
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 06 January 2023
Sec. Orthopedic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1040715
This article is part of the Research Topic New Perspectives and Innovative Techniques in Contemporary Spine Surgery View all 17 articles
Background: Alkaptonuria is a rare autosomal genetic disorder with an incidence of about 1 in 1 million per year. Spinal involvement often manifests in the later stages of the disease. However, this is the first report of the presentation of thoracolumbar spinal stenosis.
Case presentation: We report the case of a 61-year-old female patient with significant thoracolumbar stenosis symptoms. The patient had obvious kyphosis with preoperative lower extremity muscle strength grade 2/5. Symptoms and imaging signs initially suggested ankylosing spondylitis. This patient was classified into motor incomplete injury (ASIA C). However, the patient was found to have melanin deposits on the sclera and skin, and the urine was darkened at rest. CT and MRI both suggested no bone bridge connection between vertebrae, which was the key difference between ankylosing spondylitis and alkaptonuria in imaging. Most importantly, urine specimen testing and intraoperative pathology demonstrated alkaptonuria. The patient underwent spinal decompression and vertebral body fixation. Postoperative recovery was good: the patient had significantly relieved pain and could stand and walk.
Conclusion: This case is the first report of thoracolumbar spinal stenosis associated with alkaptonuria involving the spine.
Alkaptonuria (AKU) is a rare genetic disease with an incidence of about 1 in 1 million per year (1). The early manifestation of AKU involves the darkening of urine after resting. The progression appears as melanosis of the skin and sclera. In the late stages of disease progression, homogeneous acid (HGA) deposits in tissues such as cartilage, tendons, and ligaments lead to the degeneration of the spine and large peripheral joints. Spinal canal stenosis frequently occurs in the cervical and lumbar spine but rarely in the thoracolumbar segment (2).
We describe here for the first time the case of a 61-year-old patient with AKU presenting with thoracolumbar spinal stenosis.
A 61-year-old female patient complained of recurrent low back pain for more than 10 years and pain and numbness in both legs for approximately 8 months. We found that the patient's parents are married cousins, suggesting that the patient may have a genetic disorder. Physical examination revealed that the patient had significant kyphosis. Melanosis could be seen in both sclerae and both auricles. There were hypoesthesia and hypoalgesia below the T10 dermatome. The muscle strength for hip flexion, knee extension, and knee flexion was grade 3/5, while ankle dorsal extension and metatarsal flexion were grade 2/5. The patient exhibited hyperreflexia of both Achilles tendons and knees; furthermore, Babinski's sign, Gordon's sign, Oppenheim's sign, and the four-figure test were positive on both sides. This patient was classified into motor incomplete injury (ASIA C). On imaging examination, plain lumbar spine radiographs demonstrated that all the lumbar disc spaces were narrow with signs of osteoporosis. Magnetic resonance imaging (MRI) revealed spinal stenoses of the T10/11, L1/2, and L2/3 segments. Laboratory examination depicted that the fresh urine was light yellow and gradually turned dark brown after a period of time. Pathological examination revealed melanin deposits in the intervertebral disc and ligament tissues.
The patient underwent adequate intraoperative spinal canal decompression and was fixed with pedicle screws. At operation, we found that the bone junctions of the supraspinous ligament, interspinous ligament, and ligamentum flavum were blackened. Severe T10/11 disc degeneration was noted, with no significant distinction between the nucleus pulposus and the adjacent tissue.
The patient experienced remarkable relief and was discharged 1 week after the operation. The patient's muscle strength returned to grade 4/5 after surgery, and she could stand and walk independently. A review is scheduled for 3 months after surgery and lumbosacral surgery at an optional date, depending on the patient's condition.
This was the first description of alkaptonuria presenting as thoracic spinal stenosis. AKU is an autosomal recessive hereditary disease with an incidence of about 1 in 1 million per year (1, 3). This patient presented with symptoms similar to those of ankylosing spondylitis (AS) and was eventually diagnosed as an extremely rare case of alkaptonuria, which resulted in good recovery through surgery, so we feel it is important to report this case. The early clinical manifestations of AKU are mainly dark urine or deepening of urine color and hyperpigmentation, mostly in the sclera and external auricle. As the disease progresses, the deposits lead to stiffness of the connecting tissues, premature degeneration of the spinal joints, and labral osteophytes at the edges of the vertebral column (4). The characteristic imaging features include extensive intervertebral space narrowing and pancake-like disc calcification, sometimes with disc vacuum (5, 6).
