- 1School of Medicine, South China University of Technology, Guangzhou, China
- 2Department of Gastrointestinal Surgery, Department of General Surgery, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 3Department of General Surgery, Guangdong Provincial People's Hospital Ganzhou Hospital (Ganzhou Municipal Hospital), Ganzhou, China
- 4Department of Anorectal Surgery, Foresea Life Insurance Shaoguan Hospital, Shaoguan, China
- 5Second Department of General Surgery, The Sixth Affiliated Hospital, School of Medicine, South China University of Technology, Foshan, China
- 6The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
- 7The Fifth School of Clinical Medicine, Gannan Medical University, Ganzhou, China
Background: Neoadjuvant chemotherapy (NAC) could improve local tumor control of locally advanced colon cancer (LACC), but the prognostic value of yp stage in colon cancer remains unknown. Here, we aimed to ascertain yp stage as an indicator for LACC prognosis after NAC.
Methods: The data of patients diagnosed with colon adenocarcinoma between 2004 and 2015 were extracted from the Surveillance, Epidemiology, and End Results database. After 1:2 propensity score matching, cancer-specific survival (CSS) and overall survival (OS) were compared between the NAC and Non-NAC groups of different stage classifications. The correlation between clinical and pathological factors and CSS was identified.
Results: A total of 49, 149, and 81 matched pairs of stage 0–I, II, and III patients, respectively, were generated for analysis. For stage 0–I (p = 0.011) and III (p = 0.015), only CSS in the NAC groups were inferior. Receiving NAC was an independent prognostic risk factor for patients with stage 0–I (hazard ratio, 7.70; 95% confidence interval, 1.820–32.5; p = 0.006) and stage III (hazard ratio, 1.73; 95% confidence interval, 1.11–2.68; p = 0.015).
Conclusions: The CSS was poorer among LACC patients who underwent NAC than among those who did not. The yp stage of colon cancer after NAC has distinctive significance, which may contribute to predicting the prognosis and guiding the treatment of LACC patients after NAC.
Introduction
Colon cancer, among the most common malignant tumors worldwide, accounted for approximately two-thirds of new colorectal cancer cases and deaths in 2020 (1, 2). Locally advanced colon cancer (LACC) patients are still routinely treated with up-front surgery followed by adjuvant chemotherapy. Although neoadjuvant therapy (NAT) is not yet standard, it is proven to improve the tumor downstaging and margin-negative resection rates in colon cancer, resulting in local tumor control and even pathological complete remission (PCR) without excess complications (3–8). In 2016, the NCCN guidelines added neoadjuvant chemotherapy (NAC) as an optional treatment for the clinical T4b colon cancer cohort (9). In regards to pathological stage (p stage), an important factor that affects prognosis, several studies have reported that the final pathological stage after NAT (yp stage) is a significant predictive factor of survival outcomes among patients with rectal cancer who underwent chemoradiotherapy (10, 11). However, the prognostic value of yp stage in colon cancer remains unknown. Thus, we retrospectively analyzed cancer-specific survival (CSS) and overall survival (OS) of colon cancer patients treated with or without NAC in the Surveillance, Epidemiology, and End Results (SEER) database through propensity score matching (PSM) analysis, aiming for evaluating the effectiveness of yp stage as a prognostic indicator and adjuvant treatment guideline for colon cancer.
