- National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: The short-term and long-term effects of perioperative blood transfusion (PBT) on patients with gastric cancer are still intriguing. This systematic review and meta-analysis aimed to investigate the effects of blood transfusion on clinical outcomes in patients with gastric cancer undergoing gastrectomy.
Methods: We searched PubMed, Web of Science, Embase, and The Cochrane Library on December 31th 2021. The main outcomes were overall survival (OS), disease-free survival (DFS), disease-specific survival (DFS), and postoperative complications. A fixed or random-effects model was used to calculate the hazard ratio (HR) with 95% confidence intervals (CIs).
Results: Fifty-one studies with a total of 41,864 patients were included for this review and meta-analysis. Compared with patients who did not receive blood transfusions (NPBT), PBT was associated with worse 5-year OS (HR = 2.39 [95%CI: 2.00, 2.84]; p < 0.001; Multivariate HR = 1.43 [95%CI: 1.24, 1.63]; p < 0. 001), worse 5-year DFS (HR = 2.26 [95%CI: 1.68, 3.05]; p < 0.001; Multivariate HR = 1.45 [95%CI: 1.16, 1.82]; p < 0. 001), and worse 5-year DSS (HR = 2. 23 [95%CI: 1.35, 3.70]; p < 0.001; Multivariate HR = 1.24 [95%CI: 0.96, 1.60]; p < 0.001). Moreover, The PBT group showed a higher incidence of postoperative complications [OR = 2.30 (95%CI:1.78, 2. 97); p < 0.001] than that in the NPBT group, especially grade III-V complications, according to the Clavien-Dindo classification. [OR = 2.50 (95%CI:1.71, 3.63); p < 0.001].
Conclusion: In patients who underwent gastrectomy, PBT was associated with negative survival effects (OS, DFS, DSS) and a higher incidence of perioperative complications. However, more research was expected to further explore the impact of PBT. Meanwhile, strict blood transfusion management should be implemented to minimize the use of PBT.
1. Introduction
Gastric cancer is an important cause of cancer-related death, ranking fifth for incidence and fourth for mortality worldwide (1). Radical surgery remains the only opportunity to cure gastric cancer (2). Surgical trauma and perioperative anemia often induce blood transfusions in gastric cancer patients but some studies had shown that there were potential risks that can be attributed to immunosuppression (3, 4). Although blood transfusion is widely used by surgeons, the appropriate transfusion strategy of perioperative blood transfusion (PBT) in gastric cancer patients undergoing gastrectomy is not clear.
Previous studies had shown that PBT had adverse effects on patients in different cancers, like prostate cancer (5), lung cancer (6), and hepatocellular cancer (7). But conclusions about the effect of blood transfusion on the prognosis of gastric cancer were contradictory. Some studies had reported a negative association between PBT and prognosis of gastric cancer (8–34), whereas others found no association (35–58). A previous meta-analysis (59) had reported a worse prognosis of gastric cancer patients with PBT but was limited by the small sample size and low credibility of the evidence. Results concentrated on PBT in gastric cancer patients needed to be further confirmed.
Therefore, the study conducted this systematic review and meta-analysis to identify and summarize existing evidence and attempted to define the relationships between PBT and short- or long-term prognosis in patients undergoing gastrectomy. The aim of this study is to provide guidance for clinical decision-making and further optimize the perioperative transfusion management of gastric cancer patients.
2. Methods
This meta-analysis was performed according to the PRISMA Checklist (60). The protocol has been registered in the International prospective register of systematic reviews database (Prospro number: CRD42022314772, https://www.crd.york.ac.uk/PROSPERO/).
2.1. Literature search and study selection
Two authors independently search the databases. The literature was systematically searched using Pubmed, Embase, The Cochrane Library, and Web of Science database on 31st December 2021 for studies published until December 2021. The search strategy was as follows: [(“Stomach Neoplasms” OR “neoplasm stomach” OR “Stomach Neoplasm” OR “neoplasms stomach” OR “Gastric Neoplasms” OR “Gastric Neoplasm” OR “neoplasm gastric” OR “neoplasms gastric” OR “Cancer of Stomach” OR “Stomach Cancers OR Gastric Cancer” OR “cancer gastric” OR “cancers gastric” OR “cancers gastric” OR “Stomach Cancer” OR “cancer stomach” OR “cancers stomach” OR “Cancer of the Stomach”) AND (“Blood Transfusion” OR “Blood Transfusions” OR “Transfusion, Blood” OR “Transfusions, Blood”)]. We also searched the reference lists of relevant studies and previous meta-analyses. Duplicates were excluded. After a preliminary review of the title and abstract, some articles investigating related to blood transfusion were included. The full text of including articles were screened for eligibility for data extraction.
2.2. Inclusion and exclusion criteria
Inclusion criteria were described as follows: (1) Studies evaluating the association between perioperative blood transfusion and prognosis of gastric cancer patients after gastrectomy; (2) At least including one of the outcomes: overall survival (OS), disease-free survival (DFS), disease-specific survival (DSS) and postoperative complications; (3) Human studies.
Exclusion criteria were described as follows: (1) Studies about benign gastric diseases, patients with double primary cancers, without surgical treatment or underwent palliative resection; (2) Studies not in English; (3) Data cannot be extracted; (4) Sample size less than 100; (5) Conference abstract or review was excluded.
Studies based on duplicate authors or centers were excluded and we chose the latest one for inclusion.
2.3. Data extraction
Two authors independently extracted the data from the included studies. For each article included in the meta-analysis, the following information was extracted: (1) Study information: name of the first author; year of publication; data collection method; location of the research; sample size; group selection; median follow-up and time of the last follow-up; (2) Characteristics of patients: age, gender, body mass index (BMI), hemoglobin (Hb), albumin (Alb), comorbidity, tumor size, depth of invasion, lymph node metastasis, stage, tumor location, histologic grade; (3) Surgery information: operation time, American Society of Anesthesiologists(ASA) score, gastrectomy type (total/subtotal, open/laparoscopic), splenectomy, estimated blood loss (EBL), PBT trigger, the quantity of PBT, time of PBT, chemotherapy. (4) Outcomes: OS, DFS, DSS, postoperative complications.
The multivariable HRs with 95% CI for OS, DFS, DSS, and survival data under different stages of patients were extracted if available. The assessment of stage and lymph-node metastasis were based on the American Joint Committee on Cancer (AJCC) staging system (61–64).
2.4. Quality assessment
The quality of included studies was assessed by two dependent reviewers using Newcastle-Ottawa Scale (NOS) (65). The literature quality was evaluated from three dimensions: group selection, comparability, and outcomes for cohort studies. The NOS contained eight items and ranged from zero up to nine stars.
2.5. Statistical analysis
Effects were expressed as weighted mean difference (WMD) with a corresponding 95% confidence interval (CI) for continuous variables and odds ratio (OR) with a corresponding 95% CI for categorical variables (66).
Heterogeneity was tested using the Chi-square test based on the Cochran Q statistic and I2 metric, and subgroup analyses and a meta-regression model were used to explore sources of heterogeneity.
Heterogeneity between studies was assessed by the Chi-square test and I2 tests. I2 values greater than 50% indicated significant heterogeneity (67). In the case of I2 > 50%, the summary HR and the accompanying 95% CI were calculated with a random-effects model, otherwise, a fixed-effects model was used.
We used forest plots to aggregate the HRs of outcomes from individual studies and funnel plots to examine the bias. We stratified OS data by G. location, average age, publication year, gender, estimated blood loss, transfusion rate, preoperative Hb, stage, transfusion trigger or transfusion quantity. Sensitivity analyses were conducted by removing individual studies in turns. Subgroup analyses and sensitivity analyses were used to analyze sources of significant heterogeneity.
The meta-analysis was performed by Review Manager (RevMan v.5.4) and R (v.4.1.0 x64) software. P value < 0. 05 was considered significant statistically.
