- 1Department of Gastrointestinal Surgery, Second Department of General Surgery, The Sixth Affiliated Hospital, School of Medicine, South China University of Technology, Foshan, China
- 2Department of Gastrointestinal Surgery, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 3Shantou University Medical College, Shantou, China
- 4Guangdong Provincial People’s Hospital Ganzhou Hospital (Ganzhou Municipal Hospital), Ganzhou, China
- 5School of Medicine, South China University of Technology, Guangzhou, China
- 6School of Biology and Biological Engineering, South China University of Technology, Guangzhou, China
- 7The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
Background: Controversy exists over the role of upfront primary tumor resection (PTR) in asymptomatic patients with unresectable stage IV colorectal cancer (CRC). The purpose of this study was to evaluate the effect of upfront PTR on survival outcomes and adverse outcomes.
Methods: Searches were conducted on PubMed, EMBASE, Web of Science, and Cochrane Library from inception to August 2021. Studies comparing survival outcomes with or without adverse outcomes between PTR and non-PTR treatments were included. Review Manager 5.3 was applied for meta-analyses with a random-effects model whenever possible.
Results: Overall, 20 studies with 3,088 patients were finally included in this systematic review. Compared with non-PTR, upfront PTR was associated with better 3-year (HR: 0.69, 95% CI, 0.57–0.83, P = 0.0001) and 5-year overall survival (OS) (HR: 0.77, 95% CI, 0.62–0.95, P = 0.01), while subgroup analysis indicated that there was no significant difference between upfront PTR and upfront chemotherapy (CT) group. In addition, grade 3 or higher adverse effects due to CT were more frequent in the PTR group with marginal significance (OR: 1.74, 95% CI, 0.99–3.06, P = 0.05), and other adverse outcomes were comparable.
Conclusions: PTR might be related to improved OS for asymptomatic patients with unresectable stage IV CRC, whereas receiving upfront CT is a rational alternative without detrimental influence on survival or adverse outcomes compared with upfront PTR.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=272675
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed malignant tumor and the second leading cause of cancer death worldwide (1). Approximately 20% of newly diagnosed CRCs have reached stage IV with distant metastases, most of which are unresectable (2). It is practically inevitable for patients with primary tumor-related symptoms to receive primary tumor resection (PTR) or other interventional treatments such as diverting colostomy, colonic stenting, and decompression tube in case of obstruction, perforation, or hemorrhage. For asymptomatic patients, systemic therapy as the upfront treatment has shown acceptable tolerance, with only 16% of patients require intervention caused by subsequent primary tumor-related complications (3). Although it has been of great interest and actively debated in recent years, the role of upfront PTR remains controversial. On one hand, potential risks of emergency cases due to primary tumors can be prevented once it has been resected. On the other hand, the occurrence of postoperative adverse events or complications might delay the initiation of systemic therapy, and consequently impact patients' survival outcomes (4–6). Hence, the pros and cons of PTR need to be cautiously assessed in order to help guidelines development especially for a defined patient population with this specific condition.
Previously published studies reported conflicting results on this issue, with the result that the conclusions of relevant systematic reviews and meta-analyses have updated and changed from endorsement (7, 8) to reservation (9). All previously published meta-analyses included only retrospective studies due to the lack of prospective research before then. Recently, two randomized controlled trials (RCTs) from Korea (10) and Japan (11) were published respectively in 2020 and 2021, and both suggesting that PTR followed by chemotherapy (CT) showed no or no significant survival benefit over upfront CT.
With the emergence of more and more high-level evidence, an updated systematic review and meta-analysis is needed in time for current practice. This study aims to evaluate the effect of upfront PTR on survival outcomes and adverse outcomes, and so determine whether PTR should be performed in case of asymptomatic patients with unresectable stage IV CRC at the time of diagnosis.
Methods
Study selection
This systematic review and meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12). This study has been registered at the International Prospective Register of Systematic Reviews (CRD42021272675).
Comprehensive literature searches were identified in the databases of PubMed, EMBASE, Web of Science, and Cochrane Library from inception to August 2021. The full search strategy is available in Supplementary Tables S1–4. Two reviewers (Z. Liu and Z. Liang) independently screened the titles and abstracts of identified studies. After duplicate studies were excluded, the full texts of screened papers were reviewed for further inspection. In addition, we attempted to contact the authors of reports without sufficient data.
