
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 06 January 2023
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1013665
This article is part of the Research Topic 365 Days of Progress In Surgical Oncology View all 16 articles
Background: Few studies have investigated the relationship between sarcopenia and postoperative pulmonary complications (PPCs) after gastric cancer surgery. This study aimed to explore the impact of sarcopenia on PPCs in patients who had undergone gastric cancer surgery.
Methods: We included patients who underwent a transabdominal radical gastrectomy between June 2016 and October 2020. Patients were divided into two groups according to the median prevalence rate of lumbar triplane skeletal muscle index (L3 SMI): sarcopenia group (≤37.5% percentile in male and female group) and non-sarcopenia group (>37.5% percentile in male and female group). Baseline characteristics, intraoperative and postoperative conditions, pulmonary complications, and overall complications were compared between the two groups. The primary outcome was the incidence of PPCs. The secondary outcomes were overall postoperative complications and length of stay (LOS).
Results: Among the 143 patients included, 50 had sarcopenia and 93 had not. Compared to the non-sarcopenia group, the sarcopenia group had a higher the incidence of PPCs (22.0% vs. 8.6%, P = 0.024). The incidence of overall postoperative complications in the sarcopenia group was higher than that in the non-sarcopenia group (36.00% vs. 20.43%, P = 0.043). There was no significant difference in the LOS between the two groups.
Conclusions: Our research indicates that sarcopenia, preoperative comorbidities, and longer duration of intraoperative oxygen saturation <95% were risk factors for PPCs. Sarcopenia is an independent risk factor for postoperative complications. Given that our results provided a correlation rather than causation, future prospective randomized trials are needed to confirm the relationship between sarcopenia and prognosis.
Sarcopenia is a complex age-related syndrome characterized by progressive and generalized loss of skeletal muscle mass and function (1, 2), with the potential for physical disability, loss of independence, and adverse consequences, such as death (3, 4). The etiology of sarcopenia may be related to skeletal muscle disuse, endocrine changes, chronic wasting disease, systemic inflammatory responses, insulin resistance, and malnutrition (5, 6). Excessive inflammatory responses and chronic wasting disease largely contribute to sarcopenia, especially in patients with cancer (7). Thus, sarcopenia and cancer are causally related. Patients with gastric cancer usually have a certain degree of anorexia and underlying metabolic changes such as increased energy consumption, catabolism, and inflammation. These direct effects are exacerbated by the combined effects of chemotherapy and major gastrectomy, resulting in decreased nutrient intake. Decreased nutritional intake in patients with gastric cancer can further aggravate the occurrence and development of sarcopenia (8, 9).
Gastric cancer is the fifth most common cancer and third leading cause of cancer-related deaths worldwide (10). Studies have shown that sarcopenia is an independent factor for postoperative complications and overall survival in patients with gastric cancer (11). Studies have shown that sarcopenia is very common in older people, with a prevalence of 5%–13% in people aged 60–70 years and 11%–50% in those aged >80 years of age. Large differences in prevalence are related to differences in the measurements and cutoffs used to define sarcopenia (12). The prevalence of sarcopenia among community residents in China was 4.8% among women and 13.2% among men aged ≥70 years (13). The prevalence of sarcopenia in patients with cancer has significantly increased by approximately 35.7% (14). With further aggravation of population aging, the number of older patients with gastric cancer will gradually increase (15). Surgery remains the most important treatment for gastric cancer (16). However, the high incidence of postoperative complications and low survival rate in such patients have always been a concern for clinicians (17, 18). Postoperative complications have been shown to affect overall survival (19). Predicting the risk of postoperative complications and how to better intervene in order to reduce postoperative complications have become the focus of attention. Previous studies have shown that patients with sarcopenia have a higher risk of postoperative complications, longer length of stay (LOS), and higher hospital costs than patients without sarcopenia (20). Among the postoperative complications, anastomotic leakage and pulmonary complications have the greatest influence on postoperative mortality and prolonged LOS (21). Anastomotic leakage has decreased with improvements in surgical techniques. Pneumonia or lung-related complications are the most common postoperative complications in individuals under 80 years of age (22). Accurately predicting the risk of complications and actively preventing and doing everything possible to reduce the occurrence of postoperative pulmonary complications (PPCs) have become the focus of surgeons and anesthesiologists. However, few studies have investigated the relationship between sarcopenia and PPCs after gastric cancer surgery. In this study, we aimed to investigate the impact of sarcopenia on PPCs in patients undergoing gastric cancer surgery and to identify other risk factors for post-operative pneumonia.
