
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 06 January 2023
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1010050
This article is part of the Research Topic Case Reports in Surgical Oncology: 2022 View all 56 articles
 Sheng Yan1†
Sheng Yan1† Yuhua Zou1†
Yuhua Zou1† Xinzhi Liao1
Xinzhi Liao1 Cunzhi Zhong2
Cunzhi Zhong2 Shengyin Liu1
Shengyin Liu1 Sigen Huang1
Sigen Huang1 Junrong Zou3*
Junrong Zou3* Quanliang Liu1*
Quanliang Liu1*
Superficial angiomyxoma (SA) is a rare benign tumor that occurs either in the superficial dermis or subcutaneously. It often occurs in the trunk, neck, or limbs, and grows slowly. The diameter of the tumor is usually less than 5 cm. A giant SA of the perineum in men is very rare. We detailed the diagnosis and treatment of male patients with perineal SA and performed a literature review. We report a case of a 42-year-old male patient. He was admitted to hospital with a perineal mass found more than 1 year previously. A pelvic contrast-enhanced computed tomography scan in our hospital suggests that a round slightly hypointense foci of about 6.0 cm × 8.6 cm × 4.5 cm in size with still clear borders was seen below the penile corpus cavernosum in the perineum. We performed a perineal mass excision under continuous epidural anesthesia. A postoperative pathology report diagnosed perineal SA. There was no recurrence at follow-up for 27 months up to May 2022. Perineal SA is rare and should be combined with patient history and imaging to ensure complete excision of the mass margins. Adherence to long-term postoperative follow-up is the key to curing this case.
Superficial angiomyxoma (SA), also known as a cutaneous mucinous tumor, is a rare benign soft tissue tumor of the skin. SA was first reported by Carney et al. in 1985, with an incidence in the range of 0.008%–3% (1). Most SAs are isolated and can manifest themselves in association with Carney syndrome (2), which includes mucinous tumors, patchy skin pigmentation, and endocrine hyperfunctional disorders. Preoperative imaging examination has a certain significance for the estimation of tumor range and prognosis. However, imaging lacks typical features and a diagnosis is often made definitively by pathology. A giant SA in the perineum is extremely rare, and we report this case with the aim of providing a reference experience for clinical management.
A male patient aged 42 years was admitted to the hospital after “finding a perineal mass for more than 1 year.” The patient complained that he found a mass the size of peanut rice in the perineum 1 year previously, without tenderness and discomfort from ulceration. At that time, he did not pay attention to it, and the mass then gradually increased. A color Doppler ultrasound in the local hospital showed a slightly hypoechoic mass in the perineum, which was not treated at that time. During the physical examination, a hard and fixed mass of about 8.5 cm × 6 cm was palpable in the patient's perineum.
To understand the nature, blood supply, and anatomical location of the mass, a contrast-enhanced computed tomography (CE-CT) scan of the pelvis was performed. A round slightly hypointense foci of about 6.0 cm × 8.6 cm × 4.5 cm in size with still clear borders was seen below the penile corpus cavernosum in the perineum (Figure 1A), with a CT value of approximately 17 HU. During the arterial phase of enhanced scanning, an arterial vessel was seen (Figure 1B). The enhancement of the lesion was not obvious, and uneven enhancement was seen in the venous phase (Figure 1C), with a CT value of approximately 31 HU. The prostate was small, the edge was smooth, and the density in the parenchyma was uniform. The shape, size, and density of the seminal vesicle gland were normal. The bladder was well filled. The bladder wall was smooth, without thickening or nodule protrusion. The bladder seminal vesicle angle was normal. There was no effusion or enlarged lymph nodes in the pelvic cavity. Initially, a large benign tumor in the perineum was considered, but the type of tumor was not yet clear. In the past, the patient had been in good health. No abnormality was found in his chest x-ray, ECG, or routine biochemical examination after admission.
