan Pathol. (2008) 35(6):585–7. doi: 10.1111/j.1600-0560.2007.00846.x
7. Trivedi VAM, Rani R, Chauhan R, Singh U, Kuna N. Leiomyosarcoma of penis: an aggressive and exceptionally rare entity. Gulf J Oncol. (2017) 1(25):73–6. PMID: 29019335
8. Katsikas VS, Kalyvas KD, Ioannidis SS, Papathanasiou MV, Panagiotopoulou KP, Hitiroglou PM, et al. Leiomyosarcoma of the penis. Sarcoma. (2002) 6(2):75–7. doi: 10.1080/1357714021000022177
9. Khobragade KH, Tamhankar AS, Bakshi GK, Tongaonkar HB, Menon S. Leiomyosarcoma of penis. Indian J Cancer. (2015) 52(3):374–5. doi: 10.4103/0019-509X.176719
10. Dominici A, Delle Rose A, Stomaci N, Pugliese L, Posti A, Nesi G. A rare case of leiomyosarcoma of the penis with a reappraisal of the literature. Int J Urol. (2004) 11(6):440–4. doi: 10.1111/j.1442-2042.2004.00806.x
11. Levi I. On a case of primary fibrosarcoma of the skin of the penis: clinical and histological study. G Ital Dermatol. (1930) 71:1559–74.
12. Kreibig W. Beitrag zur Diagnostik der Penissarkome. Deutsche Zeitschrift für Chirurgie. (1931) 231(2):277–84. doi: 10.1007/BF02795646
14. Ashley DJ, Edwards EC. Sarcoma of the penis; leiomyosarcoma of the penis: report of a case with a review of the literature on sarcoma of the penis. Br J Surg. (1957) 45(190):170–9. doi: 10.1002/bjs.18004519011
15. Fagundes LA, Hampe O, Brentano L, Johann D. Leiomyosarcoma of the penis: a case report. J Urol. (1962) 88:803–4. doi: 10.1016/S0022-5347(17)64885-7
16. Izdebski M, Wiercinski J. A case of leiomyoscarcoma of the caveronoous bodies of the penis. Patol Pol. (1962) 13:397–402. PMID: 1395714713957147
17. Pack GT, Trinidad SS, Humphreys GA. Primary leiomyosarcoma of the penis: report of a case. J Urol. (1963) 89:839–40. doi: 10.1016/S0022-5347(17)64658-5
18. Chaudhuri S, Balasubrahmanyan M. Leiomyosarcoma of the penis. A case report. Indian J Pathol Bacteriol. (1966) 9(3):278–84. PMID: 59669725966972
19. Bakken CL, Hanna EA, Seybold HM. Leiomyosarcoma of the penis. J Urol. (1968) 99(6):769–71. doi: 10.1016/S0022-5347(17)62789-7
20. Hutcheson JB, Wittaker WW, Fronstin MH. Leiomyosarcoma of the penis: case report and review of literature. J Urol. (1969) 101(6):874–5. doi: 10.1016/S0022-5347(17)62446-7
21. Greenwood N, Fox H, Edwards EC. Leiomyosarcoma of the penis. Cancer. (1972) 29(2):481–3. doi: 10.1002/1097-0142(197202)29:2%3C481::AID-CNCR2820290237%3E3.0.CO;2-Q
22. Glucker E, Hirshowitz B, Gellei B. Leiomyosarcoma of the glans penis. Case report. Plast Reconstr Surg. (1972) 50(4):406–8. doi: 10.1097/00006534-197210000-00026
23. Nkposong EO, Osunkoya BO. Leiomyosarcoma of the penis. Report of a case. West Afr Med J Niger Pract. (1972) 21(2):34–6. PMID: 50644205064420
24. Gupta S, Gupta IM, Bhatnagar BN. A rapidly fatal leiomyosarcoma of the penis with widespread metastasis. Indian J Pathol Bacteriol. (1973) 16(4):71–3. PMID: 47915264791526
25. Prabhakar BR, Sethi RS, Singh H, Tung BS, Prabhakar H. Leiomyosarcoma of penis. Indian J Cancer. (1975) 12(1):103–6. PMID: 11840631184063
