- Department of Anesthesiology and Pain Medicine, Soonchunhyang University Hospital Seoul, Seoul, South Korea
Background: Propofol-based total intravenous anesthesia (TIVA) is considered a prophylactic approach to decrease postoperative nausea and vomiting (PONV). Despite general anesthesia commonly being performed in end-stage renal disease (ESRD) patients, PONV in ESRD patients has not been well-described. We investigated PONV in peripheral vascular surgery under general anesthesia in ESRD patients.
Methods: To compare PONV between propofol-based TIVA and anesthesia with volatile anesthetics, we collected retrospective data from patients who underwent peripheral vascular surgery under general anesthesia from July 2018 to April 2020. We performed univariable and multivariable analyses, including factors that could be associated with PONV and those previously shown to affect PONV.
Result: A total of 1,699 peripheral vascular surgeries under general anesthesia in ESRD patients were eligible for analysis. Based on the multivariable analysis, TIVA (odds ratio [OR], 0.45; 95% confidence interval [CI], 0.35–0.60; P < 0.001) significantly decreased PONV. Female sex (OR, 1.85; 95% CI, 1.44–2.38; P < 0.001) and anesthetic duration (OR, 1.01; 95% CI, 1.00–1.01; P < 0.001) were associated with increased PONV.
Conclusion: Propofol-based TIVA is the most influential factor decreasing PONV after peripheral vascular surgery in ESRD patients. Anesthesiologists can apply propofol-based TIVA as an alternative to anesthesia with volatile anesthetics.
Introduction
Postoperative nausea and vomiting (PONV) is one of the most common adverse effects of general anesthesia (1). Generally, the importance of PONV has been devalued, although it has a significant impact on postoperative care. PONV can delay discharge, disrupt oral intake, and lead to serious complications such as wound dehiscence and anatomic leaks. Therefore, it can increase treatment costs (2). Furthermore, PONV is a more common cause of patient discomfort than postoperative pain (3). Numerous factors affect the incidence of PONV, including patient characteristics, anesthetic factors, and surgical procedures (4). Among the prophylactic options for PONV, propofol-based total intravenous anesthesia (TIVA) is considered an excellent anesthetic strategy (5).
The incidence of end-stage renal disease (ESRD), the final stage of chronic kidney disease (CKD), is increasing globally. In the year 2000, approximately 1.1 million patients worldwide were being treated for CKD, showing an increase of 6%–7%, which is greater than the global population growth rate. The number of hemodialysis patients is estimated to reach 3,500,000 by 2020 (6). For these patients, hemodialysis is the most common treatment, which has increased the survival rate and improved patient quality of life (7). To achieve vascular access for chronic hemodialysis, peripheral vascular surgeries are performed in ESRD patients (8).
Although vascular access surgery (arteriovenous fistula formation) for hemodialysis can be performed under local anesthesia alone, many patients require general anesthesia for complicated peripheral vascular surgeries (e.g., graft interposition or aneurysm removal) due to the complexity of the procedures. For this reason, general anesthesia is commonly performed in ESRD patients.
Maintenance of general anesthesia should be achieved using short-acting drugs with minimal renal metabolism. Generally, short-acting volatile anesthetics such as desflurane or sevoflurane are preferred and the opiate remifentanil and the hypnotic propofol can be administered through continuous intravenous infusion as an alternative (9, 10). However, volatile agents are commonly considered the main cause of PONV, whereas TIVA with propofol is thought to decrease PONV (5, 11). Furthermore, there is a relatively high incidence of nausea and vomiting in hemodialysis patients (6). Nevertheless, PONV in peripheral vascular surgery for ESRD patients has not been well-described.
The aims of this study were to investigate PONV in peripheral vascular surgery under general anesthesia in ESRD patients and to compare the incidence between propofol-based TIVA and anesthesia with volatile anesthetics.
Materials and methods
To compare PONV after general anesthesia in ESRD patients with propofol-based TIVA or anesthesia with volatile anesthetics, retrospective data collection was performed from July 2018 to April 2020 at Soonchunhyang University Hospital, Seoul, Republic of Korea. This retrospective observational study was approved by Soonchunhyang University Hospital's institutional review board (IRB number: SCHUH2020-06-004). Written informed consent was waived because of the retrospective case-control nature of the study. Our findings are presented following the format recommended by the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (12). All methods were carried out in accordance with relevant guidelines and regulations.