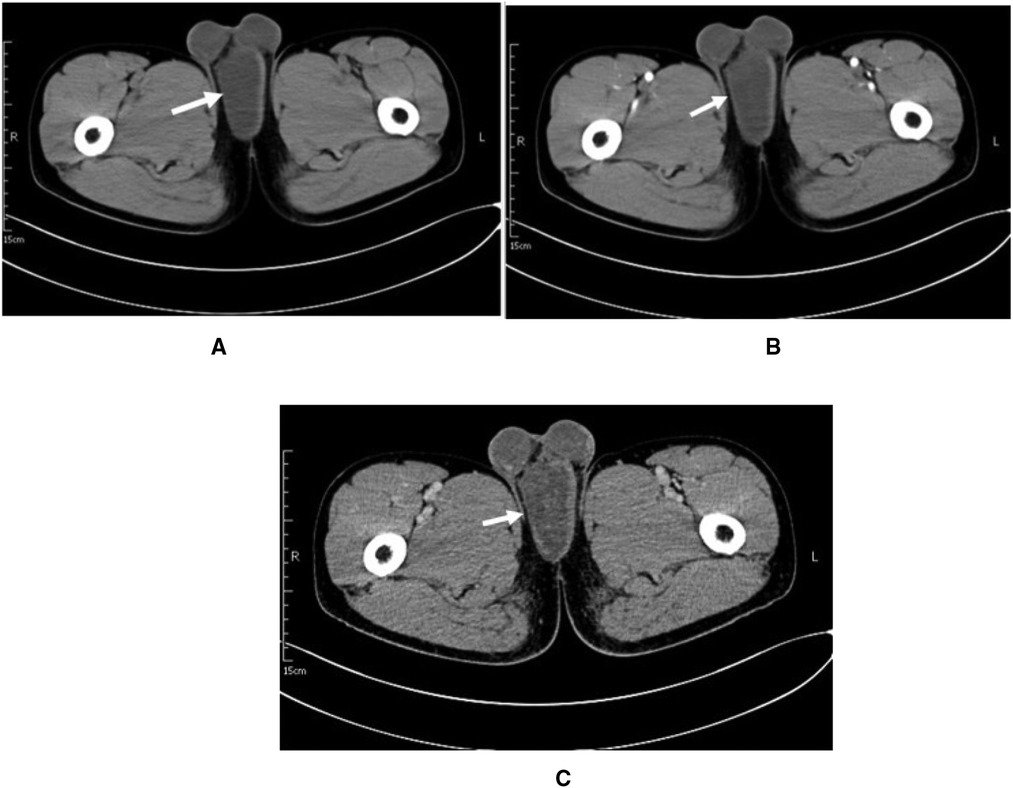
Figure 1. Patient imaging data. (A) CT plain axial image.(B) Arterial phase of the contrast. (C) Venous phase of the contrast.
After communicating with the patient, he underwent a perineal mass resection under continuous epidural anesthesia. A lithotomy position and indwelling catheterization were used. A vertical incision was made in the middle of the lower part of the scrotum. After cutting into the superficial perineal muscle layer, the tumor capsule could be seen, which was free along the tumor capsule. The tumor was close to the urethral cavernous body above the tumor, and there were tumor nutrient vessels on the inner side. After carefully ligating the vessels, the tumor was completely removed and observed. The size of the tumor was approximately 8.5 cm × 6 cm × 5 cm in size (Figure 2A); the specimen was soft. The surface was off-white, jelly-like, and rubbery after cutting (Figure 2B). The pathological report from our hospital shows that under the microscope, there were abundant interstitial mucus, fibroblasts, more thin-walled vascular hyperplasia, and no epithelial cells (Figure 3). Based on these pathological findings, the diagnosis of perineal SA was confirmed. The patient recovered successfully and was discharged on the fourth postoperative day, and is still being followed up with no recurrence, and he expressed great satisfaction with the surgical treatment.
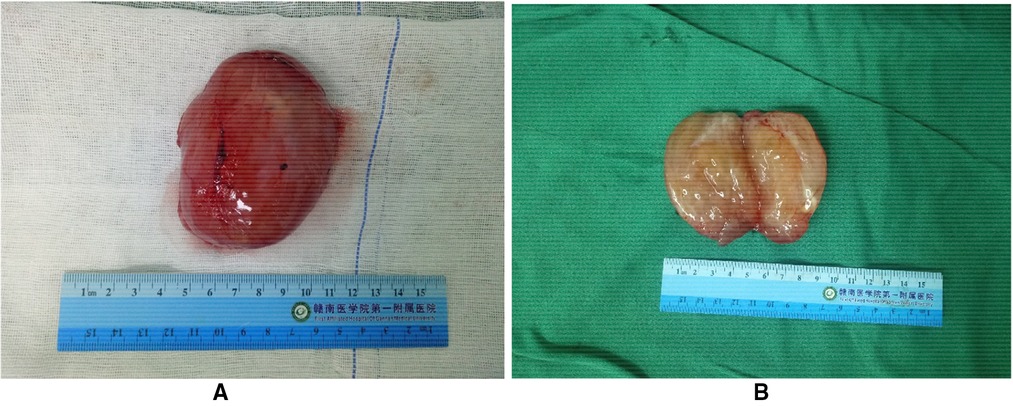
Figure 2. Patient tumor specimen. (A) The excised specimen was approximately 8.5 cm × 6 cm × 5 cm with clear margins. (B) The surface is grayish white, jelly-like, rubber-like.
