
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 06 January 2023
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1003854
Background: Anastomotic leakage (AL) is a major cause of postoperative morbidity and mortality in the treatment of colorectal cancer. The aim of this study was to investigate whether the resection of “dog-ears” in laparoscopic anterior resection of rectal cancer (called modified double-stapling technique, MDST) could reduce the rate of AL in patients with middle and high rectal cancer, as compared with the conventional double-stapling technique (DST).
Methods: The clinical data of 232 patients with middle and high rectal cancer were prospectively collected from September 2015 to October 2018. They were randomly divided into the MDST group (n = 116) and the DST group (n = 116) and the data were prospectively analyzed. Morbidity and AL rate were compared between the two groups.
Results: Patient demographics, tumor size, and time of first flatus were similar between the two groups. No difference was observed in the operation time between the two groups. The AL rate was significantly lower in the MDST group than in the DST group (3.4 vs. 11.2%, p = 0.032). The age and anastomotic technique were the factors associated with AL according to the multivariate analysis. The location of the AL in the DST group was further investigated, revealing that AL was in the same place as the “dog-ears” (11/13, 84.6%).
Conclusions: Our prospective comparative study demonstrated that MDST have a better short-term outcome in reducing AL compared with DST. Therefore, this technique could be an alternative approach to maximize the benefit of laparoscopic anterior resection on patients with middle and high rectal cancer. The “dog-ears” create stapled corners potentially ischemic, since they represent the area with high incidence of AL.
(NCT:02770911)
The mortality and morbidity due to colorectal cancer is increasing worldwide. Rectal cancer in China represents approximately two-third of all cases of colorectal cancer (1). The main treatment for rectal cancer is surgery, such as anterior resection. Several results from randomized clinical trials on rectal cancer patients demonstrated that laparoscopic surgery is consistent with open surgery regarding the long-term local recurrence, disease-free survival, and overall survival (2–5). Laparoscopic surgery for rectal cancer is less invasive and facilitate the anastomosis in the deep pelvis compared with open surgery (4–7). However, many large-scale randomized controlled trials (RCTs) compared the rate of postoperative complication between laparoscopic and open surgery, discovering no reduced the rate of anastomotic leakage (AL) (4–7).
AL in rectal cancer surgery is one of the most critical complications affecting not only the short-term outcomes but also the long-term (8–11). Moreover, recent studies revealed that the incidence of AL is from 6% to 17% after the anterior resection of the rectal cancer by laparoscopic surgery (12–14). Therefore, it is not yet possible to reduce the incidence of AL after rectal surgery. Double-stapling technique (DST) is the key step representing the technical difficulty when performing laparoscopic anterior resection for rectal cancer (15), and it is the major contributing factor of AL. DST is characterized by the placement of at least two staple lines crossing each other, creating stapled corners (called “dog-ears”) potentially ischemic, which represent the area with high incidence of AL (16).
DST for low anterior resection, reported first by Knight et al. in 1980, involves rectal transection with a linear stapler and the creation of anastomosis with a circular stapler (17). Kang et al. (18) reported a modified double-stapling technique (MDST) to remove the dog-ears in open surgery by excising the distal bowel. Their results showed that the AL rate was remarkably reduced in MDST, but their procedure was successful on sigmoid colon cancer, that was powerless for middle and high rectal cancer. Our previous study involved 110 patients with high rectal cancer and sigmoid colon cancer subjected to surgery performed entirely by MDST (19). Our previous results preliminary confirmed that MDST reduces the AL rate after laparoscopic anterior resection compared to what it was demonstrated in other previous results.
Therefore, the present study was a prospective and randomized comparison that investigates the feasibility and impact on surgical outcomes of MDST compared with DST in laparoscopic anterior resection performed on patients with middle and high rectal cancer.
The clinical data of 232 patients with middle and high rectal cancer who underwent laparoscopic anterior resection were prospectively collected between September 2015 and October 2018. Patients were randomly divided into MDST group (n = 116) and DST group (n = 116). The inclusion criteria where the following: age between 18 and 80 years, middle and high rectal cancer with the lower margin of the tumor located above the retroperitoneal fold. The exclusion criteria were as follows: patients who underwent neoadjuvant or adjuvant chemoradiotherapy, patients with an American Society of Anesthesiologists score IV, patients who had distant metastasis at initial diagnosis. All patients gave the written informed consent, and all the procedures were performed by the same team of board-certified colorectal surgeons at the Colorectal Surgery Department of Fujian Medical University, Union Hospital. The study was approved by the hospital review board (2016YF012-01). The patients flow chart is shown in Figure 1.
