
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Surg., 20 October 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1019570
 Cheng Lv1,†
Cheng Lv1,† Bin Zhou2,†
Bin Zhou2,† Donghua Zhang3,†
Donghua Zhang3,† Jiajia Lin1
Jiajia Lin1 Lingling Sun1
Lingling Sun1 Zhenzhen Zhang2
Zhenzhen Zhang2 Yuan Ding1
Yuan Ding1 Rong Sun1
Rong Sun1 Jie Zhang1
Jie Zhang1 Chuyao Zhou1
Chuyao Zhou1 Li Zhang4
Li Zhang4 Xuan Wang3
Xuan Wang3 Lu Ke1*
Lu Ke1* Weiqin Li1*
Weiqin Li1* Baiqiang Li1*
Baiqiang Li1*
Background: The ideal crystalloid fluid of choice for fluid therapy during liver transplantation is unknown. Conventional balanced crystalloids are buffered with organic anions, which requires liver metabolism to prevent matabolic acidosis and protect renal function. Therefore they can not function properly during liver transplantation. On the contrary, the bicarbonated Ringer's solution (BRS) can maintain acid-base status regardless of liver function. In this study, we aimed to test the hypothesis that, in patients undergoing orthotopic liver transplantation, compared with acetated Ringer's solutions (ARS), perioperative fluid therapy with BRS could better maintain the acid-base status.
Methods: This is a prospective, single-centre, randomised controlled trial. 72 eligible patients will be randomised to receive either BRS or ARS perioperatively. The primary endpoint is the difference in standard base excess (SBE) before and after operation. Secondary endpoints include the incidence of acute kidney injury (AKI) within 48 h post operation and free and alive days to day 14 for intensive care admission, invasive ventilation, vasopressors, and renal replacement therapy (RRT).
Discussion: Metabolic acidosis is common perioperatively, potentially leading to decreased renal blood flow and reduced glomerular filtration rate. The use of balanced solutions can prevent hyperchloremic metabolic acidosis, thereby avoiding AKI in some patients. However, during liver transplantation, when well-functioning liver metabolism is lacking, the organic anions in conventional balanced solutions may remain strong anions and thus fail to maintain the acid-base status, but no solid clinical evidence exists now. This study will, for the first time, provide evidence on the relative effects of BRS vs. ARS on acid-base status and renal injury in patients undergoing liver transplantation.
Clinical Trial Registration: The trial has been registered at the Chinese Clinical Trials Registry (ChiCTR2100046889) on 29 May 2021.
Acute kidney injury (AKI) is a common complication following liver transplantation, occurring in 29%–60% of pateints (1–4). Once it develops, regardless of the severity of AKI, it is significantly associated with increased mortality (5). A long-term cohort study conducted by the National Institute of Diabetes, Digestive and Kidney Diseases showed that, compared with patients with normal renal function, those with AKI had a hazard ratio of 1-year mortality up to 3.59 (6). The development of AKI is due to multiple factors (7–14), one of which is intraoperative and postoperative infusion of supraphysiologic chlorinated liquids (15).
During liver transplantation, fluid therapy, including crystalloids and colloids, is considered the cornerstone of perioperative management to maintain normal blood volume and preserve renal perfusion (15). The RELIEF study shows that patients undergoing liver transplantation and other major abdominal surgeries had an average fluid infusion of 3,500 ml intraoperatively and 6,146 ml within 24 h after the operation, respectively, and most of the intravenous fluids are crystalloid (16, 17).
However, the ideal crystalloid during liver transplantation remains uncertain (18). By now, normal saline (NS, 0.9% sodium chloride) is most frequently used (19, 20). Nevertheless, the chloride concentration of NS (154 mmol/L) is significantly higher than that of plasma (94–111 mmol/L), which may lower plasma strong ion difference (SID), leading to metabolic acidosis and impaired renal perfusion (21, 22). A possible alternative is balanced solution (chloride limited crystalloid) (23), which, as shown in the SMART trial, could reduce adverse kidney events in critically ill patients when compared with NS (24).
In practice, the most commonly used balanced crystalloids are buffered with organic anions, such as lactate, acetate, or malate, all of which require liver metabolism to increase SID (25). During liver transplantation, liver metabolism is suspended during the anhepatic phase and severely compromised during the early neohepatic phase (26), thus organic anions buffered solution can not function properly, thereby inducing metabolic acidosis.