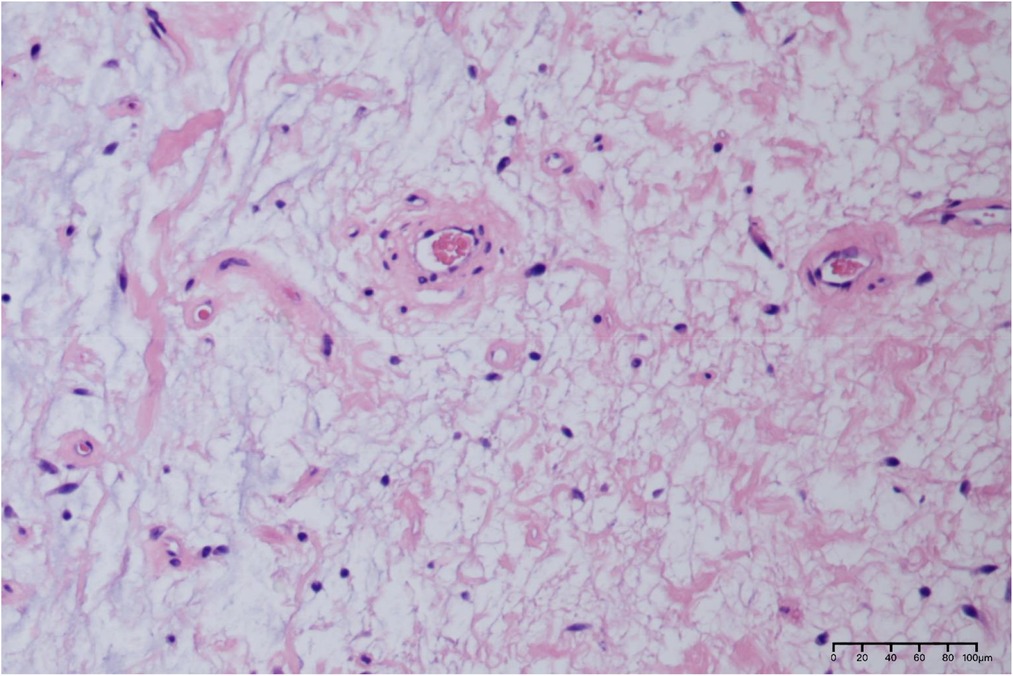
Figure 3. Pathological histological section of this case (HE × 200). Scattered spindle cells, stellate cells and perivascular inflammatory cells are visible against the background of a large amount of mucus stroma microscopically.
SA is a rare benign skin tumor, which is considered a special type of soft tissue tumor. They are difficult to diagnose because they lack unique features like fibroepithelial polyps (3). In recent years, SA has been clearly defined as an isolated soft tissue tumor entity. They can occur anywhere in superficial tissue and are painless (4), slow-growing masses. Allen et al. first reported 28 cases of SA in 1988 and named it superficial vascular mucinous adenoma (5). The clinical manifestations are mainly skin papules, nodular or polypoid masses, no pain, and a wave motion on palpation. The skin color on the surface is normal and mostly single lesion (6). In 2002, the World Health Organization classified SA as a benign tumor with undetermined differentiation (6). SA is rare in clinic and has not been reported in a large sample volume. At present, more than 30 cases have been reported in China. There is no significant gender difference in the incidence population, and the incidence is slightly higher in men than in women (4). The peak incidence is at the age of 40 years approximately (7). The disease can occur throughout the body (4, 8), mainly in the trunk, extremities, head and neck, and rarely in the perineum and vulva (3, 9). This case is a middle-aged man with a giant tumor in the perineum, which is rare.
