
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 06 January 2023
Sec. Vascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1031108
This article is part of the Research TopicAcute Mesenteric Ischemia: Novel Diagnostic Modalities and Treatment Strategies to Improve Patient OutcomeView all 5 articles
 Bo Yang1
Bo Yang1 Zhang Xiaping1,2*
Zhang Xiaping1,2*
Objective: This study explored the correlation between serum HMGB1 levels and postoperative vascular restenosis in patients with lower extremity arteriosclerosis obliterans (LEASO).
Methods: A total of 362 patients LEASO who received vascular intervention were recruited in this study. Serum HMGB1 levels were measured by enzyme-linked immunosorbent assay. Logistic regression analysis was used to identify the influencing factors associated with vascular restenosis. The R procedure was used to create nomogram model. Receiver operating characteristic (ROC) analysis was used to determine the predictive value of serum HMGB1 and nomogram model for vascular restenosis.
Results: Of the 362 LEASO patients included, 103 (28.45%) developed restenosis within 6 months of postoperative follow-up. Postoperative HMGB1 levels were significantly higher in patients with restenosis compared to those with non-restenosis. Postoperative HMGB1 levels were significantly and positively correlated with the severity of postoperative restenosis (r = 0.819). The AUC of postoperative HMGB1 for the diagnosis of postoperative restenosis was 0.758 (95% CI: 0.703–0.812), with a sensitivity and specificity of 56.31% and 82.24%, respectively. Multivariate logistic regression analysis showed that diabetes, smoking, regular postoperative medication, increased fibrinogen, decreased red blood cells, increased hs-CRP, and increased postoperative HMGB1 were independently associated with postoperative restenosis in patients with LEASO. The C-index of the nomogram prediction model constructed based on the seven influencing factors mentioned above was 0.918. The nomogram model was significantly more predictive of postoperative restenosis in LEASO patients compared with a single postoperative HMGB1 (AUC: 0.918, 95% CI: 0.757–0.934).
Conclusion: Postoperative serum HMGB1 is an independent risk factor associated with postoperative vascular restenosis in patients with LEASO, and a novel nomogram model based on postoperative serum HMGB1 combined with clinical characteristics may help to accurately predict the risk of postoperative restenosis in patients with LEASO.
Lower extremity arteriosclerosis obliterans (LEASO) is a common disease in clinical vascular surgery, mostly in middle-aged and elderly people (1). 30% of LEASO occurs in iliac artery, and the other 70% are more common in the femoral artery, popliteal artery, and distal artery (2). The clinical manifestations of arteriosclerosis occlusion of the lower extremities often show intermittent claudication, resting pain, and gangrene; and the severe progress of which may even affect the life safety of the patient (3). The treatment methods of LEASO include vascular interventional therapy and vascular bypass surgery (4). Due to the large surgical trauma, minimally invasive interventional therapy, also known as percutaneous transluminal angioplasty (PTA), is often used, including balloon dilation and stent implantation. However, restenosis after interventional treatment has become a difficult and hot topic of clinical treatment. It was found that the restenosis rate in LEASO patients was 30%–50% within 6 months after intervention, and the incidence of restenosis within 1 year after intervention was as high as 70% (5).
There are many causes of restenosis after LEASO intervention, which are generally considered to be related to vascular inflammation, endothelial cell injury, and excessive proliferation and migration of smooth muscle cells (6). A large number of released inflammatory factors cause the body to show a high inflammatory state, which will aggravate the damage to vascular function and more easily lead to the occurrence of postoperative restenosis in patients. Neointimal proliferation is thought to underlie the pathophysiological development of restenosis, triggered by the pro-inflammatory molecules released due to endothelial damage, particularly during the thrombogenic and proliferative phases of restenosis (2). However, the mechanisms by which restenosis occurs after interventional procedures in patients with LEASO are still poorly understood. The search for reliable biomarkers to predict the occurrence of postoperative restenosis is of great importance.
