- 1Department of Gynecology, The Affiliated Hospital of Medical School of Ningbo University, Ningbo, China
- 2Medical School of Ningbo University, Ningbo, China
Objective: Routine omentectomy is generally performed during surgery for patients with epithelial ovarian cancer (EOC). The current study aims to evaluate the impact of omentectomy on cause-specific survival of Stage I–IIIA EOC patients.
Methods: Patients who presented with clinical Stage I–IIIA serous, clear cell, endometrioid, and mucinous ovarian cancers were selected from the SEER Database for the period between 2004 and 2018. We extracted clinicopathological data and surgical information with the focus on the performance of omentectomy and lymphadenectomy. Binary logistic regression and recursive partitioning analyses were conducted to identify the significant factors for the performance of omentectomy during surgery. Propensity score matching (PSM) and inverse probability treatment weighting (IPTW) techniques were utilized to balance confounding factors. Multivariate, exploratory subgroup analyses and sensitivity analyses were conducted to evaluate the impact of omentectomy on cause-specific survival (CSS).
Results: A total of 13,302 patients with EOC were enrolled in the study. The cohort comprised 3,569 endometrioid, 4,915 serous, 2,407 clear cell, and 2,411 mucinous subtypes. A total of 48.62% (6,467/13,302) of patients underwent the procedure of omentectomy during primary surgery, and only 3% absolute improvement in CSS at the individual level was observed, without statistical significance based on multivariate analysis. According to the regression-tree model with recursive partitioning analysis, the procedure of lymphadenectomy was found to be the strongest factor to distinguish the performance of omentectomy, followed by the tumor stage. Patients who underwent omentectomy were more likely to be managed in Stage I than those who underwent lymphadenectomy. After PSM-IPTW adjustment, the inclusion of omentectomy in the initial surgical procedure did not demonstrate a beneficial impact on CSS compared with those who did not undergo the procedure. Exploratory subgroup analysis indicated that the performance of omentectomy improved 5-year CSS in Stage II–IIIA patients. In the sensitive analyses for various tumor stages, omentectomy appeared to benefit only Stage II patients. However, patients across various stages seemed to benefit from the performance of lymphadenectomy, irrespective of the performance of omentectomy on them.
Conclusion: Routine omentectomy may not be associated with survival benefit for patients with a grossly normal-appearing omentum, especially for those with clinical Stage I epithelial ovarian cancers.
Introduction
Epithelial ovarian cancer (EOC) represents the most lethal female reproductive system–associated malignancy, contributing to more than 300,000 incidences and approximately 200,000 deaths worldwide in 2020 (1). EOC is a heterogeneous disease, encompassing four major histological categories: serous, mucinous, endometrioid, and clear cell (2). Comprehensive surgical staging is routinely performed in presumed early-stage EOC disease, including systemic exploration, total abdominal hysterectomy (TAH) with bilateral salpingo-oophorectomy (BSO) if no fertility-sparing is required, omentectomy, peritoneal biopsy, and/or lymphadenectomy (3). Patients with EOC higher than FIGO Stage IA are commonly administered adjuvant platinum-based chemotherapy, while postoperative prognosis depends mainly on the FIGO stage, tumor grade, and residual tumor volume. Patients who are diagnosed at Stages I or II have 5-year survival rates of 70%–90%. Regretfully, about 75% of patients are diagnosed at FIGO Stage III or IV with extensive metastasis and have a 5-year survival rate of less than 50% (4, 5). Although major improvements have been achieved in patient survival rates in many types of cancer, only a modest improvement has been accomplished in EOC even after a breakthrough therapy targeting poly ADP ribose polymerase (PARP) via inhibitors (6) or the FDA-approved bevacizumab (7), possibly due to the development of platinum resistance (8). As such, the tumor microenvironment (TME) becomes an attractive therapeutic target, which has been the focus of intensive research in recent years (9). Of particular interest is the omental tumor microenvironment (10). As a visceral adipose tissue with unique immune functions, the omentum was recognized to play an important role in response to the invasion of foreign bodies, promoting wound healing and tissue recovery (11, 12). Accordingly, gynecological oncologists and researchers started to focus their attention on whether immunological properties within the omentum could be utilized to fight the recurrence of ovarian cancer (10). If so, leaving the normal omentum behind at the time of surgery for early-stage EOC may be theoretically beneficial.
Omentectomy was historically included as part of the primary surgery of EOC because these cancer cells seemed to have a predisposition to invade the omentum (13). Certainly, the majority of ovarian cancer patients with macroscopic omental metastasis, FIGO Stage IIIA2-IVB, will succumb to their disease (14). Thus, in these patients, the omentum should be removed as part of complete cytoreduction to improve their chances of survival. However, occult omental metastases in otherwise EOC confined to ovaries have led to the consideration of omentectomy both for the purpose of concise staging and for its possible therapeutic benefit (15). With the exploration of targeted therapy and precision medicine for ovarian cancer, the role of complete removal of a grossly normal omentum becomes unclear (16). Nevertheless, there is no prospective or adequate retrospective studies to answer the question whether the removal of a macroscopically normal omentum during the surgical procedure of EOC is beneficial, neutral, or even detrimental to the patient.
As an extension of these considerations, we conducted an analysis of the Surveillance, Epidemiology, and End Results (SEER) database to determine the factors associated with the performance of omentectomy and what, if any, impact omentectomy had on cause-specific survival (CSS) in patients without a macroscopic spread beyond the pelvis.