Similar to other interstitial masses in the perineum, SAs are cystic in nature and are easily misdiagnosed as polyps or epidermoid cysts during physical examination (10). The surface of the SA is covered with epithelial tissue. It has a white, gray appearance, with occasional bleeding due to skin abrasion. It is an enveloped, soft, lobulated cystic tissue. The cut surface of the mass is shiny, colloid-like, translucent, gel-like tissue (2). The same is true for the surface of the mass incision in this case. Its pathogenesis is not yet clear. SAs may be sporadic or related to Carney syndrome, which is an autosomal dominant syndrome characterized by cardiac and mucosal skin myxoma, skin pigmentation, and a variety of endocrine gland hyperthyroidism (mainly endocrine adenoma) (11). This case is an isolated perineal SA in a man. No cardiac myxoma or other endocrine abnormalities were found during the preoperative examination.
The preoperative diagnosis of SA is difficult, and imaging helps to clarify the relationship between the SA and the surrounding tissues. On ultrasound, the SA appears as a confined round or oval mass with heterogeneous internal echogenicity. The CT/magnetic resonance imaging (MRI) scan of SA mostly shows a rounded/lobular soft tissue mass with well-defined borders and superficial lesions, with hypodense/low signal foci without significant enhancement. Its substance is the mucus-rich stromal component of the tumor. The separation of collagen bundles is also seen, showing isointensity (CT)/low signal (MRI). The literature reports a slightly dense or mixed signal with focal hemorrhage (12).
Clinically, SA usually has good boundaries and often extend to subcutaneous fat (12). Histologically, SA has an extensive mucus stroma consisting of loose spindle or stellate fibroblast-like lobular aggregates with an abundant mucus stroma containing thin-walled, medium-sized, hyaline vessels in a disorganized arrangement (13). The nuclei are ovoid, slightly darkly stained or vesicular, with inconspicuous nucleoli, no obvious heterogeneity, and rare nuclear division. In addition, a small number of inflammatory cells, mainly lymphocytes, neutrophils, and eosinophils, can be seen (14, 15). The presence of neutrophils can be a diagnostic clue, especially in the absence of skin ulceration or inflammation, as neutrophils are not present in other mucinous lesions of the skin. SA should be distinguished from all malignant and benign myxoid tumors on the surface, including aggressive angiomyxoma, myxoid neurofibroma, dermal nerve sheath myxoma, and low-grade fibromyxoid sarcoma.
Complete surgical excision is the best treatment for superficial hemangio mucinous tumors. Although SA is a benign tumor of the skin, there is a 30%–40% chance of local recurrence after surgery due to incomplete excision or blurred margins (16). A regular review and close follow-up should be done after surgery. No distant metastasis or malignancy has been reported so far (17).
The incidence of SA is low and the clinical presentation lacks specificity, but SA should be considered when painless pelvic and perineal swellings without other features are found in young and middle-aged men. A pathological examination is the gold standard for diagnosing this disease. However, the relationship between the mass and the surrounding tissues and whether it invades deep tissues can be clarified by ultrasound, MRI, and CT auxiliary examination, which is a reliable guide for judging the benignity and malignancy of the tumor and its prognosis. Extensive surgical excision is currently the main method of treatment, ensuring that the edges of the mass are removed intact to avoid postoperative recurrence. Although SA has a good prognosis, patients still need to be informed of regular follow-ups.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SY prepared and wrote the article. YZ was directly involved in the management of the patients. XL, SL, and SH were responsible for the collection and organization of the literature. CZZ prepared the intraoperative images. QL and JZ revised the manuscript and acted as corresponding authors. QL and YZ were the primary surgeons. All authors contributed to the article and approved the submitted version.