Study population
We retrospectively enrolled 1,923 consecutive cases: all were ESRD patients who underwent peripheral vascular surgery under general anesthesia at age 30–90 years. Among them, emergency surgeries and cases without postoperative visit records for managing PONV were excluded.
Data collection
Medical records were reviewed retrospectively for patient characteristics, laboratory data, medical treatments, and clinical outcomes. We defined PONV as any nausea, retching, or vomiting according to the postoperative visit records. Demand for antiemetics and medical records indicating PONV in the post-anesthesia care unit (PACU) on postoperative day (POD) 1 were analyzed.
Anesthetic management
When departing for the operating theater, all patients were premedicated with 0.1 mg of glycopyrrolate intramuscularly, except when contraindicated. Upon arrival in the operating theater, standard monitoring devices were applied, including electrocardiography, pulse oximetry, and an oscillometric noninvasive blood-pressure cuff. Bispectral index monitoring (BIS System; Aspect Medical Systems, Newton, MA, United States) was performed for all participants.
In the TIVA group, general anesthesia was induced and maintained with propofol and remifentanil via effect site targeting using a target-controlled infusion system (Orchestra Primea; Fresenius Kabi AG, Bad Homburg, Germany) after intravenous lidocaine (40 mg) administration. Propofol was administered using the Schnider pharmacokinetic model and remifentanil using the Minto model. The target concentrations of propofol and remifentanil were maintained at 2–5 μg/ml and 0–6 ng/ml, respectively, according to a BIS of 40–60.
In the volatile-anesthetics group, induction was performed using intravenous lidocaine (40 mg), propofol (1–1.5 mg/kg), and rocuronium (0.6 mg/kg) for neuromuscular blockade. Anesthesia was maintained with oxygen, medical air, and volatile anesthetics, including desflurane (n = 170, 29.1%) or sevoflurane (n = 415, 70.9%). Patients were administered intravenous remifentanil as required in the same way as the TIVA group. The volatile anesthetic and remifentanil dose were adjusted to achieve target BIS values of 40–60.
Patients received intravenous ephedrine (4 mg), phenylephrine (50 μg), or an inotropic infusion as required for blood pressure values below 20% of baseline during the operation. At skin closure in both groups, patients received intravenous fentanyl (0.3–0.5 µg/kg). At the end of the surgical procedure, the neuromuscular blockade was reversed with intravenous pyridostigmine (0.2 mg/kg) and glycopyrrolate (5 µg/kg) or with sugammadex (1–2 mg/kg) as needed. Tracheal extubation was performed under a monitoring train-of-four ratio >0.9.
The agents used for anesthesia depended on the discretion of the anesthesiologist assigned to each case. In the PACU and ward, patients received opioid or anti-emetics on demand.
Statistical analysis
The Kolmogorov-Smirnov test was used to test the hypothesis of a normal distribution for continuous variables. All continuous variables were reported as means ± standard deviations and all categorical variables were reported as n values (proportion, %). Categorical variables were compared using the chi-square test or Fisher's exact test, and continuous variables were compared using the t-test or Mann-Whitney U test for intergroup comparisons of PONV and other clinical variables. To explore the relationships between PONV and other clinical variables, we performed univariable and multivariable analyses, including factors that could be associated with PONV and those previously known to have an effect on PONV. R software (version 4.0.0; April 24, 2020, R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses; a P-value <0.05 was considered significant.
Results
Among 1,923 cases, 224 were excluded because of a lack of postoperative visit records or emergency surgery. A total of 1,699 peripheral vascular surgeries under general anesthesia in ESRD patients were identified during the study period and all were included in the analysis.
