- Department of Neurosurgery, Neurological Institute, Taichung Veterans General Hospital, Taichung, Taiwan
Introduction: Spindle cell oncocytoma (SCO) of the pituitary gland is increasingly established with improvements in histological and immunohistochemical examination. However, the diagnosis was often mistaken based on imaging studies and nonspecific clinical manifestations.
Purpose: This case is presented to provide an overview of the characteristics of the rare tumor as well as to demonstrate the difficulties in diagnosis and current treatments.
Clinical discussion: The pathogenesis of SCO remains unclear, and a possible origin was described. Further research is needed to optimize pre-operative diagnosis and surgical strategy.
Conclusion: SCO should be considered when images indicate some features. Gross total resection (GTR) after surgery seems to have better long-term tumor control, and radiotherapy may help decrease tumor progression in patients with non-GTR. Regular follow-up is advised because of the higher recurrence rate.
Introduction
Spindle cell oncocytoma (SCO) of the adenohypophysis is a rare neoplasm of the pituitary gland first described by Roncaroli et al. (1), and has been included in the World Health Organization (WHO)'s classification of the central nervous system since 2007.
Clinically and radiologically, these neoplasms are often indistinguishable from nonfunctioning pituitary adenomas. It is a challenge for surgeons to evaluate and manage. We presented herein a case of SCO and reviewed the literature, focusing on tumor origin, radiological features, and treatment choice.
Case presentation
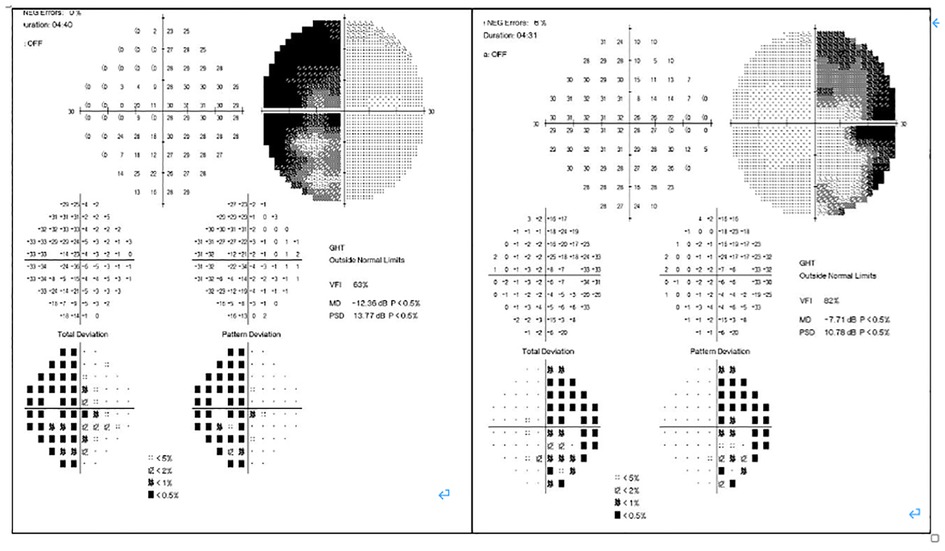
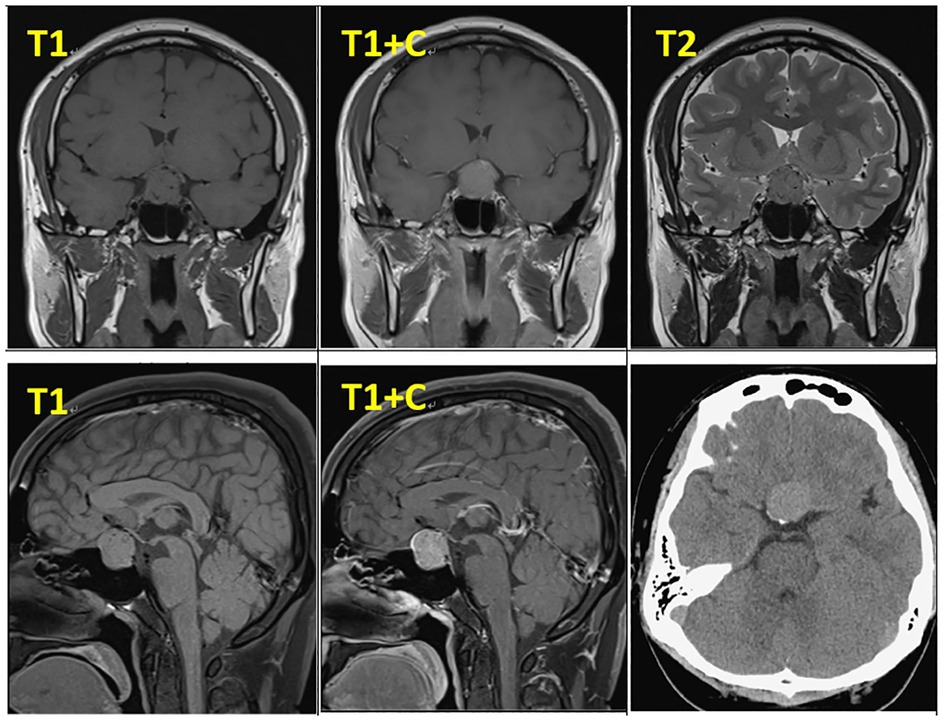