Material and methods
Patient selection
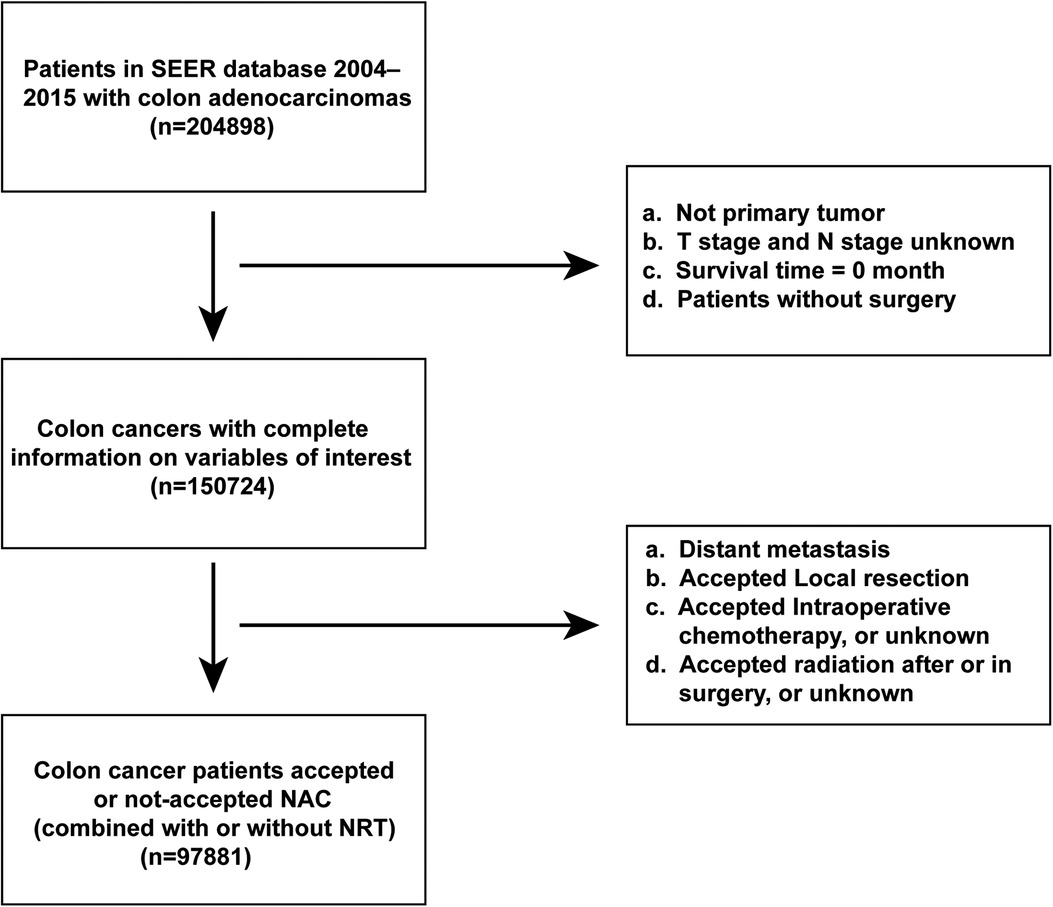
The data of patients pathologically diagnosed with primary colon adenocarcinoma between January 1, 2004 and December 31, 2015 were extracted from the SEER database (SEER*Stat Version 8.3.9). The study inclusion criteria were as follows: (1) histological type limited to colon adenocarcinoma (International Classification of Diseases for Oncology, 3rd edition codes for adenocarcinoma: 8140/3, 8143/3, 8144/3, 8210/3, 8261/3, 8263/3, 8220/3, and 8221/3; special type adenocarcinoma: 8141/3,8211/3, 8213/3, 8255/3, 8260/3, 8262/3, 8310/3, 8323/3, 8440/3, 8460/3, 8470/3, 8480/3, 8481/3; and signet ring cell carcinoma: 8490/3); (2) non-metastatic colon cancer; (3) radical intestinal resection; and (4) receipt or non-receipt of NAC (regardless of whether he/she uses preoperative radiotherapy or not). Surgery and systemic therapy sequences were limited to systemic therapy before surgery, systemic therapy both before and after surgery, surgery both before and after systemic therapy, systemic therapy after surgery, and no systemic therapy and/or surgical procedures. Surgery and radiation sequences were limited to radiation before surgery and no radiation and/or cancer-directed surgery; and (5) accurate prognostic information. The study exclusion criteria were as follows: (1) non-primary or multiple primary cancers; (2) rectosigmoid-Junction cancer; (3) unknown American Joint Committee on Cancer (AJCC) 7th pathological stage; (4) surgery including local tumor resection, none, or unknown; and (5) patients without NAC did not receive the standard treatment which the NCCN guidelines recommended to each pathological stage. (6) survival time of 0.
Variables collected
(1) Patient information: sex, age at diagnosis, year at diagnosis, race, and insurance; (2) Tumor information: primary site, tumor size (Ts), pathological grade, histological type, tumor-node-metastasis stage, number of lymph nodes (LNs) detected; (3) Treatment data: sequence of chemotherapy, whether preoperative radiotherapy was administered; (4) Follow-up data: CSS and OS. CSS was defined as the time interval between the diagnosis of colon cancer and death caused by colon cancer. OS was defined as the time interval between the diagnosis of colon cancer and death from any cause. All the included cases were re-staged by the AJCC 7th edition according to the data provided by the SEER database.
Patient classification
In this study, patients whose systemic therapy sequence was recorded as systemic therapy before surgery, systemic therapy both before and after surgery, and surgery both before and after systemic therapy were classified into the NAC group, whereas those whose systemic therapy sequence was recorded as systemic therapy after surgery or no systemic therapy and/or surgical procedures were classified into the Non-NAC group.
Statistical analysis
All data were sorted out and analyzed by R software (version 4.1.2). Continuous variables were compared using the unpaired t-test, while categorical variables were compared using the χ2 test. CSS curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Factors associated with CSS were estimated by uni- and multivariate analyses using the Cox proportional hazards model. Factors with p < 0.05 in univariate analysis were included in multivariate analysis.