This single-center, retrospective cohort study used data obtained from the discharge medical records of patients undergoing gastrointestinal surgery from June 2016 to October 2020 in the Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, Chengdu, China. This study was approved by the Biomedical Research Ethics Committee of West China Hospital, Sichuan University, and was registered at www.chictr.org.cn (ChiCTR1900026578).
The inclusion criteria were as follows: age 18–75 years; American Society of Anesthesiologists (ASA) I–III, and plan to undergo transabdominal radical gastrectomy for clinical stage I-III gastric cancer. Patients were excluded if their medical records were incomplete or inaccurate, if they had missing abdominal computed tomography (CT), or if they had a history of radical gastric resection or preoperative chemotherapy. Proximal (PG), distal (DG), or total gastrectomy (TG) was performed by specialized surgeons, according to the Japanese Gastric Cancer Treatment Guidelines (23).
For each patient, the data were collected by trained surgeons, radiologists, and anesthesiologists. The surgeons were trained by experienced surgeons until they were sufficiently skilled and precise in data collection (as judged by an experienced surgeon).
The basic information was as follows: patient sociodemographic characteristics, clinical characteristics, surgical procedures, and outcomes. The intraoperative parameters examined were as follows: the types of resection, anesthesia method, operation time (min), mechanical ventilation time (min), respiratory parameters (tidal volume [ml/kg], positive end expiratory pressure [PEEP], airway pressure, end-tidal carbon dioxide), circulation (systolic and diastolic blood pressure, heart rate, intraoperative vasoactive drug use), intraoperative pulse oximetry (SPO2) < 95% duration, intraoperative infusion volume (colloid volume, crystalloid volume), urine volume, and medication data.
After surgery, we monitored: the time of removing the tracheal tube after surgery (min), days of hospitalization after surgery (days), hospitalization expenses (yuan), time of removing gastric tube after surgery (days), postoperative complications, and pulmonary complications within 15 days after surgery (24), postoperative destination, whether patient-controlled analgesia was used, postoperative pathological diagnosis, histological type, TNM stage, and readmission within 30 days of discharge.
For primary outcomes, we measured PPCs and for secondary outcome measures, we considered the severity classification of PPCs, other postoperative complications, and intra-abdominal infections.
Sarcopenia was defined as low muscle mass, strength, and/or physical performance. Previous studies have shown that lumbar triplane skeletal muscle index (L3 SMI) on CT is the gold standard for estimating muscle quality (25). After professional training, imaging physicians identified and measured the muscle area of the L3 plane and divided it by the height squared (m2) to obtain the skeletal muscle index of L3 SMI (cm2/m2) on the syngo Multimodality Workplace software (Siemens Medical Solutions, Forchheim, Germany).
Studies have shown that the median prevalence of sarcopenia in patients with gastric cancer is approximately 35.7% (14). Therefore, in this study, we used a median of 35.7% for grouping. In all the medical records collected, ≤ 35.7% of both sexes were classified as the sarcopenia group and >35.7% as the non-sarcopenia group. A data collection flowchart is presented in Figure 1.
PPCs were defined as any of the following postoperative conditions within 15 days after operation: initial ventilation support for >48 h, re-intubation due to respiratory failure or pneumonia, respiratory infection, respiratory failure, bronchospasm, atelectasis, pleural effusion, pneumothorax, or aspiration pneumonia (26). The severity of PPCs was classified as 0–5, in which 0 indicates that PPCs have no symptoms or signals, 1–4 indicates gradual deterioration of complications, and 5 indicates death before discharge (27). A grade of at least 2 was defined as severe PPCs. Postoperative complications were defined as any deviation from the normal postoperative course and were graded according to the Clavien-Dindo classification (28).