In the currently reported patient, melanin deposition in the sclera and external auricle and darkening of the urine after resting were observed. In the operation, the T10/11 disc was found to be severely degenerated, and the nucleus pulposus was indistinguishable from the adjacent tissue. Intraoperatively, parts of the interspinous ligament, supraspinous ligament, and ligamentum flavum were found to be darkened (Figure 1). Disc herniation has been previously reported in patients with AKU (7, 8), but severe thoracolumbar spinal canal stenosis is rare (9). Alkaptonuria patients are easily misdiagnosed as AS. AS can be followed by disc fibrosis and calcification, bony ankylosis of the spine, and the characteristic bamboo spine (10). Increased brittleness of spinal bone due to bony ankylosis of the spine and vertebral osteoporosis in late AS, combined with a stress increase in the thoracolumbar segment, leads to a fracture called an Andersson lesion (11). However, imaging data revealed that the patient had multiple intervertebral degenerations, including decreased intervertebral height, destruction of the upper and lower endplates, and low signal in the nucleus pulposus region on T2 images owing to atrophy and dehydration of the nucleus pulposus. No significant vertebral fusion was found in the spine (Figure 2). This is the point of differentiation from the presentation of patients with AS.
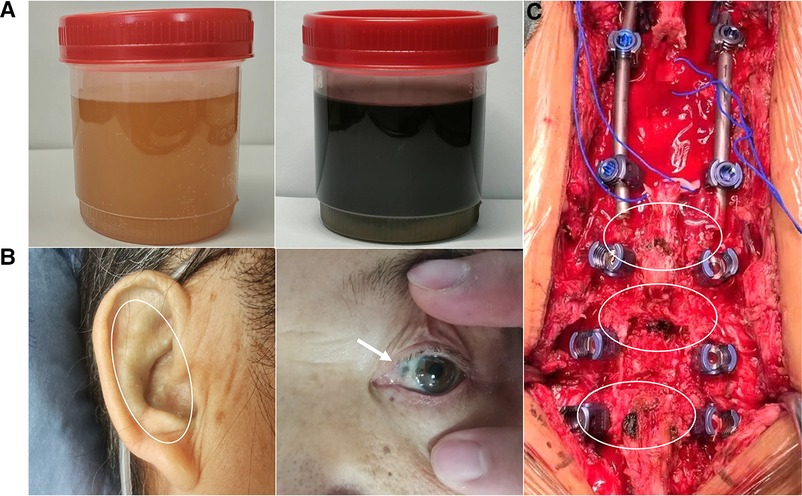
Figure 1. Patient's urine darkened after resting (A); melanin deposition in the sclera and external auricle (B); intraoperatively, the bony junction of the interspinous ligament, supraspinous ligament, and ligamentum flavum were black (C).
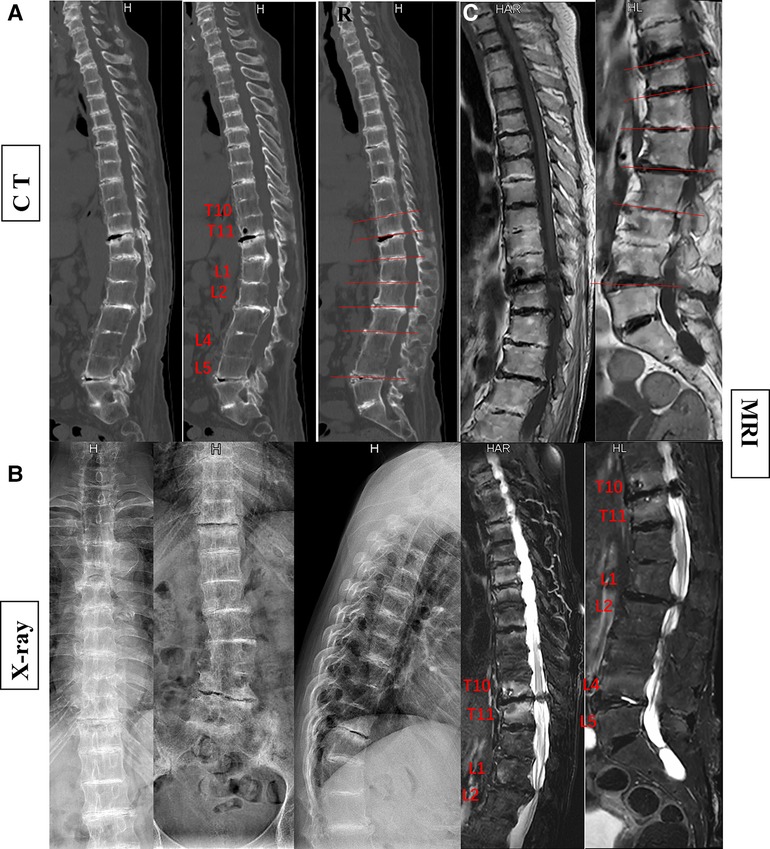
Figure 2. The patient's preoperative imaging examination revealed kyphosis and spinal stenosis. Disruption and stenosis of the intervertebral disc with labral changes. Intervertebral ossification dysplasia, vertebral body without ossification connected (A, CT; B, x-ray; C, MRI).
