- 1Graduate School, Dalian Medical University, Dalian, China
- 2Department of Spine Surgery, Dalian Municipal Central Hospital, Dalian, China
Objective: To investigate the clinical efficacy and imaging outcomes of unilateral biportal endoscopy (UBE) with unilateral laminotomy for bilateral decompression (ULBD) in the treatment of severe lumbar spinal stenosis (SLSS).
Methods: We retrospectively analyzed 50 patients with SLSS treated with UBE-ULBD from October 2018 to March 2021. Visual analog scale (VAS) for back and legs pain, Oswestry disability index (ODI), modified Macnab criteria, complications, hospital stay, preoperative and postoperative dural sac cross-sectional area (DSCA) and Schizas grade, mean angle of facetectomy and osseous lateral recess decompression rate were examined.
Results: The mean follow-up period was 10.7 months. The mean hospital stay was 2.76 ± 1.02 days. At the final follow-up, VAS for back pain and legs pain decreased from 7.22 ± 0.95 to 1.26 ± 0.44 and from 7.88 ± 0.69 to 1.18 ± 0.39, respectively; ODI decreased from 69.88 ± 6.32% to 14.96 ± 2.75%. According to the modified Macnab criteria, the results were excellent in 24 (48%), good in 22 (44%), and fair in 4 (8%). Excellent or good results (a satisfactory outcome) were obtained in 92% of the patients. There were 2 cases of complications of dural sac tear. The postoperative DSCA was significantly enlarged compared with that before surgery, from 44.74 ± 9.85 to 126.86 ± 14.81 mm2. According to Schizas grade, the stenosis grade changes from preoperative grade C in 16 cases, grade D in 34 cases, to postoperative grade A in 40 cases, and grade B in 10 cases. The mean angle of facetectomy of the ipsilateral facet joint was 70.87 ± 5.68, contralateral was 65.07 ± 4.98. The decompression rate was 70.81 ± 4.43% (ipsilateral side) and 71.22 ± 3.68% (contralateral).
Conclusions: UBE-ULBD has a good clinical effect in the treatment of SLSS, and has achieved satisfactory results in spinal canal enlargement, undercutting of facet joints, and decompression effect. It is a safe and effective surgical for SLSS.
Introduction
Lumbar spinal stenosis (LSS) is a degenerative disease of the lumbar spine, common in middle-aged and elderly (1, 2). LSS is usually associated with back pain or sciatica, of which neurogenic intermittent claudication is the typical symptom (3, 4). Anatomically, LSS can be divided into three types, including central spinal stenosis, lateral recess stenosis, and foraminal stenosis (5). Conservative treatment is the preferred treatment for most patients with LSS. In case of failure, surgery should be performed (6).
Presently, severe LSS refers to dural sac cross-sectional area (DSCA) ≤ 75 mm2 based on magnetic resonance imaging (MRI) (5), or based on the morphology of the dural sac as Schizas C or D grade (7). And such patients generally have severe osteophyte hyperplasia of facet joints, as well as hyperplasia and even ossification of ligaments, resulting in severe nerve roots or cauda equina compression. Conservative treatment of SLSS has a great risk of failure, and surgical treatment should be actively adopted. Some studies have pointed out that for SLSS, surgical treatment has a better clinical effect than non-surgical treatment (1, 8).
For LSS, traditional laminectomy decompression is a routine surgical procedure (9). This requires extensive resection of the posterior structure and facet joints, which may result in iatrogenic instability and subsequent fusion surgery (9). To reduce the trauma caused by surgery, various minimally invasive techniques have developed rapidly, including endoscopic techniques. However, for SLSS, minimally invasive surgery may not complete decompression due to technical difficulties and other factors (10).
In recent years, unilateral biportal endoscopy (UBE) has generated a wave of frenzied learning. It has an independent working and viewing portal, with the freer operation and higher decompression efficiency, which well overcomes the shortcomings of insufficient decompression in previous endoscopic surgery (11, 12). And the feasibility of UBE with unilateral laminotomy for bilateral decompression (ULBD) in the treatment of LSS has been confirmed to provide satisfactory clinical results (13, 14). In addition, Kim et al. (14) reported the feasibility of UBE technology in the treatment of SLSS, followed up 58 patients, and achieved a satisfaction rate of 93.1%. However, the authors only looked at severe central spinal stenosis in the study and lacked analysis of imaging results. In addition, there were no other reports about this in subsequent studies.
Therefore, this study aims to explore the clinical effect of UBE-ULBD in the treatment of SLSS, and further explore the decompression effect of this technology through imaging results.
Materials and methods
Patient population
A total of 50 patients were enrolled from October 2018 to October 2021 in this study. All surgeries were performed by the same senior spine surgeon with extensive experience in endoscopic surgery, who performed >200 surgeries annually.
Inclusion criteria
① Single-segment LSS
② Presenting classic neurogenic intermittent claudication with lower extremity symptoms.
③ DSCA ≤ 75 mm2 or Schizas C or D grade.
