
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 05 January 2023
Sec. Orthopedic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1039785
This article is part of the Research Topic Surgical Innovation and Advancement in Orthopedics View all 57 articles
Talar chondroblastoma, which is a benign tumor of immature cartilage cells, is an uncommon but easily missed diagnosis of foot pain. Arthroscopic treatment for this condition is a safe, powerful, and promising technique with definitive advantages of visualization and minimal invasion. Here, we report a case of a talar chondroblastoma treated by posterior ankle arthroscopic curettage, allograft bone graft, and platelet-rich plasma-fibrin glue (PRP-FG) application.
Level of evidence: Case Report. Level IV.
Chondroblastoma accounts for 1% of all bone tumors (1), which primarily affect a patient population aged 10–30 years old. Chondroblastoma is generally classified as a benign but “intermediate, rarely metastasizing” category of tumor with a recurrence rate of 4.8%–39.5% (2–4). The first case of chondroblastoma was reported in the year 1928 and the first talar chondroblastoma was reported in 1947 (5, 6). Typically, chondroblastoma occurs in the epiphysis of a long bone, but less than 10% affects the talus (7, 8). The talar chondroblastoma reported in literature were mainly located at the body of the talus, which uncommonly causes cortical erosion. Surgery is the main treatment modality for chondroblastoma, including curettage, resection, and radiofrequency ablation alone or in combination with other procedures (2, 9). Surgery for talar chondroblastoma is technically challenging due to the risk of joint surfaces breaking or fracture of the talus. Given the small size of the talus with the deep and complicated location of the tumor, it is very difficult to expose the operative field and excise it if open surgery is performed. Compared with open surgery, arthroscopic treatment has obvious advantages of visualization and minimal invasion, allowing for early mobilization and superior postsurgical functional and cosmetic results.
To the best of our knowledge, only one case of a patient who underwent posterior ankle arthroscopic treatment for a talar chondroblastoma has been reported (10). Here, we report a talar chondroblastoma patient treated by posterior ankle arthroscopic curettage, allograft bone graft, and platelet-rich plasma-fibrin glue (PRP-FG) application.
A 32-year-old man presented with pain and swelling in his right ankle on bearing weight and walking for 7 months. The only remarkable clinical finding was diffuse tenderness over the posterior ankle and deep-seated pain at maximal plantar flexion, which could not be exactly pinpointed. The Visual Analogue Scale (VAS) score was 6 points and the American Orthopedic Foot and Ankle Society (AOFAS) score was 71 points.
Preoperative x-ray and CT images showed a well-demarcated lytic lesion in the posterior half of the talar body. Preoperative MRI images showed a lesion with no cortical and articular surface breakage, measuring 2.5*2.2*2.2 cm (Figure 1). An x-ray-guided punch biopsy was performed and both histopathology and immunohistochemical results confirmed the diagnosis of chondroblastoma (Figure 2).
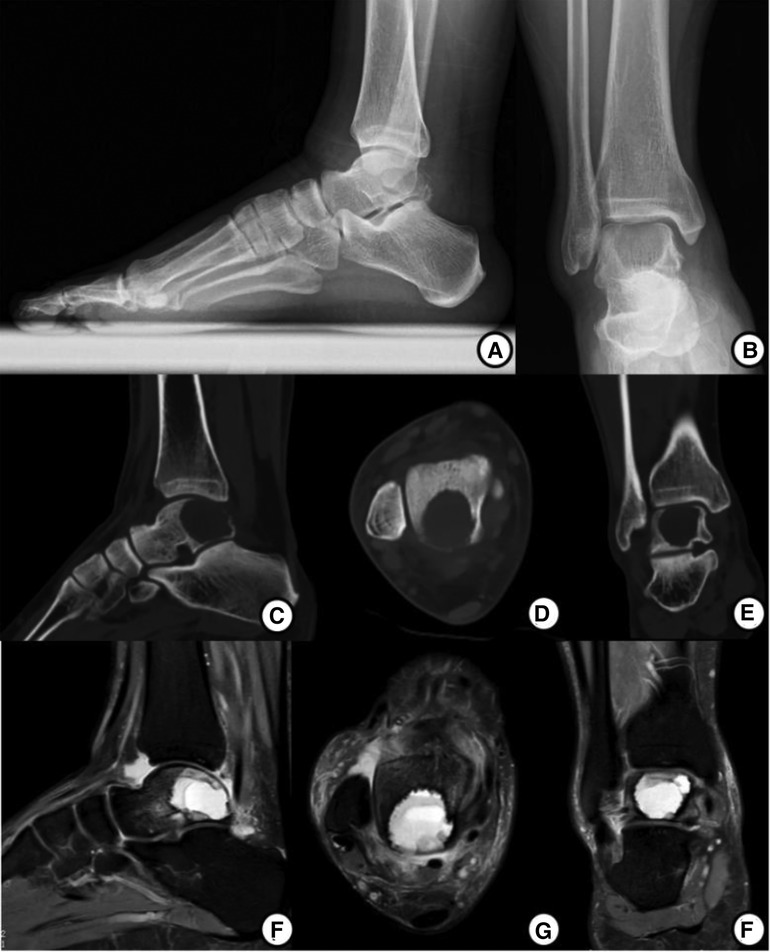
Figure 1. Preoperative imagological data. (A,B) AP and Lateral x-ray before operation show a well-demarcated and lytic lesion in the posterior talus. (C–E) Preoperative CT images show a lesion. (F–H) Preoperative MRI images show a lesion with no cortical and articular surface breakthrough, measuring 2.5*2.2*2.2 cm.