The Shapiro–Wilk test was used to test the normality of continuous variables. The Student's t-test was used for quantitative data of normal and approximately normal distribution, and the mean±standard deviation was used for quantitative data of severely skewed distributions. For such distributions, the rank sum test was used and the data were expressed as median (25% quantile, 75% quantile). Categorical variables were analyzed using the chi-squared or Fisher's exact test, and classified variable data were expressed as numbers and percentages. Univariate logistic regression analysis was used for the univariate analysis. Variables with significant trends and known prognostic values, such as age, were selected as potential parameters in the univariate analysis. Forward stepwise variable selection was used to establish a multivariate logistic regression. All tests were bilateral (except for logistic regression analysis) and were considered statistically significant at P < 0.05. The IBM SPSS statistical software version 23.0 (SPSS Inc., Chicago, IL) was used for the statistical analysis.
A total of 143 patients met the inclusion criteria and were included in the study. The basic patient information is summarized in Tables 1, 2. They were divided into sarcopenia and non-sarcopenia groups according to the abdominal CT-guided L3 SMI, and the median prevalence rate was 35.7%.
Compared with those in the non-sarcopenia group, the patients in the sarcopenia group had lower body weight (kg) (P < 0.001), BMI (kg/m2) (P < 0.001), and preoperative blood albumin (P = 0.001). In addition, serum prealbumin (mg/L) (P = 0.029), T-BIL (μM) (P = 0.001), and ALT (IU/L) (P = 0.006) levels were also lower. The intraoperative small dose of remifentanil (µg/h) was lower (P = 0.041). The intraoperative tidal volume (ml/kg) of kilogram body weight (ml/kg) in the sarcopenia group was larger than that in the non-sarcopenia group (P < 0.001). As for the types of surgical resection, most distal gastrectomies were performed in the two groups, accounting for >50% in each group, while there was no proximal gastrectomy in the sarcopenia group (P = 0.003). The postoperative use of an intravenous analgesia pump was lower in the sarcopenia group than in the non-sarcopenia group (P = 0.037). In terms of pathological diagnosis, adenocarcinoma was lower in the sarcopenia group than in the non-sarcopenia group (P = 0.007). Other preoperative and intraoperative factors were not significantly different between the two groups.
Compared with the non-sarcopenia group, the sarcopenia group had worse outcomes for the incidence of the PPCs (P = 0.024). Moreover, the incidence of postoperative complications was higher in the sarcopenia group than in the non-sarcopenia group (P = 0.043). A total of three patients were then re-admitted within 30 days after discharge (one case in the sarcopenia group and two cases in the non-sarcopenia group). There were no significant differences in hospitalization cost, LOS, postoperative gastric tube extubation time, or postoperative endotracheal tube extubation time (Table 3).
In the univariate analysis, age, sarcopenia, preoperative comorbidities, SPO2 < 95% when inhaling air under air, and duration of intraoperative SPO2 < 95% were risk factors for the PPCs. In the multivariate analysis that included these factors, sarcopenia (odds ratio [OR] 3.79, 95% confidence interval [CI] 1.27–11.34, P = 0.017), preoperative comorbidities (OR 2.86, 95% CI 1.01–8.15, P = 0.049), and duration of intraoperative SPO2 < 95% (OR 1.14, 95% CI 1.04–1.24. P = 0.005) may be risk factors for PPCs. Univariate analysis also revealed that age, sarcopenia, preoperative comorbidities, and the duration of intraoperative SPO2 < 95% were risk factors for severe PPCs. Multivariate regression analysis showed that sarcopenia (OR 5.10, 95% CI 1.63–16.00, P = 0.005) and the duration of intraoperative SPO2 < 95% (OR 1.12; 95% CI 1.02–1.22, P = 0.016) were independent risk factors for severe PPCs in patients after gastric cancer surgery (Table 4).
Univariate analyses also found that sarcopenia was the risk factor for postoperative complications. Multivariate regression analysis with age using binary logistic regression showed that sarcopenia (OR 2.19, 95% CI 1.02–4.72, P = 0.045) was an independent risk factor for postoperative complications after gastric cancer surgery (Table 5).