④ Lumbar spondylolisthesis ≤ I°, and no obvious lumbar instability.
⑤ Lumbar scoliosis <20°.
Exclusion criteria
① Multi-segmented LSS
② DSCA >75 mm2 and Schizas A or B grade.
③ Lumbar spondylolisthesis ≥ II° or lumbar instability.
④ LSS is caused by fractures, tumors, and infections.
⑤ Lumbar curvature ≥20°.
⑥ Patients with poor general status or other diseases that cannot tolerate prone surgery under general anesthesia.
Surgical technique
All patients underwent general anesthesia and were placed in prone position. The abdomen is suspended by placing the arch bridge cushion on the ventral side. The target segment was determined by C-arm fluoroscopy.
The incision design was as follows (for right-hander): (1) the incision on the left: 5 mm aside the posterior midline, the working portal (8–10 mm) was located at the upper edge of the lower lamina, and the view portal (4–6 mm) was located about 25–30 mm above the working portal (according to the soft tissue thickness). (2) The incision on the right: 5 mm aside the posterior midline and moved slightly caudally compared to the left.
Firstly, the stripper can be used to detach the multifidus from the SP base and lower edge of the upper lamina. Then the trocha was placed directing at the conjunction between the SP base and the upper lamina. A cruciate incision is made into the deep fascia of the working channel to maintain a fluent outflow. After expanded by the serial dilators, the endoscopy and instrument were inserted into the two portals respectively. Radiofrequency was used to clean the surrounding soft tissue, an “initial camp” was created as the working space.
Then resect anticlockwise from the lower edge of the upper lamina, the medial portion of the inferior articular process (IAP), the superior articular process (SAP) and the upper edge of the lower lamina. During this procedure, resect the fluffy superficial layer of the ligamentum flavum (LF) first to obtain a better view. Then expose the attachment of the LF and remove the medial part of the SAP under the lateral margin of the dura was seen. Then perform the lateral recess decompression with curette and rongeur. Decompression was supposed to be enough until the medial border of the pedicle was observed.
After ipsilateral decompression is satisfactory, resect part of the ventral bone structure (about 5 mm) of both upper and lower SP base to entry the contralateral canal. Herein, the central fissure of the LF (also called “V collar”) can be regarded as a landmark of midline of the canal. Move over the deep layer of the LF, resect part of the ventral surface of the lamina (about 3–4 mm) until the contralateral IAP was seen. Remove part of the IAP (about 5–6 mm) to reveal the SAP. The SAP removal was enough until the medial border of the pedicle was recognized. If needed, then the contralateral lateral recess decompression and foraminal decompression of both segments can be performed simultaneously. The ideal contralateral decompression extent was showed in Figure 1.
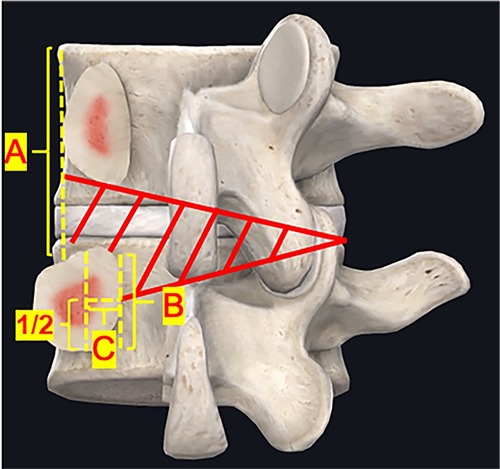
Figure 1. Secompression range of unilateral biportal endoscopy. (A) Outer border of the pedicle; (B) Inner edge of pedicle; (C) Midline border of the pedicle. The contralateral decompression extent (red area). Lateral recess decompression should reach half of the medial border of the pedicle (C), and the foraminal decompression should reach the outer border of the pedicle (A).
Then, the instrument was moved cephalically and the nerve roots in the foraminal region were probed for compression. The tip of the SAP and the LF in the foraminal region were removed. The foraminal region was decompressed to the outer edge of the pedicle. After satisfactory decompression, the disc on the ventral side of the dural sac was probed for disc herniation. If disc herniation was detected, the herniated nucleus pulposus was removed. The drainage tube was placed under endoscopic guidance (Figures 2, 3).
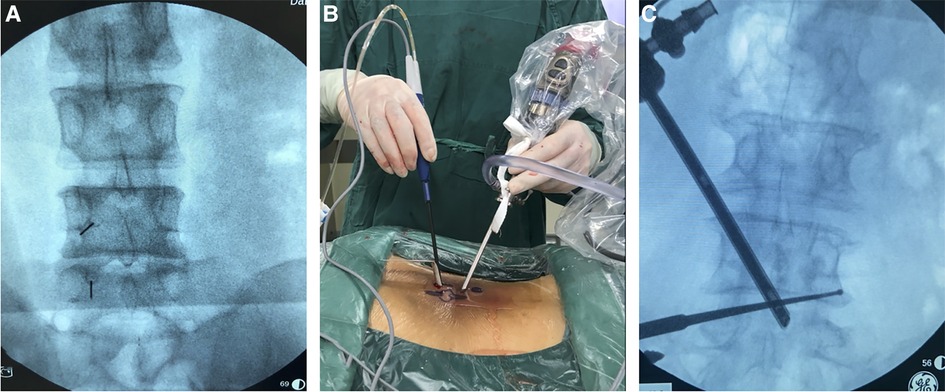
Figure 2. Operation and localization of unilateral biportal endoscopy. (A) Pictures of surgical procedures, the left hand of the surgeon is the endoscope, and the right hand is the radiofrequency probe; (B) 5 ml syringe needles were used for preoperative incision localization; (C) A nerve root probe was used to explore the foraminal region to determine the extent of decompression.