Methods
Study population
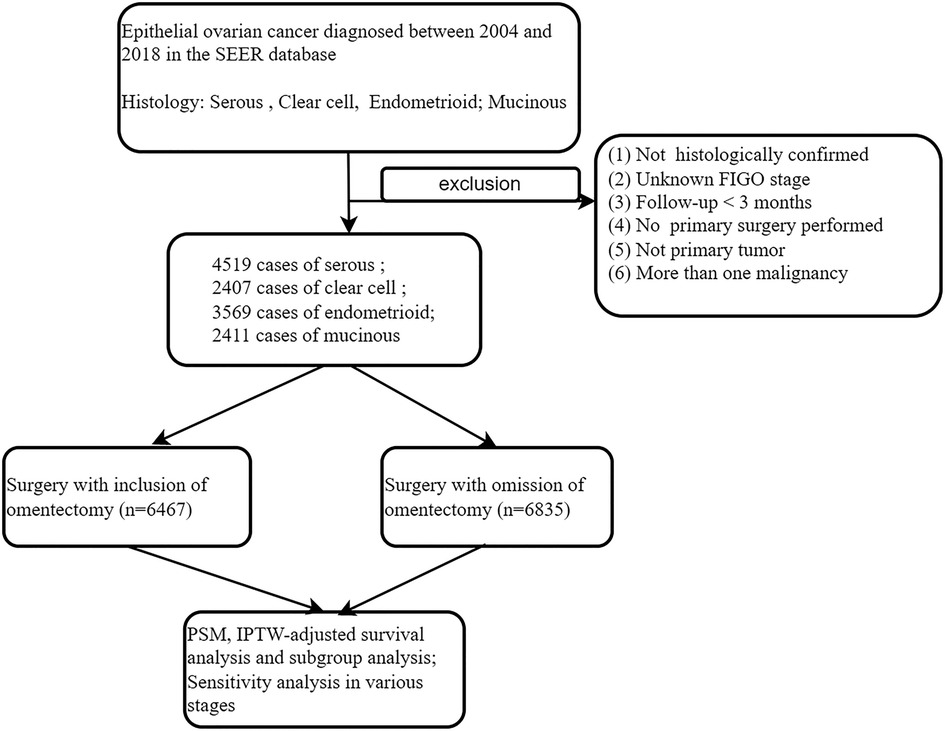
We conducted a retrospective analysis for patients with epithelial ovarian cancer of predominantly or purely serous, clear cell, endometrioid, and mucinous histology. The SEER database (SEER*Stat 8.3.9.2), which contains the data of cancer patients from 17 regional registries (https://seer.cancer.gov/seerstat/), was employed for the analysis. We queried the 2021 release of the SEER database covering the 2004–2018 period, when modern staging information became available in SEER. Ovarian cancer was confirmed by the histology of a hysterectomy specimen and based on the WHO International Classification of Diseases for Oncology, third edition (ICD-O-3) morphology codes as follows: 8441-serous cystadenocarcinoma, NOS, 8460-papillary serous cystadenocarcinoma, 8461-serous surface papillary carcinoma, 8462-papillary serous cystadenocarcinoma, 8463-serous surface papillary carcinoma, 8310-clear cell carcinoma, 8330-endometrioid carcinoma, 8382-endometrioid adenocarcinoma, secretory variant, 8383-endometrioid adenocarcinoma, ciliated cell variant, 8470-mucinous cystadenocarcinoma, 8471-papillary mucinous cystadenocarcinoma, NOS, 8472-mucinous cystadenocarcinoma, 8473-seromucinous carcinoma, 8480-mucinous adenocarcinoma, 8481-mucin-producing adenocarcinoma, and 8482-mucinous adenocarcinoma, endocervical type. Cancer stage was based on the revised FIGO stage in 2014, in which IIIA was defined as tumor involving one or both ovaries or fallopian tubes, or peritoneal cancer, with a cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes (17). Based on site-specific surgery codes, women who underwent at least unilateral salpingo-oophorectomy (site-specific surgery codes 25–80) were selected, and information including whether lymphadenectomy or omentectomy was performed on them was obtained. Performance of omentectomy during surgery was the focus of our study. Because all data included in the SEER database is publicly available online, this study did not require Institutional Review Board approval or informed consent by the study subjects. However, we obtained permission to access the SEER program data from the US National Cancer Institute (reference number: 22756-Nov2020).
The exclusion criteria are listed as follows: (i) patients with more than one malignancy or secondary tumor; (ii) missing information on patients' age, cancer stage, or survival period; (iii) those with the surgery code “local tumor excision or destruction; surgery NOS’’ were excluded, given the fact that we could not identify the scope of the surgical procedure performed. (iv) Stage IIIB–IVB patients were excluded because, in them, the omentum was usually involved. A landmark survival time of 3 months was applied in order to account for immortal time bias. These procedures were demonstrated as detailed in the diagram of Figure 1.
Variable record and cohort definition
Demographic information of the patients encompassed age (<60, 60–60, and > 70), year of diagnosis (2004–2008, 2009–2013, and 2014–2018), marital status (married, single/unmarried, divorced/separated, widowed, and unknown), race (Whites, non-Whites, and others), median household income, and serum CA125 level (elevated, normal, or not documented). Tumor characteristics included histology subtypes (endometrioid, serous, clear cell, and mucinous), stage (I–IIIA), grade (Grade I, well differentiated; Grade II, moderately differentiated; Grade III, poorly differentiated; Grade IV, undifferentiated; unknown grade), tumor size (<50, 50–100, >100 mm, unknown), and tumor laterality (unilateral, bilateral). Treatment data included hysterectomy, salpingo-oophorectomy, omentectomy, lymphadenectomy, and adjuvant chemotherapy.
Outcome measures
Cause-specific survival (CSS) was evaluated for outcome analysis. CSS was defined as the interval from final diagnosis to death due to endometrial cancer. The primary endpoints were estimated as 3-, 5-year and 10-year CSS rates. Patients who survived at the last follow-up were censored.
Statistical analysis
Continuous variables were described as median [interquartile range (IQR)], while categorical variables were demonstrated as frequency. Baseline characteristics were compared in terms of both pre- and post-matching with chi-square test analysis, in which the statistical significance in proportion differences with a p value <0.05 was considered unbalanced. Then, a binary logistic regression model was fitted, and all the pre-/intra-operative factors with a P < 0.05 in the univariable analysis were entered into the initial model, and a conditional backward method was used with a final stopping rule of P < 0.05. The Hosmer–Lemeshow test was used to assess the goodness-of-fit in the final model, and a P > 0.05 was interpreted as a good-fit model. These significant factors identified from binary logistic regression were utilized in subsequent analysis. In an attempt to identify the specific patterns for patient and tumor demographics for the performance of omentectomy, a recursive partitioning analysis was performed to construct a regression-tree model for risk patterns (18). Subsequently, those factors significant for performing omentectomy in the binary logistic regression analysis were entered in the final model, and the chi-square automatic interaction detector method was used for the model with a stopping rule of three layers. The determined nodes in this analysis were utilized in subsequent sensitive analysis.