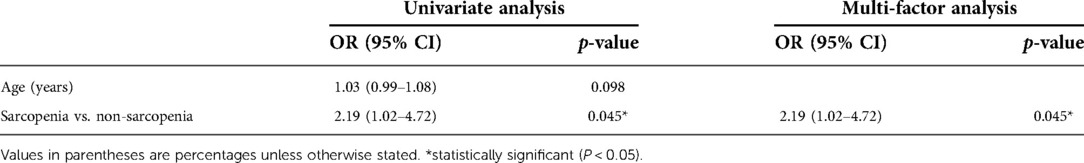
Table 5. Univariate and multivariate analysis of factors associated with postoperative complications.
We performed a single-center cohort study to investigate the effect of sarcopenia on PPCs in patients after gastric cancer surgery. We found that sarcopenia was associated with a higher incidence of postoperative complications and PPCs, compared to non-sarcopenia, in patients undergoing gastric cancer surgery. Multivariate regression analysis showed that sarcopenia, preoperative comorbidities, and the duration of intraoperative SPO2 <95% were the risk factors for PPCs in patients undergoing radical gastrectomy. Sarcopenia and intraoperative SPO2 <95% were still the risk factors for severe PPCs. In addition, sarcopenia was an independent risk factor for postoperative complications after gastrectomy.
Some studies have investigated the relationship between sarcopenia and postoperative complications following gastric cancer surgery (29–31). Patients with sarcopenia have a higher risk of postoperative complications and longer LOS. Zhou et al. indicated that sarcopenia is a strong independent risk factor for postoperative complications in older patients with gastric cancer (32). This is consistent with the results of our studies. The mechanism of sarcopenia leading to the increased risk of postoperative complications, especially pulmonary complications, remains unclear, and it is speculated that it may be related to the following possible mechanisms. Respiratory and swallowing muscles are affected by sarcopenia, which can lead to damage to the lungs and swallowing function. Impaired respiratory muscle function and swallowing function may lead to postoperative difficulty in expectoration, aspiration, postoperative pneumonia, and atelectasis (33, 34). Second, sarcopenia is associated with increased insulin resistance and increased circulation of proinflammatory cytokines, which may lead to the risk of postoperative acute lung injury (35). It has been reported that sarcopenia is associated with an increased inflammatory response to surgery (36). Increased inflammatory activity may also lead to pulmonary complications. Muscle fibers produce cytokines and other peptides such as interleukin-6, which affect the immune response by inhibiting the production of tumor necrosis factor-α and insulin resistance (37, 38). Sarcopenia may lead to immune senescence, which is characterized by impaired cellular immune function and increased inflammatory activity (39).
These factors may lead to PPCs. Fortunately, preoperative exercise through inspiratory muscle training, nutritional support, and other preoperative interventions may improve muscle function in patients with sarcopenia and effectively reduce the incidence of PPCs (25, 40). Further prospective studies are required to verify this finding. Sarcopenia can be diagnosed using a questionnaire, action ability test, or L3 SMI on CT. In the future, more attention should be paid to the diagnosis of sarcopenia in different regions, races, and populations, and the relationship between sarcopenia and prognosis should be further explored.
Our study also showed that the longer the duration of SPO2 < 95%, the higher the incidence of PPCs, especially the severe PPCs. Previous studies also found that a low SPO2 was associated with increased mortality and mortality caused by pulmonary diseases (41). The duration of intraoperative SPO2 < 95% may be related to the changes in pulmonary ventilation function caused by lung disease, mechanical ventilation lung injury, operation, and other inflammatory stimulations, which are currently recognized as indicators closely related to PPCs. This study indicated that the management of intraoperative mechanical ventilation could be further improved.
Interestingly, we found that tidal volume was larger in the sarcopenia group (P < 0.001) than in the non-sarcopenia group. In clinical practice, the tidal volume is often set according to the patient's body weight and pulmonary function. Compared to the non-sarcopenia group, the sarcopenia group had a smaller body weight but a larger tidal volume. Studies have shown that mechanical ventilation itself can induce inflammation and cooperate with surgery-induced responses. This magnifying inflammatory cascade reaction leads to lung injury and systemic multiple-organ failure.