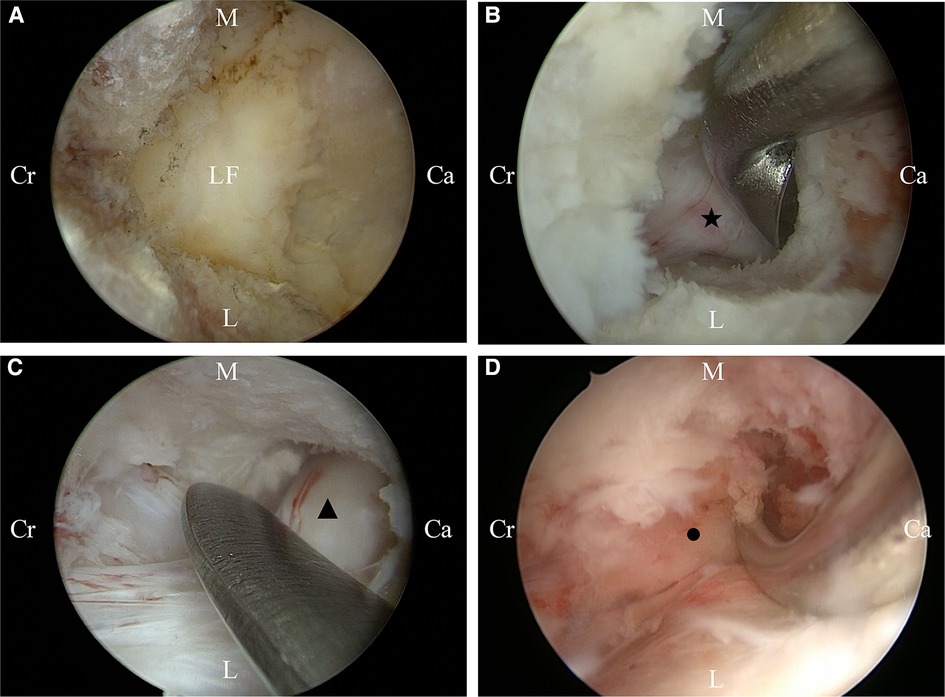
Figure 3. Intraoperative conditions of unilateral biportal endoscopy for decompression. (A) Initial camp; (B) Decompression of the ipsilateral traversing nerve root; (C) Decompression of the contralateral traversing nerve root; (D) Decompression of the contralateral foraminal. Ca, caudal sides; Cr, cranial sides; L, lateral; M, medial. LF, ipsilateral traversing nerve root; ▴, contralateral traversing nerve root; ●, contralateral exiting nerve root.
Evaluation
The hospital stays and complications of all patients were recorded. All imaging examinations were completed in all patients before surgery. Computed tomography (CT) examination was performed within 3 days after surgery and MRI examination was performed within 1 month after surgery.
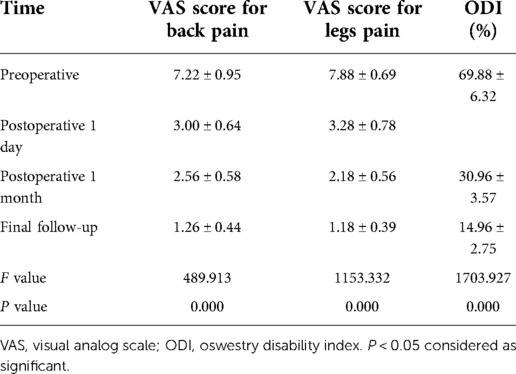
VAS for back and leg pain were used to assess the severity of back and both legs pain before surgery, 1 day and 1 month after surgery, and during the last follow-up. The ODI was used to assess low limb dysfunction before surgery, 1 month after surgery, and during the last follow-up. The modified Macnab criteria were used to examine patient satisfaction at the last follow-up.
In terms of imaging outcomes, three consecutive images were taken by two independent imaging doctors at the level of the intervertebral disc with an interval of 3 mm at axial T2-weighted MRI. DSCA was calculated by describing the boundary of the dural sac (Figure 4), and the degree of spinal stenosis was evaluated according to Schizas grade by observing the morphology of the dural sac.