To explore the performance of omentectomy on survival impact on EOC patients, multiple imputations by chained equations were conducted to control potential bias caused by confounding factors. First, we used a propensity score adjustment by inverse probability of treatment weighting (IPTW) to maximally reduce the differences between the performance and the non-performance of omentectomy. Specifically, the propensity score was calculated using a logistic regression model based on the above-analyzed characteristics. Stratified by performance or non-performance of omentectomy, the propensity score matching (PSM) method was employed through the nearest neighbor-matching with a caliper value of 0.5 for 1:1 matching. Afterward, IPTW was calculated as 1/PS (19, 20) in the omentectomy-performed group, whereas IPTW was calculated as 1/(1-PS) in the cohort with no omentectomy procedure. Stabilization of IPTW was performed by multiplying the standard IPTW by the probability of undergoing the surgery that each patient received (21). Prior to and after IPTW adjustment, univariate analysis (UVA) of the effect of patient characteristics on CSS was conducted using the Kaplan–Meier (KM) method, with the log-rank method for the evaluation of significance. Multivariable analysis (MVA) was performed by using the Cox proportional hazards regression model. Covariates included in the MVA model were selected if they were found significant in the UVA model. Next, we conducted exploratory subgroup analyses and evaluated heterogeneity as the subgroups were presumed to have been subjected to similar conditions (22). Quantification of heterogeneity was evaluated by using the I2 statistic and the Cochran Q test (23). Random-effects models were used when study heterogeneity was high (I2 > 50%) and fixed-effects models were employed when heterogeneity was low (I2 ≤ 50%) (24). In the final sensitive analysis, Kaplan–Meier plotting was used to illustrate CSS rates based on performance of omentectomy in selected subgroups. Statistical analyses were performed by using STATA-MP (version 17.0, College Station, TX, USA), SPSS (version 22.0; SPSS, Chicago, IL, USA), and R software (version 3.6.3; http://www.r-project.org/). Two-sided hypotheses were used for statistical analysis, and a P < 0.05 was considered statistically significant.
Results
Descriptive characteristics of the study population and survival outcomes among all subgroups
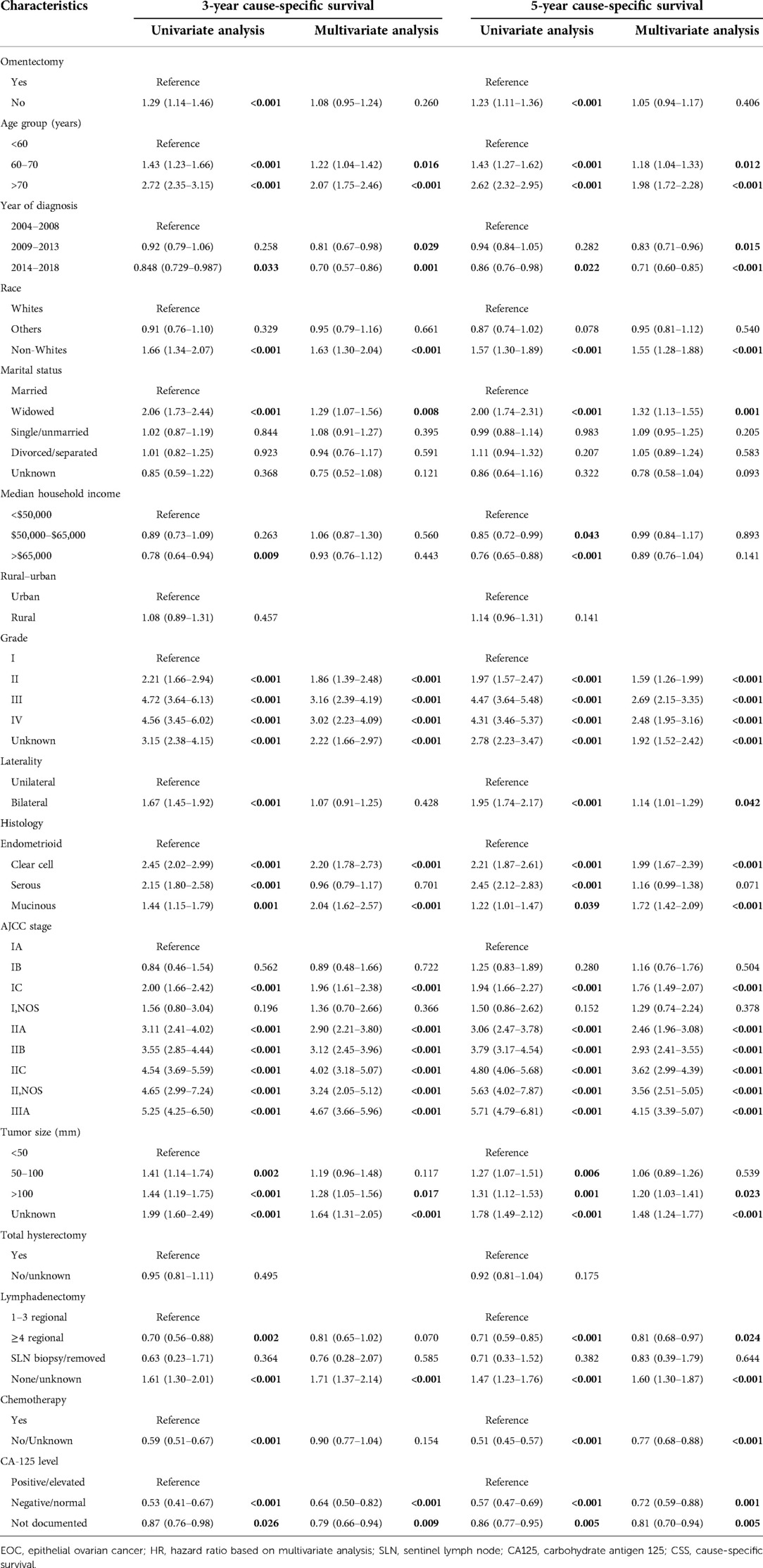
According to the set criteria, the data of a total of 13,302 patients who were diagnosed with epithelia ovarian cancer as the primary malignancy and who underwent at least unilateral salpingo-oophorectomy were extracted for the 2004 and 2018 period. Table 1 depicts the demographic and clinical characteristics of these patients and survival outcomes in the whole group and subgroups. The cohort comprised 3,569 endometrioid, 4,915 serous, 2,407 clear cell, and 2,411 mucinous cancer patients, among of which the best survival outcomes were observed for those with endometrioid cancer irrespective of stage. The median age at initial diagnosis was 56 years old [interquartile range (IQR): 47–65 years old] with a median follow-up period of 67 months [interquartile range (IQR): 33–116 months]. The 3-, 5-year, and 10-year follow-up were completed in 72.65%, 54.44%, and 23.21% of all participants, respectively. Correspondingly, the 3-, 5-year, and 10-year CSS rates were 91.35%, 85.99%, and 77.87% for the whole cohort. In multivariable analysis with correction for other covariates (Table 1), increasing tumor size and patients' age, year of diagnosis between 2004 and 2008, progression of disease stage, higher tumor grade, and bigger tumor volume were related to poor survival. Patients with white race composed of the large proportion in the whole cohort and posed better CSS outcomes than those of black race. Other covariates such as median household income and hysterectomy were not evidently associated with survival outcome. Of note, the performance of lymphadenectomy including lymph node biopsy represented the majority (69.59%) of the whole cohort, providing a beneficial effect on CSS. For instance, 5-year CSS for patients in whom more than four regional lymph nodes were removed was 89.3%, which was an almost 10% improvement compared with those in whom lymphadenectomy was not performed (79.53%). Comparably, although 48.62% (6467/13,302) of patients underwent omentectomy, only 3% absolute improvement in CSS at the 3-, 5-, and 10-year follow-up period was observed, without statistical significance based on multivariate analysis. This result prompted us to further explore the survival impact of omentectomy on EOC patients.