3. Results
3.1. Selected studies
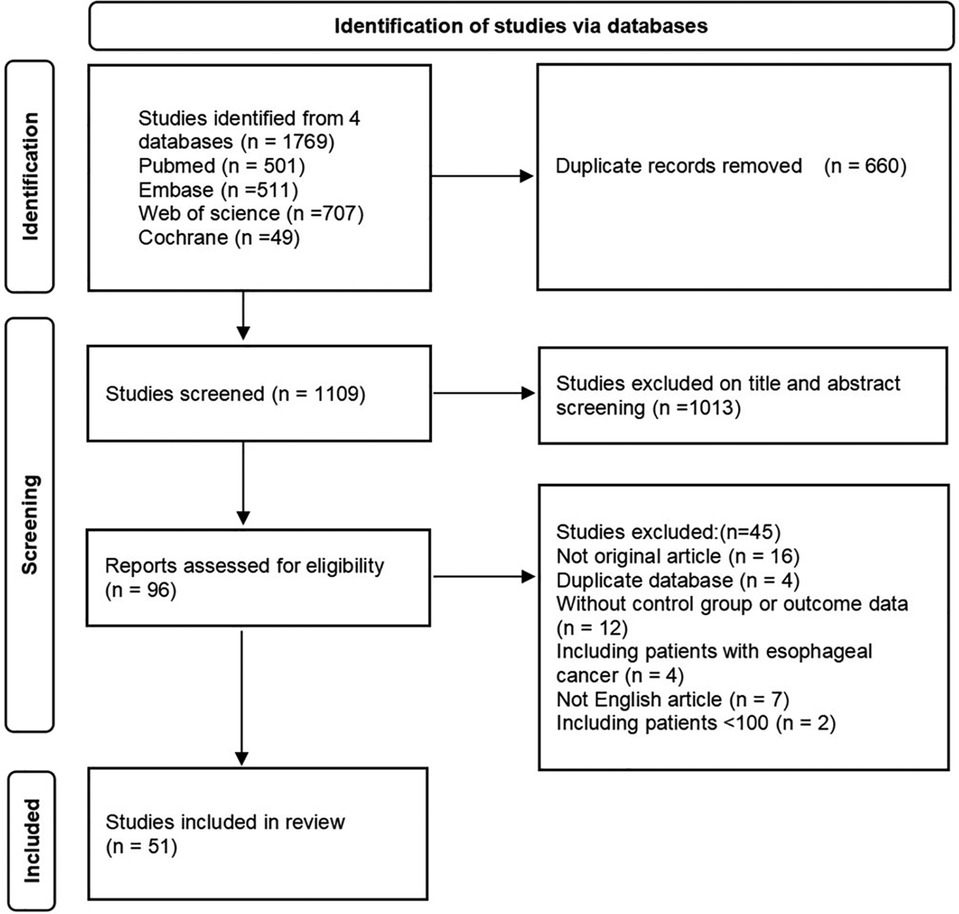
A total of 1,769 articles were retrieved by searching electronic databases (Pubmed, Web of Science, Embase, and Cochrane). After the duplicates were differentiated and excluded, there were 1,109 articles remaining. We excluded the studies which were conference abstracts, non-English articles, duplicate databases, or centers by screening the title and abstract and excluded the studies that could not be extracted valid information. Finally, 51 studies (8–58) published from 1987 to 2021 that fulfilled the inclusion criteria were included. Figure 1 showed the flow chart of the search results. The reasons for excluding studies in the screening stage were shown in Table S3.
3.2. Characteristics of the patients and studies
A total of 41,864 patients were included in this meta-analysis, which involved 10,475 patients (25%) with PBT and 31,389 patients (75%) who did not receive perioperative blood transfusion (NPBT). The follow-up period ranged from 12 to 180 months, and the median was 56.2 months. The PBT rate of studies ranged from 3% to 74%. Definition of PBT was reported in 27 studies. The characteristics of these studies and patients were presented in Supplementary Tables S1, S2.
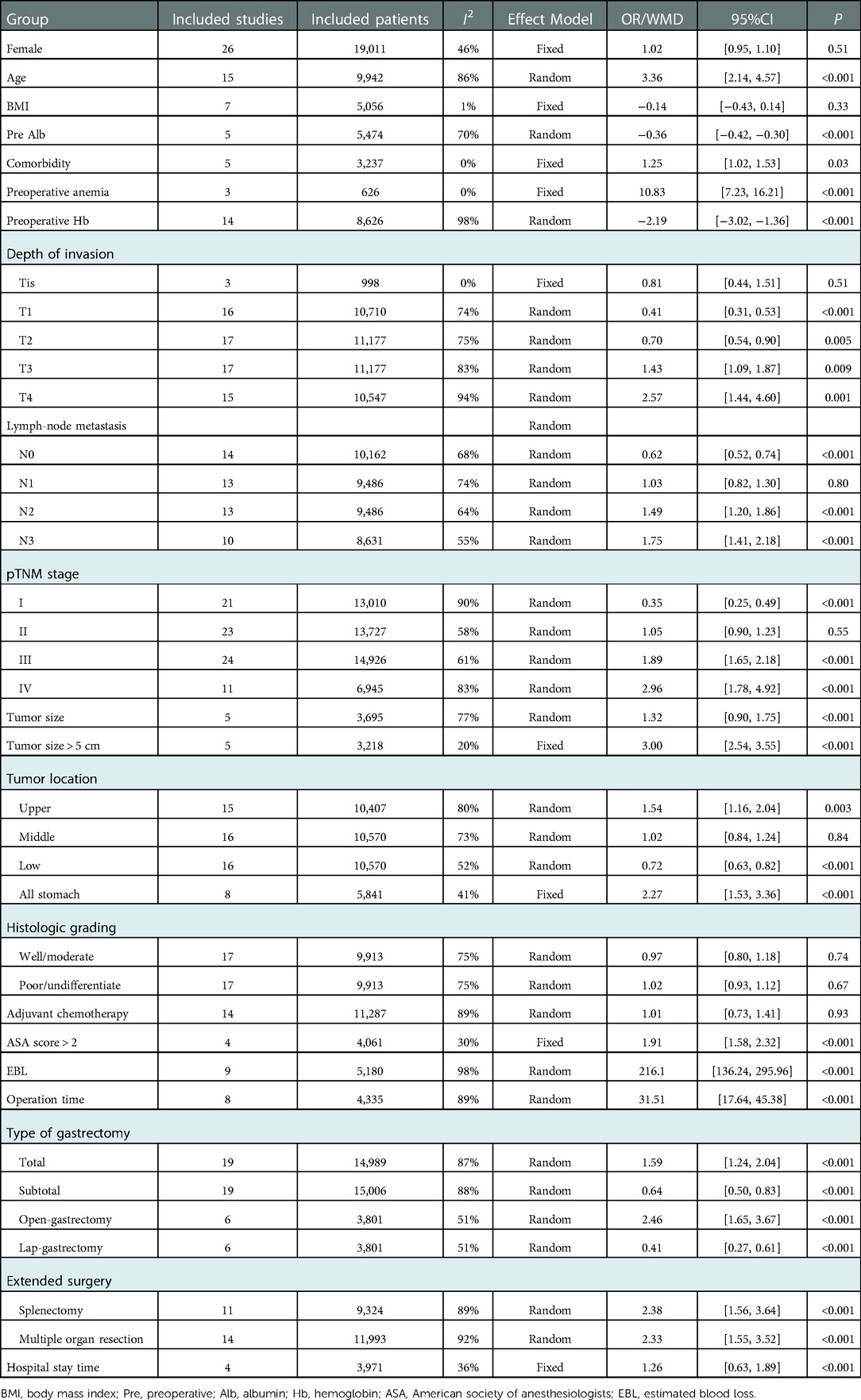
15 studies compared the age of patients and compared with the NPBT group, PBT group was older [OR: 3.36, 95%CI: (2.14, 4.57)]. 17 studies presented the preoperative Hb or anemia data, and we found patients with transfusion had a lower preoperative Hb level [OR: −2.19, 95%CI: (−3.02, −1.36)] or higher prevalence of preoperative anemia [OR: 10.83, 95%CI: (7.23, 16.21)]. Besides, PBT group have higher rate of comorbidity [OR: 1.25, 95%CI: (1.02, 1.53)] and lower preoperative albumin level [OR: −0.36, 95%CI: (−0.42, −0.30)]. There were no significant differences in different gender and BMI.