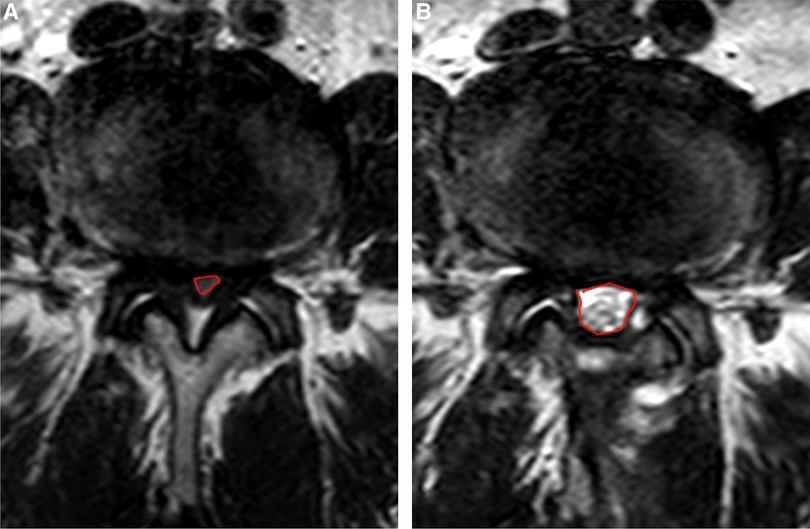
Figure 4. Dural sac cross-sectional area calculation under MRI. (A) Preoperative calculation; (B) Postoperative calculation.
The mean angle of facetectomy was calculated by measuring the angle of the inner facet of the articular process to the axial horizontal line under postoperative CT to evaluate the retention of the facet joint. The osseous lateral recess decompression rate was calculated by the formula (1- the postoperative distance from the inner edge of the SAP to the inner edge of the pedicle under CT/the preoperative distance) 100%, to assess the imaging decompression effect. To minimize the error caused by inconsistent plane selection. Three cross sections were taken consecutively at the target level and measured respectively. The results were added and averaged. (Figure 5).
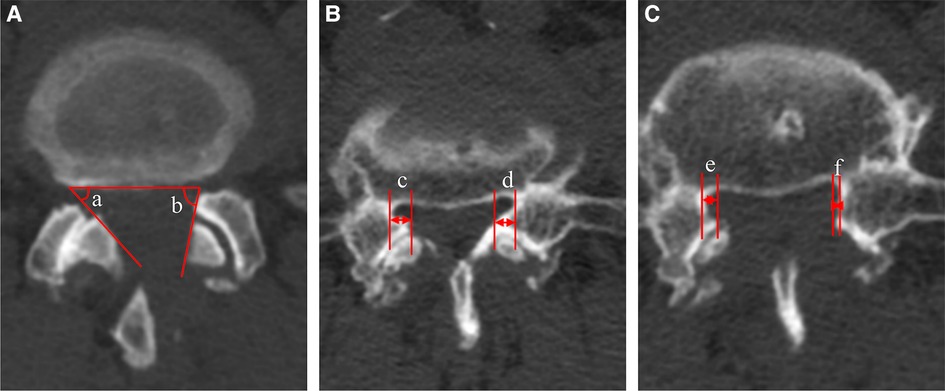
Figure 5. Measurement of resection angle of facet joint and decompression rate under CT. (A) Resection angle of facet joint, (a) contralateral side, (b) ipsilateral side; (B) The preoperative distance from the inner edge of the superior articular process to the inner edge of the pedicle, (c) contralateral side, (d) ipsilateral side; (C) The postoperative distance from the inner edge of the superior articular process to the inner edge of the pedicle, (e) contralateral side, (f) ipsilateral side.
All radiology-related measurements and calculations were performed by two independent radiologists. All data were recorded independently by three clinicians, and the average was considered for statistical analysis.
Statistical analysis
SPSS26.0 statistical software was used for statistical analysis. The data are presented as mean and standard deviation. Paired sample t-test was applied for intragroup comparison. P < 0.05 indicated a statistically significant difference.
Results
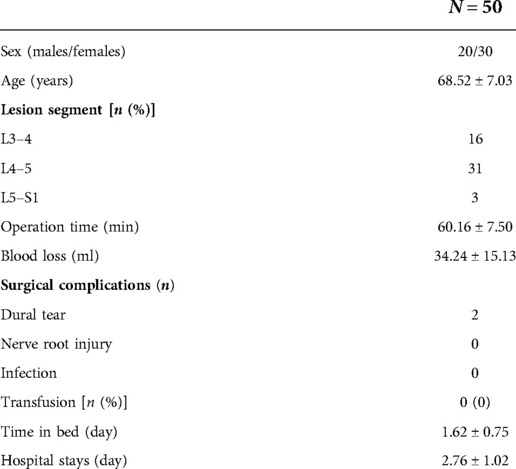
In this study, a total of 50 patients were enrolled, including 20 males and 30 females, with a mean age of 68.52 ± 7.03 years. The lesion segments were L3/4 in 16 patients, L4/5 in 31, and L5/S1 in 3 (Table 1).