On the contrary, bicarbonated Ringer's solution (BRS), introduced 11 years ago, directly replaces chloride with bicarbonate. It can increase SID regardless of liver metabolism. Previous pharmacokinetic studies showed that BRS could correct metabolic acidosis faster than lactated or acetated Ringer's solution (ARS) in shock models (26). Taken together, compared with conventional organic anion buffered balanced solutions, BRS may better maintain acid-base status during liver transplantation, but no evidence exists in the literature.
In this study, we aim to compare the effect of BRS and ARS on acid-base status and renal function in patients undergoing liver transplantation. The results of this study could also provide data to power a confirmatory Phase III study in the future.
The primary objective of this trial is to determine whether, compared to ARS, BRS can better maintain acid-based status as reflected by the change in standard base excess (SBE) levels in patients undergoing orthotopic liver transplantation.
Secondary objectives are to assess the impact of BRS on renal function and other clinical outcomes like the number of days alive and free of intensive care admission, invasive ventilation, vasopressors, and renal replacement therapy (RRT) to day 14 after operation. Laboratory results like bicarbonate, creatinine and neutrophil gelatinase-associated lipocalin (NGAL) levels will also be compared.
The present study is an investigator-initiated, single-centre, superiority, randomised controlled trial. This trial was registered at the Chinese Clinical Trials Registry (ChiCTR2100046889).
A Trial Management Committee (TMC) was formed, comprising the principal investigator and all the other co-investigators (clinical and non-clinical). The TMC is responsible for the day-to-day running of the trial. A writing and publication committee was organised for drafting the manuscript and submission of the manuscript to proper academic journals. It will also decide on the authorship of this study. An independent data safety and monitoring board (DSMB) consisting of a surgeon, an anesthesiologist, and a statistician will be organised to oversee all subjects' safety. The DSMB will review the safety report regularly and have the right to stop the trial early because of concerns about participant safety. DSMB members will not be involved in the study conduct, and all the processes will be independent of the investigators.
All adult patients undergoing orthotopic liver transplantation surgery admitted to the Jinling Hospital, Nanjing University, will be assessed for eligibility after admission. The inclusion and exclusion criteria are as follows:
1. Recipients undergoing orthotopic liver transplantation surgery;
2. Age ≥18 years.
3. Written informed consent.
1. Combined transplantation;
2. Patients receiving RRT within 1 week before operation;
3. Patients with increased serum creatinine (SCr) levels within 1 week before operation (Defined as SCr increased by more than 1.5 times or 26.5 μmol/L (0.3 mg/dL) from baseline. Baseline was defined as SCr levels obtained within 6 months, if not available, the upper limit of normal was set for baseline at 90 μmol/L for females/110 μmol/L for males);
A patient will be considered eligible if he/she meets the inclusion criteria and does not meet any of the exclusion criteria.
The randomisation sequence will be generated by an independent statistician using SAS Version 9.4 with a fixed block size (block size = 4). Randomisation will be stratified by the surgical methods adopted (classical orthotopic liver transplantation vs. piggyback orthotopic liver transplantation). Allocation will be in a 1:1 ratio.
Due to the specific double-packaging of bicarbonated Ringer's solution for stabilising the concentration of bicarbonate radical, blinding to investigators will not be applicable. However, research personnel, ICU staff and other caregivers will not have access to the randomisation schedule. Allocation concealment will be maintained by using a secure, password-protected study website to randomise consenting patients.
The only difference between the two study groups exists in the choice of perioperative intravenous isotonic crystalloid: bicarbonated Ringer's solution (BRS group) vs. acetated Ringer's solution (ARS group). The compositions of each crystalloid solution are displayed in Supplementary Table S1.
• Group 1: BRS group
Patients will receive bicarbonate Ringer's solution for fluid therapy during and within 48 h post operation.
• Group 2: ARS group
Patients will receive acetate Ringer's solution for fluid therapy during and within 48 h post operation.
First, both groups received general standard of care involving the following key components (27). The operating room temperature will be set at 22°C and standard warming measures be used for all patients. All patients will receive standard general anaesthesia induced by midazolam, sufentanil, etomidate, cisatrcurium and maintained by propofol, remifentanil, sevoflurane and additional injection of sufentanil. An arterial line and a central venous catheter will be inserted before induction of anaesthesia. Mechanical ventilation will be performed routinely. During the perioperative period, all patients will receive intravenous fluids according to a standardised protocol, which includes basiliximab, antibiotics, esomeprazole and methylprednisolone. Transfusions will be administered at the discretion of the attending anaesthetist according to the National Blood Transfusion guidelines.