Reporting meeting the criteria were considered eligible: (a) patients with stage IV CRC; (b) patients were asymptomatic or relatively symptom-free; (c) patients diagnosed and evaluated as incurable or unable to achieve curative surgery; (d) studies reporting at least survival outcomes. The exclusion criteria were as followed: (a) studies not eligible in accordance with the inclusion criteria; (b) studies without sufficient data; (c) reviews, letters, comments, conference abstracts, or reports only with a protocol.
Data collection process
Variables of characteristics included year, country, study design, enrollment interval, number of participants, age, gender, and other clinicopathological features.
The primary outcomes were long-term survival outcomes, including overall survival (OS) and progression-free survival (PFS) of the PTR group and non-PTR group. The secondary outcomes consisted of postoperative complications and adverse events of both groups.
Data were independently extracted by two reviewers (Z. Liang and Z. Liu). Discrepancies were tackled through discussion with a third reviewer (XF).
Quality assessment
The modified Jadad quality scale (13) ranging from 0 to 7 points was used for bias assessment of RCTs and the Newcastle-Ottawa scale (NOS) (14) ranging from 0 to 9 points was for non-RCTs in this systematic review, with higher scores indicating better quality. Studies scoring greater than or equal to 4 points of the modified Jadad scale or 5 points of the NOS were considered high quality.
Furthermore, funnel plots were also used to evaluate bias including selection bias, publication bias, true heterogeneity, poor methodological quality of smaller studies, and so on. Bias can be visually measured by whether the funnel diagram is symmetrically distributed, but it is subjective, and the interpretation results vary for different reviewers.
Quality assessment was rated by two review authors (Z. Liang and Z. Liu). In case of disagreements, a third author (XF) was asked to participate in discussion until a consensus is reached.
Statistical analysis
Review Manager (Revman) 5.3 (https://community.cochrane.org/help/tools-and-software/revman) was applied for meta-analyses with a random-effects model whenever possible. Survival outcomes were presented as hazard ratios (HRs) with 95% confidence intervals (CIs). If HRs of included studies were not reported directly, an estimated HR was derived from Kaplan-Meier curves based on the method raised by Tierney et. al (15). In addition, continuous variables were analyzed by weighted mean differences (WMDs) with 95% CI, and odds ratios (ORs) with 95% CIs were used to assess dichotomous variables. All results compared were considered statistically significant at a two-sided P < 0.05.
The heterogeneity was evaluated by the Cochrane Q test and Higgins I2 test. If the heterogeneity was considered high (P < 0.1 or I2 > 50%), then a subgroup analysis or sensitivity analysis would be conducted.
Results
Study selected
In total, 2005 studies were identified through databases, and 1,193 were left out after removing the duplicates. Of these, 44 studies requiring full-text assessment for eligibility remained. After further reviewing, a total of 20 published articles including 2 RCTs and 18 non-RCTs met the criteria. The flow chart demonstrating the details of the selection process was presented in Figure 1.
Quality assessment
The quality of the included non-RCTs studies ranged from four to eight according to the NOS, and the median score was six points. Both two RCTs scored five points on the modified Jadad scale. Funnel plots were depicted for 3-year OS and 5-year OS with roughly symmetric distribution, suggesting that nearly barren of publication bias existed, even though there might be certain bias and heterogeneity.
Quality assessment of included studies was presented in Supplementary Table S5 and Figure S1.
Study characteristics
In total, 20 studies (10, 11, 16–33) including 3,088 patients were finally included in this study. The baseline characteristics of each study were collected, listing in Table 1. These articles were published ranging from 1999 to 2021. The only two published RCTs (10, 11) were released in 2020 and 2021 respectively. In summary, 1,667 (54%) patients with asymptomatic and unresectable stage IV CRCs received PTR. The median follow-up period reported by included studies ranged from 7 to 46.5 months. The median age was concentrated around 60–65 years old. The proportion of males suffering from the disease was higher than that of females. Patients in studies of Benoist et al. and Galizia et al. were reported only with live metastases. Additionally, we summarized the clinicopathological characteristics of all the included studies. Notably, the clinicopathological features of eight studies (17, 18, 20, 21, 26, 28, 30–32) were not matched, which might contribute to bias or heterogeneity.