Sarcopenia negatively affects the prognosis of patients who require mechanical ventilation, increasing all-cause mortality in these patients (42). This may be related to nutritional status, chronic inflammatory reaction, changes in hormone levels, and lack of physical activity in sarcopenia. Some studies have shown that low tidal volume can reduce pulmonary and systemic inflammatory responses compared with conventional tidal volume (43, 44). Mechanical ventilation with a high tidal volume may cause injury in healthy lungs (45, 46). Although the tidal volume of the two groups in this study did not exceed 10 ml/kg, it was higher in the sarcopenia group than in the non-sarcopenia group. There was no significant difference in the average value of PEEP between the two groups (the average value was approximately 2.8–2.9). Some studies have shown that the use of lower levels of PEEP may make the small airways open and close repeatedly, resulting in atelectasis and accelerating the development of pulmonary complications (47, 48). Multifaceted lung-protective ventilation strategies for high-risk patients, combined with low tidal volume, reopening of collapsed alveoli, and moderate levels of PEEP, can prevent further collapse (49). This would help reduce the incidence of postoperative atelectasis, improve clinical results, and reduce the consumption of medical resources. Whether the existing lung-protective ventilation strategy is the best perioperative ventilation management mode for patients with sarcopenia, and how to individualize PEEP and tidal volume to reduce the increase in PPCs caused by mechanical ventilation remain open questions.
Given the adverse effects of sarcopenia on mortality and hospital outcomes, sarcopenia is often considered a treatable indicator in adult respiratory medical treatment (50). It also shows that the prognosis of patients with sarcopenia can be improved through clinical intervention. Previous studies have shown that rehabilitation exercises, nutritional support, and growth hormone supplementation can improve the muscle mass and prognosis of patients with mechanical ventilation (51–53). However, to date, accurate intervention for sarcopenia has been the focus of attention in patients with oligomyopathy. These findings suggest that doctors should pay more attention to the perioperative respiratory system, intraoperative ventilation management, and postoperative lung rehabilitation. It is not limited to the preoperative evaluation and intraoperative management of anesthesiologists, but also includes early identification and intervention by surgeons, rehabilitation doctors, and nurses to reduce its effect on the poor prognosis of patients with sarcopenia. Therefore, it is necessary to carry out a unified standard diagnostic method, larger sample sizes, and multicenter prospective studies on sarcopenia intervention.
Our study bears several limitations. First, this was a single-center retrospective study with incomplete or, in limited cases, absent medical records, and a small sample size. The conclusions of this study need to be verified in additional multicenter prospective studies, involving larger samples. Second, the definitions of sarcopenia were different. In this study, CT-guided L3 SMI was directly used as an index to evaluate sarcopenia, but it was not diagnosed using a muscle strength test. Since this was a retrospective study, we could not comprehensively evaluate skeletal muscle function. Additionally, the cutoff value was not used in this study because of disease type and other factors. The cutoff value depends on measurement techniques, reference studies, and population availability. Moreover, the definition of sarcopenia is greatly influenced by race, population, sex, and other factors. Currently, considerable controversy remains. Therefore, we used the median prevalence rate of patients with gastric cancer to divide the patients into sarcopenia and non-sarcopenia groups. Because some patients visited the local hospital for revisit after surgery, the relevant data for a long time after surgery could not be accurately collected; therefore the long-term prognosis of the patients was not analyzed in this study.
Our study demonstrates that the duration of intraoperative SPO2 <95%, sarcopenia, and preoperative comorbidities were the risk factors for PPCs, especially severe PPCs. Furthermore, sarcopenia was an independent risk factor for postoperative complications. Future large randomized controlled trials and long-term follow-ups are needed to confirm the relationship between sarcopenia and prognosis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by The Biomedical Research Ethics Committee of West China Hospital of Sichuan University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conception and design: XZ, CD and XW; Provision of study materials or patients: XZ, CD and LH; Collection and assembly of data: XZ, CD and XW; Data analysis and interpretation: XZ, CD and RZ; Manuscript writing: XZ, CD and XW. All authors contributed to the article and approved the submitted version.