A 1:2 PSM analysis without replacement was conducted via the nearest neighbor method with a caliper of 0.1 times the standard deviation of the propensity score (12). Matched variables included: age, sex, race, insurance, primary site, tumor size, pathological grade, histological type, AJCC stage, and number of LNs detected. Two-sided values of p < 0.05 were considered statistically significant.
Results
Baseline characteristics
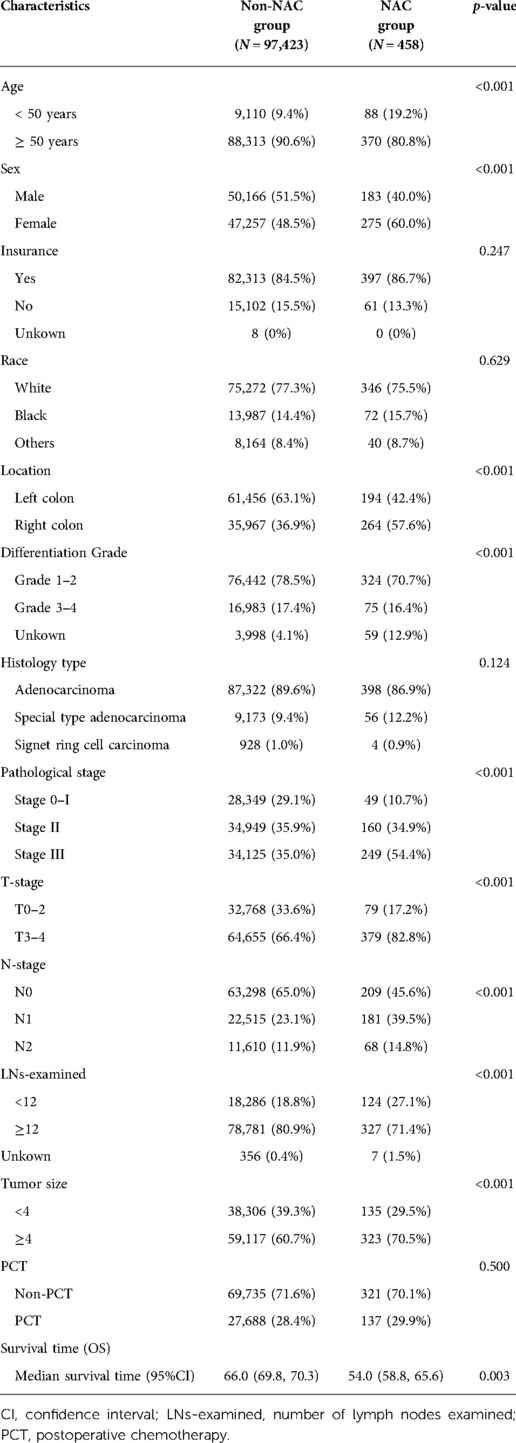
A total of 97,881 patients were included in this study (Figure 1). Of these, 458 patients underwent NAC, whereas 97,423 did not. Baseline demographic and clinicopathological characteristics were presented in Table 1. We found that the proportion of patients who received NAC for colon adenocarcinoma increased gradually from 3.635% in 2006 to 8.607% in 2015 (Figure 2). The median survival time was 66.0 [95% confidence interval (CI), 69.8–70.3] months in the Non-NAC group and 54.0 (95% CI, 58.8–65.6) months in the NAC group (p = 0.003; Table 1).
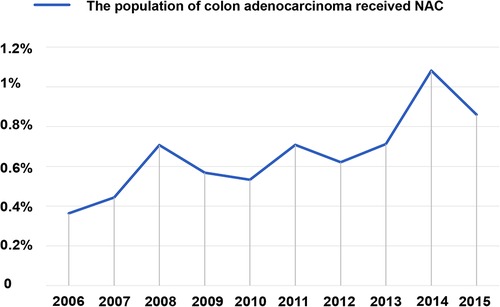
Figure 2. Rates of all patients with colon adenocarcinoma who received NAC were recorded in the SEER database from 2006 to 2015. The proportion received NAC in colon adenocarcinoma increased gradually from 3.635% in 2006 to 8.607% in 2015. NAC, neoadjuvant chemotherapy.
Propensity score matching
To minimize confounding factors, we respectively matched the Non-NAC and NAC groups in stage 0–I, II, and III cohorts to achieve a balanced distribution of these baseline covariates between the paired groups. As a result, yp stage 0–I (n = 49) and p stage 0–I (n = 98), 149 yp stage II (n = 149) and p stage II (n = 295), and 81 yp stage III (n = 81) and p stage III (n = 160) were matched (Supplementary Table S1).