Primary outcomes
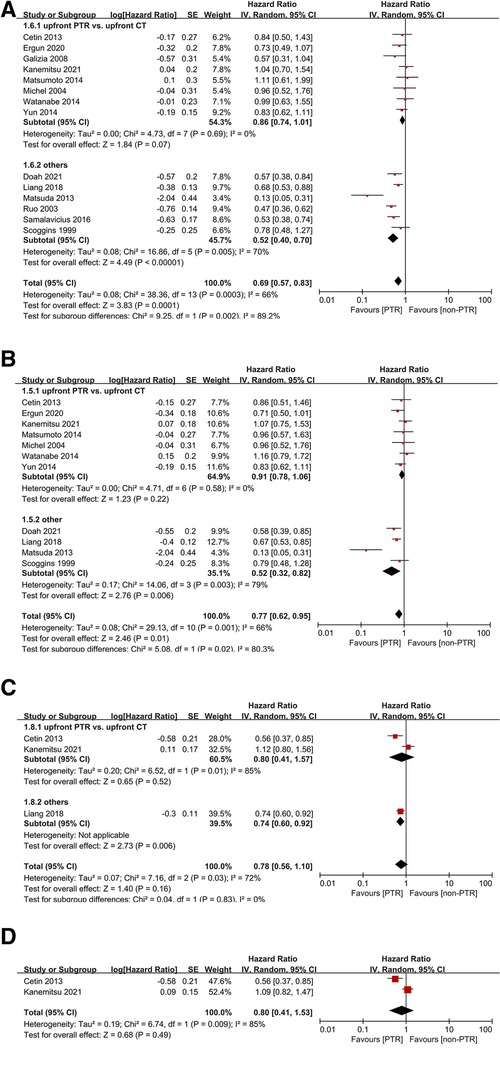
Of all included studies, six non-RCTs reported a significant advantage of OS in the PTR group. Compared with non-PTR group, upfront PTR group was associated with better 3-year (HR: 0.69, 95% CI, 0.57–0.83, P = 0.0001) (Figure 2A) and 5-year OS (HR: 0.77, 95% CI, 0.62–0.95, P = 0.01) (Figure 2B). A high level of heterogeneity was observed in both analyses (I2 = 66% for both 3-year and 5-year OS).
Subgroup analyses indicated that there was no significant difference between upfront PTR and upfront CT group (P = 0.07 and 0.22 for 3-year and 5-year OS), and no heterogeneity was observed.
Only three studies reported PFS outcomes between the two groups. There was no significant difference in either 2-year (HR: 0.78, 95% CI, 0.56–1.10, P = 0.16) (Figure 2C) or 3-year DFS (HR: 0.80, 95% CI, 0.41–1.53, P = 0.49) (Figure 2D). The heterogeneities might be also due to various types of treatment in non-PTR groups.
In addition, four studies (10, 19, 21, 22) reporting a comparison of 2-year OS between groups suggested that PTR was not significantly related to the benefit from survival. Patients in non-PTR groups of all these four studies received CT. One study (32) suggested that 2-year, 3-year, and 5-year OS all benefit from PTR, but did not provide more adequate data for a meta-analysis.
Notably, Zhang et,al (29). reported that PTR could prolonged OS in patients only with left-side CRC (P = 0.009) but showed no benefit in terms of right-side CRC (P = 0.910).
Secondary outcomes
In pooled analyses, comparable incidence of postoperative complications (P = 0.86) (Figure 3A) and 30-day mortality after upfront treatment (P = 0.79) (Figure 3B) were observed. Grade 3 or higher adverse effects due to CT were more frequent in the PTR group with marginal significance (OR: 1.74, 95% CI, 0.99–3.06, P = 0.05) (Figure 3C). Additionally, pooled analysis of two studies indicated that patients in non-PTR groups received CT earlier than those in PTR groups (WMD: 27.13 days, 95% CI, 25.20–29.05, P < 0.00001) (Figure 3D), and another study (26) similarly reported that the median time from the first visit to chemotherapy was significantly earlier in CT group.