Table 1. Estimated 3-, 5-, and 10-year cause-specific survival for EOC patients stratified by clinical–pathological characteristics.
Exploration of the performance of omentectomy among subgroups and cause-specific survival analysis after PSM–IPTW adjustment by the non-performance of omentectomy
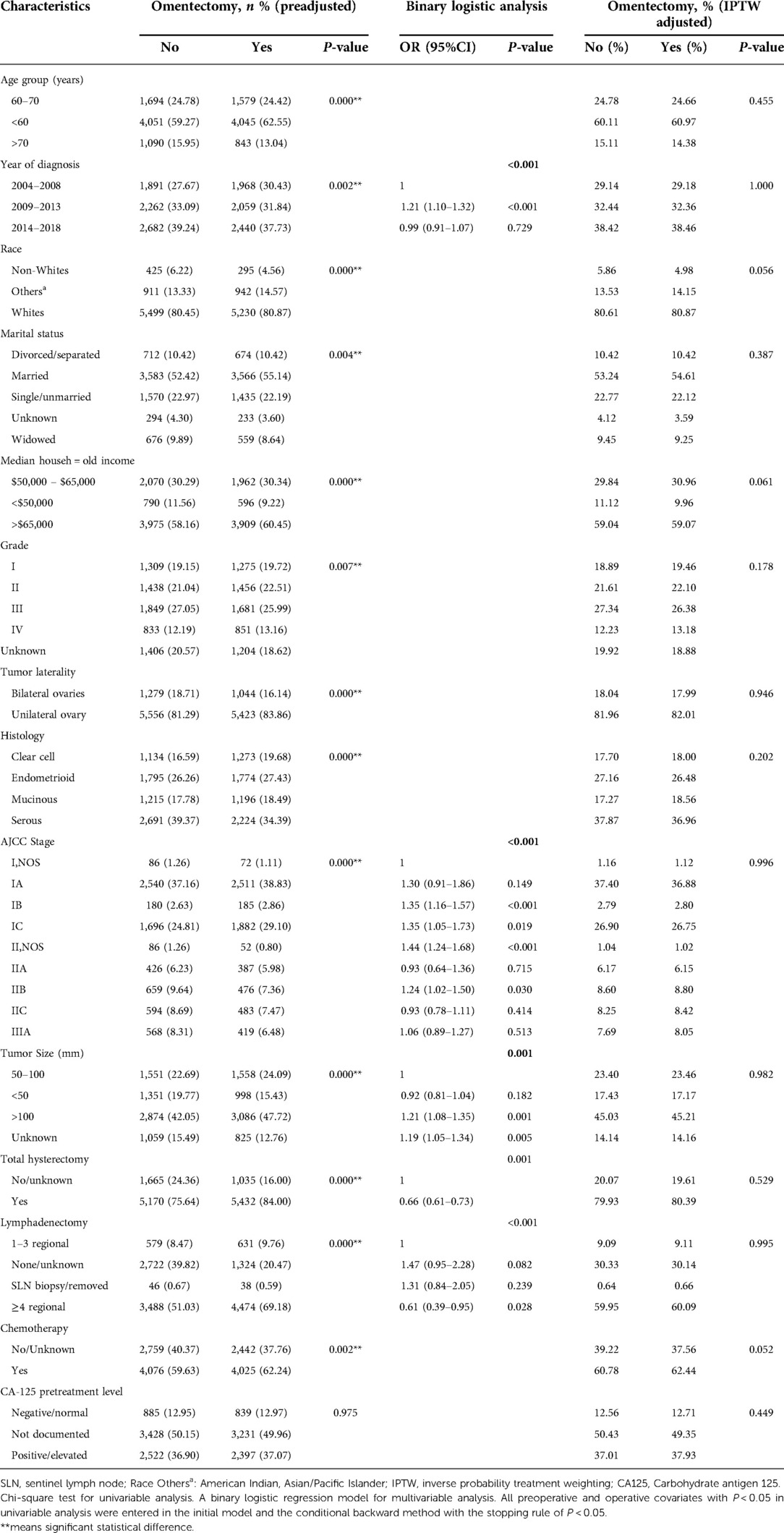
To further explore the association of omentectomy among various clinicopathologic parameters, we stratified the cohort by way of performance or non-performance of omentectomy, as illustrated in Table 2. Before PSM and IPTW adjustment, most baseline characteristics were found to be significantly unbalanced based on univariate analysis. Patients who underwent omentectomy tended to be younger than 60 years of age, were diagnosed between 2004 and 2008, were non-Whites, excluding others, had a higher household income, had tumor involving both ovaries and bigger than 50 mm in diameter, had a histology of clear cell, endometrioid and mucinous subtypes, belonged to the group of Stage I, and who underwent both lymphadenectomy and hysterectomy. In binary logistic analysis, the performance of lymphadenectomy, early-stage tumor, and a bigger tumor size was linked to an increased likelihood of omentectomy (all P < 0.05). In the regression-tree model with recursive partitioning analysis, Hosmer–Lemeshow test indicated a good-fit model (P = 0.331). The procedure of lymphadenectomy was found to be the strongest factor to distinguish the performance of omentectomy, followed by the tumor stage (Supplementary Figure S1). Notably, in the omentectomy group, lymphadenectomy patients in whom more than four regional lymph nodes were removed constituted nearly 70%. After PSM and IPTW adjustment by omentectomy, all baseline characteristics were well balanced with a P value > 0.05. The results are demonstrated in Table 2.