Surgical indicators and clinical outcomes
The mean follow-up period was 10.7 months (minimum duration of 6 months). The mean hospital stay was 2.76 ± 1.02 days. 2 patients developed symptoms of dural sac tear, which were gradually relieved after conservative treatment and recovered completely within 5 days. (Table 1).
The VAS for back pain and legs pain was significantly improved from day 1 after surgery and decreased from 7.22 ± 0.95 to 1.26 ± 0.44 and from 7.88 ± 0.69 to 1.18 ± 0.39, at the final follow-up, respectively. ODI improved from 69.88 ± 6.32% to 14.96 ± 2.75% at the final follow-up. According to the modified Macnab criteria, the results were excellent in 24 patients (48%), good in 22 (44%), and fair in 4 (8%). Excellent or good results (a satisfactory outcome) were obtained in 92% of the patients. (Table 2).
Imaging outcomes
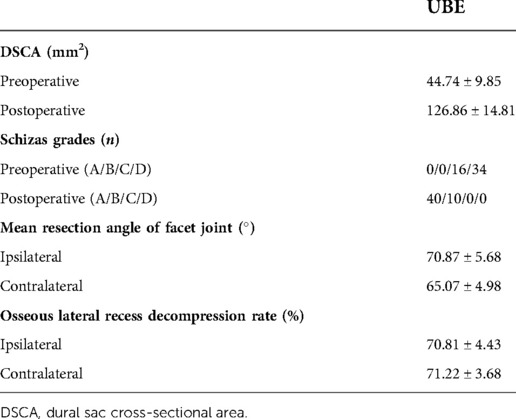
The postoperative DSCA was significantly enlarged compared with that before surgery, from 44.74 ± 9.85 to 126.86 ± 14.81 mm2. According to Schizas grade, the stenosis grade was improved from preoperative grade C in 16 cases and grade D in 34 cases to postoperative grade A in 40 cases and grade B in 10 cases. The mean angle of facetectomy of ipsilateral facet joint was 70.87 ± 5.68, contralateral was 65.07 ± 4.98, both less than 90. The decompression rate of the osseous lateral recess on the ipsilateral side was 70.81 ± 4.43%, and the contralateral was 71.22 ± 3.68%. (Table 3).
Discussion
SLSS is usually associated with central spinal stenosis, lateral recess stenosis, and foraminal stenosis, with multiple nerve root compression (15). Presently, the main purpose of surgical treatment for SLSS is to fully decompress and relieve nerve compression.
In this condition, traditional open laminectomy for whole spinal canal decompression is a preferer option. However, the large trauma and additional fusion and fixation lead to slow recovery, more complications, and further adjacent segment degeneration (9, 10). Some studies have shown that in patients (>65 years old) with SLSS, the probability of life-threatening complications (including mortality) during surgery increases with trauma (14). The ULBD technique has been proven effective and minimally invasive (retains 80% lumbar stiffness) but has not been widely performed by its technical difficulty under microscopy or full endoscopy, especially in SLSS cases (16–18). ULBD under uniportal endoscopic (UE) for LSS decompression also has been reported (13, 19, 20). The common advantages, like less trauma, less bleeding, lower complication rate and faster recovery has been proven (21). However, the hard coaxial confine the viewing and working scope, unfree manipulation results in risk of insufficient decompression. In addition, specialized surgical instruments and high learning curve limit its wide application further (22–25). However, studies showed that decompression of SLSS by ULBD under microscopy and full endoscopy may have risks such as insufficient decompression and increased complication rate (10, 15, 26). While because of the independent view-working channel, unlimited operation angle, more instruments option, and better visualization, UBE was thought to be optimal for UBLD, even for SLSS cases (14, 27). Chio et al. (9) pointed out that UBE has achieved good clinical efficacy in the short-term follow-up for the treatment of LSS and this technology has the advantages of less trauma, less bleeding, quick recovery, and a short learning curve.
Multifidus is the most easily injured paravertebral muscle in posterior spinal surgery and is only innervated by the medial branch of the posterior ramus of the spinal nerve (28). The dissection and contraction of the paravertebral muscle can lead to muscle denervation and atrophy, increasing the risk of back surgery failure (29). UBE creates working space through natural lacunae such as the multifidus triangle, which can effectively reduce the damage to the paravertebral structure (such as multifidus). The results of this study show that VAS scores for back and legs pain were significantly improved immediately after surgery and continued to improve during follow-up. At the final follow-up ODI was improved from 69.88 ± 6.32% to 23.28 ± 3.87% and 92% of patients have excellent or good outcomes according to the modified Macnab criteria. The lower postoperative back pain may be due to less trauma to the multifidus muscle, and the relief of postoperative leg pain also reflects the better effect of nerve root decompression.