26. Hamal PB. Leiomyosarcoma of penis—case report and review of the literature. Br J Urol. (1975) 47(3):319–24. doi: 10.1111/j.1464-410X.1975.tb03974.x
27. Blath RA, Manley CB. Leiomyosarcoma of the prepuce. J Urol. (1976) 115(2):220–1. doi: 10.1016/S0022-5347(17)59141-7
28. Armijo M, Herrera E, de Dulanto F, Naranjo R, Camacho F. Penile leiomyosarcoma. Ultrastructural study (author's transl). Ann Dermatol Venereol. (1978) 105(3):267–74. PMID: 677694677694
29. Elem B, Nieslanik J. Leiomyosarcoma of the penis. Br J Urol. (1979) 51(1):46. doi: 10.1111/j.1464-410X.1979.tb04244.x
30. Weinberger GI, Wajsman Z, Beckley S, Simpson CL. Primary sarcoma of penis. Urology. (1982) 19(2):193–4. doi: 10.1016/0090-4295(82)90580-5
31. Jain SS, Vohra RR, Kohli PK, Bharti . Leiomyosarcoma of penis (a case report). J Postgrad Med. (1982) 28(2):120b–2. PMID: 71313447131344
32. McDonald MW, O'Connell JR, Manning JT, Benjamin RS. Leiomyosarcoma of the penis. J Urol. (1983) 130(4):788–9. doi: 10.1016/S0022-5347(17)51464-0
33. Isa SS, Almaraz R, Magovern J. Leiomyosarcoma of the penis. Case report and review of the literature. Cancer. (1984) 54(5):939–42. doi: 10.1002/1097-0142(19840901)54:5%3C939::AID-CNCR2820540533%3E3.0.CO;2-Y
34. Smart RH. Leiomyosarcoma of the penis. J Urol. (1984) 132(2):356–7. doi: 10.1016/S0022-5347(17)49624-8
35. Valadez RA, Waters WB. Leiomyosarcoma of penis. Urology. (1986) 27(3):265–7. doi: 10.1016/0090-4295(86)90287-6
36. Kathuria S, Jablokow VR, Molnar Z. Leiomyosarcoma of penile prepuce with ultrastructural study. Urology. (1986) 27(6):556–7. doi: 10.1016/0090-4295(86)90345-6
37. Fetsch JF, Davis CJ Jr., Miettinen M, Sesterhenn IA. Leiomyosarcoma of the penis: a clinicopathologic study of 14 cases with review of the literature and discussion of the differential diagnosis. Am J Surg Pathol. (2004) 28(1):115–25. doi: 10.1097/00000478-200401000-00014
38. Koizumi H, Nagano K, Kosaka S. A case of penile tumor: combination of leiomyosarcoma and squamous cell carcinoma. Hinyokika Kiyo. (1987) 33(9):1489–91. PMID: 33247453324745
39. Pow-Sang MR, Orihuela E. Leiomyosarcoma of the penis. J Urol. (1994) 151(6):1643–5. doi: 10.1016/S0022-5347(17)35328-4
40. Dobos N, Nisenbaum HL, Axel L, Van Arsdalen K, Tomaszewski JE. Penile leiomyosarcoma: sonographic and magnetic resonance imaging findings. J Ultrasound Med. (2001) 20(5):553–7. doi: 10.7863/jum.2001.20.5.553
41. Mendis D, Bott SR, Davies JH. Subcutaneous leiomyosarcoma of the frenulum. ScientificWorldJournal. (2005) 5:571–5. doi: 10.1100/tsw.2005.76
42. Nanri M, Kondo T, Okuda H, Tanabe K, Toma H. A case of leiomyosarcoma of the penis. Int J Urol. (2006) 13(5):655–8. doi: 10.1111/j.1442-2042.2006.01376.x
43. Sundersingh S, Majhi U, Narayanaswamy K, Balasubramanian S. Primary leiomyosarcoma of the penis. Indian J Pathol Microbiol. (2009) 52(3):447–8. doi: 10.4103/0377-4929.55028