After PSM and IPTW adjustment, univariate analysis (UVA) revealed that omentectomy patients showed improved 3- and 5-year CSS outcomes; however, multivariate analysis (MVA) also revealed similar 3-year CSS (HR 1.08, 95% CI 0.95–1.25, P = 0.26) and 5-year CSS (HR1.05, 95% CI 0.94–1.17, P = 0.406) outcomes. Prognostic factors associated with CSS in patients adjusted by omentectomy persisted, similar to all other significant factors pre-adjustment. A similar prognosis was observed between endometrioid and serous histology patients, although they showed better survival rates than clear cell and mucinous subtypes. Hysterectomy was not statistically associated with prognosis. The performance of lymphadenectomy still provided a beneficial effect on CSS, with the benefit being prominent in those in whom more than four regional lymph nodes were removed. Adjusted UVA and MVA are displayed in Table 3.
Exploratory subgroup and sensitivity analyses in EOC patients stratified by the performance of omentectomy
Based on the aforementioned multivariate analysis, the performance of omentectomy was not associated with beneficial survival impact, even though survival difference was evident in univariate analysis. This inconsistent result prompted us to further explore who would possibly benefit from the procedure of omentectomy. An exploratory subgroup analysis related to prognosis was conducted in selected subgroups, as shown in the forest plot (Figure 2). Before and after matching, heterogeneity was found to be low (I2 < 10%) in the fixed-effects model; therefore, we employed the fixed-effects model to illustrate the result. Prior to matching, survival benefit was observed from the performance of omentectomy in those patients who were younger than 60 years old, diagnosed between 2004 and 2013, those with Stage IC–IIB, tumor grade II, a histology of endometrioid, and serous subtypes, and those in whom more than four regional lymph nodes were removed (Figure 2A). After IPTW adjustment, the above prognostic factors persisted; however, patients who did undergo lymphadenectomy benefited from the performance of omentectomy (Figure 2B), underlining the impact factor in terms of lymphadenectomy and omentectomy on EOC patients.
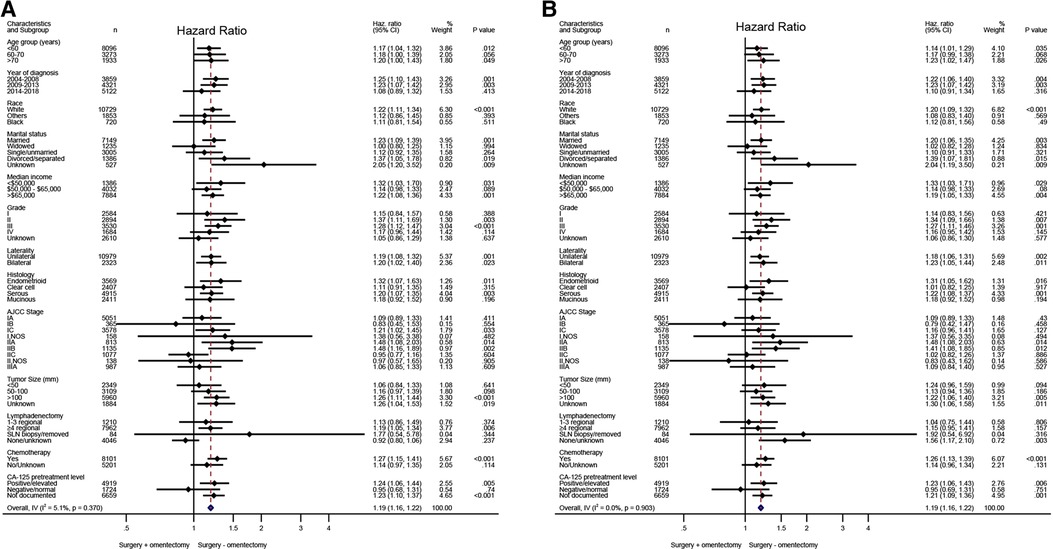
Figure 2. Exploratory subgroup analysis concerning omentectomy impact on survival outcome in the whole cohort. (A) Cause-specific survival before IPTW adjustment. (B) Cause-specific survival after IPTW adjustment. (D) Overall survival after IPTW adjustment. CI, confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weighting. The vertical solid line refers to a hazard ratio of 1.0. HR < 1 favors surgery without omentectomy and HR > 1 favors surgery with omentectomy. A score value of P < 0.05 indicates statistical significance.
As analyzed above, the performance of omentectomy was unbalanced in various stages vis-à-vis lymphadenectomy, with the two factors related to prognosis. Thus, we further performed sensitivity analysis to explore the impact of omentectomy and lymphadenectomy on patients’ survival stratified by disease stages. The patients were classified as follows: omentectomy alone, lymphadenectomy alone, both, and none. The performance of omentectomy showed a similar impact on CSS compared with those who did not undergo the procedure, irrespective of the performance of lymphadenectomy (Figure 3). In detail, lymphadenectomy showed a beneficial effect on those who underwent the procedure compared with those who did not undergo it. Stage I EOC patients benefited from lymphadenectomy alone compared with omentectomy alone; however, those with a combination of both did not show better survival than those with lymphadenectomy alone (Figure 3A). Significant CSS improvement after the performance of omentectomy alone was observed only in Stage II patients (Figure 3B). For Stage IIIA patients, lymphadenectomy with or without omentectomy promoted better survival rates than omentectomy alone or none of these, and no survival difference existed between the latter two patient groups (Figure 3C).
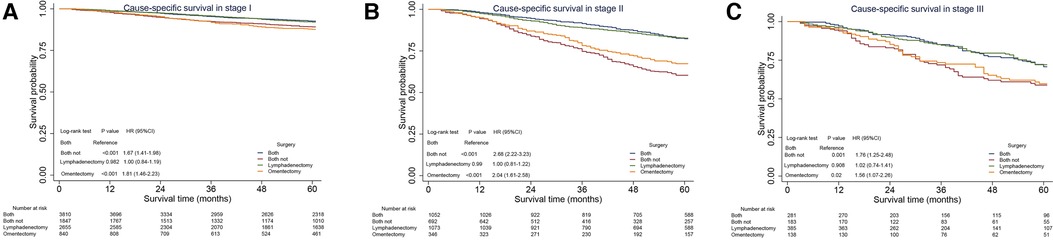
Figure 3. Sensitivity analysis for omentectomy and lymphadenectomy on cause-specific survival (CSS) in Stage I–IIIA epithelial ovarian cancer. (A) CSS in Stage I EOC. (B) CSS in Stage II EOC. (C) CSS in Stage IIIA1 EOC. CI, confidence interval; HR, hazard ratio. P < 0.05 indicates statistical significance.