According to the TNM stage system (61–64), data from eligible studies showed that pathological stages of PBT group were more likely to be stage III[OR: 1.89, 95%CI: (1.65, 2.18)] and stage IV [OR: 2.57, 95%CI: (1.44,4.60)]. 17 studies reported the depth of invasion of tumor and 14 studies reported the lymph node metastasis. PBT group had a higher ratio of T3 [OR: 1.43, 95%CI: (1.09, 1.87)], T4 [OR: 2.57, 95%CI: (1.44, 4.60)], N2[OR: 1.49, 95%CI: (1.20, 1.86)], and N3[OR: 1.75, 95%CI: (1.41, 2.18)]. Differences of tumor location (upper location: OR: 1.54, 95%CI: [1.16, 2.04]; all stomach: OR: 2.27, 95%CI: [1.53, 3.36]) and tumor size (larger tumor size: OR: 1.32, 95%CI: [0.90, 1.75]; tumor size > 5 cm: OR:3.00, 95%CI: [2.54, 3.55]) were also found. However, as for histological differentiation, there was no significant difference between the two groups.
More than two thirds of studies presented the operation data. PBT group had a higher rate of conversion to open surgery [OR: 2.46, 95%CI: (1.65, 3.67)], total gastrectomy [OR: 1.59, 95%CI: (1.24, 2.04)] and multi-organ resection [OR: 2.33, 95%CI: (1.55, 3.52)], especially splenectomy [OR: 2.38, 95%CI: (1.56, 3.64)]. Besides, patients with PBT had higher ASA scores [ASA > 2: OR: 1.91, 95%CI: (1.58, 2.32)], greater EBL [OR: 216.1, 95%CI: (136.24, 295.96)] and longer hospital stay time [OR: 1.26, 95%CI: (0.63,1.89)] when compared with patients without PBT (Table 1).
3.3. Postoperative complications
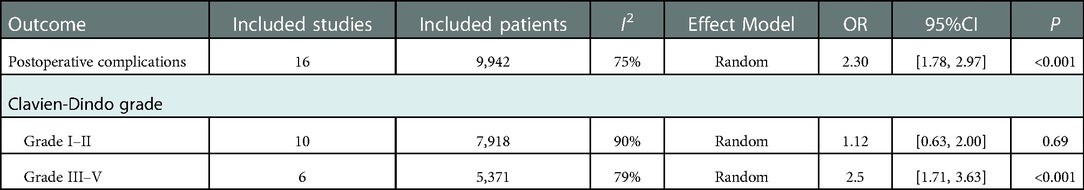
16 studies with 9,942 patients showed postoperative complications after gastrectomy. The OR of postoperative complications was 2.30 [95%CI: (1.78, 2.97)]. According to the Clavien-Dindo grade (68), the PBT group had a higher incidence rate of grade III-V complications [OR: 2.50, 95%CI: (1.71, 3.63); p < 0. 01], whereas no significant difference was seen in grade I-II [OR: 1.12, 95%CI: (0.63, 2.00); p = 0.69]. (Table 2) The forest plot and funnel plot were shown in Figure 2G, Supplementary Figure S1G.
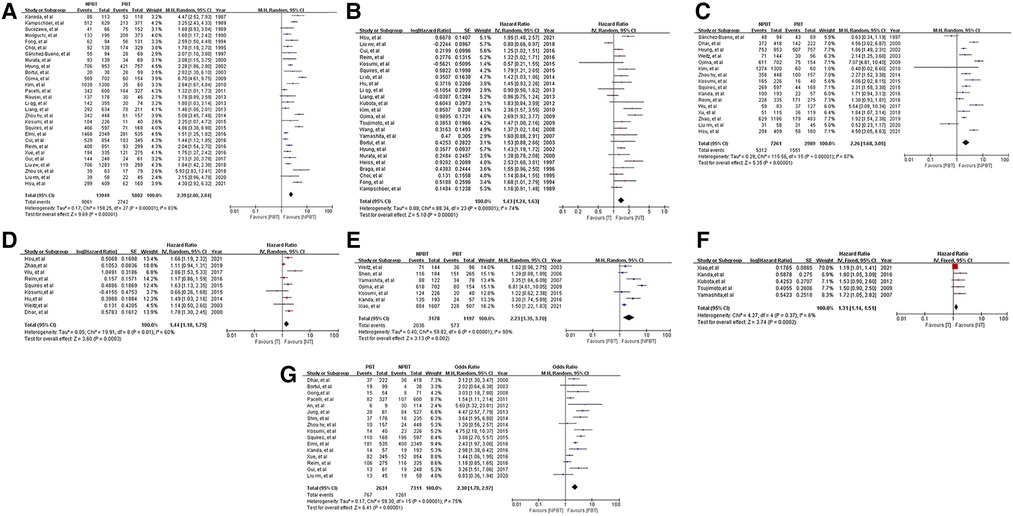
Figure 2. Forest plots and results of the meta-analysis of studies. (A) Forest plot of overall survival (OS) based on univariate results of studies. (B) Forest plot of OS based on multivariate results of studies. (C) Forest plot of disease-free survival (DFS) based on univariate results of studies. (D) Forest plot of DFS based on multivariate results of studies. (E) Forest plot of disease-specific survival (DSS) based on univariate results of studies. (F) Forest plot of DSS based on multivariate results of studies. (G) Forest plot of postoperative complications based on results of studies.