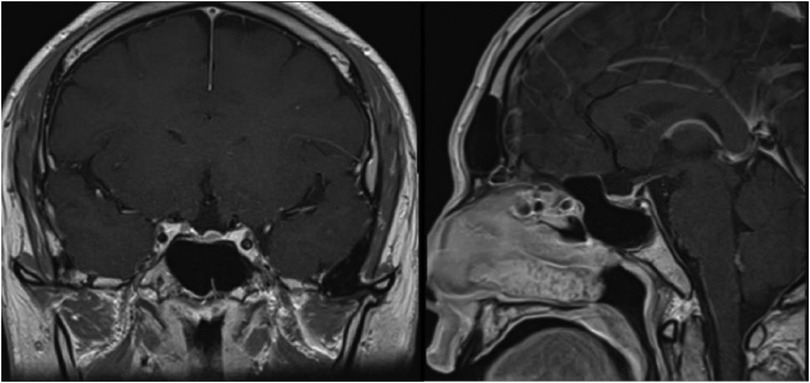
In February 2019, a 31-year-old man without medical history came to our hospital with the chief complaint of progressive bilateral vision loss for 6 months. In the outpatient department, his visual field test (Figure 1) showed a temporal defect in both eyes. Brain computed tomography (Figure 2) showed increased soft tissue density over the sellar and suprasellar regions. Brain magnetic resonance imaging (Figure 2) was further arranged, which showed a mass with enhancement measuring about 2.3 cm × 2.3 cm × 2.0 cm in size located at the sella with suprasellar extension. Under the impression of a pituitary tumor with optic chiasm compression, he was admitted for further evaluation and management. On admission, his neurological examination revealed essentially negative findings, except for loss of visual field; his hormone studies were within normal limits. Under general anesthesia, he underwent an operation using the endoscopic endonasal transsphenoidal approach for the removal of the tumor. Grossly, the tumor was yellow and soft in consistency (Figure 3). Easy bleeding of the tumor was noted and could be controlled under assistance of hemostatic agent. After surgery, his vision was subjectively improved. Histological examination (Figure 4) showed tumor growth exhibiting spindle to epithelioid cells, featuring eosinophilic and granular cytoplasm. Immunohistochemically, the neoplastic cells showed TTF-1 (+), EMA (+), and S-100 (+). Based on the results of the histopathology findings and immunohistochemical stains, SCO was diagnosed. He was discharged in good condition and has been receiving regular follow-up at our neurosurgery department (Figure 5).