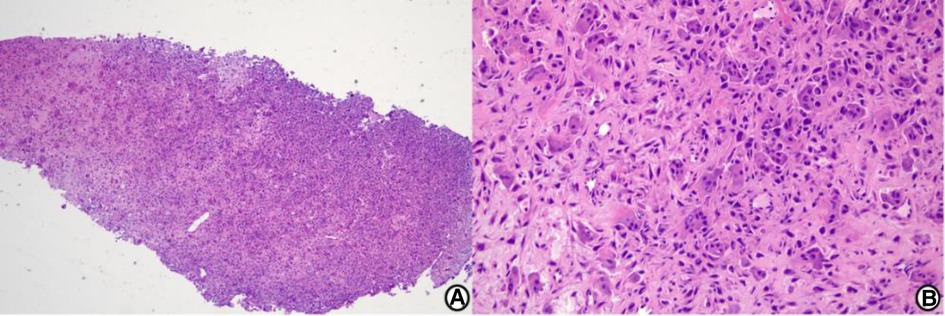
Figure 2. Histological image. Histological examination shows chondroblastoma cells and foci of the eosinophilic chondroid matrix. The immunohistochemical results show KI67(5% +), H3F3A(−), Kp1(+), CD163(partial +), H3F3B(+), P16(partial +), P63(partial +), and SATB2(+). The findings are consistent with chondroblastoma. (A) Low-power view (hematoxylin and eosin stain, origin magnification ×40). (B) High-power view (hematoxylin and eosin stain, origin magnification ×200).
After careful evaluation and discussion with the patient, we finally chose the posterior ankle arthroscopic treatment option. Arthroscopy was conducted 2 weeks after the biopsy procedure. The conditions are described in Figure 3.
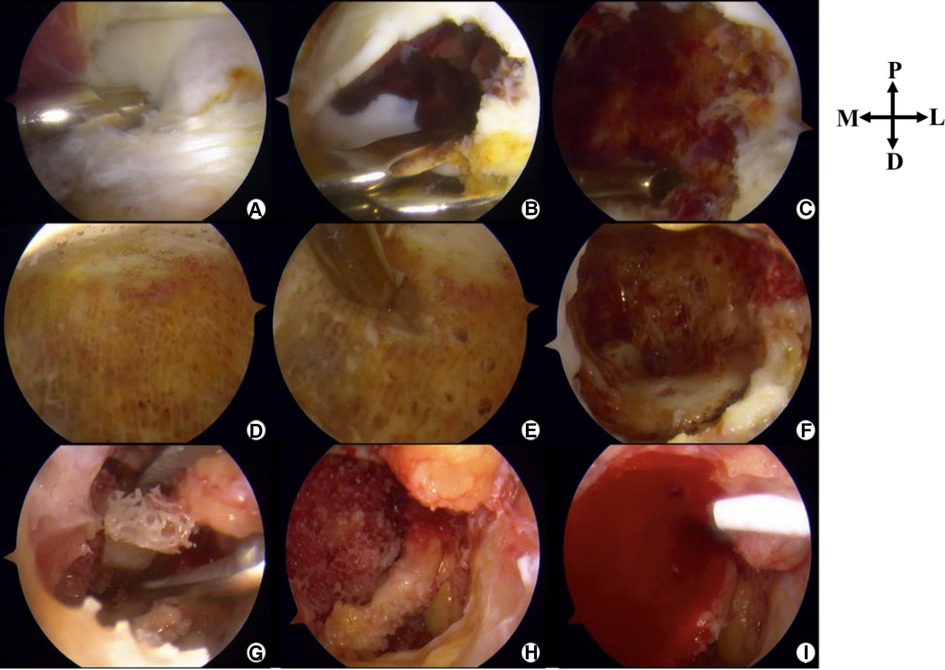
Figure 3. Intraoperative photographs (posterior ankle arthroscopy). P, Proximal; D, Distal; M, Medial; L, Lateral. (A) The lesion is located anterior to the posterior intermalleolar ligament. (B) Parts of the cartilage are removed to disclose the lesion. (C) Surgical debridement of the lesion followed by curettage. (D) Healthy cancellous bone after curettage. (E) Microfracture. (F) “Dry” arthroscopic view showing the cavity. (G) Allograft bone graft. (H) A photograph after the completion of implantation of allograft bone. (I) PRP-FG (platelet rich-plasma-fibrin glue) was injected.