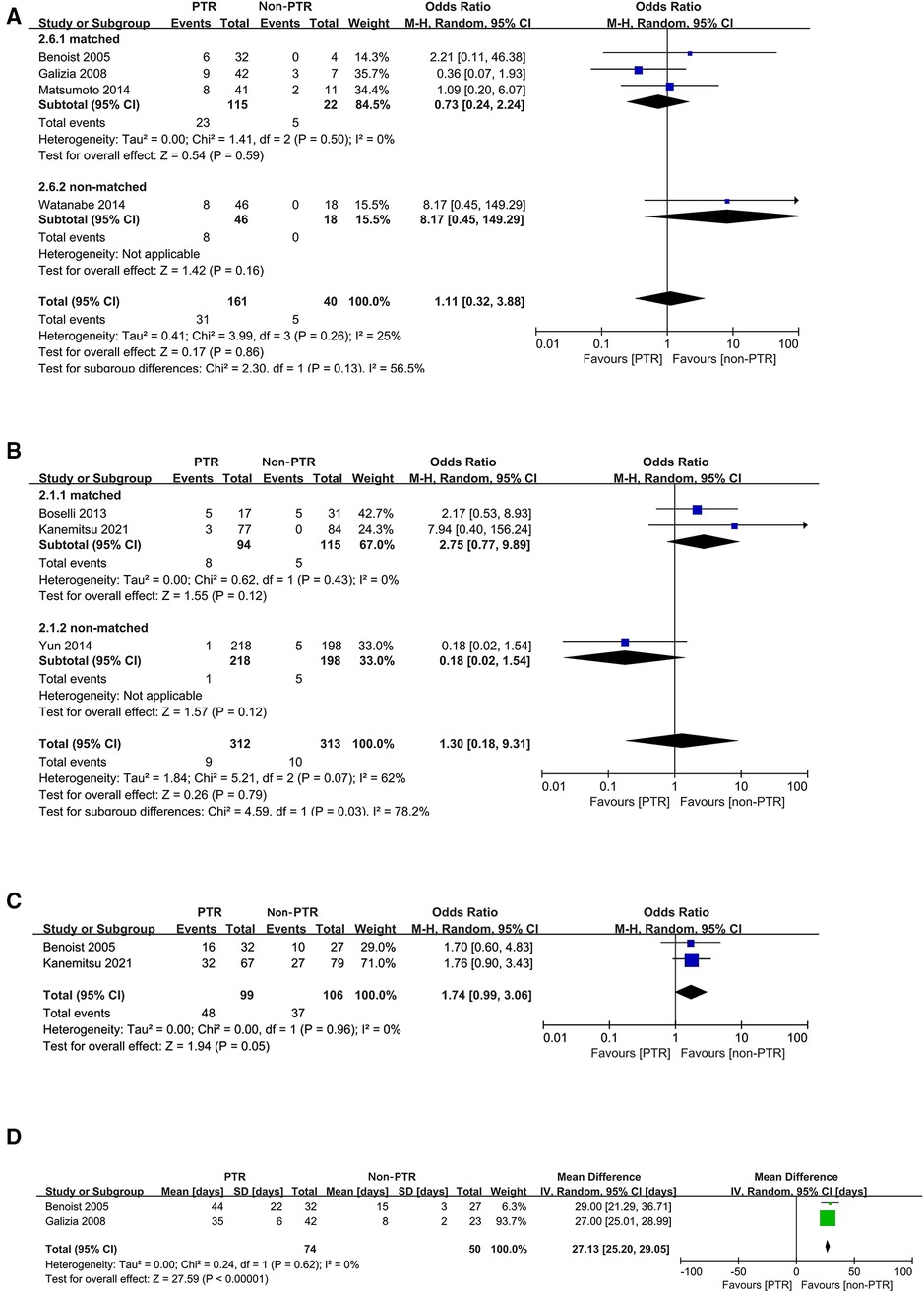
Figure 3. Forest plots for postoperative complications (A), 30-day mortality after upfront treatment (B), grade 3 or higher adverse effects due to chemotherapy (C), and interval from diagnosis to chemotherapy (D).
Several studies reported symptoms of the primary tumor over the period of palliative treatment in non-PTR groups, mainly marked by hemorrhage, perforation, and obstruction. The proportions of patients above requiring emergency surgery due to these symptoms ranged from 3% to 27%.
Discussion
Survival outcomes or the follow-up morbidities may vary considerably because of different treatment manners for asymptomatic patients suffering from unresectable stage IV CRC. Therefore, the strategy of upfront treatment is of great importance for newly diagnosed patients. Even though a significant benefit from PTR was showed in OS, subgroup analysis indicated that there was no significant difference between upfront PTR and upfront CT group. It is also an intriguing finding because a proportion of the patients in non-PTR groups were not treated with CT and some even did not receive any treatment. Besides, the follow-up status of patients without upfront resection would not be inferior to that of patients receiving upfront PTR. Even if patients treated conservatively in the initial diagnosis eventually need surgery due to symptoms of the primary tumor, the postoperative outcomes were relatively acceptable. The only issue that warranted consideration may be the significantly different start times of CT. Granted, the current guideline (34) recommends the startup of CT should be as soon as possible, but a meta-analysis (35) including 3 RCTs published in 2018 assessing survival differences with immediate vs. delayed CT for patients with asymptomatic, unresectable, and metastatic CRC suggested that giving immediate CT might make little or no difference to OS (P = 0.18). However, the researchers found it hard to draw a credible conclusion due to the limited number of trials, very sparse data, and uncertainty of the high-level evidence provided. Thus, this issue is still necessary for cautious consideration particularly in the evaluation process of surgical indications.