Discussion
The current SEER database exploration was a retrospective population-based analysis with, to our knowledge, the largest sample size to evaluate factors associated with the performance of omentectomy and its impact on CSS in patients with epithelia ovarian cancer. By way of literature review, there has never been a randomized control trial explaining whether resection of a grossly normal-appearing omentum made any difference in EOC patient outcomes. An analysis of the earlier SEER database included 5,454 EOC patients who underwent omentectomy during surgery compared with 2,404 patients who did not undergo the procedure. No statistical difference was found in terms of disease-specific survival in that cohort (25). In this study, we included a larger number of cases with a relatively long follow-up period to further explore the impact of omentectomy on the survival of EOC patients.
Omentectomy was included at the time of surgery in approximately half of EOC patients. Although a marginal increase in the estimated survival rate was observed in these omentectomy EOC patients, after controlling for confounders, it was found that the performance of omentectomy was not associated with better survival. Afterward, we utilized the PMS–IPTW method to control for confounding factors and conditional landmark analysis, reducing the possibility that this conclusion suffered from selection bias and immortality bias, respectively. In subgroup and sensitive analyses, omentectomy showed a significant survival benefit for patients in Stage II; however, there was no survival difference in those with Stage I disease.
Omentectomy was initially included as a routine component of ovarian cancer surgery in the early 1960s. At that time, it was indicated that ovarian cancer patients who had undergone extensive tumor removal during operation, fared better (26). Until now, the current clinical guidelines also included omentectomy in the standard surgical procedure for all epithelial ovarian cancer patients, even in early-stage disease (3, 17, 27). For instance, the most recent NCCN guidelines recommended that for patients with disease apparently confined to the ovaries or to the pelvis (presumed Stage I/II), omentectomy should be performed to exclude higher-stage disease. For patients with disease spreading to the upper abdomen (Stage III/IV), it was recommended that omentum be removed (3). However, neither of the abovementioned clinical guidelines spoke about the specific survival impact of omentectomy on EOC patients. Omentectomy was included in most EOC cases based on the theory that omentum may harbor micrometastases and/or may be a site of recurrent disease in the future. As such, comprehensive staging mainly contributed to the determination in terms of adjuvant chemotherapy, which was based only on the results of staging surgery. However, the percentage of microscopic metastasis in a grossly normal omentum remains uncertain. Limited studies reported that the rate of occult omental metastases in EOC confined to ovaries (Stage I) ranged from 2% to 4% and then reached a higher stage to Stage III A in 3% to 11% of patients (28). However, performing an omentectomy did not result in a prolonged survival period in this condition, according to the recent French guidelines for oncology (28). In one retrospective review of 256 ovarian cancer patients with a macroscopically normal omentum, it was observed that after routine staging omentectomy, only 7 (2.7%) patients were upstaged and only one patient received adjuvant chemotherapy based on microscopic metastasis (29). Another study that enrolled 211 patients showed that only 2% of patients were upstaged and no patients were determined to be administered with chemotherapy based only on microscopic involvement of the omentum (30). That study included patients with endometrioid (37%), serous (25%), mucinous (16%), and clear cell (14%) cancer subtypes. Our exploratory subgroup analysis found that patients with serous and endometrioid histologies may benefit from the performance of omentectomy; however, no impact was found on those with mucinous and clear cell subtypes. A literature search revealed only one research showing 1 of 35 “initial” Stages IA and 33 IC ovarian mucinous cancer patients having microscopic omentum metastasis at the final histological analysis (31). Nevertheless, in those patients in whom adjuvant chemotherapy was determined, it was uncertain whether the removal of the normal omentum resulted in any potential therapeutic effect (32–34). In addition, the pathology literature suggested that for the sole purpose of staging and detecting microscopic disease, omental biopsies may probably suffice in a grossly normal-appearing omentum (34). One group of researchers suggested that, with regard to pathologic evaluation, 10 blocks could obtain a sensitivity of as high as 95% (35). Another early study reported that, for patients with endometrial and ovarian carcinomas without a macroscopic intra-abdominal lesion, just three to five omentum samples seemed sufficient for staging (36). Based on these findings, most oncologists reasoned that a careful macroscopic examination might be the most important procedure in identifying small omental metastasis.
Given that the performance of lymphadenectomy was identified as the most prominent confounding factor for omentectomy in the present analysis, we paid particular attention to the impact of omentectomy on CSS in the subgroup of lymphadenectomy and non-lymphadenectomy patients. More importantly, we further explored the survival difference among patients who underwent lymphadenectomy, omentectomy, both, or none, with the purpose of reducing the possibility of omentectomy impact on survival confounded by lymphadenectomy. The role of lymphadenectomy in ovarian cancer has been in the realm of contradiction for a long period. Many retrospective analyses, including involving large samples of patients, have indicated a survival benefit for lymphadenectomy, and accordingly, patients have been exposed to this procedure over several decades. Lymph-node metastases detected by systematic lymphadenectomy have been reported in the range of 44 to 53% in patients across all FIGO stages (37). A higher stage is associated with an increasing frequency of lymph node metastases, approximately 3%–14% being reported in early-stage EOC patients (38, 39). The core issue here is whether removal of lymph nodes should be performed only to stage the disease or whether the removal itself improves survival (40). For EOC patients in the presumed early stage, a randomized study showed that systematic lymphadenectomy facilitated better and easier detection of metastatic nodes compared with lymph node sampling, but this was not associated with improved survival (41). Another meta-analysis reported that systematic lymphadenectomy improved OS in patients with early-stage disease, even though it did not improve progression-free survival (42). In our study, lymphadenectomy was performed in a majority of Stage I–II patients, demonstrating a significant improvement compared with those without lymph node removal. The addition of omentectomy to lymphadenectomy did not improve survival rates compared with lymphadenectomy alone. In Stage II patients in whom lymphadenectomy was performed, omentectomy showed a survival benefit. Interestingly, the performance of omentectomy persistently showed no survival improvement in Stage IIIA patients, which was partly upstaged by lymphadenectomy when tumors confined to the ovaries. Chemotherapy is usually administered in Stage IIIA EOC patients, which makes the influence of omentectomy on such patients more uncertain.