After spinal anesthesia, the patient was placed in a prone position with a thigh tourniquet. All bony prominences were protected with cushion jelly to prevent pressure sore. A total of 40 ml blood was collected from his arm vessel. PRP was prepared using the package produced by the Shandong Weigao Group Medical Polymer Company. A quantity of 4 ml of concentrated PRP was prepared by the double centrifugation method at 2,000 r/min for 10 min with a centrifugation radius of 15 cm. Concentrated PRP was set aside.
The 2-portal posterior ankle arthroscopy technique, first introduced by Van Dijk (11), was used with a saline gravity irrigation system. A 4.0-mm, 30° arthroscope was inserted via the posterolateral portal and a shaver was placed in the posteromedial portal. By identifying the flexor hallucis longus by protecting its medial aspect, the neurovascular bundle was kept safe. After debridement of the posterior ankle capsule and posterior intermalleolar ligament, the surface of the lesion with overlying soft cartilage was identified. The soft cartilage at the most posterior part of the talar dome (avoiding the weight-bearing area) was removed to expose the lesion, which was soft and friable and grey in color, mixed with a dark red–stained hematoma. It was curetted until the healthy cancellous bone was exposed. Microfracture was conducted on the wall of the cavity. After thorough lavage, the saline inflow was stopped and the intra-articular fluid was drained out. Then, we switched to “dry” transosseous arthroscopy. Under direct vision, 30 g allograft granular cancellous bone was packed into the lesion and gently tamped down. Once the bone graft was done, concentrated PRP fibrinogen and thrombin (Zhejiang Hangkang Pharmaceutical Co., Ltd.) were mixed. Then, the injection was applied to the surface defect and fibrin glue (Harbin Pacific Biopharmaceutical Co., Ltd.) was used for sealing. The portals were closed with a suture, and a dry, sterile compression dressing was applied. The foot and ankle were immobilized with a splint.
The suture was removed 2 weeks after the surgery. From the first day to postoperative 2 weeks, the patient remained on non-weight-bearing crutches with the ankle was placed in 90° dorsiflexion. Active muscle contraction was encouraged, and the range of motion was restricted within the first 2 weeks. Ankle plantar flexion and dorsiflexion exercises were started after 2 weeks, with the number increased by 30 times a day until it reached 150 times a day. Then, the partial weight-bearing process with a postoperative walking boot and crutch was gradually increased from 2 to 6 weeks. This process started from a weight of 20 lbs, with the weekly weight increased by 20 lbs. At the postoperative 6 weeks (the first follow-up visit), an x-ray revealed an initially incorporated bone graft. We encouraged the patient to increase his weight-bearing walking exercise and activities as tolerated. Instructions on rehabilitation exercises were given to the patient during follow-up visits.
We found that there were no postoperative complications. The first follow-up was conducted at 6 weeks. Since then, follow-ups have been conducted every 3 months. The most recent follow-up was performed one year after the surgery. The patient was satisfied with their full motor function recovery and pain-free condition. The VAS score was 0 points, and the AOFAS score was 100 points. Ankle flexion and extension range of motion was 70°. One-year postoperative CT images showed good bone remodeling and osteointegration with no tumor recurrence (Figure 4).