High mobility group box protein B1 (HMGB1) is a key stress signal currently located in the nucleus with functions such as involvement in DNA transcription, induction of inflammatory responses, and regulation of vascular endothelial cell function (7). Studies have shown that HMGB1 is involved in the pathogenesis of atherosclerotic diseases. HMGB1 can be released from various cell types of atherosclerotic plaques, including smooth muscle cells, endothelial cells, and macrophages (8). Once released, HMGB1 exerts a variety of inflammatory effects on these cells. The pro-inflammatory effect of HMGB1 is mainly due to its structural characteristics and derived cellular biological effects (9). A variety of receptors play a role in HMGB1 signaling, including receptors for advanced non-enzymatic glycation end products (RAGEs) and members of the Toll-like receptors (TLRs) (10). TLRs bind to RAGEs to amplify the inflammatory response, an important mechanism that promotes atherosclerosis (11), and HMGB1 is the only known receptor with the highest affinity. Additionally, HMGB1 is also considered to be a neoendothelial mediator that stimulates vascular smooth muscle cell proliferation and migration (12). These data suggest that HMGB1 may be involved in the pathological process of restenosis after intervention. However, to date, studies on the relationship between circulating HMGB1 and restenosis after intervention in patients with LEASO remain unavailable.
In this study, we investigated the relationship between serum HMGB1 levels and postoperative vascular restenosis in 362 patients with LEASO who underwent vascular intervention. We also screened for risk factors associated with postoperative vascular restenosis based on Logistic regression analysis. Additionally, based on the results of logistic regression analysis, we developed a nomogram model combining serum HMGB1 with clinical characteristics to predict postoperative vascular restenosis in patients with LEASO. Our results suggested that serum HMGB1 levels may be a valuable biomarker for predicting restenosis after LEASO, which may provide insights for better understanding of the mechanisms of vascular restenosis.
This study was approved by the ethics committees of The Second Xiangya Hospital, Central South University was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. LEASO patients hospitalized from 2016 to 2021 were collected. All patients met the following criteria: patients satisfied the diagnostic criteria for arteriosclerosis obliterans of the lower extremities and were confirmed to have superficial femoral artery disease, and lower extremity arterial intervention was performed; patients with Rutherford III–VI, manifesting as intermittent claudication, rest pain, tissue ulcers and gangrene; patients aged 40–80 years; patients with ankle brachial index (ABI) < 0.9; patients with regularly lower extremity arterial ultrasound or CT angiography after operation; patients not using ARBs, β receptor blockers or angiotensin-converting enzyme inhibitors ACEIs. The following patients were excluded: patients with a history of lower extremity trauma or surgery; patients with amputation or death after surgery; patients with severe hepatic or renal insufficiency; patients with residual stenosis probability >30% after surgery; patients who need traditional bypass surgery; patients with malignant tumors, autoimmune diseases, mental diseases, and acute and chronic inflammatory diseases.
Femoral artery puncture was performed on the patient under local anesthesia. Then angiography of the lower extremity arteries was performed. Depending on the results of lower extremity computed tomography angiography (CTA), different interventional treatment options were adopted. If the stenosis of the vessel was greater than 50%, the appropriate balloon was selected to dilate the stenotic vessel; if the residual stenosis after balloon dilatation was greater than 30% (or endothelial stripping), endovascular stenting was performed after balloon dilatation of the stenotic vessel. The infrapopliteal artery was dilated using a 1.5–3.0 mm diameter, 120 mm length balloon (INV Atech) at a pressure of 8–14 atmospheres (1 atmosphere = 101.325 kPa) for 180 s, and a SMART self-expanding stent (Cordis, Miami Lakes, FL) was used in this study. After angiography, the luminal stenosis at the lesion site was less than 30%, and there was no significant arterial entrapment or serious complications related to the procedure, and the treatment was judged to be successful. The patient was given heparin 4000 U for 3 days; oral clopidogrel 75 mg/d for 1 month, followed by a long-term oral administration of aspirin 100 mg/d.
The basic information of the patients’ first admission was collected, including age, gender, course of disease, underlying diseases, smoking history, and drinking history.
The baseline biochemical indicators of patients before vascular interventional surgery were recorded, including systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood glucose (FBG), high-sensitivity C-reactive protein (hs-CRP), total cholesterol (TC), triacylglycerol (TG), high density lipoprotein cholesterol (H-DLC), low density lipoprotein cholesterol (L-DLC), red blood cell count, white blood cell count, hemoglobin, and fibrinogen. Fasting venous blood was collected from patients before and 12 h after intervention. The level of HMGB1 was detected using a commercial human HMGB1 ELISA kit (Beyotime, Shanghai, China).