For analysis of subgroups, we also matched the p stage 0–I group with the postoperative chemotherapy (PCT) subgroup (n = 10) and the non-PCT subgroup (n = 39) in the yp 0–I group separately. Moreover, the PCT subgroup (n = 4) and non-PCT subgroup (n = 6) of the yp stage 0–I group were also subjected to PSM matching. In stage II, non-PCT cohort in NAC group (n = 116) PSM matched with non-PCT cohort in Non-NAC group (n = 229), while PCT cohort in NAC group (n = 33) matched with PCT cohort in Non-NAC group (n = 66). The patient and tumor characteristics were well-balanced between the matched cohorts (p > 0.05).
CSS and OS stratified by preoperative therapy
Among stage 0–I patients, CSS was significantly poorer in the NAC group than in the Non-NAC group (p = 0.011) (Figure 3A). Interesting, the CSS of the matched NAC -PCT group (n = 10) and Non-NAC group (n = 20) was similar (p = 0.140) (Supplementary Figure S1A). However, the NAC -non-PCT group (n = 39) had significantly worse CSS than the Non-NAC group (n = 78) (p = 0.012) (Supplementary Figure S1B). Moreover, the 5-year CSS was 50% in the matched Non-NAC -PCT group (n = 4) vs. 33.3% in the matched Non-NAC -non-PCT group (n = 6), the difference of which was not statistically significant on the univariate log-rank test (p = 0.410) (Supplementary Figure S1C). Among stage II patients, there was no significant difference in CSS (p = 0.890) between patients who received NAC and those who did not (Figure 3B). Additionally, the stratified analysis showed that the CSS of the NAC -non-PCT group (n = 116) was similar to that of the Non-NAC -non-PCT group (n = 229) (p = 0.650) (Supplementary Figure S1D). Consistently, the CSS of patients who received PCT (including T4b) in the yp stage II group (n = 33) was similar to that of patients in the p stage II group (n = 66) (p = 0.660) (Supplementary Figures S1E,F). Moreover, among the stage III patients, the CSS was significantly worse for the NAC vs. Non-NAC group (p = 0.015) (Figure 3C). Nevertheless, there was no significant difference in OS among the stage 0–I (p = 0.870), stage II (p = 0.074), and stage III groups (p = 0.130) groups (Supplementary Figure 2).
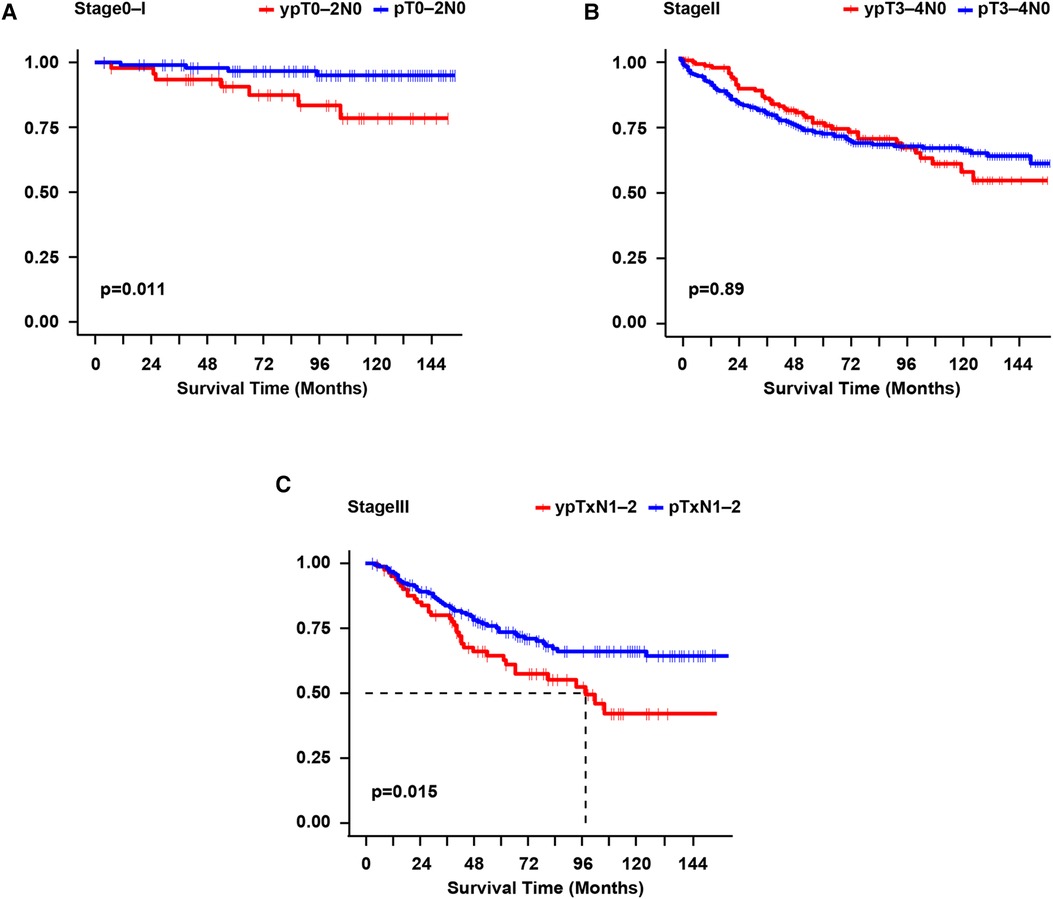
Figure 3. Survival curves were constructed per the Kaplan-Meier method for cause-specific survival for each pathological stage. Log-rank test for p-value. (A): yp stage 0-I vs. p stage 0-I; (B): yp stage II vs. p stage II; (C): yp stage III vs. p stage III. yp, the final pathological stage after neoadjuvant chemotherapy; p, pathological stage.