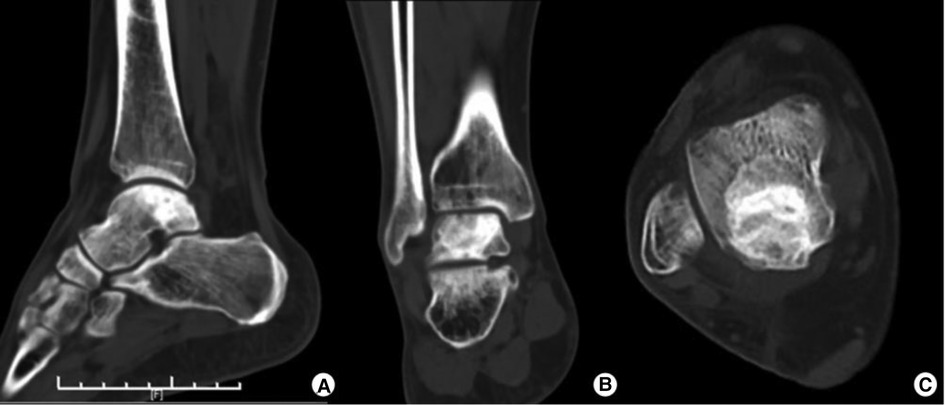
Figure 4. One-year postoperative CT images showing good bone remodeling and osteointegration with no tumor recurrence.
To the best of our knowledge, only one case of a patient with posterior ankle arthroscopic treatment for a talar chondroblastoma has been reported. Yonter et al. (10) reported a patient with a 1.3*2.0*0.8 = 2.08 cm3 chondroblastoma in the posterior talus via arthroscopic curettage, allograft bone graft, and augmentation with a cell-free matrix. Our case presented a challenging larger-sized talar chondroblastoma (2.5*2.2*2.2 = 12.1 cm3), and we combined posterior ankle arthroscopy, including wet and “dry” transosseous arthroscopy, with curettage, allograft bone graft, and PRP-FG. Our patient showed satisfactory results without any recurrence at a 1-year follow-up.
Although talar chondroblastoma is uncommon, almost 50% of foot chondroblastoma occurs in the talus (12). Interestingly, Angelini et al. (12) suggested that the recurrence rate of chondroblastoma in the foot was even lower than that in other body locations. There are few reports about appropriate treatments and clear outcomes for talar chondroblastoma. Therefore, we review and summarized the literature in Table 1. We found that most of the cases reported a generally good prognosis, except the case of a patient who had a massive tumor with local invasion (talus, calcaneus, tibiotalar joint, and the adjacent soft tissue).
Shears et al. (26) claimed that it is unnecessary to do a bone graft after benign talar tumor excision. However, in this small number of case series, we note that only two out of eight patients had a tumor volume larger than 10 cm3. Thus, this triggered concern about whether such findings also hold for those with relatively large-size lesions. In addition, it took 6–12 months for us to fully consolidate the defect, which delayed the recommendation of rehabilitation exercises to our patient. Moreover, early weight-bearing walking without the reconstruction of bony defects might cause talar collapse or fracture. Therefore, caution must be exercised because our patient had a large-sized talar chondroblastoma with a bony defect of 12.1 cm3. If a talar collapse or fracture happens, management would become much more difficult. Among multiple bone graft materials, autograft bone has the advantages of osteoconductivity, osteoinductivity, and potential osteogenicity (27–29). Therefore, it remains the gold standard for bone defect reconstruction. Similarly, autologous bone transplantation is the most widely used procedure in bone defect repair for talar chondroblastoma (10, 15–18, 21). Furthermore, a study reported that vascularized bone autograft was used to construct larger defects of the talar caused by bone tumor (19, 25). Although all these procedures are effective, potential pain and complications at the donor sites are worrying (30). Under such conditions, an allograft bone graft may be a good alternative (14, 20). Moreover, artificial bone graft substitutes such as the demineralized bone matrix or platelet-derived growth factor augmented ceramic granules showed good results compared with autologous grafts (31). In addition, bone cement implantation has been reported (22, 23). However, the major disadvantages of this procedure are the occurrence of thermal injury, secondary fracture, and osteoarthritis (32). It is of note that joint degeneration tends to be the case when the tumor is in close proximity to the articular cartilage (33). Van et al. (34) found that if the tumor–cartilage distance was ≤3 mm, the use of bone cement was more likely to result in joint degeneration. Thus, we did not use bone cement in our patient, as the remainder of the subchondral bone was extremely thin. We chose an allograft bone graft after considering the patient's apprehensions about extra donor site surgery and the resultant morbidities. On this basis, we combined allograft bone with PRP-FG to enhance tissue regeneration and repair (35). PRP-FG offsets the drawbacks of PRP, which lacks fixation and has poor tissue adhesive ability (36, 37). The final follow-up results of our case demonstrate the effectiveness of this procedure. However, a more prolonged observational follow-up is required to assess long-term results.