We would like to thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. (2010) 95:139–59. doi: 10.1093/bmb/ldq008
2. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48(4):601. doi: 10.1093/ageing/afz046
3. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-Associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. (2003) 95(5):1851–60. doi: 10.1152/japplphysiol.00246.2003
4. Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. (2007) 55(8):1216–23. doi: 10.1111/j.1532-5415.2007.01259.x
5. Douglas PJ, Short KR, Campbell WW, Elena V, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr (2008) (5):1562S. doi: 10.1093/ajcn/87.5.1562S
6. Sayer AA, Dennison EM, Syddall HE, Jameson K, Martin HJ, Cooper C. The developmental origins of sarcopenia: using peripheral quantitative computed tomography to assess muscle size in older people. J Gerontol-Biol Sci Med Sci. (2008) 63(8):835–40. doi: 10.1093/gerona/63.8.835
7. Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. (2011) 62(1):265. doi: 10.1146/annurev-med-061509-131248
8. Stojcev Z, Matysiak K, Duszewski M, Banasiewicz T. The role of dietary nutrition in stomach cancer. Contemp Oncol. (2013) 17(4):343–5. doi: 10.5114/wo.2013.37213
9. Takiguchi S, Takata A, Murakami K, Miyazaki Y, Yanagimoto Y, Kurokawa Y, et al. Clinical application of ghrelin administration for gastric cancer patients undergoing gastrectomy. Gastric Cancer. (2014) 17(2):200. doi: 10.1007/s10120-013-0300-8
10. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: Cancer J Clin. (2016) 66(1):7–30. doi: 10.3322/caac.21332
11. Tamura T, Sakurai K, Nambara M, Miki Y, Toyokawa T, Kubo N, et al. Adverse effects of preoperative sarcopenia on postoperative complications of patients with gastric cancer. Anticancer Res. (2019) 39(2):987–92. doi: 10.21873/anticanres.13203
12. Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. (2008) 12(7):452–6. doi: 10.1007/BF02982705
13. Cheng Q, Zhu X, Zhang X, Li H, Du Y, Hong W, et al. A cross-sectional study of loss of muscle mass corresponding to sarcopenia in healthy Chinese men and women: reference values, prevalence, and association with bone mass. J Bone Miner Metab. (2014) 32(1):78–88. doi: 10.1007/s00774-013-0468-3
14. McGovern J, Dolan RD, Horgan PG, Laird BJ, McMillan DC. Computed tomography-defined low skeletal muscle Index and density in cancer patients: observations from a systematic review. J Cachexia Sarcopenia Muscle. (2021) 12(6):1408–17. doi: 10.1002/jcsm.12831
15. Kota K, Hiroko YS. Comparison of time trends in stomach cancer incidence (1973–2002) in Asia, from cancer incidence in five continents, vols iv–ix. Jpn J Clin Oncol. (2009) 39(1):71–2. doi: 10.1093/jjco/hyn150
16. Thrumurthy SG, Chaudry MA, Hochhauser D, Mughal M. The diagnosis and management of gastric cancer. BMJ. (2013) 347, f6367. doi: 10.1136/bmj.f6367
17. Takeshita H, Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, et al. Surgical outcomes of gastrectomy for elderly patients with gastric cancer. World J Surg. (2013) 37(12):2891–8. doi: 10.1007/s00268-013-2210-7
18. Zhou CJ, Chen FF, Zhuang CL, Pang WY, Zhang FY, Huang DD, et al. Feasibility of radical gastrectomy for elderly patients with gastric cancer. Eur J Surg Oncol. (2016) 42(2):303–11. doi: 10.1016/j.ejso.2015.11.013
19. Yan-Mei B, Xin-Zu C, Cheng-Kun J, Ru-Bai Z, Yu-Fei G, Li-Bo Y, et al. Safety and survival benefit of surgical management for elderly gastric cancer patients. Hepato-gastroenterol. (2014) 61(134):1801–5.