Patient characteristics
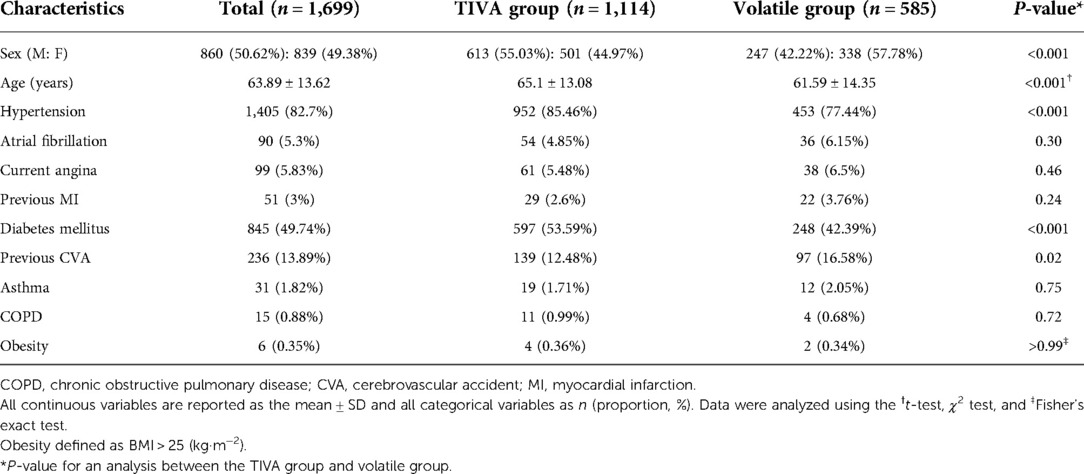
The clinical characteristics of the patients are presented in Table 1. Age (P < 0.001), hypertension (P < 0.001), and diabetes mellitus (P < 0.001) were higher in the TIVA group than in the volatile group. The proportion of female sex (P < 0.001) and previous cerebrovascular accidents were higher in the volatile group (P = 0.02) than in the TIVA group. There were no significant differences in atrial fibrillation, current angina, previous myocardial infarction, asthma, chronic obstructive lung disease, or obesity between the two groups.
Anesthetic management and PONV
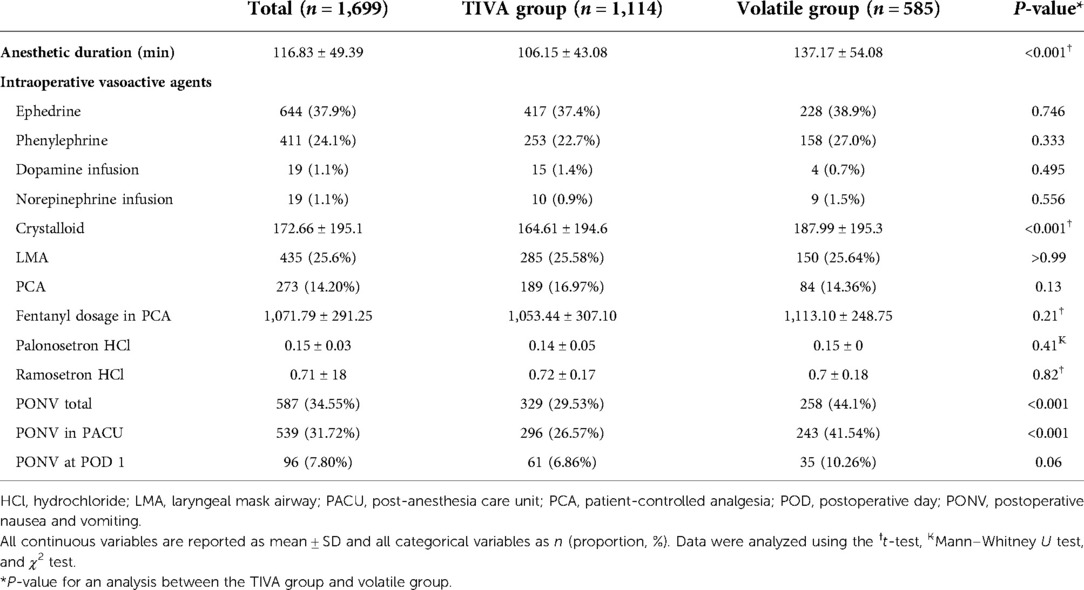
Table 2 shows anesthetic management and PONV. The incidence of PONV was significantly higher in the volatile group in total (P < 0.001) and in the PACU (P < 0.001) than in the TIVA group (Figure 1). Anesthetic duration (P < 0.001) and the volume of intraoperative crystalloid infusion (P < 0.001) were higher in the volatile group than in the TIVA group. PONV at POD 1, use of intraoperative vasoactive agents, use of postoperative inotropic agents, laryngeal mask airway, patient-controlled analgesia (PCA), fentanyl dosage in PCA, and dosage of administered antiemetics (palonosetron hydrochloride and ramosetron hydrochloride) did not differ significantly between the two groups.