Considering the heterogeneities observed in meta-analyses of the survival outcomes, we divided the studies that all participants in the non-PTR group receiving CT into separate subgroups and performed sensitivity analyses of the results, consequently yielding a contrary result in OS outcome. Tests for heterogeneity showed no heterogeneity in the CT subgroups in terms of survival analyses, indicating that the results in CT subgroups were more convincing. As for the incidence of postoperative complications and 30-day mortality after upfront treatment, sensitivity analyses were performed that heterogeneity was eliminated by excluding studies whose clinicopathological characteristics were not matched. But in any case, the results still showed comparable between the two groups.
Some of the included studies also yielded interesting findings through further analyses. There were five studies (17, 18, 21, 28, 32) reporting significant differences of primary tumor sites between PTR and non-PTR groups. Patients with right-sided colon cancer in four of these studies (17, 18, 28, 32) were more likely to receive PTR, and they all achieved better OS except for studies reported by Michel et al. On the other hand, patients with rectal cancer in another study (21) tended to receive PTR, and the results did not support the significant advantage of PTR. A study (28) reported that the site of the tumor in the rectum was statistically significant independent prognostic factors for better survival. Besides, Liang (30) and his colleges found that wild-type RAS gene was a favorable factor for patients experiencing PTR with asymptomatic unresectable stage IV CRCs by multivariate regression analysis (P = 0.039), while the survival outcomes were comparable between the PTR group and non-PTR group for patients with mutant-type RAS (P = 0.102). Similarly, BRAF mutation, which is also an essential prognostic marker in stage IV CRC, should be assessed in this type of patient. Additionally, when patients were classified according to the volume of liver parenchyma replaced by metastases, the level of unresectable liver tumor load was not found to favor primary tumor resection (16). As expected by the researchers, the survival outcome was correlated with the size of the liver tumor load. These findings demonstrated that resection of asymptomatic primary lesions did not provide a survival advantage in incurable stage IV disease. However, it is a great pity that no more studies focused on this issue from then on.
Two included studies (19, 20) reported interval between diagnosis and the start of systemic CT in the CT group and period between PTR and the start of systemic CT in the PTR group. Both indicated that PTR would significantly lead to CT delay. One of them (19) reported that 18.9% of patients resulted in postoperative complication after PTR including wound infection, pleural effusion, pulmonary embolism, urinary tract infection, and intra-abdominal abscess. The other (20) showed that 21.4% of patients with PTR experienced slight postoperative complications, but all could be discharged from hospital and were able to undergo CT as scheduled. According to our subgroup analyses, PTR prior to the initiation of systemic CT was not necessarily required. Instead, PTR could bring complications which might delay the application of CT. Furthermore, wasted finances of unnecessary surgery should also be considered as a significant issue from the perspective of health economics.
With the publication of RCTs, an updated systematic review and meta-analysis was urgently required. In recent years, original articles concerning the role of upfront PTR in asymptomatic and unresectable stage IV CRC emerged with conflicting results. Due to the retrospective nature of these reports with discrepancies of clinicopathological features between groups and the lack of randomization applied, significant heterogeneities were observed in previous meta-analyses. Notably, some of these relevant meta-analyses mixed asymptomatic cases with symptomatic or unclear ones. Moreover, we further compared the differences between upfront PTR and upfront CT groups and found that upfront PTR was not necessary for patients who were willing to receive CT. This finding meets the conclusions of both RCTs.
The selection of target participants for further studies may need to be more precise. For example, the inclusion of patients with no or slight symptoms is demanded. Lam-Boer and his colleges suggested an OS benefit for patients with unresectable stage IV CRCs who underwent PTR as the upfront treatment after diagnosis in a nationwide population-based propensity-score adjusted study, but they also analyzed that confounding by indication could not be avoided as in most observational studies. Without available data on clinical symptoms, it could be assumed that patients with symptomatic CRCs were more likely to undergo PTR. Besides, with the innovation of anti-cancer drugs, a variety of alternatives can thus opt for conservative treatment without PTR. To determine which treatment approach is the key to providing better survival outcomes, subgroup analyses involving specific treatment regimens should be explored.