Patients were followed up monthly for 6 months. CTA or ultrasound examination should be performed at least once a month during the 6 months of follow-up. Criteria for restenosis after interventional therapy: CTA and color Doppler ultrasound of the lower extremity arteries showed that the degree of vascular stenosis was >50%. The severity of restenosis of each patient was calculated according to the following formula (15): severity (%) = (B-A)/A × 100%, where A was the minimum lumen area at the stenosis area after interventional therapy and B was the minimum lumen area at the stenosis area at the end of the 6th month after interventional therapy. For patients with multiple vascular stenoses, the mean value was adopted to represent the severity of restenosis. The severity of restenosis was divided into four classes: I, 0%–20%; II, 20%–40%; III, >40%–60%; IV, >60%.
The Kolmogorov-Smimov test was used to test whether the measurement data conformed to a normal distribution. The normally distributed measurement data were expressed as mean centerSD, and the independent samples t test was performed in parallel. Non-normally distributed measurement data were expressed as medians (quartiles), and the Mann-Whitney U test was performed. The enumeration data were analyzed by the 2 test. Based on independent risk factors in multivariate Logistic regression analysis. R 4.2.1 (http://www.r-project.org) was used to perform all the graphics based on R packages “rms”, “ggplot2”, “pROC”, and “car”. Program on R package was provided as Supplementary file. The performance of the nomogram was evaluated by the Harrell Concordance Index (C-index). The receiver operating characteristic curve (ROC) was used to evaluate the predictive value of HMGB1 level and nomogram model for postoperative restenosis, and the results were expressed as the area under the curve (AUC) and the 95% confidence interval (95% CI) of the area. Generally, the diagnostic accuracy with an AUC equal or above 0.6 is considered acceptable (16). P < 0.05 was considered to represent a statistically significant difference.
A total of 362 patients with LEASO, including 103 patients (28.45%) with postoperative restenosis, were included in this study. Compared with the non-Restenosis group, the proportion of patients with postoperative restenosis who had diabetes, smoking history and irregular postoperative medication significantly increased, and the levels of FBG, TC, fibrinogen and hs-CRP were significantly increased, while the level of red blood cell count was significantly lower (Table 1).
Subsequently, preoperative and postoperative serum HMGB1 levels were compared between Restenosis group and non-Restenosis group. There was no significant difference in preoperative HMGB1 levels between the two groups (Figure 1). The postoperative HMGB1 level in the Restenosis group was significantly higher than that in the non-Restenosis group (Figure 1). Spearman correlation analysis showed that postoperative HMGB1 levels were significantly and positively correlated with the severity of postoperative restenosis (Table 2). Subsequently, the efficacy of preoperative and postoperative serum HMGB1 in the diagnosis of postoperative restenosis in LEASO patients was evaluated by ROC (Figure 2). The AUC of postoperative HMGB1 for the diagnosis of postoperative restenosis was 0.758 (95%CI: 0.703–0.812), and the sensitivity and specificity were 56.31% and 82.24%, respectively.
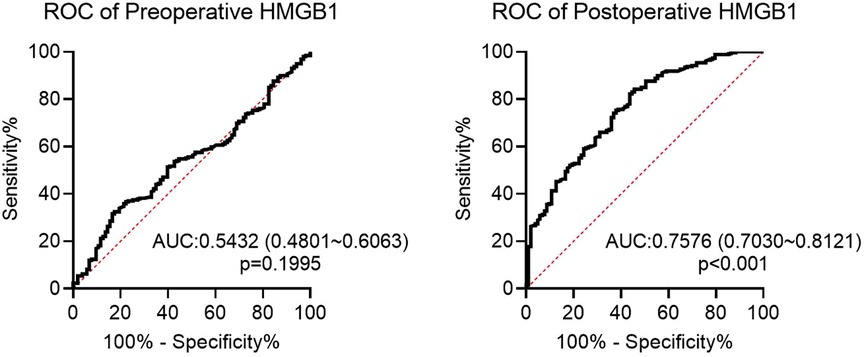
Figure 2. ROC curve of preoperative or postoperative HMGB1 for diagnosis of restenosis in LEASO patients.