Prognostic factors for CSS of different stage classifications
As shown in Supplementary Table S2, uni- and multivariate Cox analyses demonstrated that, in the NAC group, not receiving PCT (hazard ratio [HR], 7.70; 95% confidence interval [CI], 1.820–32.500; p = 0.006) was an independent prognostic risk factor of CSS for patients. In the matched stage 0-I groups. In the matched stage II groups, age ≥ 50 years (HR, 2.436; 95% CI, 1.350–5.225; p = 0.011), T4a stage (HR, 3.065; 95% CI, 1.779–5.282; p < 0.001), T4b stage (HR,3.065; 95% CI, 2.308–5.110; p < 0.001) and poor histological differentiation (HR, 1.971; 95% CI, 1.244–3.123; p = 0.004) were independent risk factors, while ≥12 detected LNs (HR, 0.38; 95% CI, 0.326–0.652; p < 0.001) was an independent protective factor for CSS. In the matched stage III groups, only receiving NAC (HR, 1.657; 95% CI, 1.069–2.631; p = 0.024) was independently and significantly associated correlated with CSS.
Discussion
Due to the advantages of NAC of improving tumor downstaging, improving the R0 resection rate, and even prolonging disease-free survival time, its application in LACC is gradually increasing (3, 4, 6, 7, 13–15). Nonetheless, it remains unknown whether the final pathological stage (yp stage) of LACC patients who received NAC has a similar prognostic value to that of the usual postoperative pathological stage (p stage). To our knowledge, this is the first study to focus on the prognostic significance of yp stage in LACC patients after NAC. As the large difference in sample size between the NAC and Non-NAC groups, we balanced the clinical characteristics of the two groups using PSM for more reliable results. With the PSM and analysis of the survival time among stages 0–I, II, and III, respectively, we found that the CSS of LACC patients who underwent NAC was poorer than that of patients at the same pathological stage who did not, which indicated that yp stage of colon adenocarcinoma after NAC has significantly prognostic value and may provide evidence for PCT following curative surgery.
The present study found that young patients, those with right colon cancer, and those with poorly differentiated cancer were more likely to receive NAC. The shorter survival time in the NAC group may be due to worse histological grade, fewer detected LNs, later TN stage, and greater tumor bulk burden in the NAC group. As reported, p stage I colon cancer has good prognosis after radical resection alone. Compared with pT1-2N0 patients who did not receive chemoradiotherapy, ypT1-2N0 patients who received NAT preparation were more likely to relapse, and the recurrence rates of ypT0N0 and ypT1-2N0 were 2.7% and 12.3%, respectively (16). Moreover, several studies reported that, compared with p stage I patients, those with yp stage I disease had many risk factors leading to poor outcomes, such as poorer tissue differentiation, later T stage, and higher carcinoembryonic antigen levels (17, 18).
As for the stage 0–I patients in our study, the NAC group showed an inferior CSS to the Non-NAC group, which was also consistent with previous reports in NAT among rectal malignant tumors. Furthermore, the stratified analysis showed that the CSS was significantly worse in the yp stage 0–I non-PCT vs. p stage 0–I group, while the CSS of the yp stage 0–I with PCT group was similar to that of the p stage0–I group. Consistently, the 5-year CSS of the yp stage 0–I with PCT group was better than that of the yp stage 0–I non-PCT group, but the difference was not statistically significant. These results suggested that patients with yp stage 0–I colon cancer may benefit from PCT, which supports the findings of Collette et al. regarding rectal cancer in that LARC patients downgraded to ypT0-2 after preoperative radiotherapy can benefit from PCT (19).Other recent studies also demonstrated that patients with rectal cancer who reached PCR after NAT also benefited from PCT (20, 21). Patients who achieved descending stage after NAT also responded to adjuvant treatment (19). PCT for patients who respond to NAT may be beneficial by potentially eradicating residual micrometastatic disease (19, 22). Therefore, receiving adjuvant chemotherapy may contribute to the prognosis of these patients. A further randomized trial with a larger sample size is warranted to compensate for the limited sample size, especially in the PCT and non-PCT groups, of yp stage 0–I patients in our study.