There were serval limitations in this study. Firstly, the majority of the included studies were retrospective studies leading to the lack of randomization and control of baseline data. Secondly, both RCTs drawing conclusions that PTR showed no survival benefit were based on East Asian populations, which has the potential for racial bias. Thirdly, radiofrequency ablation for liver metastasis (36) and radiotherapy for rectal cancer (37) may play a certain role for unresectable metastatic CRC patients. Although these treatments were partially addressed in the included studies, they were not analyzed independently, making it difficult to clarify their effects. Lastly, significant heterogeneities were shown, which might be blamed on differences in study design, sample sizes, treatment approaches in non-groups, and clinicopathological features including the primary site of tumor and severity of tumor metastases.
In any case, the role of PTR in outcomes improvement will remain an area of ongoing debate for years. It is worth noting that there are still ongoing trials such as the CAIRO4 study (38), but more prospective and randomized studies are urgently needed for better assessment.
Conclusions
PTR might be related to improved OS for asymptomatic patients with unresectable stage IV CRC, whereas receiving upfront CT is a rational alternative without detrimental influence on survival or adverse outcomes compared with upfront PTR.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
Study concepts: ZL, ZL, XF. Study design: ZL, ZL, XF. Data acquisition: ZZ, MX. Quality control of data and algorithms: ZL, ZL, XY. Data analysis and interpretation: XC, CH, WH. Statistical analysis: XC, JW, XF. Manuscript preparation: ZL. Manuscript editing: XY. Manuscript review: XF, XY. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Science and Technology Planning Project of Guangdong Province, China (2017A030223006, 2016A020215128), the Science and Technology Planning Project of Guangzhou, China (201704020077), the Second Batch of Scientific Research Projects of Dengfeng Plan (DFJH201913), the Research Fund of CSCO-Roche Oncology (Y-2019Roche-190), the Research Fund of CSCO-Hansoh Oncology (Y-HS2019/2-050), the Research Fund of Guangdong General Hospital (No. y012015338), the Yuexiu Science and Information Center of Guangzhou Scientific Foundation (2012-GX-046), the Medical Scientific Research Foundation of Guangdong Province, China (A2020019), and the Science and Technology Plan of Guangzhou, Guangdong Province, China (202102080230).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1047373/full#supplementary-material.
Abbreviations
CRC, colorectal cancer; PTR, primary tumor resection; RCT, randomized controlled trial; CT, chemotherapy; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; OS, overall survival; PFS, progression-free survival; NOS, Newcastle-Ottawa scale; HR, hazard ratio; CI, confidence interval; WMD, weighted mean difference; OR, odds ratio.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Leufkens AM, van den Bosch MA, van Leeuwen MS, Siersema PD. Diagnostic accuracy of computed tomography for colon cancer staging: a systematic review. Scand J Gastroenterol. (2011) 46(7–8):887–94. doi: 10.3109/00365521.2011.574732
3. McCahill LE, Yothers G, Sharif S, Petrelli NJ, Lai LL, Bechar N, et al. Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of NSABP trial C-10. J Clin Oncol. (2012) 30(26):3223–8. doi: 10.1200/JCO.2012.42.4044
4. Sarela AI, Guthrie JA, Seymour MT, Ride E, Guillou PJ, O’Riordain DS. Non-operative management of the primary tumour in patients with incurable stage IV colorectal cancer. Br J Surg. (2001) 88(10):1352–6. doi: 10.1046/j.0007-1323.2001.01915.x
5. Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. (2009) 27(20):3379–84. doi: 10.1200/JCO.2008.20.9817
6. Alawadi Z, Phatak UR, Hu CY, Bailey CE, You YN, Kao LS, et al. Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer. (2017) 123(7):1124–33. doi: 10.1002/cncr.30230
7. Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg. (2010) 34(4):797–807. doi: 10.1007/s00268-009-0366-y