Patients were recruited at the participating hospital before the start of any treatment and randomly divided (1:1) by ProMISe data management system (version 4.0) using a stratified and randomly varying block design applied to both the experimental group (MDST) and the standard group (DST). The stratifying factors were institution, ECOG scale of performance status (0 or 1), cT stage (cT2–cT3 or cT4), and cN stage (cN– or cN+). Randomization was coordinated by the Clinical Research Center. The investigators were blinded to the treatment assignment during the evaluation of the primary endpoint until the prespecified number of events was reached. However, patients and clinical staff were not blinded to the assigned group due to the nature of the intervention.
Our technique of laparoscopic surgery for the treatment of the left-sided colorectal cancer was previously described in detail (20, 21). Patient preparation and the dissection method before anastomosis were not different between the MDST group and the DST group. The mesorectum at the point where the rectal wall was transected, was mobilized for a clean colorectal anastomosis before the application of the laparoscopic linear stapler (ECR60G, Ethicon Endo-Surgery Inc.) to the distal bowel, and the surgeon kept the layer of the longitudinal muscle intact. The transection of the distal rectum was performed using one cartridge. The assistant inserted the circular stapler device (CDH33, Ethicon Endo-Surgery Inc.) through the anus up to the close rectal lumen after the removal of the anvil and the formation of the staple line forming the dog-ears, as clearly shown on the rectal stump. Then the trocar was extended, and the rectal wall was perforated by gently rotating the instrument. The surgeon made a laparoscopic suture on the two dog-ears in the patients of the MDST group using 3-0 monofilament sutures, then the trocar was fully extended, and the dog-ears were removed from the staple line around the trocar by means of a suture tied through them (Figure 2A). In this way, the staple line was kept within the circular knife when the circular stapler was closed. Then a true end-to-end anastomosis was performed after stapler firing (Figures 2B,C). However, the surgeon did not make a laparoscopic suture on the two dog-ears in the patients of the DST group (Figure 2D). After anastomosis, the air leak test was performed in the patients of both groups. A drain was routinely placed in the pelvic cavity, near the anastomosis, before the closure of the abdominal incision. No protective stoma was made.
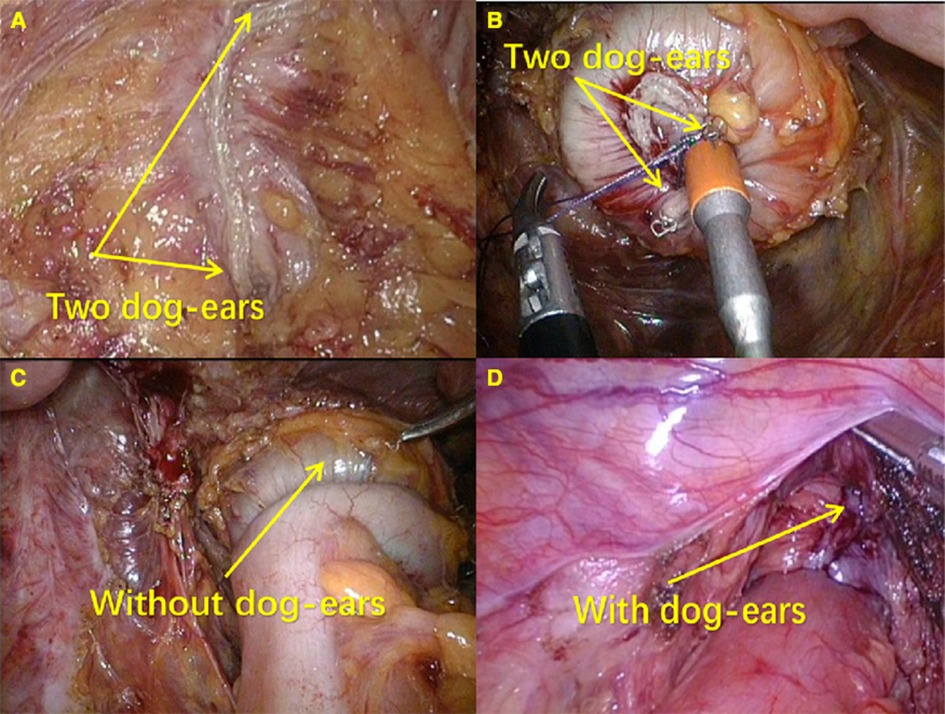
Figure 2. End-to-end anastomosis by DST and MDST. (A) Suturing two corners of the staple line around the trocar; (B) Without “dog ears” after anastomosis; (C) Distal rectum with “dog ears”; (D) With “dog ears” after anastomosis.