As for stage II, CSS and OS were similar in the NAC and Non-NAC groups. This finding suggests that the risk of yp stage II patients may be classifiable according to the same criteria as and receive the same treatment regimen for p stage II. However, there was wide heterogeneity among stage II colon cancer patients, and the 5-year OS ranged from 58.4% (IIc) to 87.5% (IIa). Therefore, stage II patients require stratification to distinguish between high- and low-risk groups and guide the choice of treatment. However, the relevant information was not available in the SEER database.
Our study also found that the CSS of yp stage III patients was significantly poorer than that of p stage III patients, aligned with previous researches that patients with N+ after chemotherapy had a poor prognosis (23, 24). However, it is difficult to determine whether patients with a poor NAT response will benefit from PCT (25–27). Collette et al. reported that patients with a poor NAC response (ypN2) could not benefit from PCT and that the poor response may indicate resistance to treatment (19). Thus, we inferred that patients with yp stage III colon cancer may require adjustment to the adjuvant chemotherapy regimen. As another study reported that patients with p stage III disease had a better survival rate than those with yp stage III rectal cancer, the recurrence-free survival rate of yp stage III patients was intended to increase after treatment with second-line chemotherapy (28). Notably, the loss of DNA mismatch repair protein expression may occur in fluorouracil-based chemotherapy-insensitive colon cancer patients (29). Therefore, combination treatment with immunotherapy may be a promising research direction to improve the prognosis of patients with yp stage III colon cancer.
We also identified some independent risk factors for CSS. Poor tumor differentiation was an independent risk factor for prognosis in stage II patients. Poorly differentiated and undifferentiated tumors are more likely to metastasize distantly, the main cause of tumor death. Another independent risk factor found for CSS in this research was T4 for stage IIpatients. Regardless of LN status, colon cancer with advanced local invasion (T4) is more prone to local recurrence and distant metastasis, resulting in a low survival rate. Besides, according to the American Society of Clinical Oncology and the European Society of Medical Oncology, the number of LNs dissected (<12) is a risk factor for recurrence in patients with stage II colorectal cancer, consistent with our study findings (30, 31).
Another finding was that CSS was poorer for patients who underwent NAC than for those who did not undergo NAC, while the OS was similar between the two groups. Acknowledgedly, CSS is only related to tumor death, while OS is related to death caused by any reason. Although we adjusted variables that may lead to OS differences, including age, gender, diagnosis year, etc., we did not balance many other potential confounders between the NAC group and Non-NAC group, such as basic disease and economic status. Therefore, we speculated that there was no difference in OS because more patients in the Non-NAC group died of other causes, thus offsetting the difference in CSS between the two groups. This retrospective study had possible selective bias despite the PSM analysis and the fact that we selected patients treated with standard therapy whenever possible. Also, the SEER database lacks several important characteristics, such as chemotherapy or radiotherapy dosage, mismatch repair/micro-satellite instability status, perineural invasion, and lymphatic vascular invasion. Therefore, we cannot adjust for these potential confounding factors, especially in stage II patients, which made it impossible to distinguish the low- and high-risk groups for a further stratified analysis. Thus, large-scale prospective randomized studies are needed that explore the prognostic value of the yp stage in LACC.
Conclusions
The CSS was poorer for patients who underwent NAC than for those who did not undergo NAC in the same pathological stage, while the OS was similar between the two groups. Our results suggest that the final pathological stage of colon cancer after NAC has different clinical significance from the usual postoperative pathological stage and may be used to predict prognosis and guide treatment for LACC patients after NAC.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.
Ethics statement
Our institution does not require the ethical approval for reporting case which from the public database, such as SEER database.
Author contributions
XY and XF designed and supervised the study. MX and ZL extracted and analyzed the data. MX and YG wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Leading Innovation Specialist Support Program of Guangdong Province, the Science and Technology Planning Project of Ganzhou (No. 202101074816), National Key Clinical Specialty Construction Project (2021-2024, No. 2022YW030009), the Science and Technology Planning Project of Guangdong Provincial People's Hospital (Guangdong Academy of Medical Sciences, No. DFJH201913), CSCO-Roche Cancer Research Foundation (No. Y-2019Roche-190) and CSCO-Haosen Research Foundation (No. YHS2019/2 -050).