Although we included the largest sample of patients in whom omentectomy was performed during surgery to investigate the impact of omentectomy on the CSS of patients with clinical Stage I–IIIA EOC, we recognized several inherent methodological limitations in this study. Five questions need to be addressed in a future study. First, the selection bias of a retrospective study design represented the main limitation of this study. Our findings remained primarily hypothesis-generating, and going forward, they must be evaluated in the context of randomized evidence, when available. Second, our data lacked detailed information regarding tumor margin status and the intraoperative omental and peritoneal assessment. Because of the surgical codes used, we could not analyze the scope of the performed omentectomy or the mode of surgery, that is whether it was open or minimally invasive. Furthermore, based on the coding schema used by the SEER database, we could not determine whether patients who did not undergo omentectomy received omental biopsies. Third, the database did not contain data regarding the chemotherapy regimen, course, as well as response. Fourth, our analysis focused primarily on CSS without providing any details about local recurrence and distant metastasis after initial treatment. This could be attributed to the unavailability of such details in the SEER database, which could have important implications for studying the impact of adjuvant therapy on this patient population.
Conclusion
Patients with clinical Stage I epithelial ovarian carcinomas managed by surgery with the inclusion of omentectomy did not have any survival benefit. Thus, routine omentectomy could be potentially omitted when staging EOC patients when grossly omental abnormalities and extra-ovarian disease spread were not identified. The performance of omentectomy should still be a routine practice when tumor spreads beyond the surface of the ovaries. Further, multi-institutional studies, focusing on the incidence of isolated microscopic omental metastases as well as on the oncologic outcomes of patients having a normal-appearing omentum and not undergoing omentectomy, are required to validate our results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Author contributions
SY was responsible for the study planning. ZH was responsible for data collection, statistical analysis, and manuscript writing. YY made a potential contribution to statistical analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Ningbo Natural Science Foundation (grant no. 2018A610331).
Acknowledgments
The authors sincerely thank all the staff of the SEER program for their important work and diligent effort.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1052788/full#supplementary-material.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
2. Köbel M, Kang EY. The evolution of ovarian carcinoma subclassification. Cancers (Basel). (2022) 14:400–16. doi: 10.3390/cancers14020416
3. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:191–226. doi: 10.6004/jnccn.2021.0007
4. Steinberga I, Jansson K, Sorbe B. Quality indicators and survival outcome in stage IIIB–IVB epithelial ovarian cancer treated at a single institution. In Vivo. (2019) 33:1521–30. doi: 10.21873/invivo.11632
5. Chehade H, Tedja R, Ramos H, Bawa TS, Adzibolosu N, Gogoi R, et al. Regulatory role of the adipose microenvironment on ovarian cancer progression. Cancers (Basel). (2022) 14:2267–82. doi: 10.3390/cancers14092267
6. Taylor KN, Eskander RN. PARP inhibitors in epithelial ovarian cancer. Recent Pat Anticancer Drug Discov. (2018) 13:145–58. doi: 10.2174/1574892813666171204094822
7. Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. (2019) 381:2416–28. doi: 10.1056/NEJMoa1911361
8. Kim S, Han Y, Kim SI, Kim HS, Kim SJ, Song YS. Tumor evolution and chemoresistance in ovarian cancer. NPJ Precis Oncol. (2018) 2:20–9. doi: 10.1038/s41698-018-0063-0
9. Cummings M, Freer C, Orsi NM. Targeting the tumour microenvironment in platinum-resistant ovarian cancer. Semin Cancer Biol. (2021) 77:3–28. doi: 10.1016/j.semcancer.2021.02.007
10. Bella Á, Arrizabalaga L, Di Trani CA, Fernández-Sendin M, Teijeira A, Russo-Cabrera JS, et al. Omentum: friend or foe in ovarian cancer immunotherapy? Int Rev Cell Mol Biol. (2022) 371:117–31. doi: 10.1016/bs.ircmb.2022.04.017
11. Shah S, Lowery E, Braun RK, Martin A, Huang N, Medina M, et al. Cellular basis of tissue regeneration by omentum. PLoS One. (2012) 7:e38368. doi: 10.1371/journal.pone.0038368
12. Meza-Perez S, Randall TD. Immunological functions of the omentum. Trends Immunol. (2017) 38:526–36. doi: 10.1016/j.it.2017.03.002
13. Redondo A, Guerra E, Manso L, Martin-Lorente C, Martinez-Garcia J, Perez-Fidalgo JA, et al. SEOM clinical guideline in ovarian cancer (2020). Clin Transl Oncol. (2021) 23:961–8. doi: 10.1007/s12094-020-02545-x
14. Iwagoi Y, Motohara T, Hwang S, Fujimoto K, Ikeda T, Katabuchi H. Omental metastasis as a predictive risk factor for unfavorable prognosis in patients with stage III–IV epithelial ovarian cancer. Int J Clin Oncol. (2021) 26:995–1004. doi: 10.1007/s10147-021-01866-3
15. Arie A B, McNally L, Kapp DS, Teng NN. The omentum and omentectomy in epithelial ovarian cancer: a reappraisal. Part I – omental function and history of omentectomy. Gynecol Oncol. (2013) 131:780–3. doi: 10.1016/j.ygyno.2013.09.014
16. Bilbao M, Aikins JK, Ostrovsky O. Is routine omentectomy of grossly normal omentum helpful in surgery for ovarian cancer? A look at the tumor microenvironment and its clinical implications. Gynecol Oncol. (2021) 161:78–82. doi: 10.1016/j.ygyno.2020.12.033
17. Berek JS, Renz M, Kehoe S, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet. (2021) 155(Suppl 1):61–85. doi: 10.1002/ijgo.13878
18. Chen PG, Lee SY, Barnett GH, Vogelbaum MA, Saxton JP, Fleming PA, et al. Use of the Radiation Therapy Oncology Group recursive partitioning analysis classification system and predictors of survival in 19 women with brain metastases from ovarian carcinoma. Cancer. (2005) 104:2174–80. doi: 10.1002/cncr.21472