The technical difficulty of treating a talar chondroblastoma is that it lies in a deep and complicated position and is occluded by the tibial mortise and soft tissue of the posterior ankle. Open surgery, sometimes requiring osteotomy for better exposure of the lesion, may cause more trauma and induces subsequent tissue adhesions. In our case, posterior ankle arthroscopy offered a direct visualization of the surgical field, allowing for subsequent refined operation. At 2 weeks after arthroscopy, our patient was given the freedom of early partial weight-bearing and early mobilization. The patient was allowed to carry out the full weight-bearing activities of daily living at an earlier stage than that of open surgery. Our experience demonstrated the technical feasibility and superiority of arthroscopy. Arthroscopy is a safe, powerful, and minimally invasive technique offering good postoperative results. Studies have shown that arthroscopy has produced inspiring outcomes in benign bone tumors (38–42). More importantly, full functional recovery after a period of 8–12 weeks after arthroscopy has been observed. The application of arthroscopic treatment in chondroblastoma is listed in Table 2. In our case, we are pleased to report that arthroscopic treatment in talar chondroblastoma yielded good initial results. However, there is insufficient evidence of whether arthroscopic treatment of chondroblastoma won't decrease the tumor recurrence rate. Therefore, orthopedic surgeons must remain vigilant. A larger number of studies and a higher level of evidence are warranted to support this potential indication.
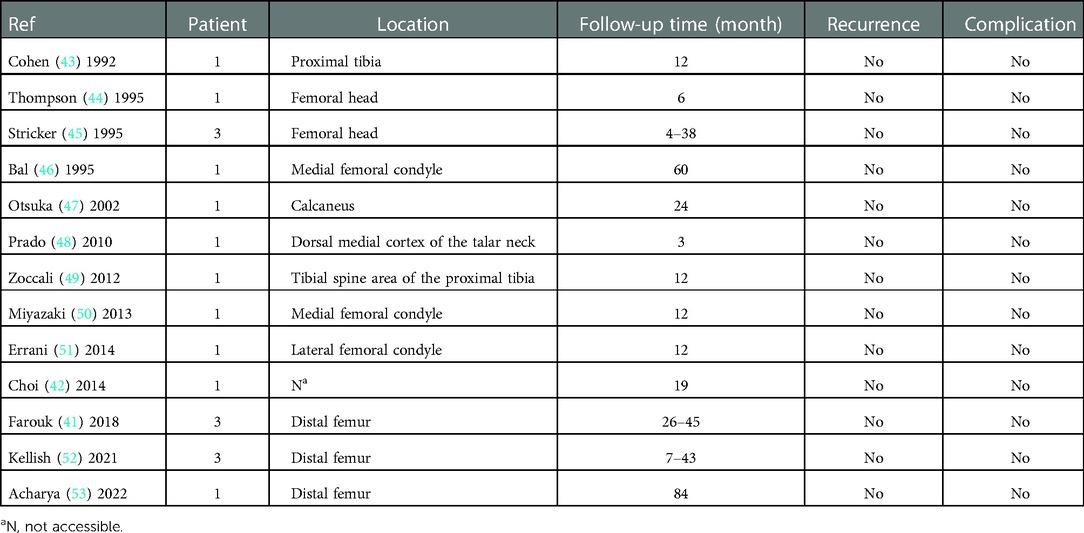
Table 2. Application of arthroscopic treatment in chondroblastoma previously reported in the literature.
Posterior ankle arthroscopy with allograft bone graft and PRP-FG is a secure, motivating, and promising technique to treat chondroblastoma in the posterior talus, offering clinicians an excellent alternative to open surgery.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CC and ZL conceptualized the study. JX collected patient information and reviewed the literature. CC and ZL drafted the manuscript. ZS and JX revised the manuscript. All authors contributed to the article and approved the submitted version.