3.4. Long-term outcomes
3.4.1. Overall survival
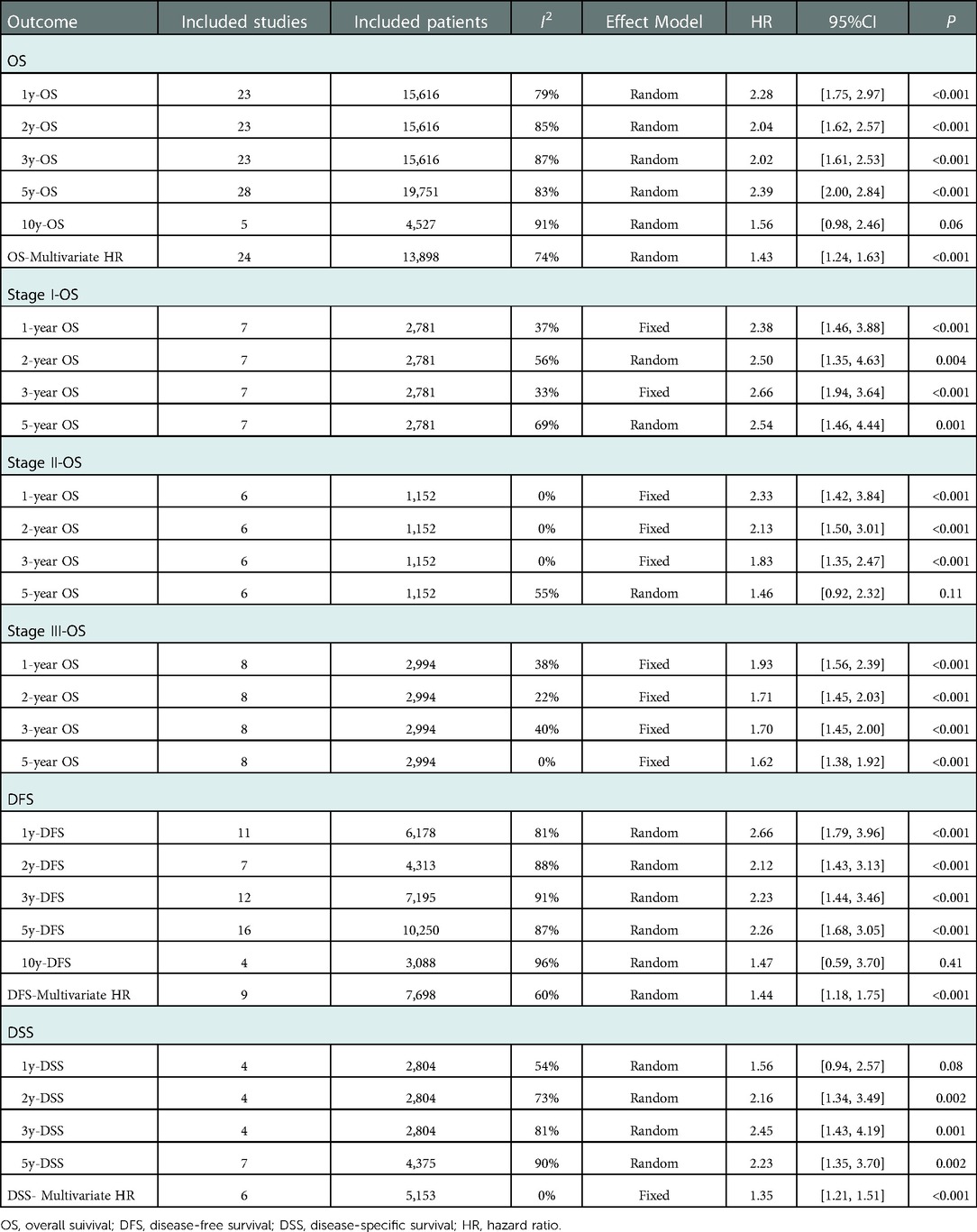
36 studies reported data on OS. Data on 5-year OS was available from 28 studies and HRs after multivariable analyses were extracted from 24 studies. The total number of enrolled patients was 25,122, with individual samples ranging from 103 to 2,884 (median 699). The HR of 5-year OS was 2.39 [95% CI: (2.00, 2.84), P < 0.01] and the summary of the multivariable HR was 1.43 [95% CI: (1.24, 1.63)]. Measure of heterogeneity indicates a high degree of variability about 5-year OS (HR: I2 = 83%, P < 0.01; multivariable HR: I2 = 74%, P < 0.01). The random-effects model was used to obtain estimates. The forest plots of OS were shown in Figures 2A,B.
A stratified analysis of OS was performed and the results were shown in Table 3. Publication years (before or after 2010), NOS score (≤7 stars or >7 stars), geographical location (west or east), average age (≤60 or >60), EBL (≤500 ml or >500 ml), PBT trigger (Hb < 7 g/L or Hb < 8 g/L), PBT rate (≤40% or >40%) and quantity (4U ≤ 50% or 4U > 50%) did not change the outcome significantly, which showed the result was robust.
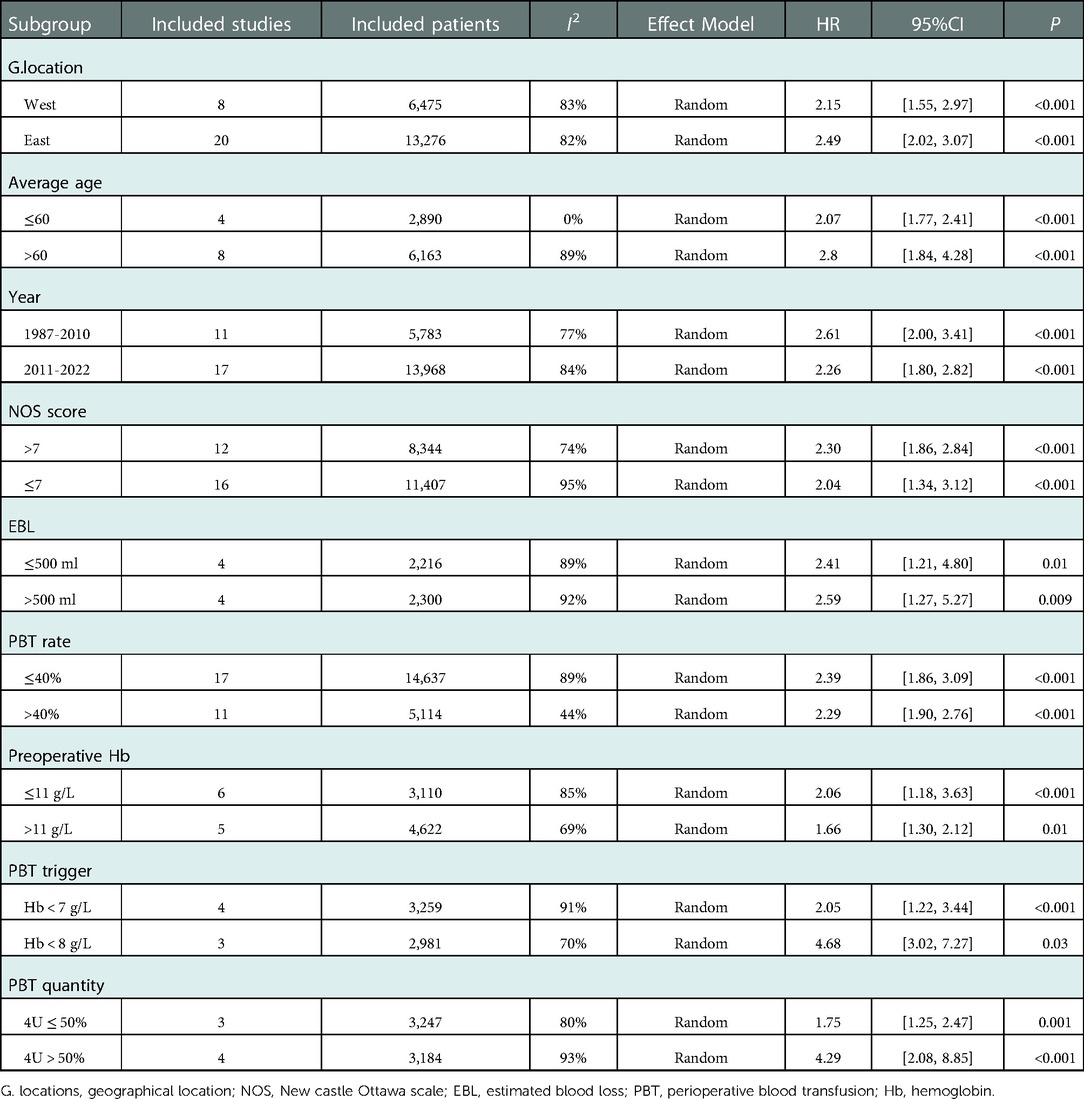
Table 3. Stratified meta-analysis of overall survival comparison between the PBT group and NPBT group.
Sensitivity analysis, which explored the effect on overall results by sequentially omitting individual studies, and a baujat plot was conducted to explore the source of heterogeneity between studies. (Supplementary Figure S2). 6 studies (9, 13, 21, 32, 42, 50) might be the main reason for the high heterogeneity. The funnel plot showed obvious asymmetry and publication bias was detected (Supplementary Figures S1A,B).