The risk factors with significant statistical significance in univariate regression analysis were included in multivariate Logistic regression. The results showed that diabetes, smoking, increased fibrinogen, decreased red blood cells, elevated hs-CRP, and increased postoperative HMGB1 were independent risk factors for postoperative restenosis in patients with LEASO, while regular postoperative medication was a protective risk factor (Table 3).
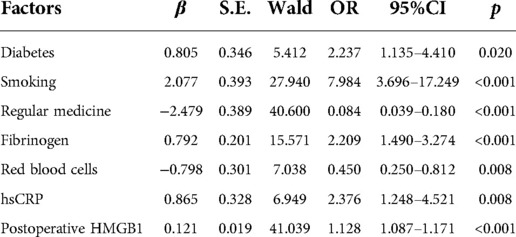
Table 3. Logistic regression analysis for risk factors for postoperative restenosis in LEASO patients.
We incorporated the seven independent risk factors mentioned above in an individualized nomogram prediction model for restenosis risk based on Logistic regression analysis (Figure 3). The nomogram model was evaluated by Harrell concordance index and ROC curve. Internal validation showed that the nomogram could accurately predict the C-index of restenosis, which was 0.918. A ROC curve was generated to verify the predictive accuracy of the nomogram, with an AUC of 0.918 (95%CI: 0.757–0.934; P < 0.001) (Figure 4). The consistency of the nomogram model for predicting postoperative restenosis in LEASO patients was evaluated by calibration curve. The results showed that the nomogram model predicted the probability of postoperative restenosis in LEASO patients was in good agreement with the actual probability (Figure 5).
Lower extremity arteries are one of the common arteries prone to atherosclerosis in addition to carotid and coronary arteries (13, 14). Vascular intervention has become the main treatment for LEASO because of its minimally invasive and significant efficacy (15, 16). It is well known that endovascular restenosis after interventional treatment is a difficult aspect of clinical treatment (17). A meta-analysis on the outcome of ischemic restenosis treatment of lower extremity arteries in patients with LEASO showed that the 3-year primary patency rate of stenting for long-segment iliac or superficial femoral artery occlusions was only 50% (18). It is important to explore biomarkers associated with the occurrence of restenosis. The present study demonstrates for the first time that elevated serum HMGB1 levels are a risk factor for restenosis after LEASO intervention. In addition, this study combined postoperative serum HMGB1 and clinical characteristics to construct a nomogram to predict the occurrence of restenosis after LEASO intervention.
In human atherosclerotic lesions from the aorta, carotid and coronary arteries, HMGB1 expression was significantly increased in the nucleus and cytoplasm of macrophages and smooth muscle cells located near the intima compared with normal human arteries (19). Strong expression of HMGB1 was also observed in regions adjacent to the necrotic core of atherosclerotic lesions (20). Intimal hyperplasia and restenosis following carotid balloon injury have been shown to be associated with enhanced HMGB1 expression (21, 22). Inhibition of HMGB1 can prolong scaffold life by regulating smooth muscle cell proliferation and neointima formation (23). Moreover, regulation of the inflammatory response by HMGB1 also plays a role in the response to stent injury (24, 25). The present study showed that postoperative serum HMGB1 levels were significantly higher in patients with restenosis than in patients without restenosis. However, there was no significant difference in preoperative serum HMGB1 levels between the two groups. Additionally, postoperative HMGB1 levels were positively correlated with the severity of postoperative restenosis. These data suggest that HMGB1 is involved in the occurrence of restenosis after the intervention. Higher serum HMGB1 levels are considered to be a potential marker of subclinical atherosclerosis (26). In the correlation study between Gensini score of coronary heart disease angiography and serum HMGB1 level, serum HMGB1 level was increased with disease progression, speculating that HMGB1 was a new inflammatory marker to predict the evolution of coronary stenosis degree in atherosclerotic heart disease (27). In the current study, ROC analysis showed that postoperative HMGB1 levels had a high predictive value for the occurrence of restenosis after interventional therapy in LEASO patients. Furthermore, Logistic regression analysis showed that postoperative serum HMGB1 level was an independent risk factor for restenosis in LEASO patients after interventional therapy. Collectively, postoperative serum HMGB1 can be used as a new potential serum biomarker for predicting restenosis after interventional treatment in patients with LEASO.