Narrowing of the spinal canal or foramina is a common finding in spine imaging of the elderly. Only when symptoms of neurogenic claudication and/or cervical myelopathy are present is a spinal stenosis diagnosis made, of the lumbar spine, cervical spine, or both (only very rarely is the thoracic spine involved) (2). The patient in the case report had significant preoperative spinal cord symptoms, was unable to walk independently, was pushed in a wheelchair, and had preoperative muscle strength of grade 2/5. Postoperatively, she could stand and walk, and her muscle strength returned to grade 4/5. Thoracic spinal stenosis is a rare entity for which the incidence is unknown (12). Spinal stenosis can be classified etiologically into two categories: congenital and acquired (13). Most patients will have acquired canal stenosis, often due to degenerative causes, systemic illness or postsurgical pathology (2). This patient has significant thoracolumbar stenosis. Intraoperatively, we found darkening of the ligamentum flavum with significant melanin deposition, which suggests that the cause of T10/11 segment stenosis is mainly the calcification of the ligamentum flavum (Figure 3). The reason for ossification of the ligamentum flavum may be the deposition of HGA. Additionally, disc degeneration and loss of height may force invagination of the ligamentum flavum and subsequent pressure on the dural dorsal capsule, contributing to hypertrophy of the ligamentum flavum (2).
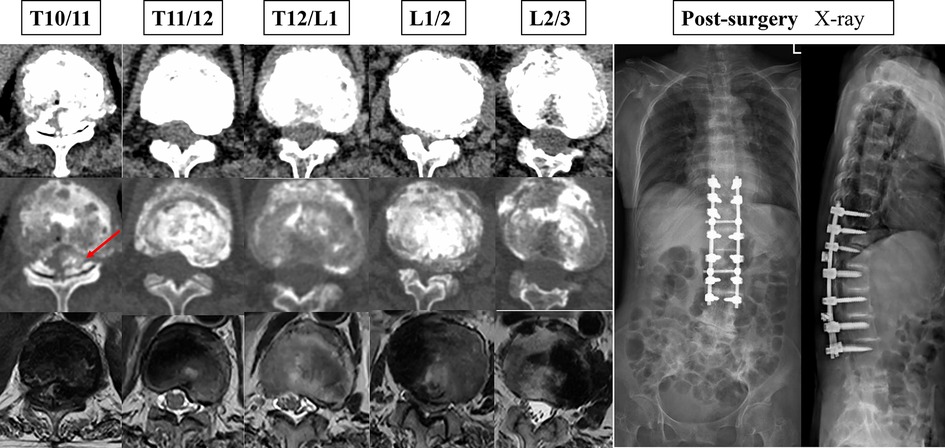
Figure 3. The patient's preoperative cross-sectional imaging examination and postoperative x-ray maps.
Currently, there is no effective therapy for AKU, and symptomatic supportive treatment is the mainstay. Dietary restrictions on phenylalanine and tyrosine intake and oral vitamin C are suggested to reduce the production and deposition of HGA (14–16). It has been reported that nitisinone can reduce HGA production and has a therapeutic effect on AKU (17, 18). Surgery is considered feasible for patients with spinal or peripheral large joint involvement to improve the quality of survival (19, 20).