Facet joints, as part of the “three-joint complex” of the spine, are extremely important in maintaining spine stability. Thus, extensive resection of facet joints may lead to postoperative segmental instability (11, 30). Studies have demonstrated that preservation of facet joint integrity can reduce postoperative instability and avoid additional fusion (18). In this study, the ipsilateral and contralateral mean facetectomy angles were both less than 90 which even smaller than the previous study which reported a mean facetectomy angle <90 indicating better preservation of facet joints (25). We thought that may be because of the special view of 30-degree endoscopy which allows an easier undercutting than 0-degree endoscopy (14). Pao et al. pointed out that in SLSS cases, due to the structural malformations and narrow space, full decompression may need more damage to the ipsilateral facet joint. To deal with this problem, performing contralateral decompression first or resecting more bone of the SP base shall be suitable (11).
DSCA and Schizas grade is commonly used in the clinical evaluation of the degree of spinal stenosis. In this study, the postoperative DSCA was significantly enlarged and the degree of spinal stenosis was recovered to grade A or grade B postoperatively. Considering the individual differences of spinal canal size in different patients, we developed the decompression rate of the osseous lateral recess and other detection parameters according to the pathological factors of stenosis to evaluate the decompression effect of lateral recess. However, this parameter has not been mentioned in the international community, and the rationality of this parameter needs to be further explored and verified by large sample data.
In terms of decompression strategy, considering the complexity of severe lumbar spinal stenosis, there may be central spinal stenosis, lateral recess stenosis, and foraminal stenosis at the same time, so they are generally separated during decompression. For central spinal stenosis, partial laminectomy and resection of the ligamentum flavum are required to achieve enlargement of the spinal canal, and management of the ventral disc herniation of the dural sac is required (5). In the management of lateral recess stenosis, adequate excision of the inner edge of the SAP and IAP is necessary, but excessive excision should be avoided to affect the stability of the spine (11, 30). For foraminal stenosis, the tip of the SAP should be removed and the ligaments in the foraminal area should be fully released. The SAP should be removed in pieces. The excised area should be <50% of the SAP. If it is >50%, the translational and rotational stabilities of the movable segment may be affected (31). In addition, avoiding nerve root damage and protecting the radicular artery during decompression is essential.
For decompression extent, Wang et al. (32) clarified the definition of lumbar lateral recess and proposed the West China classification, dividing the lumbar lateral recess into 6 zones. The study also pointed out that the degeneration occurs mostly in zones 1, and 2 (about 81.5%). Wherein, zone 2A is the most frequent site of nerve root compression. As for foraminal stenosis, Murata et al. pointed out that stenosis was mainly concentrated at the outer edge of the pedicle (outside the pedicle's center), reaching 94% (33). Therefore, in UBE-ULBD for SLSS, the ideal decompression extent shall be: (1) transversely, the inner edge of the bilateral pedicle was used as the boundary. (2) longitudinally, the proximal decompression reached the ventral surface of the cephalad lamina (the cranial attachment of the LF) and the distal decompression reached the midline of the pedicle to achieve sufficient decompression of zone 2 (Figure 5).
In this study, 2 patients developed dural sac tears and no patients developed an infection. Continuous irrigation with saline helps control bleeding and reduce postoperative infection (34). To avoid intracranial hypertension caused by high-pressure saline, controlling the saline pressure at 20–30 mmHg is recommended. The total dural tear rate reported in UBE was 1.9%–5.8% and occurred mostly on the dorsal side, caused by water pressure, instrument friction, limited field of vision, difficulty in anatomical identification, and the learning curve (35). It has been reported the dural tear rate significantly decreases after 50 surgeries (36). In addition, in SLSS, calcification or ossification of the LF sometimes may adhere to the dural sac, as well as the central folding of the dura sac hidden in epidural fat tissue. According to the preoperative MRI and intraoperative observation, we found that epidural fat was significantly reduced or even disappeared. Therefore, it is supposed to be more careful to identify the adhesion and separate the dural sac and LF.
Nevertheless, the present study has several limitations. First, this study lacked a control group, which led to the failure to fully clarify the advantages and disadvantages of UBE in the treatment of SLSS. Second, this was a retrospective study with small sample size and short follow-up time, which failed to evaluate the long-term efficacy of patients.
Conclusions
UBE-ULBD is a safe and feasible surgical method in the treatment of SLSS. Due to its unique technical advantages, UBE-ULBD can achieve satisfactory whole spinal canal decompression. Meanwhile, it preserves more stabilization structures of the spine to the greatest extent and avoids further fusion and internal fixation. It has the advantages of less trauma, less bleeding, faster recovery, lower complication rate, and lower operation cost.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Dalian Central Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YH, DY and WX set up the conception and designed the research. YH, HF and DY collected and interpreted patient's clinical information. YH and HF drafted the manuscript. WX and DY critically reviewed, revised the manuscript and supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Key R&D program of China (project number 2019YFC0121400).
Acknowledgments
The authors are grateful to the patients and their family members for their participation. Thanks for all doctors and nursing staff involved in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviation
UBE, unilateral biportal endoscopy; ULBD, unilateral laminotomy for bilateral decompression; SLSS, severe lumbar spinal stenosis; LSS, lumbar spinal stenosis; VAS, visual analog scale; ODI, oswestry disability index; DSCA, dural sac cross-sectional area; MRI, magnetic resonance imaging; SP, spinous process; IAP, inferior articular process; SAP, superior articular process; LF, ligamentum flavum; CT, computed tomography.