Figure 1. Incidence rate of postoperative nausea and vomiting (PONV) between total intravenous anesthesia (TIVA) group and volatile anesthesia group. *P value <0.05 between two groups.
Univariable and multivariable analyses of factors associated with PONV
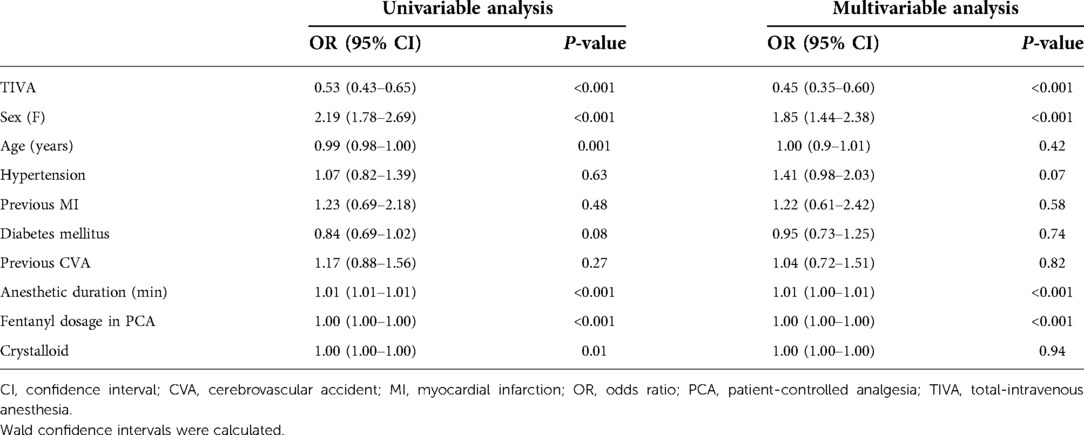
Based on our univariable analysis, TIVA (P < 0.001), female sex (P < 0.001), age (P = 0.001), anesthetic duration (P < 0.001), fentanyl dosage in PCA (P < 0.001), and volume of intraoperative crystalloid infusion (P = 0.01) were significant factors affecting PONV (Table 3). Among these factors, TIVA (odds ratio [OR], 0.53; 95% confidence interval [CI], 0.43–0.65) and age (OR: 0.99; 95% CI, 0.98–1.00) were associated with decreased PONV. Female sex (OR, 1.85; 95% CI, 1.78–2.69) and anesthetic duration (OR, 1.01; 95% CI, 1.01–1.01) were associated with increased PONV.
Based on our multivariable analysis, TIVA (P < 0.001), female sex (P < 0.001), anesthetic duration (P < 0.001), and fentanyl dosage in PCA (P < 0.001) were significant factors affecting PONV. Multivariable analysis showed that TIVA (OR, 0.45; 95% CI, 0.35–0.60) decreased PONV. Female sex (OR, 1.85; 95% CI, 1.44–2.38) and anesthetic duration (OR, 1.01; 95% CI, 1.00–1.01) were associated with increased PONV.
Discussion
In this retrospective observational study, TIVA was the most influential factor decreasing PONV after peripheral vascular surgery in ESRD patients. Female sex and anesthetic duration were factors that increasing PONV. The total incidence of PONV was 34.55%. Our study shows that propofol-based TIVA could be considered an alternative anesthetic method to reduce PONV in peripheral vascular surgery for ESRD patients.
Several independent factors are thought to be associated with PONV. These factors can be divided into multiple categories, including patient-specific (age, sex, smoking status, history of motion sickness or previous PONV), anesthetic (volatile anesthetics, intraoperative use of opioids, hydration, anesthetic duration), surgical (type and postoperative use of opioid), and other (mask ventilation, body mass index, pain) (4, 13). Among these, the most reliable risk factors of PONV were female sex, history of PONV or motion sickness, non-smoker, younger age, volatile anesthetics and postoperative opioids (14).
In ESRD patients, previous studies reported a higher prevalence of upper gastrointestinal (GI) symptoms such as nausea (74%), vomiting (68%), and anorexia (64%) (15). The reason for the high prevalence of GI symptoms in ESRD patients is unclear. Nevertheless, multiple etiologies such as treatments for the digestive system, the patient's diet, medication regimen, and developed disabilities are considered major causes of nausea and vomiting (16, 17).