Timely detection and control of risk factors related to restenosis or even occlusion of blood vessels or lumen heads in postoperative patients can improve the postoperative prognosis of LEASO patients (16, 28). There are many factors that affect postoperative restenosis. Previous studies have shown that diabetes, hypertension, and smoking status are risk factors for postoperative restenosis in LEASO patients (29, 30). hs-CRP is a common indicator reflecting the inflammatory state (31), and its elevation has been confirmed to be a risk factor for recurrence after intervention (32). Fibrinogen is a new indicator of oxidative stress and inflammation in the body in recent years (33). A higher plasma fibrinogen concentration, as a separate variable, had a similar effect on cardiovascular risk as well-known risk factors such as smoking cigarette, obesity, arterial hypertension, and diabetes (34). In our study, diabetes, smoking, regular medicine, fibrinogen, red blood cells, hs-CRP were also confirmed to be independent risk factors for restenosis after interventional therapy in LEASO patients.
Predictor identification and risk assessment are critical for effective medical decision-making to prevent restenosis (35). The risk of major cardiovascular events and mortality in patients with diabetic nephropathy was significantly improved by multifactorial intervention (36). Therefore, treatment of relevant risk factors will help to reduce the incidence of restenosis in patients with LEASO undergoing interventional therapy. Nomogram-based predictive models have been widely used in clinical research (37). In the present study, the aforementioned independent risk factors were included in the prediction model we constructed with an optimal C-index of 0.918. Moreover, the nomogram-based prediction model was effective in predicting the occurrence of restenosis. Furthermore, the combination of six independent influencing factors associated with postoperative restenosis predicted the occurrence of restenosis more accurately than postoperative serum HMGB1 alone.
This study has several limitations. First, it was a single-center retrospective study with a small sample size. Second, the follow-up period of this study was short and the analysis of the influencing factors was not comprehensive enough. Because the mechanisms of restenosis after lower extremity arterial vascular intervention are very complex, it is necessary to expand the sample size, extend the follow-up time, and add different influencing factors (such as different drugs) to further explore the mechanisms in the future. In addition, the present study only analyzed the HMGB1 level at 12 h after intervention, and it is not yet possible to determine whether it is the best indicator for evaluation. The dynamic changes of HMGB1 still need to be observed in the follow-up, and the nomogram model still needs to be supported by data from the validation group.
In conclusion, this study demonstrates for the first time that HMGB1 levels in postoperative serum samples from patients with LEASO are significantly elevated after intervention and that postoperative HMGB1 levels have a high predictive value for the development of restenosis. In addition, elevated postoperative serum HMGB1 levels were an independent risk factor for restenosis after interventional treatment. A nomogram model constructed from postoperative HMGB1 combined with clinical characteristics can effectively assess the occurrence of restenosis after intervention in patients with LEASO. HMGB1 may become a therapeutic target for revascularization.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by This study was approved by the ethics committees of The Second Xiangya Hospital, Central South University was performed in accordance with the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
BY: conducted most of the experiments and wrote the manuscript; ZXP: conducted the experiments and designed the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mansori K, Allahmoradi M. Comments on preoperative sepsis is a predictive factor for 30-day mortality after major lower limb amputation among patients with arteriosclerosis obliterans and diabetes. J Ortho Sci. (2020) 25:1132. doi: 10.1016/j.jos.2019.09.021