Throughout the operation, arterial blood gas will be measured every 60 min during the dissection phase, every 30 min during the anhepatic phase (including a 5-min pre-reperfusion blood gas) and every 30 min during the neohepatic phase for 1 h (including a 5-min post-reperfusion blood gas), then hourly until surgical closure. When pH is less than 7.2 or SBE is less than −10 mmol/L, additional 5% sodium bicarbonate will be infused with a total amount of 5% NaHCO3(ml) = (−2.3-SBE)*weight (kg)*0.42 to correct acidosis. The infusion will be started at a rate of 90 ml/h and discontinued with a targeted pH level of 7.35.
When the operation is completed, all the patients will be transferred to the surgical Intensive Care Units for postsurgical care. Both groups will receive standard treatment according to the guidelines after admission (27), including continuous vital signs recording, adequate fluid therapy, routine medical treatment (blood glucose control, antibiotics if needed and sedatives if required), organ support measures, etc. Organ failure would be assessed on a daily basis according to the SOFA score.
Initiation of RRT should be based on the criteria described by Bellomo et al. (28). Patients who have AKI (at least 1.5 times increase in creatinine from known baseline value) and meet predefined specific criteria will be eligible for initiation of RRT.
The primary outcome measure is the change of SBE during the operation, reflected by the difference in SBE measure before and after the operation (ΔSBE = SBEpost-SBEpre). SBEpre will be measured before introduction of anaesthesia, and SBEpost will be measured shortly before transportation to the surgical ICU.
1. The amount of BRS/ARS used during operation and within the first 2 days post operation;
2. Additional sodium bicarbonate used during operation and within the first 2 days post operation;
3. Serum acetic acid concentration before operation, 5 min post reperfusion of the donor liver, 0, 6, 12, 24 and 48 h post operation.
All the baselines mentioned below refer to data obtained within 24 h before operation.
1. Incidence of AKI within 48 h after operation. AKI is defined as current serum creatinine (SCr) increased 1.5 times baseline value OR with an increase greater than 26.5 µmol/L.
2. Serum Scr, Cys C levels at 0, 24 and 48 h post operation;
3. Serum NGAL levels at 0, 12, 24 and 48 h post operation;
4. Arterial pH, SBE, HCO3−, Lac, Cl− at 5 min post reperfusion of the donor liver, 0, 6, 12, 24 and 48 h post operation;
5. Effective SID (eSID = [Na+] + [K+] + [Ca2+] + [Mg2+]-[Cl−]-[Lac−]) at 5 min post reperfusion of the donor liver, 0, 6, 12, 24 and 48 h post operation;
6. Requirement of new RRT within 14 days post operation;
7. RRT free and alive days to 14 days post operation.
1. New receipt of organ support within 14 days post operation (new receipt of RRT; new receipt of mechanical ventilation (non-invasive included); new receipt of vasoactive agents);
2. New-onset organ failure within 14 days post operation;
3. ICU free and alive days within 14 days post operation;
4. Mortality to 14 days post operation.
A web-based electronic database (Unimed Scientific, Wuxi, China) is used for data collection and storage. All data are input by the designated coordinator. Training for data entry was arranged by the provider of the electronic database before study commencement. The data required to be collected during different phases are shown in Figure 1.