Moreover, further survival analyses were performed under different tumor stages according to the pTNM stage system. There were 7 studies, 8 studies, and 6 studies that showed survival rates between different groups at stages I, II, and III respectively. Compared to the NPBT patients, the PBT group was associated with lower 1-, 2-, 3-year OS at stages I, II, and III and lower 5-year OS at stage I [HR:2.54, 95%CI: (1.46, 4.44); p < 0.001;], III [HR:1.62, 95%CI: (1.38, 1.92); p < 0.001] whereas there was no significant difference in 5-year OS among stage II patients [HR:1.46, 95%CI: (0.92, 2.32); p = 0.11]. (Table 4).
3.4.2. Disease-free survival
17 studies reported data on DFS. Data on 5-year DFS were available from 16 studies and HRs after multivariable analyses were extracted from 9 studies. The 5-year DFS was lower in patients with PBT than NPBT patients. (HR = 2.26, 95% CI: [1.68, 3.05]; multivariable HR = 1.44, 95% CI: [1.18, 1.75]). I(2) as shown in Table 4. The funnel plot showed obvious asymmetry (Supplementary Figures S1C,D). The forest plots of DFS were shown in Figures 2C,D.
3.4.3. Disease-specific survival
9 studies reported data on DSS. Data on 5-year DSS were available from 7 studies and HRs after multivariable analyses were extracted from 6 studies. The 5-year DSS was lower in patients with PBT than NPBT patients. (HR = 2.23, 95% CI: [1.35, 3.70]; multivariable HR = 1.35, 95% CI: [1.21, 1.51]). I2 as shown in Table 4. The funnel plot showed obvious asymmetry (Supplementary Figures S1E,F). The forest plots of DFS were shown in Figures 2E,F.
4. Discussion
To date, the effects of PBT on the prognosis of gastric cancer patients undergoing gastrectomy were still controversial, and consensus had not yet been reached finally. The review and meta-analysis involved 51 studies with 41,864 gastric cancer patients. To our best knowledge, this analysis represented the largest assessment of current research that targeted the impact of PBT on the long- and short-term outcomes. A primary finding was that PBT was associated with worse prognosis than the NPBT group.
Specifically, the results of the meta-analysis showed that PBT was associated with worse 1-,2-,3- and 5-year OS (82% vs. 91%; 66% vs. 80%; 57% vs. 72%; 47% vs. 65%), DFS (76% vs. 88%; 61% vs. 76%; 53% vs. 74%; 52% vs. 73%), and DSS (86% vs. 89%; 64% vs. 74%; 53% vs. 66%; 48% vs. 64%). The results were similar to the conclusions of previous research (59, 69–72). Similar results were found in other meta-analyses of other solid cancer, including colorectal cancer (73, 74), hepatic cancer (75), esophageal cancer (76, 77), and pancreatic cancer (78). Further, we conducted stratified analysis and sensitivity analysis of OS and the results were consistent and credible. The mechanism could be partially attributed to the suppression of the immune system induced by blood transfusion (79). Firstly, some studies showed that the patients with previous blood transfusions experienced changes in the immune system (80–82) involving inhibition of T cells and alteration in T cell subsets (83). Secondly, transfusion could trigger a series of a cascade of the immune system, including inhibition of the immunoregulatory cytokine IL-2, and the release of immunosuppressive prostaglandins 3. Besides, blood transfusion could induce transfusion-related immunomodulation (TRIM), further inhibits the function of macrophages and monocytes (84), and might lead to the decline of immune surveillance and enhance the potential for tumor growth and cellular metastasis.
Significant differences in the clinicopathological characteristics were found between the PBT group and NPBT group, which were consistent with previous studies 69. Compared with the NPBT group, the PBT group was more likely to be anemic and had lower Hb levels. Previous studies had shown that preoperative anemia was a powerful predictor of the need for blood transfusion and independently associated with an increased risk of mortality in patients undergoing surgery, even to a mild degree (85, 86). Besides, the PBT group had more advanced tumor stages, more open surgery or total gastrectomy, and more EBL. Intraoperative blood transfusion was more likely to result from the complicated operation, especially large EBL (87). In addition, patients with transfusion were older and had more comorbidities, which might also be one of the important reasons for the poor prognosis in the PBT group.
Moreover, our findings showed that the PBT group had a higher postoperative complication rate. After grading the complications according to the Clavien-Dindo grade system, PBT was particularly related to grade III-V complications, but there was no significant difference in grade I-II when compared with the NPBT group. To date, the mechanisms that targeted the association between PBT and postoperative complications were unclear. Previous studies showed that the clinicopathological features of the patients in two groups might independently influence the postoperative complications (44, 47, 88). Compared with the NPBT group, patients with PBT were prone to suffer from more surgical trauma and had less tolerance for surgery because of their poor clinical condition. These clinicopathological factors, including old, advanced tumor stage, and complicated type of surgery, might be also associated with postoperative complications (89–91). Relevant mechanisms were expected to be demonstrated further.
Strengths and limitations should be considered when interpreting the study results. In our literature review, we retrieved 3 meta-analysis and systematic reviews related to the effect of perioperative blood transfusion in gastric cancer patients published in 2015 (70, 71) or 2018 (59). These studies were limited by the small number of articles included, univariate analysis or high heterogeneity. In this meta-analysis, the number of studies included was the largest, and adopting the multivariable HR to overcome the potential bias, which made the results more reliable. Besides, we focused on the relationship between the PBT and OS, DFS, DSS, and postoperative complications of gastric cancer patients, and found the relationship between PBT and severe postoperative complications. Nevertheless, there were some limitations to this meta-analysis. For obvious ethical reasons, no randomized controlled trial (RCT) was searched and included in this meta-analysis. The heterogeneity of some results was high in this meta-analysis, which might be attributed to the wide span of publication years, different transfusion triggers, and lacking PBT guideline. In addition, few studies presented the data on the amount and components of blood transfusion and the time of PBT, this meta-analysis failed to conduct further research. More research was expected to explore the role of PBT and the appropriate PBT management strategy.
5. Conclusions
In conclusion, PBT was associated with adverse effects on the prognosis of gastric cancer patients undergoing gastrectomy, including OS, DFS, and DSS in this meta-analysis. In addition, PBT had a negative impact on postoperative complications in gastric cancer patients, especially grade III-V complications. The quality of the evidence was not high and bias were detected, which might lead to more significant results. But these results indicated that strict patient blood management strategies aimed at minimizing PBT were necessary. Future studies should be performed to further define the role of PBT and explore the guideline of PBT in gastric cancer patients.
Author contributions
(I) Conception and design: YC, LZ; (II) Administrative support: DZ, YC; (III) Collection and assembly of data: WW, XL; (IV) Data analysis and interpretation: LZ, PN; (V) Manuscript writing: All authors; (VI) Final approval of manuscript: All authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from National Key R&D Program of China [grant No. 2017YFC0908300].