References
1. Shen J, Wang Q, Wang Y, Min N, Wang L, Wang F, et al. Comparison between fusion and non-fusion surgery for lumbar spinal stenosis: a meta-analysis. Adv Ther. (2021) 38:1404–14. doi: 10.1007/s12325-020-01604-7
2. Costa F, Sassi M, Cardia A, Ortolina A, De Santis A, Luccarell G, et al. Degenerative lumbar spinal stenosis: analysis of results in a series of 374 patients treated with unilateral laminotomy for bilateral microdecompression. J Neurosurg Spine. (2007) 7:579–86. doi: 10.3171/SPI-07/12/579
3. Zaina F, Tomkins-Lane C, Carragee E, Negrini S. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst Rev. (2016) 2016(1):CD010264. doi: 10.1002/14651858.CD010264.pub2
4. Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. (2008) 358:794–810. doi: 10.1056/NEJMoa0707136
5. Schroeder GD, Kurd MF, Vaccaro AR. Lumbar spinal stenosis: how is it classified? J Am Acad Orthop Surg. (2016) 24:843–52. doi: 10.5435/JAAOS-D-15-00034
6. Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. (2008) 358:818–25. doi: 10.1056/NEJMcp0708097
7. Schizas C, Theumann N, Burn A, Tansey R, Wardlaw D, Smith FW, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine. (2010) 35:1919–24. doi: 10.1097/BRS.0b013e3181d359bd
8. Chang W, Yuwen P, Zhu Y, Wei N, Feng C, Zhang Y, et al. Effectiveness of decompression alone versus decompression plus fusion for lumbar spinal stenosis: a systematic review and meta-analysis. Arch Orthop Trauma Surg. (2017) 137:637–50. doi: 10.1007/s00402-017-2685-z
9. Choi DJ, Kim JE. Efficacy of biportal endoscopic spine surgery for lumbar spinal stenosis. Clin. Orthop. Surg. (2019) 11:82–8. doi: 10.4055/cios.2019.11.1.82
10. Wang C, Yin X, Zhang L, Xue X, Xiang Y, Jin H, et al. Posterolateral fusion combined with posterior decompression shows superiority in the treatment of severe lumbar spinal stenosis without lumbar disc protrusion or prolapse: a retrospective cohort study. J Orthop Surg Res. (2020) 15:26. doi: 10.1186/s13018-020-1552-8
11. Pao JL, Lin SM, Chen WC, Chang CH. Unilateral biportal endoscopic decompression for degenerative lumbar canal stenosis. J Spine Surg. (2020) 6:438–46. doi: 10.21037/jss.2020.03.08
12. Pao JL. A review of unilateral biportal endoscopic decompression for degenerative lumbar canal stenosis. Int J Spine Surg. (2021) 15:S65–71. doi: 10.14444/8165
13. Soliman HM. Irrigation endoscopic decompressive laminotomy. A new endoscopic approach for spinal stenosis decompression. Spine J. (2015) 15:2282–9. doi: 10.1016/j.spinee.2015.07.009
14. Kim N, Jung SB. Percutaneous unilateral biportal endoscopic spine surgery using a 30-degree arthroscope in patients with severe lumbar spinal stenosis: a technical note. Clin Spine Surg. (2019) 32:324–9. doi: 10.1097/BSD.0000000000000876
15. Zhang B, Kong Q, Yang J, Feng P, Ma J, Liu J. Short-term effectiveness of percutaneous endoscopic transforaminal bilateral decompression for severe central lumbar spinal stenosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2019) 33:1399–405. doi: 10.7507/1002-1892.201904131
16. Ghogawala Z, Dziura J, Butler WE, Dai F, Terrin N, Magge SN, et al. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med. (2016) 374:1424–34. doi: 10.1056/NEJMoa1508788
17. Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. (2014) 21:179–86. doi: 10.3171/2014.4.SPINE13420
18. Hamasaki T, Tanaka N, Kim J, Okada M, Ochi M, Hutton WC. Biomechanical assessment of minimally invasive decompression for lumbar spinal canal stenosis: a cadaver study. J. Spinal Disord. Tech. (2009) 22:486–91. doi: 10.1097/BSD.0b013e31818d7dc9
19. Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine. (2002) 27:432–8. doi: 10.1097/00007632-200202150-00021
20. Komp M, Hahn P, Merk H, Godolias G, Ruetten S. Bilateral operation of lumbar degenerative central spinal stenosis in full-endoscopic interlaminar technique with unilateral approach: prospective 2-year results of 74 patients. J Spinal Disord Tech. (2011) 24:281–7. doi: 10.1097/BSD.0b013e3181f9f55e
21. Polikandriotis JA, Hudak EM, Perry MW. Minimally invasive surgery through endoscopic laminotomy and foraminotomy for the treatment of lumbar spinal stenosis. J Orthop. (2013) 10:13–6. doi: 10.1016/j.jor.2013.01.006