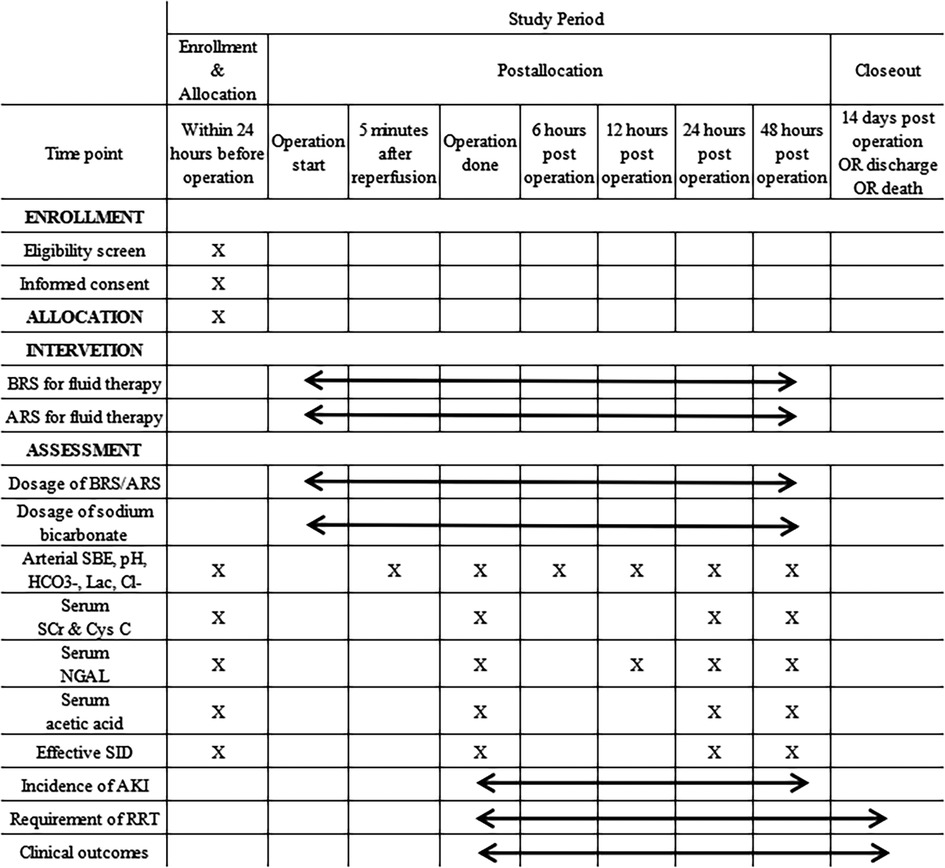
Figure 1. Schedule of enrolment, interventions, and assessments. BRS, bicarbonated Ringer's solution; ARS, acetated Ringer's solution; SBE, standard base excess; Lac, lactate; SCr, serum creatinine; Cys C, cystatin C; NGAL, neutrophil gelatinase-associated lipocalin; SID, strong ion difference; AKI, acute kidney injury; RRT, renal replacement therapy. *This figure is created by CL, JL and LK.
The principal investigator will be responsible for data management, safety, privacy, and quality. The reporting and presentation of this trial will follow the CONSORT guidelines (29). Based on the principle of intention to treat (ITT), a full-analysis set (FAS) will be performed on the population with outcome reporting. FAS will be used for the analysis of baseline characteristics and main therapeutic interventions. The safety set (SS) will include all enrolled patients to assess the safety profile of the study intervention.
According to the previous study (30) and our unpublished data, the change of SBE during operation is approximately −2 mmol/L with a standard deviation of 2.5 mmol/L in patients receiving ARS. We estimated that a sample size of 72 participants (36 per group) could provide 80% power at a two-sided alpha level of 0.05 to detect ≥1.7 mmol/L increase in the primary endpoint results from the use of BRS with a potential loss of 5% recruitments.
Descriptive statistics will be used to assess any marked baseline differences in demographics or outcome measures between the two groups. Comparisons of binary outcomes will be expressed as relative risk with 95% confidence intervals and comparisons of continuous outcomes as mean differences together with 95% confidence intervals. Comparisons will be made using t-test and ANOVA for repeated-measures or Wilcoxon rank-signed test and Kruskall-Wallis according to the underlying distribution for continuous data and Chi-square or Fisher's exact T test for categorical data, as appropriate. Two-sided 5% significance levels will be used to identify statistically significant results. All confidence intervals reported will be 95% confidence intervals.
All adverse events (AE) are required to be recorded and the DSMB will review all the safety profiles regularly during the study period. AEs will be reported in a uniform format through the electronic data capture system. The detection and report of the AEs will depend on the physicians involved in this trial.
Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Metabolic acidosis is common perioperatively, potentially leading to decreased renal blood flow and reduced glomerular filtration rate (31). The use of balanced solutions can prevent hyperchloremic metabolic acidosis, thereby avoiding AKI in some patients. However, in patients suffering hemorrhagic shock or undergoing major surgery, where the liver metabolism is impaired, the organic anions in conventional balanced solutions might remain strong anions and thus fail to maintain acid-base status. BRS, a novel balanced crystalloid buffered with bicarbonate rather than organic anions, may perform better in maintaining acid-base status under such circumstances. Paul et al. demonstrated that compared with ARS, BRS significantly reduced large acetate surges in cardiac surgical patients (32). Animal experiments and small clinical studies also observed similar phenomenons (33–35), but the impact on renal function was not assessed.