Acknowledgments
All authors made substantial contributions to the intellectual content of this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1011005/full#supplementary-material.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Johnston FM, Beckman M. Updates on management of gastric cancer. Curr Oncol Rep. (2019) 21(8):67. doi: 10.1007/s11912-019-0820-4
3. Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. (2013) 110(5):690–701. doi: 10.1093/bja/aet068
4. Fragkou PC, Torrance HD, Pearse RM, Ackland GL, Prowle JR, Owen HC, et al. Perioperative blood transfusion is associated with a gene transcription profile characteristic of immunosuppression: a prospective cohort study. Crit Care. (2014) 18(5):541. doi: 10.1186/s13054-014-0541-x
5. Pushan Z, Manbiao C, Sulai L, Jun L, Ruidong Z, Hanshen Y. The impact of perioperative blood transfusion on survival and recurrence after radical prostatectomy for prostate cancer: a systematic review and meta-analysis. J Cancer Res Ther. (2018) 14(Supplement):S701–s707. doi: 10.4103/0973-1482.193115
6. Wang T, Luo L, Huang H, Yu J, Pan C, Cai X, et al. Perioperative blood transfusion is associated with worse clinical outcomes in resected lung cancer. Ann Thorac Surg. (2014) 97(5):1827–37. doi: 10.1016/j.athoracsur.2013.12.044
7. Xun Y, Tian H, Hu L, Yan P, Yang K, Guo T. The impact of perioperative allogeneic blood transfusion on prognosis of hepatocellular carcinoma after radical hepatectomy: a systematic review and meta-analysis of cohort studies. Medicine (Baltimore). (2018) 97(43):e12911. doi: 10.1097/MD.0000000000012911
8. Dhar DK, Kubota H, Tachibana M, Kotoh T, Kinugasa S, Shibakita M, et al. A tailored perioperative blood transfusion might avoid undue recurrences in gastric carcinoma patients. Dig Dis Sci. (2000) 45(9):1737–42. doi: 10.1023/A:1005538429420
9. Elmi M, Mahar A, Kagedan D, Law CH, Karanicolas PJ, Lin Y, et al. The impact of blood transfusion on perioperative outcomes following gastric cancer resection: an analysis of the American college of surgeons national surgical quality improvement program database. Can J Surg. (2016) 59(5):322–9. doi: 10.1503/cjs.004016
10. Fong Y, Karpeh M, Mayer K, Brennan MF. Association of perioperative transfusions with poor outcome in resection of gastric adenocarcinoma. Am J Surg. (1994) 167(2):256–60. doi: 10.1016/0002-9610(94)90087-6
11. Gui R, Tang H, Gao M, Liu J, Huang R, Zhao G, et al. Impact of perioperative blood transfusion on survival of patients undergoing laparoscopic gastrectomy for gastric cancer. J Buon. (2017) 22(2):396–402.28534361
12. Heiss MM, Allgayer H, Gruetzner KU, Tarabichi A, Babic R, Mempel W, et al. Prognostic influence of blood transfusion on minimal residual disease in resected gastric cancer patients. Anticancer Res. (1997) 17(4a):2657–61.9252697
13. Hsu FK, Chang WK, Lin KJ, Liu CY, Fang WL, Chang KY. The associations between perioperative blood transfusion and long-term outcomes after stomach cancer surgery. Cancers (Basel). (2021) 13(21):5438. doi: 10.3390/cancers13215438
14. Hyung WJ, Noh SH, Shin DW, Huh J, Huh BJ, Choi SH, et al. Adverse effects of perioperative transfusion on patients with stage III and IV gastric cancer. Ann Surg Oncol. (2002) 9(1):5–12. doi: 10.1245/aso.2002.9.1.5
15. Kanda M, Kobayashi D, Tanaka C, Iwata N, Yamada S, Fujii T, et al. Adverse prognostic impact of perioperative allogeneic transfusion on patients with stage II/III gastric cancer. Gastric Cancer. (2016) 19(1):255–63. doi: 10.1007/s10120-014-0456-x
16. Kaneda M, Horimi T, Ninomiya M, Nagae S, Mukai K, Takeda I, et al. Adverse affect of blood transfusions on survival of patients with gastric cancer. Transfusion. (1987) 27(5):375–7. doi: 10.1046/j.1537-2995.1987.27587320526.x
17. Kim SH, Lee SI, Noh SM. Prognostic significance of preoperative blood transfusion in stomach cancer. J Gastric Cancer. (2010) 10(4):196–205. doi: 10.5230/jgc.2010.10.4.196
18. Kosumi K, Baba Y, Harada K, Yoshida N, Watanabe M, Baba H. Perioperative blood transfusion, age at surgery, and prognosis in a database of 526 upper gastrointestinal cancers. Dig Surg. (2015) 32(6):445–53. doi: 10.1159/000433609
19. Liu X, Ma M, Huang H, Wang Y. Effect of perioperative blood transfusion on prognosis of patients with gastric cancer: a retrospective analysis of a single center database. BMC Cancer. (2018) 18(1):649. doi: 10.1186/s12885-018-4574-4
20. Maeta M, Shimizu N, Oka A, Kondo A, Yamashiro H, Tsujitani S, et al. Perioperative allogeneic blood transfusion exacerbates surgical stress-induced postoperative immunosuppression and has a negative effect on prognosis in patients with gastric cancer. J Surg Oncol. (1994) 55(3):149–53. doi: 10.1002/jso.2930550304
21. Ojima T, Iwahashi M, Nakamori M, Nakamura M, Naka T, Katsuda M, et al. Association of allogeneic blood transfusions and long-term survival of patients with gastric cancer after curative gastrectomy. J Gastrointest Surg. (2009) 13(10):1821–30. doi: 10.1007/s11605-009-0973-9
22. Shen JG, Cheong JH, Hyung WJ, Kim J, Choi SH, Noh SH. Adverse effect of splenectomy on recurrence in total gastrectomy cancer patients with perioperative transfusion. Am J Surg. (2006) 192(3):301–5. doi: 10.1016/j.amjsurg.2006.04.014
23. Shin HS, Oh SJ, Suh BJ. Factors related to morbidity in elderly gastric cancer patients undergoing gastrectomies. J Gastric Cancer. (2014) 14(3):173–9. doi: 10.5230/jgc.2014.14.3.173
24. Squires MH 3rd, Kooby DA, Poultsides GA, Weber SM, Bloomston M, Fields RC, et al. Effect of perioperative transfusion on recurrence and survival after gastric cancer resection: a 7-institution analysis of 765 patients from the US gastric cancer collaborative. J Am Coll Surg. (2015) 221(3):767–77. doi: 10.1016/j.jamcollsurg.2015.06.012
25. Tsujimoto H, Ichikura T, Ono S, Sugasawa H, Hiraki S, Sakamoto N, et al. Impact of postoperative infection on long-term survival after potentially curative resection for gastric cancer. Ann Surg Oncol. (2009) 16(2):311–8. doi: 10.1245/s10434-008-0249-8
26. Wang X, Wan F, Pan J, Yu GZ, Chen Y, Wang JJ. Tumor size: a non-neglectable independent prognostic factor for gastric cancer. J Surg Oncol. (2008) 97(3):236–40. doi: 10.1002/jso.20951
27. Wu G, Zhang DY, Duan YH, Zhang YQ, Cui XN, Luo Z. Correlations of hemoglobin level and perioperative blood transfusion with the prognosis of gastric cancer: a retrospective study. Med Sci Monit. (2017) 23:2470–8. doi: 10.12659/MSM.900907
28. Xiao H, Xiao Y, Chen P, Quan H, Luo J, Huang G. Association among blood transfusion, postoperative infectious complications, and cancer-specific survival in patients with stage II/III gastric cancer after radical gastrectomy: emphasizing benefit from adjuvant chemotherapy. Ann Surg Oncol. (2021) 28(4):2394–404. doi: 10.1245/s10434-020-09102-4
29. Xu D, Fang X, Li Y, Zhang Z, Li Q. Perioperative blood transfusion is one of the factors that affect the prognosis of gastric cancer. J Buon. (2018) 23(3):672–7.30003736