20. Wang SL, Zhuang CL, Huang DD, Pang WY, Lou N, Chen FF, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: a prospective study. Ann Surg Oncol. (2016) 23(2):556–64. doi: 10.1245/s10434-015-4887-3
21. Gertsen EC, Goense L, Brenkman HJF, van Hillegersberg R, Ruurda JP. Identification of the clinically most relevant postoperative complications after gastrectomy: a population-based cohort study. Gastric Cancer. (2020) 23(2):339–48. doi: 10.1007/s10120-019-00997-x
22. Wong JU, Tai FC, Huang CC. An examination of surgical and survival outcomes in the elderly (65-79 years of age) and the very elderly (≥80 years of age) who received surgery for gastric cancer. Curr Med Res Opin. (2020) 36(2):229–33. doi: 10.1080/03007995.2018.1520083
23. Suo J, Wei LI. Interpretation of Japanese gastric cancer association(jgca) gastric cancer treatment guidelines 2018-the 5th edition. Chin J Pract Surg. (2018) 24(1):1–21. doi: 10.1007/s10120-020-01042-y
24. Clavien PA, Barkun J, Oliveira MLD, Vauthey JN, Makuuchi M. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250(2):187. doi: 10.1097/SLA.0b013e3181b13ca2
25. Phillips SM. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr. (2019) 6(4):452–60. doi: 10.3945/an.115.008367
26. Canet J, Gallart L, Gomar C, Paluzie G, Vallès J, Castillo J, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiol. (2010) 113(6):1338. doi: 10.1097/ALN.0b013e3181fc6e0a
27. Hulzebos EH, Helders PJ, Favié NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing cabg surgery: a randomized clinical trial. Jama. (2006) 296(15):1851–7. doi: 10.1001/jama.296.15.1851
28. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
29. Yang Z, Zhou X, Ma B, Xing Y, Jiang X, Wang Z. Predictive value of preoperative sarcopenia in patients with gastric cancer: a meta-analysis and systematic review. J Gastrointest Surg. (2018) 22(11):1890–902. doi: 10.1007/s11605-018-3856-0
30. Kuwada K, Kuroda S, Kikuchi S, Yoshida R, Nishizaki M, Kagawa S, et al. Sarcopenia and comorbidity in gastric cancer surgery as a useful combined factor to predict eventual death from other causes. Ann Surg Oncol. (2018) 25(5):1160–6. doi: 10.1245/s10434-018-6354-4
31. Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Nagatsuma Y, Nakayama T, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer. (2016) 19(3):986–93. doi: 10.1007/s10120-015-0546-4
32. Zhou CJ, Zhang FM, Zhang FY, Yu Z, Chen XL, Shen X, et al. Sarcopenia: a new predictor of postoperative complications for elderly gastric cancer patients who underwent radical gastrectomy. J Surg Res. (2017) 211:137. doi: 10.1016/j.jss.2016.12.014
33. Wakabayashi H, Sakuma K. Rehabilitation nutrition for sarcopenia with disability: a combination of both rehabilitation and nutrition care management. J Cachexia Sarcopenia Muscle. (2014) 5(4):269–77. doi: 10.1007/s13539-014-0162-x
34. Bahat G, Tufan A, Ozkaya H, Tufan F, Akpinar TS, Akin S, et al. in Male nursing home residents. Aging Male. (2014) 17(3):136–40. doi: 10.3109/13685538.2014.936001
35. Nishigori T, Okabe H, Tanaka E, Tsunoda S, Sakai Y. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J Surg Oncol. (2016) 113(6):678–84. doi: 10.1002/jso.24214
36. Reisinger KW, Derikx JPM, Vugt JLAV, Meyenfeldt MFV, Hulsewé KW, Damink SWMO, et al. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr. (2016) 35(4):924–7. doi: 10.1016/j.clnu.2015.07.005
37. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. (2012) 8(8):457–65. doi: 10.1038/nrendo.2012.49
38. Pedersen BK, Bruunsgaard H. Possible beneficial role of exercise in modulating low-grade inflammation in the elderly. Scand J Med Sci Sports. (2003) 13(1):56–62. doi: 10.1034/j.1600-0838.2003.20218.x