Figure 3. Intraoperative picture. P, normal pituitary gland after tumor removal. T, the gross appearance of the tumor.
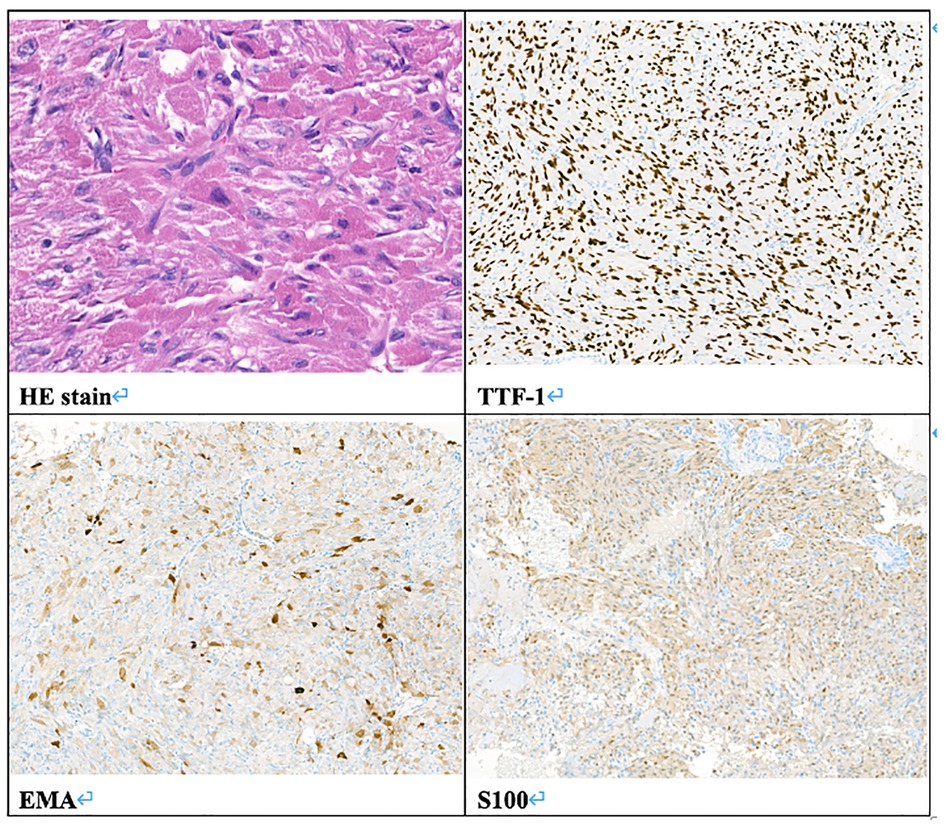
Figure 4. Pathologic report. Histology of the tumor. HE stain, original magnification 400×. Immunohistochemistry stain, TTF-1 (+), EMA (+), S100 (+), original magnification 100×.
Discussion
SCO is a rare tumor of the adenohypophysis and has been characterized as spindle to epithelioid cells with an oncocytic appearance (1). SCO has similar clinical and radiographic features to nonfunctioning pituitary adenoma and is a challenge to diagnose pre-operatively. To date, fewer than 100 cases of pituitary SCO have been reported in the literature.
Its pathogenesis and natural history have not yet been fully clarified. Nevertheless, there are distinct features of SCO that are becoming increasingly apparent in previously documented case reports.
SCO can be characterized by its ultrastructural/histologic findings. There are typically elongated or polygonal spindle cells with numerous mitochondria with lamellar cristae, small numbers of lysosomes, and rough endoplasmic reticulum (1, 2). These neoplastic cells are linked by intermediate junctions and desmosomes (1). Mitosis is rare, and necrosis is not seen (1).