8. Clancy C, Burke JP, Barry M, Kalady MF, Calvin Coffey J. A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Ann Surg Oncol. (2014) 21(12):3900–8. doi: 10.1245/s10434-014-3805-4
9. Simillis C, Kalakouti E, Afxentiou T, Kontovounisios C, Smith JJ, Cunningham D, et al. Primary tumor resection in patients with incurable localized or metastatic colorectal cancer: a systematic review and meta-analysis. World J Surg. (2019) 43(7):1829–40. doi: 10.1007/s00268-019-04984-2
10. Park EJ, Baek JH, Choi GS, Park WC, Yu CS, Kang SB, et al. The role of primary tumor resection in colorectal cancer patients with asymptomatic, synchronous, unresectable metastasis: a multicenter randomized controlled trial. Cancers (Basel). (2020) 12(8):2306. doi: 10.3390/cancers12082306
11. Kanemitsu Y, Shitara K, Mizusawa J, Hamaguchi T, Shida D, Komori K, et al. Primary tumor resection plus chemotherapy versus chemotherapy alone for colorectal cancer patients with asymptomatic, synchronous unresectable metastases (JCOG1007; iPACS): a randomized clinical trial. J Clin Oncol. (2021) 39(10):1098–107. doi: 10.1200/JCO.20.02447
12. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007
13. Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement Geriatr Cogn Disord. (2001) 12(3):232–6. doi: 10.1159/000051263
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
15. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
16. Scoggins CR, Meszoely IM, Blanke CD, Beauchamp RD, Leach SD. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol. (1999) 6(7):651–7. doi: 10.1007/s10434-999-0651-x
17. Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg. (2003) 196(5):722–8. doi: 10.1016/S1072-7515(03)00136-4
18. Michel P, Roque I, Di Fiore F, Langlois S, Scotte M, Teniere P, et al. Colorectal cancer with non-resectable synchronous metastases: should the primary tumor be resected? Gastroenterol Clin Biol. (2004) 28(5):434–7. doi: 10.1016/S0399-8320(04)94952-4
19. Benoist S, Pautrat K, Mitry E, Rougier P, Penna C, Nordlinger B. Treatment strategy for patients with colorectal cancer and synchronous irresectable liver metastases. Br J Surg. (2005) 92(9):1155–60. doi: 10.1002/bjs.5060
20. Galizia G, Lieto E, Orditura M, Castellano P, Imperatore V, Pinto M, et al. First-line chemotherapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch Surg. (2008) 143(4):352–8; discussion 8. doi: 10.1001/archsurg.143.4.352
21. Seo GJ, Park JW, Yoo SB, Kim SY, Choi HS, Chang HJ, et al. Intestinal complications after palliative treatment for asymptomatic patients with unresectable stage IV colorectal cancer. J Surg Oncol. (2010) 102(1):94–9. doi: 10.1002/jso.21577
22. Boselli C, Renzi C, Gemini A, Castellani E, Trastulli S, Desiderio J, et al. Surgery in asymptomatic patients with colorectal cancer and unresectable liver metastases: the authors’ experience. Onco Targets Ther. (2013) 6:267–72. doi: 10.2147/OTT.S39448
23. Cetin B, Kaplan MA, Berk V, Tufan G, Benekli M, Isikdogan A, et al. Bevacizumab-containing chemotherapy is safe in patients with unresectable metastatic colorectal cancer and a synchronous asymptomatic primary tumor. Jpn J Clin Oncol. (2013) 43(1):28–32. doi: 10.1093/jjco/hys175
24. Matsuda K, Hotta T, Takifuji K, Yokoyama S, Oku Y, Hashimoto T, et al. Clinical outcome of up-front surgery in patients with asymptomatic, incurable synchronous peritoneal carcinomatosis. Surg Today. (2013) 43(9):984–9. doi: 10.1007/s00595-012-0348-9
25. Matsumoto T, Hasegawa S, Matsumoto S, Horimatsu T, Okoshi K, Yamada M, et al. Overcoming the challenges of primary tumor management in patients with metastatic colorectal cancer unresectable for cure and an asymptomatic primary tumor. Dis Colon Rectum. (2014) 57(6):679–86. doi: 10.1097/DCR.0000000000000025
26. Watanabe A, Yamazaki K, Kinugasa Y, Tsukamoto S, Yamaguchi T, Shiomi A, et al. Influence of primary tumor resection on survival in asymptomatic patients with incurable stage IV colorectal cancer. Int J Clin Oncol. (2014) 19(6):1037–42. doi: 10.1007/s10147-014-0662-x
27. Yun JA, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, et al. The role of palliative resection for asymptomatic primary tumor in patients with unresectable stage IV colorectal cancer. Dis Colon Rectum. (2014) 57(9):1049–58. doi: 10.1097/DCR.0000000000000193