The study was sponsored by the Key Research and Development Program of National Ministry of Science and Technology (2018YFC2001504), Bio-medical Engineering Program of “Shanghai Jiao Tong University Star” Plan (YG2022ZD018), Shanghai Artificial Intelligence Innovation and Development Program (2020-RGZN-02006), and Biomedicine Supporting Program of Shanghai “Science and Technology Innovation Plan” (19441902400).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chen W, Difrancesco LM. Chondroblastoma: an update. Arch Pathol Lab Med. (2017) 141(6):867–71. doi: 10.5858/arpa.2016-0281-RS
2. Xu H, Nugent D, Monforte HL, Binitie OT, Ding Y, Letson GD, et al. Chondroblastoma of bone in the extremities: a multicenter retrospective study. J Bone Joint Surg Am. (2015) 97(11):925–31. doi: 10.2106/jbjs.N.00992
3. Suneja R, Grimer RJ, Belthur M, Jeys L, Carter SR, Tillman RM, et al. Chondroblastoma of bone: long-term results and functional outcome after intralesional curettage. J Bone Joint Surg Br. (2005) 87(7):974–8. doi: 10.1302/0301-620x.87b7.16009
4. Choi JH, Ro JY. The 2020 WHO classification of tumors of bone: an updated review. Adv Anat Pathol. (2021) 28(3):119–38. doi: 10.1097/pap.0000000000000293
5. Breck LW, Emmett JE. Chondroblastoma of the talus: a case report. Clin Orthop. (1956) 7:132–5.13356528
7. Ramappa AJ, Lee FY, Tang P, Carlson JR, Gebhardt MC, Mankin HJ. Chondroblastoma of bone. J Bone Joint Surg Am. (2000) 82(8):1140–5. doi: 10.2106/00004623-200008000-00011
8. Laitinen MK, Stevenson JD, Evans S, Abudu A, Sumathi V, Jeys LM, et al. Chondroblastoma in pelvis and extremities – a single centre study of 177 cases. J Bone Oncol. (2019) 17:100248. doi: 10.1016/j.jbo.2019.100248
9. Xie C, Jeys L, James SL. Radiofrequency ablation of chondroblastoma: long-term clinical and imaging outcomes. Eur Radiol. (2015) 25(4):1127–34. doi: 10.1007/s00330-014-3506-1
10. Yonter SN, Aslan L, Can A, Ogut T. A case of arthroscopic treatment of chondroblastoma-induced chondropathy situated at the posterior talus. J Am Podiatr Med Assoc. (2021) 111(5). doi: 10.7547/20-125
11. Van Dijk CN. Hindfoot endoscopy. Foot Ankle Clin. (2006) 11(2):391–414, vii. doi: 10.1016/j.fcl.2006.03.002
12. Angelini A, Arguedas F, Varela A, Ruggieri P. Chondroblastoma of the foot: 40 cases from a single institution. J Foot Ankle Surg. (2018) 57(6):1105–9. doi: 10.1053/j.jfas.2018.05.005
13. Hull MT, Gonzalez-Crussi F, Derosa GP, Graul RS. Aggressive chondroblastoma. Report of a case with multiple bone and soft tissue involvement. Clin Orthop Relat Res. (1977) 126:261–5.
14. Moore TM, Roe JB, Harvey JP Jr. Chondroblastoma of the talus: a case report. J Bone Joint Surg Am. (1977) 59(6):830–1. doi: 10.2106/00004623-197759060-00020
15. Ochsner PE, Von Hochstetter AR, Hilfiker B. Chondroblastoma of the talus: natural development over 9.5 years. Case report. Arch Orthop Trauma Surg (1978). (1988) 107(2):122–5. doi: 10.1007/bf00454501
16. Yu GV, Sellers CS. Chondroblastoma of the talus. J Foot Ankle Surg. (1996) 35(1):72–7. doi: 10.1016/s1067-2516(96)80016-0
17. Sterling G, Wilson A. Chondroblastoma of the talus: a case report. J Foot Ankle Surg. (2002) 41(3):178–82. doi: 10.1016/s1067-2516(02)80068-0
18. Anderson AF, Ramsey JR. Chondroblastoma of the talus treated with osteochondral autograft transfer from the lateral femoral condyle. Foot Ankle Int. (2003) 24(3):283–7. doi: 10.1177/107110070302400315
19. Hassenpflug J, Ulrich HW, Liebs T, Lankes JM, Terheyden H, Kreusch T, et al. Vascularized iliac crest bone graft for talar defects: case reports. Foot Ankle Int. (2007) 28(5):633–7. doi: 10.3113/fai.2007.0633
20. Zhang K, Gao Y, Dai H, Zhang S, Li G, Yu B. Chondroblastoma of the talus: a case report and literature review. J Foot Ankle Surg. (2012) 51(2):262–5. doi: 10.1053/j.jfas.2011.10.006