SCO has no immunoreactivity for neuroendocrine markers and hormones, but shows simultaneous positivity to vimentin, galectin-3, S-100 protein, TTF-1, antimitochondrial antibody MU213-UC clone 131, and frequently EMA. These tumor cells do not express GFAP, cytokeratins CAM2.5, CD34, CD68, synaptophysin, chromogranin, BCL-2, or smooth muscle actin (1, 3). Similar expression of vimentin, S-100 protein, EMA, and galectin-3 together with the presence of desmosomes and intermediate junctions suggests their possible derivation from folliculostellate cells of the adenohypophysis, which are capable of divergent differentiation as a type of stem cell (4, 5).
Recently, reports have described diffuse thyroid transcription factor-1 (TTF-1) expression, a specific marker in neurohypophysis (posterior pituitary gland), in SCO, pituicytoma, and granular cell tumors, indicating a pituicyte lineage (6). A new subtype, primary papillary epithelial tumor of the sella (PPETS), has been defined as a tumor entity with papillary architecture and TTF-1 expression (7).
Evidence suggests circumventricular organs, including the neurohypophysis, organum vasculosum of the lamina terminalis, and median eminence, exhibit stem cell properties, which may differentiate toward PPETS (2). However, folliculostellate cells are negative for TTF-1 (2). The pathogenesis of SCO remains a subject of debate.
Given its oncocytic features, the differential diagnosis for SCO may include pituitary neoplasms capable of oncocytic change (especially gonadotrophinomas, null cell adenomas, and rarely silent corticotroph adenomas) (8), metastases from extrahypophyseal oncocytic tumors (1), paragangliomas, meningiomas with oncocytic change, sellar schwannomas (3), and other posterior pituitary tumors.
Pre-operative symptoms are not diagnostic, and the most common symptoms are the results of the mass effect on parasellar structures. The most clinical complaint is visual impairment (9), as in our case.
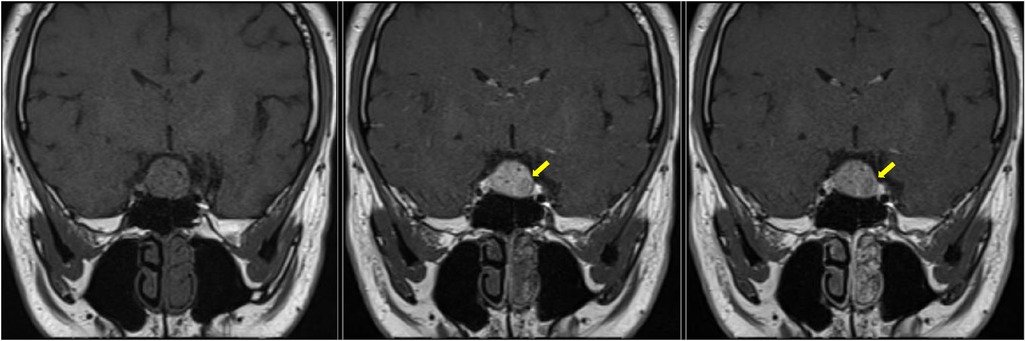
Radiologically, there are generally no definitive signs for the diagnosis of SCO, but some radiological clues can be found. SCOs are usually isointense on T1-weighted imaging with flow voids (10). They tend to have a missing posterior pituitary spot, as the posterior pituitary gland cannot be seen separately from the tumor (11). Borges et al. detailed the features of multifocal intratumoral bleeding with the appearance of a blooming artifact (heterogeneous) in the tumor on MRI (12). Hasiloglu's sign described intense contrast enhancement in the early stage of dynamic contrast-enhanced MRI (DCE-MRI) with some hypointense millimetric foci, which is consistent with hypervascularity and non-enhancing hemosiderin deposits (2). As in our case, the tumor exhibits an early wash-in phenomenon with a subsequent plateau (Figure 6), which unlike most pituitary microadenoma (13). Cavernous sinus invasion, sellar floor destruction, and calcification have rarely been reported (9) (Table 1).