19. Kane LT, Fang T, Galetta MS, Goyal DKC, Nicholson KJ, Kepler CK, et al. Propensity score matching: a statistical method. Clin Spine Surg. (2020) 33:120–2. doi: 10.1097/bsd.0000000000000932
20. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. (2008) 168:656–64. doi: 10.1093/aje/kwn164
21. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. (2015) 34:3661–79. doi: 10.1002/sim.6607
22. Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg. (2018) 27:317–21. doi: 10.1093/icvts/ivy163
23. Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. (2011) 11:41. doi: 10.1186/1471-2288-11-41
24. Foreman J, Salim AT, Praveen A, Fonseka D, Ting DSW, Guang He M, et al. Association between digital smart device use and myopia: a systematic review and meta-analysis. Lancet Digit Health. (2021) 3:e806–e18. doi: 10.1016/s2589-7500(21)00135-7
25. McNally L, Teng NN, Kapp DS, Karam A. Does omentectomy in epithelial ovarian cancer affect survival? An analysis of the surveillance, epidemiology, and end results database. Int J Gynecol Cancer. (2015) 25:607–15. doi: 10.1097/igc.0000000000000412
26. Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. (1975) 42:101–4.1234624
27. Komiyama S, Katabuchi H, Mikami M, Nagase S, Okamoto A, Ito K, et al. Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of ovarian cancer including primary peritoneal cancer and fallopian tube cancer. Int J Clin Oncol. (2016) 21:435–46. doi: 10.1007/s10147-016-0985-x
28. Bolze PA, Collinet P, Golfier F, Bourgin C. Surgery in early-stage ovarian cancer: Article drafted from the French Guidelines in oncology entitled “Initial management of patients with epithelial ovarian cancer” developed by FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY under the aegis of CNGOF and endorsed by INCa. Gynecol Obstet Fertil Senol. (2019) 47:168–79. doi: 10.1016/j.gofs.2018.12.007
29. Lee JY, Kim HS, Chung HH, Kim JW, Park NH, Song YS. The role of omentectomy and random peritoneal biopsies as part of comprehensive surgical staging in apparent early-stage epithelial ovarian cancer. Ann Surg Oncol. (2014) 21:2762–6. doi: 10.1245/s10434-014-3648-z
30. Powless CA, Bakkum-Gamez JN, Aletti GD, Cliby WA. Random peritoneal biopsies have limited value in staging of apparent early stage epithelial ovarian cancer after thorough exploration. Gynecol Oncol. (2009) 115:86–9. doi: 10.1016/j.ygyno.2009.06.037
31. Gouy S, Saidani M, Maulard A, Faron M, Bach-Hamba S, Bentivegna E, et al. Staging surgery in early-stage ovarian mucinous tumors according to expansile and infiltrative types. Gynecol Oncol Rep. (2017) 22:21–5. doi: 10.1016/j.gore.2017.08.006
32. Cress RD, Bauer K, O'Malley CD, Kahn AR, Schymura MJ, Wike JM, et al. Surgical staging of early stage epithelial ovarian cancer: results from the CDC-NPCR ovarian patterns of care study. Gynecol Oncol. (2011) 121:94–9. doi: 10.1016/j.ygyno.2010.12.359
33. Dizon DS, Restivo A, Lomme M, Charbonneau N, Brard L, Hughes T, et al. For women receiving chemotherapy for clinically apparent early ovarian cancer, is there a benefit to surgical staging? Am J Clin Oncol. (2008) 31:39–42. doi: 10.1097/COC.0b013e318134ee6f
34. Arie AB, McNally L, Kapp DS, Teng NN. The omentum and omentectomy in epithelial ovarian cancer: a reappraisal: part II – the role of omentectomy in the staging and treatment of apparent early stage epithelial ovarian cancer. Gynecol Oncol. (2013) 131:784–90. doi: 10.1016/j.ygyno.2013.09.013
35. Skala SL, Hagemann IS. Optimal sampling of grossly normal omentum in staging of gynecologic malignancies. Int J Gynecol Pathol. (2015) 34:281–7. doi: 10.1097/pgp.0000000000000148
36. Usubütün A, Ozseker HS, Himmetoglu C, Balci S, Ayhan A. Omentectomy for gynecologic cancer: how much sampling is adequate for microscopic examination? Arch Pathol Lab Med. (2007) 131:1578–81. doi: 10.5858/2007-131-1578-ofgchm
37. Harter P, Gnauert K, Hils R, Lehmann TG, Fisseler-Eckhoff A, Traut A, et al. Pattern and clinical predictors of lymph node metastases in epithelial ovarian cancer. Int J Gynecol Cancer. (2007) 17:1238–44. doi: 10.1111/j.1525-1438.2007.00931.x
38. Lago V, Minig L, Fotopoulou C. Incidence of lymph node metastases in apparent early-stage low-grade epithelial ovarian cancer: a comprehensive review. Int J Gynecol Cancer. (2016) 26:1407–14. doi: 10.1097/igc.0000000000000787
39. Svolgaard O, Lidegaard O, Nielsen ML, Nedergaard L, Mosgaard BJ, Lidang M, et al. Lymphadenectomy in surgical stage I epithelial ovarian cancer. Acta Obstet Gynecol Scand. (2014) 93:256–60. doi: 10.1111/aogs.12322
40. Benedetti Panici P, Giannini A, Fischetti M, Lecce F, Di Donato V. Lymphadenectomy in ovarian cancer: is it still justified? Curr Oncol Rep. (2020) 22:22–6. doi: 10.1007/s11912-020-0883-2
41. Maggioni A, Benedetti Panici P, Dell'Anna T, Landoni F, Lissoni A, Pellegrino A, et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br J Cancer. (2006) 95:699–704. doi: 10.1038/sj.bjc.6603323
Keywords: epithelial ovarian cancer, omentectomy, lymphadenectomy, PSM, IPTW, regression tree model
Citation: Hao Z, Yu Y and Yang S (2022) The impact of omentectomy on cause-specific survival of Stage I–IIIA epithelial ovarian cancer: A PSM–IPTW analysis based on the SEER database. Front. Surg. 9:1052788. doi: 10.3389/fsurg.2022.1052788
Received: 24 September 2022; Accepted: 25 November 2022;
Published: 29 December 2022.
Edited by:
Stefano Cianci, University of Messina, ItalyReviewed by:
Ruiqing Ma, Aerospace Center Hospital, ChinaFerdinando Antonio Gulino, Azienda di Rilievo Nazionale e di Alta Specializzazione (Arnas) Garibaldi, Italy
© 2022 Hao, Yu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhimin Hao ZnloYW96aGltaW5AbmJ1LmVkdS5jbg== Sufen Yang eWFuZ3N1ZmVuZWR1QHNpbmEuY29t
Specialty Section: This article was submitted to Obstetrics and Gynecological Surgery, a section of the journal Frontiers in Surgery
 Zhimin Hao
Zhimin Hao Yangli Yu
Yangli Yu Sufen Yang
Sufen Yang