Finally, this case report is a further reminder that orthopedic surgeons must be thorough in their examination and diagnosis and not abandon any suspected diagnoses. Surgery remains an important treatment for this condition.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee on Biomedical Research, West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
L-ML conceived, designed, and planned the study. HD wrote the manuscript. LW collected patient data. G-JF and Y-MS modified the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported in part by the Projects of the Science & Technology Department of Sichuan Province (2022ZDZX0029), the National Natural Science Foundation of China (81871772 and 82172495), and the 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (2021HXFH003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. (2014) 119:541–9. doi: 10.1016/B978-0-7020-4086-3.00035-7
3. Gaines JJ Jr. The pathology of alkaptonuric ochronosis. Hum Pathol. (1989) 20(1):40–6. doi: 10.1016/0046-8177(89)90200-1
4. Kitahara Y, Kaku N, Tagomori H, Tsumura H. Alkaptonuria with rapidly destructive arthropathy of the hip: a case report and literature review. Acta Orthop Traumatol Turc. (2021) 55(6):563–8. doi: 10.5152/j.aott.2021.21205
5. Reddy DR, Prasad VS. Alkaptonuria presenting as lumbar disc prolapse: case report and review of literature. Spinal Cord. (1998) 36(7):523–4. doi: 10.1038/sj.sc.3100562
6. Gürkanlar D, Daneyemez M, Solmaz I, Temiz C. Ochronosis and lumbar disc herniation. Acta Neurochir. (2006) 148(8):891–4. doi: 10.1007/s00701-006-0774-9
7. Farzannia A, Shokouhi G, Hadidchi S. Alkaptonuria and lumbar disc herniation. Report of three cases. J Neurosurg. (2003) 98(1 Suppl):87–9. doi: 10.3171/spi.2003.98.1.0087
8. Krishnan P, Chowdhury SR. Lumbar disc herniation in a patient of alkaptonuria: case report and review of literature. Neurol India. (2012) 60(6):667–9. doi: 10.4103/0028-3886.105218
9. Alkasem W, Boissiere L, Obeid I, Bourghli A. Management of a pseudarthrosis with sagittal malalignment in a patient with ochronotic spondyloarthropathy. Eur Spine J. (2019) 28(10):2283–9. doi: 10.1007/s00586-019-06020-2
10. Golder V, Schachna L. Ankylosing spondylitis: an update. Aust Fam Physician. (2013) 42(11):780–4.24217097
11. Bron JL, de Vries MK, Snieders MN, van der Horst-Bruinsma IE, van Royen BJ. Discovertebral (Andersson) lesions of the spine in ankylosing spondylitis revisited. Clin Rheumatol. (2009) 28(8):883–92. doi: 10.1007/s10067-009-1151-x
12. Dützmann S, Fernandez R, Rosenthal D. Thoracic spinal stenosis: etiology, pathogenesis, and treatment. Orthopade. (2019) 48(10):844–8. doi: 10.1007/s00132-019-03731-8
13. Arabmotlagh M, Sellei RM, Vinas-Rios JM, Rauschmann M. Classification and diagnosis of lumbar spinal stenosis. Orthopade. (2019) 48(10):816–23. doi: 10.1007/s00132-019-03746-1
14. Hughes JH, Wilson PJM, Sutherland H, Judd S, Hughes AT, Milan AM, et al. Dietary restriction of tyrosine and phenylalanine lowers tyrosinemia associated with nitisinone therapy of alkaptonuria. J Inherit Metab Dis. (2020) 43(2):259–68. doi: 10.1002/jimd.12172
15. Ranganath LR, Jarvis JC, Gallagher JA. Recent advances in management of alkaptonuria (invited review; best practice article). J Clin Pathol. (2013) 66(5):367–73. doi: 10.1136/jclinpath-2012-200877
16. Zatkova A, Ranganath L, Kadasi L. Alkaptonuria: current perspectives. Appl Clin Genet. (2020) 13:37–47. doi: 10.2147/TACG.S186773
17. Davison AS, Norman BP, Ross GA, Hughes AT, Khedr M, Milan AM, et al. Evaluation of the serum metabolome of patients with alkaptonuria before and after two years of treatment with nitisinone using LC-QTOF-MS. JIMD Rep. (2019) 48(1):67–74. doi: 10.1002/jmd2.12042
18. Ranganath LR, Psarelli EE, Arnoux JB, Braconi D, Briggs M, Bröijersén A, et al. Efficacy and safety of once-daily nitisinone for patients with alkaptonuria (SONIA 2): an international, multicentre, open-label, randomised controlled trial. Lancet Diabet Endocrinol. (2020) 8(9):762–72. doi: 10.1016/S2213-8587(20)30228-X
19. Emel E, Karagöz F, Aydín IH, Hacísalihoğlu S, Seyithanoğlu MH. Alkaptonuria with lumbar disc herniation: a report of two cases. Spine. (2000) 25(16):2141–4. doi: 10.1097/00007632-200008150-00021
Keywords: alkaptonuria, thoracolumbar spinal stenosis, case report, surgery, kyphosis
Citation: Ding H, Wang L, Feng G, Song Y and Liu L (2023) Case report: Thoracolumbar spinal stenosis associated with alkaptonuria. Front. Surg. 9:1040715. doi: 10.3389/fsurg.2022.1040715
Received: 9 September 2022; Accepted: 8 November 2022;
Published: 6 January 2023.
Edited by:
Luca Ambrosio, Campus Bio-Medico University, ItalyReviewed by:
Panagiotis Korovessis, Olympion Medical Center, Greece© 2023 Ding, Wang, Feng, Song and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Min Liu bGl1bGltaW5fc3BpbmVAMTYzLmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.