Although SCOs are considered benign tumors based on the absence of mitosis and necrosis (2), one case of malignant change with an increasing ki-67 index was reported (14). A review of the literature found SCOs have a significant risk of progression or higher rate of recurrence than other pituitary adenomas, which is associated with greater tumor size at baseline, duration of follow-up, cavernous invasion, and incomplete resection (8). Patients with subtotal resection were associated with a significantly higher risk for recurrence as compared with the gross total resection (15).
The generally accepted treatment of choice is surgical total resection, via the transcranial or transsphenoidal approach. Pre-operative embolization followed by combination with the endonasal approach and the pterional approach was presented in one case of SCO in preparation for bleeding (16). In our case, the extended transsphenoidal approach could provide better exposure compared with the standard approach in dealing with hemostasis. Total resection may be difficult to perform if any of the following occurs: intraoperative bleeding, dura adhesion, unclear margin, firm and elastic tissue texture, invasion of adjacent structures. Other managements involving radiotherapy (external beam radiation, proton beam radiation, CyberKnife, and Gamma Knife radiotherapy) tend to be promising solutions for control of tumor progression (17). The effectiveness and timing of adjuvant radiotherapy have not been well established, though it has been successfully used to achieve remission after mean follow-up of 52 months (18). However, tumor recurrence after radiotherapy was also reported and the sensitivity of SCO to radiotherapy was uncertain (9). Therefore, no clear recommendation is suggested and radiation-related complications should be taken into consideration.
For patients with a radiographically confirmed complete resection, long-term surveillance by annual MRI examination for 5 years and biannually thereafter was preferred (8). Although there is no established therapeutic protocol, long-term follow-up is essential.
Conclusion
SCO is often initially misdiagnosed as a nonfunctional pituitary adenoma due to its rarity. Comprehensive evaluation, including histopathological, immunohistochemical, radiological features, and intraoperative findings are necessary. Regular follow-up is advised as SCO is associated with a higher recurrence rate. Further research is needed to clarify the pathogenesis and risk factors of SCO.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
CNC: Drafting, organization. Both authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roncaroli F, Scheithauer BW, Cenacchi G, Horvath E, Kovacs K, Lloyd RV, et al. “Spindle cell oncocytoma” of the adenohypophysis: a tumor of folliculostellate cells? Am J Surg Pathol. (2002) 26(8):1048–55. doi: 10.1097/00000478-200208000-00010
2. Hasiloglu ZI, Ure E, Comunoglu N, Tanriover N, Oz B, Gazioglu N, et al. New radiological clues in the diagnosis of spindle cell oncocytoma of the adenohypophysis. Clin Radiol. (2016) 71(9):937.e935–937.e911. doi: 10.1016/j.crad.2016.04.022
3. Romero-Rojas AE, Melo-Uribe MA, Barajas-Solano PA, Chinchilla-Olaya SI, Escobar LI, Hernandez-Walteros DM. Spindle cell oncocytoma of the adenohypophysis. Brain Tumor Pathol. (2011) 28(4):359–64. doi: 10.1007/s10014-011-0051-3
4. Yip CM, Lee HP, Hsieh PP. Pituitary spindle cell oncocytoma presented as pituitary apoplexy. J Surg Case Rep. (2019) 6:rjz179. doi: 10.1093/jscr/rjz179
5. Matyja E, Maksymowicz M, Grajkowska W, Olszewski W, Zieliński G, Bonicki W. Spindle cell oncocytoma of the adenohypophysis—a clinicopathological and ultrastructural study of two cases. Folia Neuropathol. (2010) 48(3):175–84. PMID: 2092500120925001