In patients undergoing liver transplantation, liver metabolism, essential for all organic anion buffered balanced solutions to function properly, is suspended in the anhepatic phase and severely compromised during the early neohepatic phase. BRS, therefore, is likely to benefit these patients by better sustaining the acid-base status and thereby protecting their renal function. However, no clinical study has investigated that yet. This study will, for the first time, provide evidence on the relative effects of BRS vs. ARS on acid-base state and renal injury in patients undergoing liver transplantation.
As the infusion of sodium bicarbonate to correct metabolic acidosis could significantly increase the pH and SBE, we worked out a standardised protocol for the use of sodium bicarbonate. The amount of sodium bicarbonate used perioperatively would also be recorded. Moreover, it is known that, compared with the piggyback technique, the classical surgical procedure involves excision of the recipient inferior vena cava (IVC), which requires cross-clamping of the IVC above and below the liver, as well as the portal vein. This results in a marked reduction in venous return and consequent haemodynamic instability (36). Therefore, patients undergoing classical liver transplantations would inevitably suffer more severe metabolic acidosis. However, both surgical approaches are widely used in the study site at the discretion of the surgical teams. Therefore, we adopted stratified randomisation to compensate for the confounding effect caused by surgical methods.
This trial has several strengths and limitations. This is the first randomised controlled trial providing high-level evidence evaluating the effect of bicarbonated Ringer's solution in patients undergoing liver transplantation. And the data will be reviewed by an independent data safety monitoring board to ensure the participants' safety. However, As a phase II RCT, the study has a relatively small sample size recruited from one centre and not sufficiently powered to detect a difffference in patient-centered outcomes like incidence of AKI post operation.
The present trial is sponsored by Jinling Hospital of Nanjing University, which is the municipal centre for liver transplantation performing more than 120 liver transplantations annually. Thus the study is expected to be concluded within a year.
The BETTER trial (protocol version 1.0, February 2021) will be conducted between 1 June 2021 and 31 December 2022.
The studies involving human participants were reviewed and approved by the ethics committee of Jinling Hospital, Nanjing University (DZQH-KYLL-21-07). The patients/participants provided their written informed consent to participate in this study.
All authors were involved in the study design, and read and approved the final manuscript. During the study, CL, JL, ZZ and DZ are responsible for randomising the patients and ensuring the blinding. CL, LK, BZ, LS, ZZ, DZ, YD, RS, JZ and CZ are responsible for carrying out recruitment, managing the treatment of the patients and collecting data. LK, BZ, BL and XW are members of the Trial Steering Committee. CL, JL, LK, BZ, BL and XW drafted the manuscript. All authors contributed to the article and approved the submitted version.
The study was funded by the National Natural Science Foundation of China (No. 82070665) and China Primary Health Care Foundation (YLGX-WS-2020020). The funders had no role in the study's design, data collection, and interpretation, or preparation of the manuscript. The decision to submit the manuscript was also made by the investigators.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1019570/full#supplementary-material.
AE, adverse events; AKI, acute kidney injury; ARS, acetated Ringer's solution; BRS, bicarbonated Ringer's solution; DSMB, data safety and monitoring board; FAS, full-analysis set; ITT, intention to treat; IVC, inferior vena cava; NGAL, neutrophil gelatinase-associated lipocalin; NS, normal saline; RRT, renal replacement therapy; SBE, standard base excess; SCr, serum creatinine; SID, strong ion difference; TMC, trial management committee.