Among the available anesthetics, propofol is commonly used for the induction and maintenance of general anesthesia because it is a rapid-onset and short-acting hypnotic agent. Moreover, propofol is known to have an antiemetic effect and TIVA with propofol is effective to reduce the incidence of PONV (11). General anesthesia with volatile anesthetics is largely responsible for PONV and avoidance of volatile anesthetics alone reduced the incidence of PONV by 19% (5). In our study, propofol-based TIVA reduced the incidence of PONV, even in ESRD patients.
For patient characteristics, female sex was considered the most important risk factor for PONV in several previous reports (3, 18–20). In these articles, female patients suffered from PONV three times more often than male patients. This may be due to hormone status, since this difference between the sexes begins at puberty. Nevertheless, the menstrual cycle does not have an impact on the occurrence of PONV (21). Although the mechanism of high PONV incidence in females remains unclear, our study showed the same results with female sex increasing the incidence of PONV.
Anesthetic duration is believed to increase PONV (18, 22). Correlation between anesthetic duration and PONV was same in our study. Some studies demonstrated that sufficient intravenous fluid administration might effectively prevent PONV (23, 24). But, there was no difference according to the amount of crystalloid infusion in our study.
There are some limitations to our study. First, similar to other retrospective studies, the data were incomplete so it may have introduced unrecognized bias into the results. In addition, some baseline characteristics of the two groups were significantly different. It might cause selection bias. Second, we did not evaluate patient-specific risk factors such as smoking status, history of motion sickness, and previous PONV. Despite these factors being strongly associated with PONV, our study did not reveal a correlation. Third, we analysed the data only up to POD 1 because of most patients were discharged at POD 2; therefore, we did not compare subsequent days. Finally, nausea is a subjective symptom so the collected data relied on patient answers.
In conclusion, propofol-based TIVA is the most influential factor in decreasing PONV after peripheral vascular surgery in ESRD patients. Additionally, female sex and anesthetic duration might be increasing factors of PONV. Considering the strong prevalence of PONV in ESRD patients, anesthesiologists can apply propofol-based TIVA as an alternative to anesthesia with volatile anesthetics.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Soonchunhyang University Hospital's institutional review board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HBC and SYP: Writing original draft; NK and SJC: Data analysis; SS and JHY: Writing review & editing; MGK: Data curation; JWC: Supervision and conceptualization. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Soonchunhyang University Research Fund and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2017R1C1B5076787).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Weibel S, Rücker G, Eberhart LH, Pace NL, Hartl HM, Jordan OL, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis. Cochrane Database Syst Rev. (2020) 10(10):Cd012859. doi: 10.1111/anae.15295
2. Gan TJ, Sloan F, de L Dear G, El-Moalem HE, Lubarsky DA. How much are patients willing to pay to avoid postoperative nausea and vomiting? Anesth Analg. (2001) 92(2):393–400. doi: 10.1213/00000539-200102000-00022
3. Koivuranta M, Läärä E, Snåre L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. (1997) 52(5):443–9. doi: 10.1111/j.1365-2044.1997.117-az0113.x
4. Apfel CC, Stoecklein K, Lipfert P. PONV: a problem of inhalational anaesthesia? Best Pract Res Clin Anaesthesiol. (2005) 19(3):485–500. doi: 10.1016/j.bpa.2005.03.001
5. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. (2004) 350(24):2441–51. doi: 10.1056/NEJMoa032196
6. Asgari MR, Asghari F, Ghods AA, Ghorbani R, Motlagh NH, Rahaei F. Incidence and severity of nausea and vomiting in a group of maintenance hemodialysis patients. J Renal Inj Prev. (2017) 6(1):49. doi: 10.15171/jrip.2017.09