39. Bruunsgaard H, Pedersen BK. Effects of exercise on the immune system in the elderly population. Immunol & Cell Biol. (2000) 78(5):523–31. doi: 10.1046/j.1440-1711.2000.00965.x
40. N S. Preoperative inspiratory muscle training and postoperative complications. J Am Med Assoc JAMA. (2007) 297(7):697–9. doi: 10.1001/jama.297.7.697-a
41. Vold ML, Aasebø U, Wilsgaard T, Melbye H. Low oxygen saturation and mortality in an adult cohort: the tromsø study. BMC Pulm Med. (2015) 15:9. doi: 10.1186/s12890-015-0003-5
42. Jiang T, Lin T, Shu X, Song Q, Dai M, Zhao Y, et al. Prevalence and prognostic value of preexisting sarcopenia in patients with mechanical ventilation: a systematic review and meta-analysis. Critical Care. (2022) 26(1):140. doi: 10.1186/s13054-022-04015-y
43. Michelet P, D'Journo XB, Roch A, Doddoli C, Marin V, Papazian L, et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiol. (2006) 105(5):911–9. doi: 10.1097/00000542-200611000-00011
44. Determann RM, Royakkers A, Wolthuis EK, Vlaar AP, Choi G, Paulus F, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Critical Care. (2010) 14(1):R1. doi: 10.1186/cc8230
45. Weingarten TN, Whalen FX, Warner DO, Gajic O, Schears GJ, Snyder MR, et al. Comparison of two ventilatory strategies in elderly patients undergoing Major abdominal surgery. Br J Anaesth. (2010) 104(1):16–22. doi: 10.1093/bja/aep319
46. Neto AS, Cardoso SO, Manetta JA, Pereira VGOM, Espósito DC, Manoela DOPP, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndromes. Surv Anesthesiol. (2014) 58(3):108–9. doi: 10.1097/01.SA.0000446366.05578.70
47. Benidixen HH, Hedley-Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation. A concept of atelectasis. Surv Anesthesiol. (1964) 8(6):571. doi: 10.1056/NEJM196311072691901
48. Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiol. (2005) 102(4):838–54. doi: 10.1097/00000542-200504000-00021
49. Futier E, Constantin JM, Pelosi P, Chanques G, Jaber S. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiol. (2011) 114(6):1354–63. doi: 10.1097/ALN.0b013e31821811ba
50. McDonald VM, Osadnik CR, Gibson PG. Treatable traits in acute exacerbations of chronic airway diseases. Chron Respir Dis. (2019) 16:1479973119867954. doi: 10.1177/1479973119867954
51. Medrinal C, Combret Y, Prieur G, Robledo Quesada A, Bonnevie T, Gravier FE, et al. Comparison of exercise intensity during four early rehabilitation techniques in sedated and ventilated patients in icu: a randomised cross-over trial. Critical Care. (2018) 22(1):110. doi: 10.1186/s13054-018-2030-0
52. van Zanten ARH, De Waele E, Wischmeyer PE. Nutrition therapy and critical illness: practical guidance for the icu, post-icu, and long-term convalescence phases. Critical Care. (2019) 23(1):368. doi: 10.1186/s13054-019-2657-5
Keywords: sarcopenia, skeletal muscle index (SMI), anesthesia, postoperative pulmonary complications (PPCs), gastric cancer
Citation: Zhang X, Deng C, Wan Q, Zhao R, Han L and Wang X (2023) Impact of sarcopenia on postoperative pulmonary complications after gastric cancer surgery: A retrospective cohort study. Front. Surg. 9:1013665. doi: 10.3389/fsurg.2022.1013665
Received: 7 August 2022; Accepted: 31 October 2022;
Published: 6 January 2023.
Edited by:
Aali Jan Sheen, Manchester Royal Infirmary, United KingdomReviewed by:
Pietro Fransvea, UOC Chirurgia d'Urgenza e del Trauma, Fondazione Policlinico Universitario A. Gemelli, IRCCS, Italy© 2023 Zhang, Deng, Wan, Zhao, Han and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Wang MTQ5MDgyOTExNkBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.