30. Xue L, Chen XL, Wei-Han Z, Yang K, Chen XZ, Zhang B, et al. Impact of perioperative blood transfusion on postoperative complications and prognosis of gastric adenocarcinoma patients with different preoperative hemoglobin value. Gastroenterol Res Pract. (2016) 2016:6470857. doi: 10.1155/2016/6470857
31. Yamashita K, Sakuramoto S, Kikuchi S, Katada N, Kobayashi N, Watanabe M. Transfusion alert for patients with curable cancer. World J Surg. (2007) 31(12):2315–22. doi: 10.1007/s00268-007-9237-6
32. Zhou HY, Yi W, Wang J, Zhang J, Wang WJ, Hu ZQ. Association of perioperative allogeneic blood transfusions and prognosis of patients with gastric cancer after curative gastrectomy. Am J Surg. (2014) 208(1):80–7. doi: 10.1016/j.amjsurg.2013.08.029
33. Zhou SK, Yang LL, Chen R, Lu Y, Zheng YH. HLA-DQB1*03 genotype and perioperative blood transfusion are not conducive to the prognosis of patients with gastric cancer. J Clin Lab Anal. (2018) 32(7):e22443. doi: 10.1002/jcla.22443
34. Hu Y, Ying M, Huang C, Wei H, Jiang Z, Peng X, et al. Oncologic outcomes of laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective cohort study from China. Surg Endosc. (2014) 28(7):2048–56. doi: 10.1007/s00464-014-3426-9
35. An JY, Kim KM, Kim YM, Cheong JH, Hyung WJ, Noh SH. Surgical complications in gastric cancer patients preoperatively treated with chemotherapy: their risk factors and clinical relevance. Ann Surg Oncol. (2012) 19(8):2452–8. doi: 10.1245/s10434-012-2267-9
36. Bortul M, Calligaris L, Roseano M, Leggeri A. Blood transfusions and results after curative resection for gastric cancer. Suppl Tumori. (2003) 2(5):S27–30.12914386
37. Braga M, Molinari M, Zuliani W, Foppa L, Gianotti L, Radaelli G, et al. Surgical treatment of gastric adenocarcinoma: impact on survival and quality of life. A prospective ten year study. Hepatogastroenterology. (1996) 43(7):187–93.8682460
38. Choi JH, Chung HC, Yoo NC, Lee HR, Lee KH, Kim JH, et al. Perioperative blood transfusions and prognosis in patients with curatively resected locally advanced gastric cancer. Oncology. (1995) 52(2):170–5. doi: 10.1159/000227452
39. Cui J, Deng J, Ding X, Zhang L, Zhang R, Wu W, et al. Blood transfusion does not affect survival of gastric cancer patients. J Surg Res. (2016) 200(1):98–104. doi: 10.1016/j.jss.2015.07.019
40. Gong DJ, Miao CF, Bao Q, Jiang M, Zhang LF, Tong XT, et al. Risk factors for operative morbidity and mortality in gastric cancer patients undergoing total gastrectomy. World J Gastroenterol. (2008) 14(42):6560–3. doi: 10.3748/wjg.14.6560
41. Jung DH, Lee HJ, Han DS, Suh YS, Kong SH, Lee KU, et al. Impact of perioperative hemoglobin levels on postoperative outcomes in gastric cancer surgery. Gastric Cancer. (2013) 16(3):377–82. doi: 10.1007/s10120-012-0196-8
42. Kampschöer GH, Maruyama K, Sasako M, Kinoshita T, van de Velde CJ. The effects of blood transfusion on the prognosis of patients with gastric cancer. World J Surg. (1989) 13(5):637–43. doi: 10.1007/BF01658891
43. Kubota T, Hiki N, Nunobe S, Kumagai K, Aikou S, Watanabe R, et al. Significance of the inflammation-based Glasgow prognostic score for short- and long-term outcomes after curative resection of gastric cancer. J Gastrointest Surg. (2012) 16(11):2037–44. doi: 10.1007/s11605-012-2036-x
44. Li QG, Li P, Tang D, Chen J, Wang DR. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J Gastroenterol. (2013) 19(25):4060–5. doi: 10.3748/wjg.v19.i25.4060
45. Li X, Cao B, Liu Y, Mei L, Che X, Zhao Z. Multivariate analysis of prognostic factors in 549 patients undergoing surgical treatment of gastric cancer. Hepatogastroenterology. (2014) 61(130):535–42.
46. Liang YX, Guo HH, Deng JY, Wang BG, Ding XW, Wang XN, et al. Impact of intraoperative blood loss on survival after curative resection for gastric cancer. World J Gastroenterol. (2013) 19(33):5542–50. doi: 10.3748/wjg.v19.i33.5542
47. Liu RM, Liu XH, Cheng GM, Wang WQ, Zhang YF. Effect of perioperative blood transfusion on the survival of gastric cancer patients undergoing laparoscopic gastrectomy. Indian J Pharm Sci. (2020) 82:115–20. doi: 10.36468/pharmaceutical-sciences.spl.69
48. Moriguchi S, Maehara Y, Akazawa K, Sugimachi K, Nose Y. Lack of relationship between perioperative blood transfusion and survival time after curative resection for gastric cancer. Cancer. (1990) 66(11):2331–5. doi: 10.1002/1097-0142(19901201)66:11%3C2331::AID-CNCR2820661113%3E3.0.CO;2-H
49. Murata N, Idezuki Y, Konishi T, Watanabe H, Ushirokoji Y, Shinohara K, et al. Influence of perioperative blood transfusion on the prognosis of patients with gastric cancer receiving anticancer chemotherapy. Gastric Cancer. (2000) 3(1):24–7. doi: 10.1007/PL00011685
50. Pacelli F, Rosa F, Marrelli D, Pedrazzani C, Bossola M, Zoccali M, et al. Do perioperative blood transfusions influence prognosis of gastric cancer patients? Analysis of 927 patients and interactions with splenectomy. Ann Surg Oncol. (2011) 18(6):1615–23. doi: 10.1245/s10434-010-1543-9
51. Rausei S, Ruspi L, Galli F, Tirotta F, Inversini D, Frattini F, et al. Peri-operative blood transfusion in gastric cancer surgery: prognostic or confounding factor? Int J Surg. (2013) 11(Suppl 1):S100–103. doi: 10.1016/S1743-9191(13)60027-8
52. Reim D, Strobl AN, Buchner C, Schirren R, Mueller W, Luppa P, et al. Perioperative transfusion of leukocyte depleted blood products in gastric cancer patients negatively influences oncologic outcome: a retrospective propensity score weighted analysis on 610 curatively resected gastric cancer patients. Medicine (Baltimore). (2016) 95(29):e4322. doi: 10.1097/MD.0000000000004322
53. Sánchez-Bueno F, García-Marcilla JA, Pérez-Abad JM, Vicente R, Aranda F, Lujan JA, et al. Does perioperative blood transfusion influence long-term prognosis of gastric cancer? Dig Dis Sci. (1997) 42(10):2072–6. doi: 10.1023/A:1018818517811
54. Sugezawa A, Kaibara N, Sumi K, Ohta M, Kimura O, Nishidoi H, et al. Blood transfusion and the prognosis of patients with gastric cancer. J Surg Oncol. (1989) 42(2):113–6. doi: 10.1002/jso.2930420209
55. Weitz J, D'Angelica M, Gonen M, Klimstra D, Coit DG, Brennan MF, et al. Interaction of splenectomy and perioperative blood transfusions on prognosis of patients with proximal gastric and gastroesophageal junction cancer. J Clin Oncol. (2003) 21(24):4597–603. doi: 10.1200/JCO.2003.12.136
56. Yamaguchi K, Tokui N, Maeda S, Kominami T, Nakamura K, Kitamura K. Perioperative blood transfusion and gastric cancer: adverse effects or unfavourable conditions of pretreatment? Aust N Z J Surg. (1990) 60(10):765–72. doi: 10.1111/j.1445-2197.1990.tb07471.x
57. Zhao B, Huang X, Lu H, Zhang J, Luo R, Xu H, et al. Intraoperative blood loss does not independently affect the survival outcome of gastric cancer patients who underwent curative resection. Clin Transl Oncol. (2019) 21(9):1197–206. doi: 10.1007/s12094-019-02046-6
58. Kouyoumdjian A, Trepanier M, Al Shehhi R, Cools-Lartigue J, Ferri LE, Lee L, et al. The effect of preoperative anemia and perioperative transfusion on surgical outcomes after gastrectomy for gastric cancer. J Surg Res. (2021) 259:523–31. doi: 10.1016/j.jss.2020.10.003