1. Zhu M, Li Y, Xia Q, Wang S, Qiu Y, Che M, et al. Strong impact of acute kidney injury on survival after liver transplantation. Transplant Proc. (2010) 42(9):3634–8. doi: 10.1016/j.transproceed.2010.08.059
2. Karapanagiotou A, Kydona C, Dimitriadis C, Sgourou K, Giasnetsova T, Fouzas I, et al. Acute kidney injury after orthotopic liver transplantation. Transplant Proc. (2012) 44(9):2727–9. doi: 10.1016/j.transproceed.2012.09.096
3. O'Riordan A, Wong V, McQuillan R, McCormick PA, Hegarty JE, Watson AJ. Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation. Am J Transplant. (2007) 7(1):168–76. doi: 10.1111/j.1600-6143.2006.01602.x
4. Hu J, Chen R, Liu S, Yu X, Zou J, Ding X. Global incidence and outcomes of adult patients with acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. (2016) 30(1):82–9. doi: 10.1053/j.jvca.2015.06.017
5. Barri YM, Sanchez EQ, Jennings LW, Melton LB, Hays S, Levy MF, et al. Acute kidney injury following liver transplantation: definition and outcome. Liver Transpl. (2009) 15(5):475–83. doi: 10.1002/lt.21682
6. Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. (2010) 10(6):1420–7. doi: 10.1111/j.1600-6143.2010.03126.x
7. Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. (2017) 13(11):697–711. doi: 10.1038/nrneph.2017.119
8. Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. (2003) 9(7):741–7. doi: 10.1053/jlts.2003.50113
9. Chen J, Singhapricha T, Hu KQ, Hong JC, Steadman RH, Busuttil RW, et al. Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: a matched study. Transplantation. (2011) 91(3):348–53. doi: 10.1097/TP.0b013e31820437da
10. Sanchez EQ, Gonwa TA, Levy MF, Goldstein RM, Mai ML, Hays SR, et al. Preoperative and perioperative predictors of the need for renal replacement therapy after orthotopic liver transplantation. Transplantation. (2004) 78(7):1048–54. doi: 10.1097/01.TP.0000137176.95730.5B
11. Bilbao I, Charco R, Balsells J, Lazaro JL, Hidalgo E, Llopart L, et al. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin Transplant. (1998) 12(2):123–9. PMID: 9575400
12. Lima EQ, Zanetta DM, Castro I, Massarollo PC, Mies S, Machado MM, et al. Risk factors for development of acute renal failure after liver transplantation. Ren Fail. (2003) 25(4):553–60. doi: 10.1081/JDI-120022546
13. Lebron Gallardo M, Herrera Gutierrez ME, Seller Perez G, Curiel Balsera E, Fernandez Ortega JF, Quesada Garcia G. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transpl. (2004) 10(11):1379–85. doi: 10.1002/lt.20215
14. Cabezuelo JB, Ramirez P, Rios A, Acosta F, Torres D, Sansano T, et al. Risk factors of acute renal failure after liver transplantation. Kidney Int. (2006) 69(6):1073–80. doi: 10.1038/sj.ki.5000216
15. Nadeem A, Salahuddin N, El Hazmi A, Joseph M, Bohlega B, Sallam H, et al. Chloride-liberal fluids are associated with acute kidney injury after liver transplantation. Crit Care. (2014) 18(6):625. doi: 10.1186/s13054-014-0625-7
16. Myles P, Bellomo R, Corcoran T, Forbes A, Wallace S, Peyton P, et al. Restrictive versus liberal fluid therapy in major abdominal surgery (RELIEF): rationale and design for a multicentre randomised trial. BMJ Open. (2017) 7(3):e015358. doi: 10.1136/bmjopen-2016-015358
17. Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. (2018) 378(24):2263–74. doi: 10.1056/NEJMoa1801601
18. Schumann R, Mandell S, Michaels MD, Klinck J, Walia A. Intraoperative fluid and pharmacologic management and the anesthesiologist's supervisory role for nontraditional technologies during liver transplantation: a survey of US academic centres. Transplant Proc. (2013) 45(6):2258–62. doi: 10.1016/j.transproceed.2013.03.026
19. Working Group IAPAPAAPG. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. (2013) 13(4 Suppl 2):e1–15. doi: 10.1016/j.pan.2013.07.063
20. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. (2017) 43(3):304–77. doi: 10.1007/s00134-017-4683-6
21. Suetrong B, Pisitsak C, Boyd JH, Russell JA, Walley KR. Hyperchloremia and moderate increase in serum chloride are associated with acute kidney injury in severe sepsis and septic shock patients. Crit Care. (2016) 20(1):315. doi: 10.1186/s13054-016-1499-7