6. Gupta RK, Batra VV, Singh D, Sharma MC, Kumar V. Is spindle cell oncocytoma a true entity or a variant of pituicytoma? A case report with review of literature. Neurol India. (2018) 66(5):1413–18. doi: 10.4103/0028-3886.241353
7. Roncaroli F, Chatterjee D, Giannini C, Pereira M, La Rosa S, Brouland JP, et al. Primary papillary epithelial tumour of the sella: expanding the spectrum of TTF-1-positive sellar lesions. Neuropathol Appl Neurobiol. (2020) 46(5):493–505. doi: 10.1111/nan.12622
8. Das L, Vaiphei K, Rai A, Ahuja CK, Singh P, Mohapatra I, et al. Posterior pituitary tumours: patient outcomes and determinants of disease recurrence or persistence. Endocr Connect. (2021) 10(4):387–400. doi: 10.1530/EC-20-0621
9. Samadian M, Mousavinejad SA, Khoshsirat S, Dehghan M, Sharifi G, Ebrahimzadeh K, et al. Trans-nasal trans-sphenoidal endoscopic resection of spindle cell oncocytoma of adenohypophysis: the first case report in a child and a review of literature. Asian J Neurosurg. (2020) 15(1):210–3. doi: 10.4103/ajns.AJNS_166_19
10. Hasegawa H, Van Gompel JJ, Oushy SH, Pollock BE, Link MJ, Meyer FB, et al. A comprehensive study of spindle cell oncocytoma of the pituitary gland: series of 6 cases and meta-analysis of 85 cases. World Neurosurg. (2021) 149:e197–e216. doi: 10.1016/j.wneu.2021.02.051
11. Borg A, Jaunmuktane Z, Dorward N. Tumors of the neurohypophysis: one unit's experience and literature review. World Neurosurg. (2020) 134:e968–78. doi: 10.1016/j.wneu.2019.11.043
12. Borges MT, Lillehei KO, Kleinschmidt-DeMasters BK. Spindle cell oncocytoma with late recurrence and unique neuroimaging characteristics due to recurrent subclinical intratumoral bleeding. J Neurooncol. (2011) 101(1):145–54. doi: 10.1007/s11060-010-0229-2
13. Chaudhary V, Bano S. Imaging of the pituitary: recent advances. Indian J Endocrinol Metab. (2011) 15(Suppl. 3):216–18. doi: 10.4103/2230-8210.84871
14. Kong X, Li D, Kong Y, Zhong D. Malignant adenohypophysis spindle cell oncocytoma with repeating recurrences and a high Ki-67 index. Medicine. (2017) 96(4):e5657. doi: 10.1097/MD.0000000000005657
15. Vuong HG, Nguyen TPX, Pham N, Dunn IF. Risk factors for tumor recurrence and progression of spindle cell oncocytoma of the pituitary gland: a systematic review and pooled analysis. Pituitary. (2021) 24:429–37. doi: 10.1007/s11102-020-01110-7
16. Yoshida K, Toda M, Akiyama T, Takahashi S, Nishimoto M, Ozawa H, et al. Combined endoscopic endonasal and video-microscopic transcranial approach with preoperative embolization for a posterior pituitary tumor. World Neurosurg. (2018) 119:201–8. doi: 10.1016/j.wneu.2018.07.245
17. Oushy S, Graffeo CS, Perry A, Stafford SL, Link MJ, Pollock BE. Single-fraction stereotactic radiosurgery for spindle cell oncocytoma: preliminary experience and systematic review of the literature. J Neurooncol. (2019) 144(2):325–32. doi: 10.1007/s11060-019-03231-x
Keywords: pituitary tumor, spindle cell, benigh tumors, TTF-1 gene, surgical resection
Citation: Chang CN and Shen CC (2023) Spindle cell oncocytoma of the pituitary tumor: A rare case report and literature reviews. Front. Surg. 9:1021680. doi: 10.3389/fsurg.2022.1021680
Received: 17 August 2022; Accepted: 28 December 2022;
Published: 30 January 2023.
Edited by:
Mario Ganau, Oxford University Hospitals NHS Trust, United KingdomReviewed by:
Salvatore Chibbaro, Strasbourg University Hospital, FranceGoktug Akyoldas, Koç University, Turkey
© 2023 Chang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chia Ning Chang bmluZzE5OTAwMUBnbWFpbC5jb20=
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Chia Ning Chang
Chia Ning Chang Chiung Chyi Shen
Chiung Chyi Shen