22. Kim HS, Paudel B, Jang JS, Oh SH, Lee S, Park JE, et al. Percutaneous full endoscopic bilateral lumbar decompression of spinal stenosis through uniportal-contralateral approach: techniques and preliminary results. World Neurosurg. (2017) 103:201–9. doi: 10.1016/j.wneu.2017.03.130
23. Yang F, Chen R, Gu D, Ye Q, Liu W, Qi J, et al. Clinical comparison of full-endoscopic and microscopic unilateral laminotomy for bilateral decompression in the treatment of elderly lumbar spinal stenosis: a retrospective study with 12-month follow-up. J Pain Res. (2020) 13:1377–84. doi: 10.2147/JPR.S254275
24. Yoshikane K, Kikuchi K, Okazaki K. Clinical outcomes of selective single-level lumbar endoscopic unilateral laminotomy for bilateral decompression of multilevel lumbar spinal stenosis and risk factors of reoperation. Global Spine J. (2021):21925682211033575. doi: 10.1177/21925682211033575
25. Heo DH, Lee DC, Park CK. Comparative analysis of three types of minimally invasive decompressive surgery for lumbar central stenosis: biportal endoscopy, uniportal endoscopy, and microsurgery. Neurosurg Focus. (2019) 46:E9. doi: 10.3171/2019.2.FOCUS197
26. Chen T, Zhou G, Chen Z, Yao X, Liu D. Biportal endoscopic decompression vs. microscopic decompression for lumbar canal stenosis: a systematic review and meta-analysis. Exp Ther Med. (2020) 20:2743–51. doi: 10.3892/etm.2020.9001
27. Park SM, Kim GU, Kim HJ, Choi JH, Chang BS, Lee CK, et al. Is the use of a unilateral biportal endoscopic approach associated with rapid recovery after lumbar decompressive laminectomy? A preliminary analysis of a prospective randomized controlled trial. World Neurosurg. (2019) 128:e709–18. doi: 10.1016/j.wneu.2019.04.240
28. Vialle R, Court C, Khouri N, Olivier E, Miladi L, Tassin JL, et al. Anatomical study of the paraspinal approach to the lumbar spine. Eur Spine J. (2005) 14:366–71. doi: 10.1007/s00586-004-0802-5
29. Sihvonen T, Herno A, Paljarvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. (1993) 18:575–81. doi: 10.1097/00007632-199304000-00009
30. Guha D, Heary RF, Shamji MF. Iatrogenic spondylolisthesis following laminectomy for degenerative lumbar stenosis: systematic review and current concepts. Neurosurg. Focus. (2015) 39:E9. doi: 10.3171/2015.7.FOCUS15259
31. Ahn JS, Lee HJ, Choi DJ, Lee KY, Hwang SJ. Extraforaminal approach of biportal endoscopic spinal surgery: a new endoscopic technique for transforaminal decompression and discectomy. J Neurosurg Spine. (2018) 28:492–8. doi: 10.3171/2017.8.SPINE17771
32. Wang Y, Kong Q, Chen Z. Reconsideration of lumbar spinal stenosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2019) 33:789–94. doi: 10.7507/1002-1892.201904027
33. Murata S, Minamide A, Iwasaki H, Nakagawa Y, Hashizume H, Yukawa Y, et al. Microendoscopic decompression for lumbosacral foraminal stenosis: a novel surgical strategy based on anatomical considerations using 3D image fusion with MRI/CT. J Neurosurg Spine. (2020): 1–7. doi: 10.3171/2020.5.SPINE20352
34. Kim JE, Choi DJ, Park EJJ, Lee HJ, Hwang JH, Kim MC, et al. Biportal endoscopic spinal surgery for lumbar spinal stenosis. Asian Spine J. (2019) 13:334–42. doi: 10.31616/asj.2018.0210
35. Lee HG, Kang MS, Kim SY, Cho KC, Na YC, Cho JM, et al. Dural injury in unilateral biportal endoscopic spinal surgery. Global Spine J. (2021) 11:845–51. doi: 10.1177/2192568220941446
Keywords: unilateral biportal endoscopy (UBE), unilateral laminotomy for bilateral decompression (ULBD), severe lumbar spinal stenosis, clinical outcomes, imaging outcomes
Citation: Hu Y, Fu H, Yang D and Xu W (2023) Clinical efficacy and imaging outcomes of unilateral biportal endoscopy with unilateral laminotomy for bilateral decompression in the treatment of severe lumbar spinal stenosis. Front. Surg. 9:1061566. doi: 10.3389/fsurg.2022.1061566
Received: 4 October 2022; Accepted: 10 November 2022;
Published: 6 January 2023.
Edited by:
Yutong Gu, Fudan University, China© 2023 Hu, Fu, Yang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weibing Xu eHV3ZWliaW5nc21tdUAxNjMuY29t Dongfang Yang MTMwNzg4MTI3QHFxLmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Yutong Hu
Yutong Hu Hao Fu1,2†
Hao Fu1,2† Weibing Xu
Weibing Xu