2. Donners MM, Daemen MJ, Cleutjens KB, Heeneman S. Inflammation and restenosis: implications for therapy. Ann Med. (2003) 35:523–31. doi: 10.1080/07853890310014876
3. Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL, et al. Society for vascular surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. (2015) 61:2s–41s. doi: 10.1016/j.jvs.2014.12.009
4. Cui L, Chen L, Dai Y, Ou J, Qiu M, Wang S. Increased level of tim-3(+)PD-1(+)CD4(+)T cells with altered function might be associated with lower extremity arteriosclerosis obliterans. Front Immunol. (2022) 13:871362. doi: 10.3389/fimmu.2022.871362
5. Liistro F, Porto I, Angioli P, Grotti S, Ricci L, Ducci K, et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation. (2013) 128:615–21. doi: 10.1161/CIRCULATIONAHA.113.001811
6. Fu W, Yan L, Hou Z, Yu Y, Zhang W, Cui R, et al. Impact of cerebral small vessel disease on symptomatic in-stent restenosis in intracranial atherosclerosis. J Neurosurg. (2022) 12:1–10. doi: 10.3171/2022.6.JNS221103
7. Satoh TK. The role of HMGB1 in inflammatory skin diseases. J Dermatol Sci. (2022) 107:58–64. doi: 10.1016/j.jdermsci.2022.07.005.35907655
8. Inoue K, Kawahara K, Biswas KK, Ando K, Mitsudo K, Nobuyoshi M, et al. HMGB1 Expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc Pathol. (2007) 16:136–43. doi: 10.1016/j.carpath.2006.11.006
9. Aulin C, Larsson S, Vogl T, Roth J, Åkesson A, Swärd P, et al. The alarmins high mobility group box protein 1 and S100A8/A9 display different inflammatory profiles after acute knee injury. Osteoarthritis Cartilage. (2022) 30:1198–209. doi: 10.1016/j.joca.2022.06.009
10. Tao Z, Helms MN, Leach BCB, Wu X. Molecular insights into the multifaceted functions and therapeutic targeting of high mobility group box 1 in metabolic diseases. J Cell Mol Med. (2022) 26:3809–15. doi: 10.1111/jcmm.17448
11. van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis. (2008) 11:91–9. doi: 10.1007/s10456-008-9093-5
12. Filiberto AC, Spinosa MD. Endothelial pannexin-1 channels modulate macrophage and smooth muscle cell activation in abdominal aortic aneurysm formation. Nat Commun. (2022) 13:1521. doi: 10.1038/s41467-022-29233-4
13. Liang HL. Doppler Flow measurement of lower extremity arteries adjusted by pulsatility Index. AJR Am J Roentgenol. (2020) 214:10–7. doi: 10.2214/AJR.19.21280
14. Lin CH, Hsieh YH, Lin CH. The medial sural artery perforator flap in lower extremity reconstruction. Clin Plast Surg. (2021) 48:249–57. doi: 10.1016/j.cps.2021.01.003
15. Elmore JB, Mehanna E, Parikh SA, Zidar DA. Restenosis of the coronary arteries: past, present, future directions.. Interv Cardiol Clin. (2016) 5:281–93. doi: 10.1016/j.iccl.2016.03.002
16. Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. (2007) 100:3k–9k. doi: 10.1016/j.amjcard.2007.06.002
17. Singh AD, Singal AK, Mian A, Kapadia SR, Hedrick DP, Kanaa'N A, et al. Recurrent drug-eluting stent in-stent restenosis: a state-of-the-art review of pathophysiology, diagnosis, and management. Cardiovasc Revasc Med. (2020) 21:1157–63. doi: 10.1016/j.carrev.2020.01.005
18. Almasri J, Adusumalli J, Asi N, Lakis S, Alsawas M, Prokop LJ, et al. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J Vasc Surg. (2018) 68:624–33. doi: 10.1016/j.jvs.2018.01.066
19. Kim EJ, Park SY, Baek SE, Jang MA, Lee WS, Bae SS, et al. HMGB1 Increases IL-1β production in vascular smooth muscle cells via NLRP3 inflammasome. Front Physiol. (2018) 9:313. doi: 10.3389/fphys.2018.00313
20. Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, et al. Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol. (2004) 24:2320–5. doi: 10.1161/01.ATV.0000145573.36113.8a
21. Liu H, Dong S, Xiong W, Liu Q, Liao B. Effects of high mobility group box 1 and nuclear factor κB on neointimal hyperplasia after common carotid artery balloon injury. Int J Clin Exp Med. (2015) 8:13323–8. PMID: 2655026026550260