21. Ningegowda RV, Subramanian K, Suresh I. Chondroblastoma of the talus. J Foot Ankle Surg. (2013) 52(5):673–7. doi: 10.1053/j.jfas.2013.02.020
22. Sun B, Li XY, Zhao XY, Feng W, Liu JG. Chondroblastoma with associated aneurysmal bone cyst of the talus: a case report and review of relative literatures. Zhongguo Gu Shang. (2015) 28(7):657–9.26399111
23. Bahamonde Munoz L, Escudero Heldt M. Massive chondroblastoma of the talus: treatment with en bloc talectomy and tibiocalcaneal arthrodesis: long-term follow-up of a case. Foot Ankle Spec. (2017) 10(3):274–7. doi: 10.1177/1938640016676339
24. Ryu JJ, Kim W, Lee JS, Kim YK, Lee HS, Seo SG. Combined autograft and bone cement for painful chondroblastoma: a case report. J Foot Ankle Surg. (2018) 57(2):396–400. doi: 10.1053/j.jfas.2017.08.019
25. Wagener J, Schweizer C, Horn Lang T, Zwicky L, Schaefer DJ, Hintermann B. Vascularized bone autograft for the treatment of chondroblastoma of the talus at imminent risk of joint breakdown: three case reports. J Foot Ankle Surg. (2019) 58(2):363–7. doi: 10.1053/j.jfas.2018.08.053
26. Shears E, Dehne K, Murata H, Abudu A, Grimer RJ, Tillman RM, et al. Healing of ungrafted bone defects of the talus after benign tumour removal. Foot Ankle Surg. (2008) 14(3):161–5. doi: 10.1016/j.fas.2008.01.006
27. Bow A, Anderson DE, Dhar M. Commercially available bone graft substitutes: the impact of origin and processing on graft functionality. Drug Metab Rev. (2019) 51(4):533–44. doi: 10.1080/03602532.2019.1671860
28. Danilkowicz R, Murawski C, Pelligrini M, Walther M, Valderrabano V, Angthon C, et al. Nonoperative and operative soft-tissue and cartilage regeneration and orthopaedic biologics of the foot and ankle: an orthoregeneration network (ON) foundation review. Arthroscopy. (2022) 38(7):2350–8. doi: 10.1016/j.arthro.2022.04.018
29. Gillman CE, Jayasuriya AC. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater Sci Eng C Mater Biol Appl. (2021) 130:112466. doi: 10.1016/j.msec.2021.112466
30. Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. (1989) 3(3):192–5. doi: 10.1097/00005131-198909000-00002
31. Müller MA, Frank A, Briel M, Valderrabano V, Vavken P, Entezari V, et al. Substitutes of structural and non-structural autologous bone grafts in hindfoot arthrodeses and osteotomies: a systematic review. BMC Musculoskelet Disord. (2013) 14:59. doi: 10.1186/1471-2474-14-59
32. Benevenia J, Rivero SM, Moore J, Ippolito JA, Siegerman DA, Beebe KS, et al. Supplemental bone grafting in giant cell tumor of the extremity reduces nononcologic complications. Clin Orthop Relat Res. (2017) 475(3):776–83. doi: 10.1007/s11999-016-4755-x
33. Jamshidi K, Bagherifard A, Mohaghegh MR, Mirzaei A. Fibular strut allograft or bone cement for reconstruction after curettage of a giant cell tumour of the proximal femur: a retrospective cohort study. Bone Joint J. (2022) 104-b(2):297–301. doi: 10.1302/0301-620x.104b2.Bjj-2021-1322.R1
34. Van Der Heijden L, Van De Sande MA, Heineken AC, Fiocco M, Nelissen RG, Dijkstra PD. Mid-term outcome after curettage with polymethylmethacrylate for giant cell tumor around the knee: higher risk of radiographic osteoarthritis? J Bone Joint Surg Am. (2013) 95(21):e159. doi: 10.2106/jbjs.M.00066
35. Fang J, Wang X, Jiang W, Zhu Y, Hu Y, Zhao Y, et al. Platelet-rich plasma therapy in the treatment of diseases associated with orthopedic injuries. Tissue Eng Part B Rev. (2020) 26(6):571–85. doi: 10.1089/ten.TEB.2019.0292
36. Wang M, Gao W. Fixation of platelet-rich plasma and fibrin gels on knee cartilage defects after microfracture with arthroscopy. Int Orthop. (2022) 46(8):1761–6. doi: 10.1007/s00264-022-05377-2
37. Kaplonyi G, Zimmerman I, Frenyo AD, Farkas T, Nemes G. The use of fibrin adhesive in the repair of chondral and osteochondral injuries. Injury. (1988) 19(4):267–72. doi: 10.1016/0020-1383(88)90043-5