59. Agnes A, Lirosi MC, Panunzi S, Santocchi P, Persiani R, D'Ugo D. The prognostic role of perioperative allogeneic blood transfusions in gastric cancer patients undergoing curative resection: a systematic review and meta-analysis of non-randomized, adjusted studies. Eur J Surg Oncol. (2018) 44(4):404–19. doi: 10.1016/j.ejso.2018.01.006
60. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Br Med J. (2015) 350:g7647. doi: 10.1136/bmj.g7647
61. Kumagai K, Sano T. Revised points and disputed matters in the eighth edition of the TNM staging system for gastric cancer. Jpn J Clin Oncol. (2021) 51(7):1024–7. doi: 10.1093/jjco/hyab069
62. Washington K. 7th Edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. (2010) 17(12):3077–9. doi: 10.1245/s10434-010-1362-z
63. Sobin LH, Fleming ID. TNM classification of malignant tumors, fifth edition (1997). union internationale contre le cancer and the American joint committee on cancer. Cancer. (1997) 80(9):1803–4. doi: 10.1002/(SICI)1097-0142(19971101)80:9%3C1803::AID-CNCR16%3E3.0.CO;2-9
64. Schmoll HJ, Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, et al. AJCC Cancer staging manual, 6th edition. Ann Oncol. (2003) 14(2):345–6. doi: 10.1093/annonc/mdg077
65. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
66. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
67. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. doi: 10.1002/sim.1186
68. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–213.15273542
69. Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg. (2015) 13:102–10. doi: 10.1016/j.ijsu.2014.11.044
70. Li L, Zhu D, Chen X, Huang Y, Ouyang M, Zhang W. Perioperative allogenenic blood transfusion is associated with worse clinical outcome for patients undergoing gastric carcinoma surgery: a meta-analysis. Medicine (Baltimore). (2015) 94(39):e1574. doi: 10.1097/MD.0000000000001574
71. Vamvakas EC. Perioperative blood transfusion and cancer recurrence: meta-analysis for explanation. Transfusion. (1995) 35(9):760–8. doi: 10.1046/j.1537-2995.1995.35996029162.x
72. Goel R, Tobian AAR, Shaz BH. Noninfectious transfusion-associated adverse events and their mitigation strategies. Blood. (2019) 133(17):1831–9. doi: 10.1182/blood-2018-10-833988
73. Pang QY, An R, Liu HL. Perioperative transfusion and the prognosis of colorectal cancer surgery: a systematic review and meta-analysis. World J Surg Oncol. (2019) 17(1):7. doi: 10.1186/s12957-018-1551-y
74. McSorley ST, Tham A, Dolan RD, Steele CW, Ramsingh J, Roxburgh C, et al. Perioperative blood transfusion is associated with postoperative systemic inflammatory response and poorer outcomes following surgery for colorectal cancer. Ann Surg Oncol. (2020) 27(3):833–43. doi: 10.1245/s10434-019-07984-7
75. Tai YH, Wu HL, Mandell MS, Tsou MY, Chang KY. The association of allogeneic blood transfusion and the recurrence of hepatic cancer after surgical resection. Anaesthesia. (2020) 75(4):464–71. doi: 10.1111/anae.14862
76. Boshier PR, Ziff C, Adam ME, Fehervari M, Markar SR, Hanna GB. Effect of perioperative blood transfusion on the long-term survival of patients undergoing esophagectomy for esophageal cancer: a systematic review and meta-analysis. Dis Esophagus. (2018) 31(4). doi: 10.1093/dote/dox134
77. Lee J, Chin JH, Kim JI, Lee EH, Choi IC. Association between red blood cell transfusion and long-term mortality in patients with cancer of the esophagus after esophagectomy. Dis Esophagus. (2018) 31(2). doi: 10.1093/dote/dox123
78. Mavros MN, Xu L, Maqsood H, Gani F, Ejaz A, Spolverato G, et al. Perioperative blood transfusion and the prognosis of pancreatic cancer surgery: systematic review and meta-analysis. Ann Surg Oncol. (2015) 22(13):4382–91. doi: 10.1245/s10434-015-4823-6
79. Kormi SMA, Seghatchian J. Taming the immune system through transfusion in oncology patients. Transfus Apher Sci. (2017) 56(3):310–6. doi: 10.1016/j.transci.2017.05.017
80. Salvatierra O Jr, Vincenti F, Amend W, Potter D, Iwaki Y, Opelz G, et al. Deliberate donor-specific blood transfusions prior to living related renal transplantation. A new approach. Ann Surg. (1980) 192(4):543–52. doi: 10.1097/00000658-198010000-00012
81. Opelz G, Terasaki PI. Dominant effect of transfusions on kidney graft survival. Transplantation. (1980) 29(2):153–8. doi: 10.1097/00007890-198002000-00013
82. Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. (1973) 5(1):253–9.4572098
83. Siemionow M, Agaoglu G. Role of blood transfusion in transplantation: a review. J Reconstr Microsurg. (2005) 21(8):555–63. doi: 10.1055/s-2005-922436
84. Youssef LA, Spitalnik SL. Transfusion-related immunomodulation: a reappraisal. Curr Opin Hematol. (2017) 24(6):551–7. doi: 10.1097/MOH.0000000000000376
85. Clevenger B, Richards T. Pre-operative anaemia. Anaesthesia. (2015) 70(Suppl 1):20–8. e26-28. doi: 10.1111/anae.12918
86. Abeysiri S, Chau M, Richards T. Perioperative anemia management. Semin Thromb Hemost. (2020) 46(1):8–16. doi: 10.1055/s-0039-1697933
87. Unal D, Senayli Y, Polat R, Spahn DR, Toraman F, Alkis N, et al. Peri-operative blood transfusion in elective major surgery: incidence, indications and outcome - an observational multicentre study. Blood Transfus. (2020) 18(4):261–79. doi: 10.2450/2020.0011-20
88. Orman S, Cayci HM. Gastric cancer: factors affecting survival. Acta Chir Belg. (2019) 119(1):24–30. doi: 10.1080/00015458.2018.1453437
89. Inokuchi M, Otsuki S, Ogawa N, Tanioka T, Okuno K, Gokita K, et al. Postoperative complications of laparoscopic total gastrectomy versus open total gastrectomy for gastric cancer in a meta-analysis of high-quality case-controlled studies. Gastroenterol Res Pract. (2016) 2016:2617903. doi: 10.1155/2016/2617903
90. Mizuno A, Kanda M, Kobayashi D, Tanaka C, Iwata N, Yamada S, et al. Adverse effects of intraoperative blood loss on long-term outcomes after curative gastrectomy of patients with stage II/III gastric cancer. Dig Surg. (2016) 33(2):121–8. doi: 10.1159/000443219
Keywords: gastric cancer, perioperative blood transfusions, overall survival, disease-free survival, disease-special survival, postoperative complications
Citation: Wang W, Zhao L, Niu P, Zhang X, Luan X, Zhao D and Chen Y (2023) Effects of perioperative blood transfusion in gastric cancer patients undergoing gastrectomy: A systematic review and meta-analysis. Front. Surg. 9:1011005. doi: 10.3389/fsurg.2022.1011005
Received: 9 August 2022; Accepted: 12 December 2022;
Published: 17 January 2023.
Edited by:
Manuel Barberio, Pia Fondazione di Culto e Religione Card. G. Panico, ItalyReviewed by:
Qun Zhao, Fourth Hospital of Hebei Medical University, ChinaAudrius Dulskas, National Cancer Institute (Lithuania), Lithuania
Satvinder Singh Mudan, The London Clinic, United Kingdom
© 2023 Wang, Zhao, Niu, Zhang, Luan, Zhao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingtai Chen eWluZ3RhaWNoZW5AMTI2LmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Wanqing Wang†
Wanqing Wang† Penghui Niu
Penghui Niu Yingtai Chen
Yingtai Chen