Acknowledgments
We would like to thank Yuqing Chen for her intellectual contributions and discussions on the study design. We would like to thank Guanrong Zhang, Ph.D. for his intellectual contributions to the statistics in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1022025/full#supplementary-material.
Abbreviations
AJCC, American Joint Committee on Cancer; CSS, cancer-specific survival; LACC, locally advanced colon cancer; LNs, number of lymph nodes; NAC, neoadjuvant chemotherapy; NAT, neoadjuvant therapy; NCCN, National Comprehensive Cancer Network; OS, overall survival; PCR, pathological complete remission; PCT, postoperative chemotherapy; PSM, propensity score matching; P stage, pathological stage; SEER, Surveillance, Epidemiology, and End Results database; Ts, tumor size; Yp stage, the final pathological stage after neoadjuvant therapy.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Lu L, Mullins CS, Schafmayer C, Zeissig S, Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun. (2021) 41(11):1137–51. doi: 10.1002/cac2.12220
3. Foxtrot Collaborative G. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. (2012) 13(11):1152–60. doi: 10.1016/S1470-2045(12)70348-0
4. Morton D. FOxTROT: an international randomised controlled trial in 1052 patients (pts) evaluating neoadjuvant chemotherapy (NAC) for colon cancer. Ann Oncol. (2019) 30(Supplement 5):198–252. doi: 10.1093/annonc/mdz246.001
5. Karoui M, Rullier A, Piessen G, Legoux JL, Barbier E, De Chaisemartin C, et al. Perioperative FOLFOX 4 versus FOLFOX 4 plus cetuximab versus immediate surgery for high-risk stage II and III colon cancers: a phase II multicenter randomized controlled trial (PRODIGE 22). Ann Surg. (2020) 271(4):637–45. doi: 10.1097/SLA.0000000000003454
6. Karoui M, Gallois C, Piessen G, Legoux JL, Barbier E, De Chaisemartin C, et al. Does neoadjuvant FOLFOX chemotherapy improve the prognosis of high-risk Stage II and III colon cancers? Three years’ follow-up results of the PRODIGE 22 phase II randomized multicentre trial. Colorectal Dis. (2021) 23(6):1357–69. doi: 10.1111/codi.15585
7. Karoui M, Rullier A, Luciani A, Bonnetain F, Auriault ML, Sarran A, et al. Neoadjuvant FOLFOX 4 versus FOLFOX 4 with Cetuximab versus immediate surgery for high-risk stage II and III colon cancers: a multicentre randomised controlled phase II trial–the PRODIGE 22–ECKINOXE trial. BMC Cancer. (2015) 15:511. doi: 10.1186/s12885-015-1507-3
8. Liu F, Yang L, Wu Y, Li C, Zhao J, Keranmu A, et al. CapOX as neoadjuvant chemotherapy for locally advanced operable colon cancer patients: a prospective single-arm phase II trial. Chin J Cancer Res. (2016) 28(6):589–97. doi: 10.21147/j.issn.1000-9604.2016.06.05
9. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2021) 19(3):329–59. doi: 10.6004/jnccn.2021.0012
10. Paulson EC, Wirtalla C, Armstrong K, Mahmoud NN. Gender influences treatment and survival in colorectal cancer surgery. Dis Colon Rectum. (2009) 52(12):1982–91. doi: 10.1007/DCR.0b013e3181beb42a
11. Drew EM, Schoenberg NE. Deconstructing fatalism: ethnographic perspectives on women's Decision making about cancer prevention and treatment. Med Anthropol Q. (2011) 25(2):164–82. doi: 10.1111/j.1548-1387.2010.01136.x
12. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. (1983) 70:41–55. doi: 10.1093/biomet/70.1.41
13. Body A, Prenen H, Latham S, Lam M, Tipping-Smith S, Raghunath A, et al. The role of neoadjuvant chemotherapy in locally advanced colon cancer. Cancer Manag Res. (2021) 13:2567–79. doi: 10.2147/CMAR.S262870
14. Gosavi R, Chia C, Michael M, Heriot AG, Warrier SK, Kong JC. Neoadjuvant chemotherapy in locally advanced colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. (2021) 36(10):2063–70. doi: 10.1007/s00384-021-03945-3
15. Jung F, Lee M, Doshi S, Zhao G, Cheung KLT, Chesney T, et al. Neoadjuvant therapy versus direct to surgery for T4 colon cancer: meta-analysis. Br J Surg. (2021) 109(1):30–6. doi: 10.1093/bjs/znab382
16. Govindarajan A, Reidy D, Weiser MR, Paty PB, Temple LK, Guillem JG, et al. Recurrence rates and prognostic factors in ypN0 rectal cancer after neoadjuvant chemoradiation and total mesorectal excision. Ann Surg Oncol. (2011) 18(13):3666–72. doi: 10.1245/s10434-011-1788-y
17. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American pathologists consensus statement 1999. Arch Pathol Lab Med. (2000) 124(7):979–94. doi: 10.5858/2000-124-0979-PFICC
18. Nissan A, Stojadinovic A, Shia J, Hoos A, Guillem JG, Klimstra D, et al. Predictors of recurrence in patients with T2 and early T3, N0 adenocarcinoma of the rectum treated by surgery alone. J Clin Oncol. (2006) 24(25):4078–84. doi: 10.1200/JCO.2006.06.2968
19. Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European organisation for research and treatment of cancer radiation oncology group. J Clin Oncol. (2007) 25(28):4379–86. doi: 10.1200/JCO.2007.11.9685
20. Dossa F, Acuna SA, Rickles AS, Berho M, Wexner SD, Quereshy FA, et al. Association between adjuvant chemotherapy and overall survival in patients with rectal cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA Oncol. (2018) 4(7):930–7. doi: 10.1001/jamaoncol.2017.5597
21. Turner MC, Keenan JE, Rushing CN, Gulack BC, Nussbaum DP, Benrashid E, et al. Adjuvant chemotherapy improves survival following resection of locally advanced rectal cancer with pathologic complete response. J Gastrointest Surg. (2019) 23(8):1614–22. doi: 10.1007/s11605-018-04079-8
22. Petrelli F, Coinu A, Lonati V, Barni S. A systematic review and meta-analysis of adjuvant chemotherapy after neoadjuvant treatment and surgery for rectal cancer. Int J Colorectal Dis. (2015) 30(4):447–57. doi: 10.1007/s00384-014-2082-9
23. Bujko K, Michalski W, Kepka L, Nowacki MP, Nasierowska-Guttmejer A, Tokar P, et al. Association between pathologic response in metastatic lymph nodes after preoperative chemoradiotherapy and risk of distant metastases in rectal cancer: an analysis of outcomes in a randomized trial. Int J Radiat Oncol Biol Phys. (2007) 67(2):369–77. doi: 10.1016/j.ijrobp.2006.08.065
24. Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Saltz LB, et al. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer. (2008) 113(1):57–64. doi: 10.1002/cncr.23516
25. Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. (2015) 16(2):200–7. doi: 10.1016/S1470-2045(14)71199-4
26. Breugom AJ, van Gijn W, Muller EW, Berglund Ã, van den Broek CBM, Fokstuen T, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. (2015) 26(4):696–701. doi: 10.1093/annonc/mdu560
27. Bujko K, Glimelius B, Valentini V, Michalski W, Spalek M. Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo)therapy: a meta-analysis of randomized trials comparing surgery Âą a fluoropyrimidine and surgery+a fluoropyrimidine Âą oxaliplatin. Eur J Surg Oncol. (2015) 41(6):713–23. doi: 10.1016/j.ejso.2015.03.233
28. Hwang K, Park IJ, Yu CS, Lim SB, Lee JL, Yoon YS, et al. Impression of prognosis regarding pathologic stage after preoperative chemoradiotherapy in rectal cancer. World J Gastroenterol. (2015) 21(2):563–70. doi: 10.3748/wjg.v21.i2.563
29. Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. (2010) 28(20):3219–26. doi: 10.1200/JCO.2009.27.1825
30. Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. (2012) 23(10):2479–516. doi: 10.1093/annonc/mds236
Keywords: prognostic value, neoadjuvant chemotherapy, colon cancer, yp stage, survival
Citation: Xiang M, Liang Z, Gao Y, Feng X and Yao X (2022) Prognostic value of final pathological stage in colon adenocarcinoma after neoadjuvant chemotherapy: A propensity score-matched study. Front. Surg. 9:1022025. doi: 10.3389/fsurg.2022.1022025
Received: 18 August 2022; Accepted: 3 October 2022;
Published: 26 October 2022.
Edited by:
Luca Saadeh, University Hospital of Padua, ItalyReviewed by:
Wei Wang, Sun Yat-sen University, ChinaYe Zai Sheng, Fujian Provincial Cancer Hospital, China
© 2022 Xiang, Liang, Gao, Feng and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingyu Feng ZmVuZ3hpbmd5dUBnZHBoLm9yZy5jbg== Xueqing Yao c3l5YW94dWVxaW5nQHNjdXQuZWR1LmNu
†These authors contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Meijuan Xiang
Meijuan Xiang Zongyu Liang
Zongyu Liang Yuan Gao
Yuan Gao Xingyu Feng
Xingyu Feng Xueqing Yao
Xueqing Yao