38. Khapchik V, O'donnell RJ, Glick JM. Arthroscopically assisted excision of osteoid osteoma involving the hip. Arthroscopy. (2001) 17(1):56–61. doi: 10.1053/jars.2001.8022
39. Dai L, Zhang X, Mei Y, Gao G, Huang H, Wang C, et al. Arthroscopic excision of intra-articular osteoid osteoma of the hip: a case series. Arthroscopy. (2021) 37(10):3104–12. doi: 10.1016/j.arthro.2021.03.060
40. Mastboom MJL, Staals EL, Verspoor FGM, Rueten-Budde AJ, Stacchiotti S, Palmerini E, et al. Surgical treatment of localized-type tenosynovial giant cell tumors of large joints: a study based on a multicenter-pooled database of 31 international sarcoma centers. J Bone Joint Surg Am. (2019) 101(14):1309–18. doi: 10.2106/jbjs.18.01147
41. Farouk HA, Saladin M, Senna WA, Ebeid W. All-endoscopic management of benign bone lesions; a case series of 26 cases with minimum of 2 years follow-up. SICOT J. (2018) 4:50. doi: 10.1051/sicotj/2018041
42. Choi Y, Kwak JM, Chung SH, Jung GH, Kim JD. Tumor treated by endoscopy. Clin Orthop Surg. (2014) 6(1):72–9. doi: 10.4055/cios.2014.6.1.72
43. Cohen B, Khan TH, Dandy DJ. Arthroscopic resection of chondroblastoma of the knee. Arthroscopy. (1992) 8(3):370–2. doi: 10.1016/0749-8063(92)90071-i
44. Thompson MS, Woodward JS Jr. The use of the arthroscope as an adjunct in the resection of a chondroblastoma of the femoral head. Arthroscopy. (1995) 11(1):106–11. doi: 10.1016/0749-8063(95)90097-7
45. Stricker SJ. Extraarticular endoscopic excision of femoral head chondroblastoma. J Pediatr Orthop. (1995) 15(5):578–81. doi: 10.1097/01241398-199509000-00005
46. Bal BS, Jones L Jr. Arthroscopic resection of a chondroblastoma in the knee. Arthroscopy. (1995) 11(2):216–9. doi: 10.1016/0749-8063(95)90070-5
47. Otsuka T, Kobayashi M, Yonezawa M, Kamiyama F, Matsushita Y, Matsui N. Treatment of chondroblastoma of the calcaneus with a secondary aneurysmal bone cyst using endoscopic curettage without bone grafting. Arthroscopy. (2002) 18(4):430–5. doi: 10.1053/jars.2002.31967
48. Prado MP, Mendes AA, Amodio DT. Benign bone tumors subperiosteal on the talar neck resected anthroscopically: case reports. Einstein (Sao Paulo). (2010) 8(3):354–7. doi: 10.1590/s1679-45082010rc1530
49. Zoccali C, Teori G, Salducca N, Di Paola B, Adriani E. Arthroscopic guided biopsy and radiofrequency thermoablation of a benign neoplasm of the tibial spines area: a treatment option. BMC Musculoskelet Disord. (2012) 13:52. doi: 10.1186/1471-2474-13-52
50. Miyazaki T, Uchida K, Yayama T, Nakajima H, Honjoh K, Itoh H, et al. Chondroblastoma of the distal femur resected through a small fenestra via computed tomography navigation and endoscopy: a case report. J Med Case Rep. (2013) 7:164. doi: 10.1186/1752-1947-7-164
51. Errani C, Traina F, Chehrassan M, Donati D, Faldini C. Minimally invasive technique for curettage of chondroblastoma using endoscopic technique. Eur Rev Med Pharmacol Sci. (2014) 18(22):3394–8.25491613
52. Kellish AS, Qureshi M, Mostello A, Kim TW, Gutowski CJ. “Dry arthroscopy” is a valuable tool in the excisional curettage of chondroblastoma: a case series. J Orthop Case Rep. (2021) 11(1):82–6. doi: 10.13107/jocr.2021.v11.i01.1974
Keywords: arthroscopy, chondroblastoma, talus, benign bone tumor, platelet-rich plasma-fibrin glue, allograft
Citation: Chen C, Li Z, Xue J and Shi Z (2023) Posterior ankle arthroscopic treatment of a talar chondroblastoma with allograft and a platelet-rich plasma-fibrin glue: A case report and literature review. Front. Surg. 9:1039785. doi: 10.3389/fsurg.2022.1039785
Received: 8 September 2022; Accepted: 29 November 2022;
Published: 5 January 2023.
Edited by:
Paphon Sa-ngasoongsong, Mahidol University, ThailandReviewed by:
Odion Binitie, Moffitt Cancer Center, United States© 2023 Chen, Li, Xue and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: JianFeng Xue ZHJ4dWVqZkAxNjMuY29t ZhongMin Shi bGl1eXVhbnp1aHVhaUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.