22. Yang B, Gao P, Wu X, Yu J, Li Y, Meng R, et al. Epigallocatechin-3-gallate attenuates neointimal hyperplasia in a rat model of carotid artery injury by inhibition of high mobility group box 1 expression. Exp Ther Med. (2017) 14:1975–82. doi: 10.3892/etm.2017.4774
23. Chen J, Zhang J, Xu L, Xu C, Chen S, Yang J, et al. Inhibition of neointimal hyperplasia in the rat carotid artery injury model by a HMGB1 inhibitor. Atherosclerosis. (2012) 224:332–9. doi: 10.1016/j.atherosclerosis.2012.07.020
24. Rauvala H, Rouhiainen A. RAGE As a receptor of HMGB1 (amphoterin): roles in health and disease. Curr Mol Med. (2007) 7:725–34. doi: 10.2174/156652407783220750
25. Meiners S, Laule M, Rother W, Guenther C, Prauka I, Muschick P, et al. Ubiquitin-proteasome pathway as a new target for the prevention of restenosis. Circulation. (2002) 105:483–9. doi: 10.1161/hc0402.102951
26. de Souza AW, Westra J, Limburg PC, Bijl M, Kallenberg CG. HMGB1 In vascular diseases: its role in vascular inflammation and atherosclerosis. Autoimmun Rev. (2012) 11:909–17. doi: 10.1016/j.autrev.2012.03.007
27. Hu X, Jiang H, Bai Q, Zhou X, Xu C, Lu Z, et al. Increased serum HMGB1 is related to the severity of coronary artery stenosis. Clin Chim Acta. (2009) 406:139–42. doi: 10.1016/j.cca.2009.06.016
28. Kumar R, Batchelder A, Saratzis A, AbuRahma AF, Ringleb P, Lal BK, et al. Restenosis after carotid interventions and its relationship with recurrent ipsilateral stroke: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. (2017) 53:766–75. doi: 10.1016/j.ejvs.2017.02.016
29. Zhang B, Yao Y, Sun QF, Liu SQ, Jing B, Yuan C, et al. Circulating mircoRNA-21 as a predictor for vascular restenosis after interventional therapy in patients with lower extremity arterial occlusive disease. Biosci Rep. (2017) 37:BSR20160502. doi: 10.1042/bsr20160502
30. Yu ZH, Wang HT, Tu C. Diagnostic value of microRNA-143 in predicting in-stent restenosis for patients with lower extremity arterial occlusive disease. Eur J Med Res. (2017) 22:2. doi: 10.1186/s40001-016-0240-y
31. Pham Vu T, Nguyen Thi Thuy D, Truong Quy K, Nguyen Thi Thu H, Nguyen Van D, Diem Thi V, et al. Serum hs-CRP measured prior transplantation predicts of new-onset diabetes after transplantation in renal transplant recipients. Transpl Immunol. (2021) 66:101392. doi: 10.1016/j.trim.2021.101392
32. Aksu U, Gulcu O, Aksakal E, Kalkan K, Öztürk M, Korkmaz AF, et al. The association between CRP/albumin ratio and in-stent restenosis development in patients with ST-segment elevation myocardial infarction. J Clin Lab Anal. (2019) 33:e22848. doi: 10.1002/jcla.22848
33. Kaido T, Yoda M, Kamijo T, Taira C, Higuchi Y, Okumura N. Heterozygous variant fibrinogen γA289V (Kanazawa III) was confirmed as hypodysfibrinogenemia by plasma and recombinant fibrinogens. Int J Lab Hematol. (2020) 42:190–7. doi: 10.1111/ijlh.13152
34. Surma S, Banach M. Fibrinogen and atherosclerotic cardiovascular diseases-review of the literature and clinical studies. Int J Mol Sci. (2021) 23:193. doi: 10.3390/ijms23010193
35. Omeh DJ, Restenosis SE. Statpearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC (2022).
36. Sasso FC, Pafundi PC, Simeon V, De Nicola L, Chiodini P, Galiero R, et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: a randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc Diabetol. (2021) 20:145. doi: 10.1186/s12933-021-01343-1
Keywords: lower extremity arteriosclerosis obliterans, vascular interventional therapy, vascular restenosis, HMGB1, nomogram model
Citation: Yang B and XiaPing Z (2023) The clinical significance of serum HMGB1 in patients with lower extremity arteriosclerosis obliterans after interventional vascular restenosis. Front. Surg. 9:1031108. doi: 10.3389/fsurg.2022.1031108
Received: 29 August 2022; Accepted: 28 November 2022;
Published: 6 January 2023.
Edited by:
Matthias Mehdorn, Leipzig University, GermanyReviewed by:
George Galyfos, National and Kapodistrian University of Athens, Greece© 2023 Yang and XiaPing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang XiaPing emhhbmd4aWFwaW5nNDE3QGNzdS5lZHUuY24=
Specialty Section: This article was submitted to Vascular Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.