28. Samalavicius NE, Dulskas A, Baltruskeviciene E, Smailyte G, Skuciene M, Mikelenaite R, et al. Asymptomatic primary tumour in incurable metastatic colorectal cancer: is there a role for surgical resection prior to systematic therapy or not? Wideochir Inne Tech Maloinwazyjne. (2016) 11(4):274–82. doi: 10.5114/wiitm.2016.64981
29. Zhang R, Ma W, Gu Y, Zhang T, Huang Z, Lu Z, et al. Primary tumor location as a predictor of the benefit of palliative resection for colorectal cancer with unresectable metastasis. World J Surg Oncol. (2017 Jul 27) 15(1):138. doi: 10.1186/s12957-017-1198-0
30. Liang L, Tian J, Yu Y, Wang Z, Peng K, Liu R, et al. An analysis of relationship between RAS mutations and prognosis of primary tumour resection for metastatic colorectal cancer patients. Cell Physiol Biochem. (2018) 50(2):768–82. doi: 10.1159/000494242
31. Ergun Y, Bal O, Dogan M, Ucar G, Dirikoc M, Acikgoz Y, et al. Does primary tumor resection contribute to overall survival in unresectable synchronous metastatic colorectal cancer? J Res Med Sci. (2020) 25:14. doi: 10.4103/jrms.JRMS_1056_18
32. Urvay S, Eren T, Civelek B, Kilickap S, Yetiysigit T, Ozaslan E. The role of primary tumor resection in patients with stage IV colorectal cancer with unresectable metastases. J Buon. (2020) 25(2):939–44. PMID: 32521889.32521889
33. Doah KY, Shin US, Jeon BH, Cho SS, Moon SM. The impact of primary tumor resection on survival in asymptomatic colorectal cancer patients with unresectable metastases. Ann Coloproctol. (2021) 37(2):94–100. doi: 10.3393/ac.2020.09.15.1
34. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19(3):329–59. doi: 10.6004/jnccn.2021.0012
35. Claassen YH, van der Valk MJ, Breugom AJ, Frouws MA, Bastiaannet E, Liefers GJ, et al. Survival differences with immediate versus delayed chemotherapy for asymptomatic incurable metastatic colorectal cancer. Cochrane Database Syst Rev. (2018) 11:CD012326. doi: 10.1002/14651858.CD012326.pub2
36. Vavra P, Nowakova J, Ostruszka P, Hasal M, Jurcikova J, Martinek L, et al. Colorectal cancer liver metastases: laparoscopic and open radiofrequency-assisted surgery. Wideochir Inne Tech Maloinwazyjne. (2015) 10(2):205–12. doi: 10.5114/wiitm.2015.52082
37. Liu Q, Shan Z, Luo D, Cai S, Li Q, Li X. Palliative beam radiotherapy offered real-world survival benefit to metastatic rectal cancer: a large US population-based and propensity score-matched study. J Cancer. (2019) 10(5):1216–25. doi: 10.7150/jca.28768
38. t Lam-Boer J, Mol L, Verhoef C, de Haan AF, Yilmaz M, Punt CJ, et al. The CAIRO4 study: the role of surgery of the primary tumour with few or absent symptoms in patients with synchronous unresectable metastases of colorectal cancer–a randomized phase III study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer. (2014) 14:741. doi: 10.1186/1471-2407-14-741
Keywords: primary tumor resection, colorectal cancer, asymptomatic, unresectable, overall survival
Citation: Liang Z, Liu Z, Huang C, Chen X, Zhang Z, Xiang M, Hu W, Wang J, Feng X and Yao X (2023) The role of upfront primary tumor resection in asymptomatic patients with unresectable stage IV colorectal cancer: A systematic review and meta-analysis. Front. Surg. 9:1047373. doi: 10.3389/fsurg.2022.1047373
Received: 18 September 2022; Accepted: 1 December 2022;
Published: 6 January 2023.
Edited by:
Tomas Poskus, Vilnius University, LithuaniaReviewed by:
Vincenzo Lizzi, Azienda Ospedaliero-Universitaria Ospedali Riuniti di Foggia, ItalyXiaozhun Huang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
© 2023 Liang, Liu, Huang, Chen, Zhang, Xiang, Hu, Wang, Feng and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingyu Feng ZmVuZ3hpbmd5dUBnZHBoLm9yZy5jbg==; Xueqing Yao c3l5YW94dWVxaW5nQHNjdXQuZWR1LmNu
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Zongyu Liang
Zongyu Liang Zhiyuan Liu
Zhiyuan Liu Chengzhi Huang
Chengzhi Huang Xin Chen
Xin Chen Zhaojun Zhang
Zhaojun Zhang Meijuan Xiang
Meijuan Xiang Weixian Hu
Weixian Hu Junjiang Wang
Junjiang Wang Xingyu Feng
Xingyu Feng Xueqing Yao
Xueqing Yao