We would like to thank QL for his guidance on this paper and for editing and proofreading this manuscript in English.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore). (1985) 64(4):270–83. doi: 10.1097/00005792-198507000-00007
2. Allen PW. Myxoma is not a single entity: a review of the concept of myxoma. Ann Diagn Pathol. (2000) 4(2):99–123. doi: 10.1016/s1092-9134(00)90019-4
3. Lee SH, Cho YJ, Han M, Bae JW, Park JW, Oh SR, et al. Superficial angiomyxoma of the vulva in a postmenopausal woman: a case report and review of literature. J Menopausal Med. (2016) 22(3):180–3. doi: 10.6118/jmm.2016.22.3.180
4. Calonje E, Guerin D, McCormick D, Fletcher CD. Superficial angiomyxoma: clinicopathologic analysis of a series of distinctive but poorly recognized cutaneous tumors with tendency for recurrence. Am J Surg Pathol. (1999) 23(8):910–7. doi: 10.1097/00000478-199908000-00008
5. Allen PW, Dymock RB, MacCormac LB. Superficial angiomyxomas with and without epithelial components. Report of 30 tumors in 28 patients. Am J Surg Pathol. (1988) 12(7):519–30. doi: 10.1097/00000478-198807000-00003
6. Murphey MD. World Health Organization classification of bone and soft tissue tumors: modifications and implications for radiologists. Semin Musculoskelet Radiol. (2007) 11(3):201–14. doi: 10.1055/s-2008-1038310
7. Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. (2014) 59(5):529. doi: 10.4103/0019-5154.139893
8. Anehosur V, Adirajaiah S, Ghosh R. Intraoral superficial angiomyxoma: a case report. J Maxillofac Oral Surg. (2016) 15(Suppl 2):371–4. doi: 10.1007/s12663-016-0901-y
9. Wang YC, Li XM, Zhong GP, Xing Z, Wang ZP. Superficial angiomyxoma of penis: a case report of a 6-year follow-up. Asian J Androl. (2017) 19(2):262–3. doi: 10.4103/1008-682x.175784
10. Satter EK. Solitary superficial angiomyxoma: an infrequent but distinct soft tissue tumor. J Cutan Pathol. (2009) 36(Suppl 1):56–9. doi: 10.1111/j.1600-0560.2008.01216.x
11. Yun YI, Lee KS, Khwarg SI, Kim N. Rare case of isolated superficial angiomyxoma of the eyelid. Korean J Ophthalmol. (2020) 34(3):262–4. doi: 10.3341/kjo.2020.0010
12. Kim HS, Kim GY, Lim SJ, Ki KD, Kim HC. Giant superficial angiomyxoma of the vulva: a case report and review of the literature. J Cutan Pathol. (2010) 37(6):672–7. doi: 10.1111/j.1600-0560.2009.01333.x
13. Aberdein G, Veitch D, Perrett C. Mohs micrographic surgery for the treatment of superficial angiomyxoma. Dermatol Surg. (2016) 42(8):1014–6. doi: 10.1097/dss.0000000000000782
14. Hamzelou S, Ghanadan A, Daneshpazhooh M, Kiani A, Mahmoudi H. Superficial plantar angiomyxoma in a young man. Australas J Dermatol. (2017) 58(3):241–2. doi: 10.1111/ajd.12523
15. Zhu L, Zhao W, Shi Y, Lin B. Superficial angiomyxoma of the vulva complicated with condyloma acuminatum and Staphylococcus hominis infection. Int J Dermatol. (2014) 53(6):756–8. doi: 10.1111/j.1365-4632.2012.05572.x
16. Lee CU, Park SB, Lee JB, Park HJ, Kim MK, Chang IH. Sonographic findings of prescrotal superficial angiomyxoma. Jpn J Radiol. (2015) 33(4):216–9. doi: 10.1007/s11604-015-0395-4
Keywords: superficial angiomyxoma, perineal tumor, case report, surgical removal, clinical pathology
Citation: Yan S, Zou Y, Liao X, Zhong C, Liu S, Huang S, Zou J and Liu Q (2023) Giant superficial angiomyxoma of the male perineum: A case report. Front. Surg. 9:1010050. doi: 10.3389/fsurg.2022.1010050
Received: 2 August 2022; Accepted: 7 November 2022;
Published: 6 January 2023.
Edited by:
Riccardo Bertolo, Hospital San Carlo di Nancy, ItalyReviewed by:
Nguyen Minh Duc, Pham Ngoc Thach University of Medicine, Vietnam© 2023 Yan, Zou, Liao, Zhong, Liu, Huang, Zou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junrong Zou eWR6anJAZ211LmVkdS5jbg== Quanliang Liu bGl1cXVhbmxpYW5nMjAwOEAxNjMuY29t
†These authors share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.