44. Lacarrière E, Galliot I, Gobet F, Sibert L. Leiomyosarcoma of the corpus cavernosum mimicking a Peyronie’s plaque. Urology. (2012) 79(4):e53–4. doi: 10.1016/j.urology.2011.07.1410
45. Brisciani A, Brassetti A, Lauretti S, Mosca A, D’Alfonso V. Primary penile leiomyosarcoma: case report and review of the literature. Urologia J. (2012) 79(4):271–7. doi: 10.5301/RU.2012.9940
46. D'Cruze L, Boobala A, Balasubramanian S, Rajendiran S, Joseph LD. Primary leiomyosarcoma of the penis: a case report. J Clin Diagn Res. (2014) 8(1):162–3. doi: 10.7860/JCDR/2014/6723.3808
47. Romero Gonzalez EJ, Marenco Jiménez JL, Mayorga Pineda MP, Martínez Morán A, Castiñeiras Fernández J. Leiomyosarcoma of the penis, an exceptional entity. Urol Case Rep. (2015) 3(3):63–4. doi: 10.1016/j.eucr.2014.12.007
48. Rabinovich J. Leiomyosarcoma of the foreskin: a rare case of mesenchymal foreskin tumor. Urol A. (2018) 57(5):591–3. doi: 10.1007/s00120-018-0577-7
49. Ajmal Z, Khan AM, Zahra FT, McCarthy L, O'Malley R, Mehdi S. Leiomyosarcoma of the penis: a case report and re-appraisal. Fed Pract. (2022) 39(Suppl 2):S58–S61. doi: 10.12788/fp.0232
50. da Costa Junior RNG, Teixeira Júnior AAL, Rocha TMS, Sobrinho TBM, Barbosa LO, Silva RC, et al. Primary leiomyosarcoma of the glans. Front Oncol. (2022) 12:851003. doi: 10.3389/fonc.2022.851003
51. Goyal N, Menon S, Pal M. Primary leiomyosarcoma of the penis: a rare penile neoplasm. Med J Armed Forces India. (2022) 78(3):365–7. doi: 10.1016/j.mjafi.2022.02.004
53. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. (2013) 27(5):957–74. doi: 10.1016/j.hoc.2013.07.002
54. Tsutsumida A, Yoshida T, Yamamoto Y, Itoh T, Minakawa H, Sugihara T. Management of superficial leiomyosarcoma: a retrospective study of 10 cases. Plast Reconstr Surg. (2005) 116(1):8–12. doi: 10.1097/01.PRS.0000169711.70525.10
55. Zacher M, Heppt MV, Brinker TJ, Hayani KM, Flaig MJ, Berking C. Primary leiomyosarcoma of the skin: a comprehensive review on diagnosis and treatment. Med Oncol. (2018) 35(10):135. doi: 10.1007/s12032-018-1196-2
56. Hensley ML, Maki R, Venkatraman E, Geller G, Lovegren M, Aghajanian C, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol. (2002) 20(12):2824–31. doi: 10.1200/JCO.2002.11.050
Keywords: penis, leiomyosarcoma (LMS), case report, malignant mesenchymal tumors, urogenital neoplasms
Citation: Hao Y, Xia L, Lu M, Liu C, Zhang F, Yan Y, Huang Y and Zhang S (2023) Case report and literature review: Primary leiomyosarcoma of the penis. Front. Surg. 9:1068935. doi: 10.3389/fsurg.2022.1068935
Received: 13 October 2022; Accepted: 5 December 2022;
Published: 9 January 2023.
Edited by:
Zhaolun Cai, Sichuan University, ChinaReviewed by:
Lin Xiaokun, Wenzhou Medical University, ChinaAgneta Nordenskjöld, Karolinska University Hospital, Sweden
© 2023 Hao, Xia, Lu, Liu, Zhang, Yan, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Huang aHVhbmd5aUBtZWRtYWlsLmNvbS5jbg== Shudong Zhang c2hvb3RvbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Yichang Hao
Yichang Hao Li Xia1,†
Li Xia1,† Min Lu
Min Lu