22. Ke L, Calzavacca P, Bailey M, May CN, Li WQ, Bertolini J, et al. Systemic and renal haemodynamic effects of fluid bolus therapy: sodium chloride versus sodium octanoate-balanced solution. Crit Care Resusc. (2014) 16(1):29–33. PMID: 24588433
23. Yunos NM, Bellomo R, Story D, Kellum J. Bench-to-bedside review: chloride in critical illness. Crit Care. (2010) 14(4):226. doi: 10.1186/cc9052
24. Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. (2018) 378(9):829–39. doi: 10.1056/NEJMoa1711584
25. Guidet B, Soni N, Della Rocca G, Kozek S, Vallet B, Annane D, et al. A balanced view of balanced solutions. Crit Care. (2010) 14(5):325. doi: 10.1186/cc9230
26. Satoh K, Ohtawa M, Katoh M, Okamura E, Satoh T, Matsuura A, et al. Pharmacological study of BRS, a new bicarbonated Ringer's solution, in haemorrhagic shock dogs. Eur J Anaesthesiol. (2005) 22(9):703–11. doi: 10.1017/S026502150500116X
27. Millson C, Considine A, Cramp ME, Holt A, Hubscher S, Hutchinson J, et al. Adult liver transplantation: UK clinical guideline - part 2: surgery and post-operation. Frontline Gastroenterol. (2020) 11(5):385–96. doi: 10.1136/flgastro-2019-101216
28. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. (2019) 394(10212):1949–64. doi: 10.1016/S0140-6736(19)32563-2
29. Hemming K, Taljaard M, McKenzie JE, Hooper R, Copas A, Thompson JA, et al. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ. (2018) 363:k1614. doi: 10.1136/bmj.k1614
30. Weinberg L, Broad J, Pillai P, Chen G, Nguyen M, Eastwood GM, et al. Sodium bicarbonate infusion in patients undergoing orthotopic liver transplantation: a single centre randomised controlled pilot trial. Clin Transplant. (2016) 30(5):556–65. doi: 10.1111/ctr.12721
31. Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomised, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. (2012) 256(1):18–24. doi: 10.1097/SLA.0b013e318256be72
32. Davies PG, Venkatesh B, Morgan TJ, Presneill JJ, Kruger PS, Thomas BJ, et al. Plasma acetate, gluconate and interleukin-6 profiles during and after cardiopulmonary bypass: a comparison of plasma-lyte 148 with a bicarbonate-balanced solution. Crit Care. (2011) 15(1):R21. doi: 10.1186/cc9966
33. Ma J, Han S, Liu X, Zhou Z. Sodium bicarbonated Ringer's solution effectively improves coagulation function and lactic acid metabolism in patients with severe multiple injuries and traumatic shock. Am J Transl Res. (2021) 13(5):5043–50. PMID: 34150090; PMCID: 8205763
34. Shimada Y, Kitamura A, Nakanishi K, Hongo T, Kim C, Sakamoto A., effect of bicarbonated Ringer's solution on the acid-base balance in patients undergoing abdominal aortic aneurysm repair. J Nippon Med Sch. (2005) 72(6):364–9. doi: 10.1272/jnms.72.364
35. Wang L, Lou J, Cao J, Wang T, Liu J, Mi W. Bicarbonate Ringer's solution for early resuscitation in hemorrhagic shock rabbits. Ann Transl Med. (2021) 9(6):462. doi: 10.21037/atm-21-97
Keywords: bicarbonated Ringer's solution, liver transplantation, acid-base status, acute kidney injury, acetated Ringer's solution
Citation: Lv C, Zhou B, Zhang D, Lin J, Sun L, Zhang Z, Ding Y, Sun R, Zhang J, Zhou C, Zhang L, Wang X, Ke L, Li W and Li B (2022) The effects of bicarbonated versus acetated Ringer's solutions on acid-base status and kidney injury following orthotopic liver transplantation: Protocol for a single-centre, randomised controlled trial (The BETTER trial). Front. Surg. 9:1019570. doi: 10.3389/fsurg.2022.1019570
Received: 15 August 2022; Accepted: 3 October 2022;
Published: 20 October 2022.
Edited by:
Sami Akbulut, İnönü University, TurkeyReviewed by:
Reyhan Arslantaş, Taksim Training and Research Hospital, Turkey© 2022 Lv, Zhou, Zhang, Lin, Sun, Zhang, Ding, Sun, Zhang, Zhou, Zhang, Wang, Ke, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baiqiang Li bGlfYmFpcWlhbmdAYWxpeXVuLmNvbQ== Weiqin Li Y3RnY2hpbmFAbWVkYml0LmNu Lu Ke Y3Rna2VsdUBuanUuZWR1LmNu
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.