7. Morsch CM, Gonçalves LF, Barros E. Health-related quality of life among haemodialysis patients–relationship with clinical indicators, morbidity and mortality. J Clin Nurs. (2006) 15(4):498–504. doi: 10.1111/j.1365-2702.2006.01349.x
8. Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. (1996) 7(4):523–35. doi: 10.1681/ASN.V74523
9. Shemesh D, Raikhinstein Y, Orkin D, Goldin I, Olsha O. Anesthesia for vascular access surgery. J Vasc Access. (2014) 15(7_suppl):38–44. doi: 10.5301/jva.5000233
10. Eger EI, Koblin DD, Bowland T, Ionescu P, Laster MJ, Fang Z, et al. Nephrotoxicity of sevoflurane versus desflurane anesthesia in volunteers. Anesth Analg. (1997) 84(1):160–8. doi: 10.1213/00000539-199701000-00029
11. Sneyd J, Carr A, Byrom W, Bilski A. A meta-analysis of nausea and vomiting following maintenance of anaesthesia with propofol or inhalational agents. Eur J Anaesthesiol. (1998) 15(4):433–45. doi: 10.1097/00003643-199807000-00009
12. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. (2007) 147(8):573–7. doi: 10.7326/0003-4819-147-8-200710160-00010
13. Watcha MF, White PF. Postoperative nausea and vomitingits etiology, treatment, and prevention. Anesthesiology. (1992) 77(1):162–84. doi: 10.1097/00000542-199207000-00023
14. Apfel C, Heidrich F, Jukar-Rao S, Jalota L, Hornuss C, Whelan R, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. (2012) 109(5):742–53. doi: 10.1093/bja/aes276
15. Abu Farsakh N, Roweily E, Rababaa M, Butchoun R. Evaluation of the upper gastrointestinal tract in uraemic patients undergoing haemodialysis. Nephrol Dial Transplant. (1996) 11(5):847–50. doi: 10.1093/oxfordjournals.ndt.a027411
16. Chong VH, Tan J. Prevalence of gastrointestinal and psychosomatic symptoms among a sian patients undergoing regular hemodialysis. Nephrology. (2013) 18(2):97–103. doi: 10.1111/nep.12000
17. Barth C, Boer W, Garzoni D, Kuenzi T, Ries W, Schaefer R, et al. Characteristics of hypotension-prone haemodialysis patients: is there a critical relative blood volume? Nephrol Dial Transplant. (2003) 18(7):1353–60. doi: 10.1093/ndt/gfg171
18. Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: assessing risk factors for nausea and vomiting. Anesth Analg. (1994) 78(1):7–16. doi: 10.1213/00000539-199401000-00004
19. Apfel CC, Läärä E, Koivuranta M, Greim C-A, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting conclusions from cross-validations between two centers. J Am Soc Anesthesiologists. (1999) 91(3):693. doi: 10.1097/00000542-199909000-00022
20. Stadler M, Bardiau F, Seidel L, Albert A, Boogaerts JG. Difference in risk factors for postoperative nausea and vomiting. J Am Soc Anesthesiologists. (2003) 98(1):46–52. doi: 10.1097/00000542-200301000-00011
21. Eberhart L, Morin A, Georgieff M. The menstruation cycle in the postoperative phase. Its effect of the incidence of nausea and vomiting. Anaesthesist. (2000) 49(6):532–5. doi: 10.1007/s001010070095.10928257
22. Apfel C, Greim C, Haubitz I, Goepfert C, Usadel J, Sefrin P, et al. A risk score to predict the probability of postoperative vomiting in adults. Acta Anaesthesiol Scand. (1998) 42(5):495–501. doi: 10.1111/j.1399-6576.1998.tb05157.x
23. Maharaj CH, Kallam SR, Malik A, Hassett P, Grady D, Laffey JGJA, et al. Preoperative intravenous fluid therapy decreases postoperative nausea and pain in high risk patients. Anesth Analg. (2005) 100(3):675–82. doi: 10.1213/01.ANE.0000148684.64286.36
Keywords: ESRD, PONV, peripheral vascular surgery, propofol, TIVA
Citation: Cho HB, Park SY, Kim N, Choi SJ, Song S, Yoo JH, Kim MG and Chung JW (2022) Effect of anesthetics on postoperative nausea and vomiting after peripheral vascular surgery in end-stage renal disease patients: A retrospective observational study. Front. Surg. 9:1054670. doi: 10.3389/fsurg.2022.1054670
Received: 27 September 2022; Accepted: 28 October 2022;
Published: 23 November 2022.
Edited by:
Piergiorgio Settembrini, University of Milan, Italy© 2022 Cho, Park, Kim, Choi, Song, Yoo, Kim and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji Won Chung d29sZnRvbUBzY2htYy5hYy5rcg==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Vascular Surgery, a section of the journal Frontiers in Surgery
 Ho Bum Cho
Ho Bum Cho Sun Young Park†
Sun Young Park† Sanghoon Song
Sanghoon Song Ji Won Chung
Ji Won Chung