Perioperative outcomes including duration of the operation, length of the resection margin, time to first flatus, resumption of a soft diet, length of hospital stay, and morbidity were compared between the two groups. AL was diagnosed based on clinical signs or image studies. The clinical signs included abdominal pain or fever, production of pus or feces through the indwelling drain, and local or generalized peritonitis. Radiologic evaluation was performed to confirm the clinical suspicion.
Statistical analysis was performed using SPSS (version 22; IBM, Armonk, NY).
The χ2 test or Fisher's exact test was used to compare the categorical variables, while Student's t-test or Mann–Whitney U test was used to compare continuous variables. A p value less than 0.05 was considered statistically significant.
A total of 387 middle and high rectal cancer patients accepted to be subjected to the anterior resection by our surgery team. In addition, distant metastases were found in 50 patients and 12 patients accepted the emergency resection. Fifteen patients suffered from familial adenomatous polyposis or Lynch syndrome, 20 patients had previous or concurrent malignancies, and 24 patients refused to participate to the clinical trial. A total of the 268 patients were enrolled in the clinical trial. However, during the surgery 12 patients were transferred to open surgery and 22 patients accepted the left hemi-colectomy. The above patients were excluded from the clinical trial. Finally, 232 patients were randomly assigned (1:1) to the DST and MDST group. Age, gender, previous history of abdominal surgery, body mass index, American Society of Anesthesiologists grade, and tumor location in patients who underwent MDST were similar to those who underwent DST. No difference was found on pathologic outcomes such as tumor size, number of metastatic lymph nodes and pathological TNM stage between the two groups (Table 1).
The perioperative outcomes and postoperative recovery were the important results to evaluate the anastomotic techniques. In the present study, the perioperative outcomes and postoperative recovery of each group were compared. The result demonstrated that the operation time and blood loss did not increase in the MDST group (p = 0.581 and p = 0.209). Moreover, the result of the postoperative complication revealed that the total rate of complication between the two groups was similar (DST, 12.9% vs. MDST, 6.9%; p = 0.124). However, the AL rate was significantly lower in the MDST group compared with the DST group (DST, 11.2% vs. MDST, 3.4%; p = 0.032). Other postoperative complications such as anastomotic hemorrhage, ileus, wound infection, chyle leakage and urinary tract infection were not different between the two groups (all p > 0.05). The result of the postoperative recovery demonstrated that the length of hospital stay and the time of first bowel movement in the patients of the MDST group were significantly decreased (p = 0.032 and p = 0.005, respectively). No difference in the time of first flatus, time of resuming a soft diet, and time of resuming a semi-liquid diet was found between the two groups (Table 2).
AL in rectal cancer surgery is one of the most critical complications, and it affects not only short-term outcomes but also the long-term ones such as local recurrence and overall survival (8–11). The risk factors for the AL were evaluated, and the univariate analysis demonstrated that age (p = 0.014) and MDST (p = 0.032) were the protective factors for AL. The multivariate analysis, revealed that younger (OR = 0.945, CI: 0.900–0.993, p = 0.024) and MDST (OR = 0.308, CI: 0.096–0.987, p = 0.047) were the protect factors associated with AL (Table 3).
The patients with AL in the DST group were subjected to colonoscopy one week after the surgery to further confirm the location of AL in the DST group (Table 4 and Figure 3). AL was located were the “dog-ears” were located in the DST groups (11/13, 84.6%). The above result revealed that the “dog-ears” as the two staple lines crossing each other, created potentially ischemic stapled corners with high incidence of AL (16).
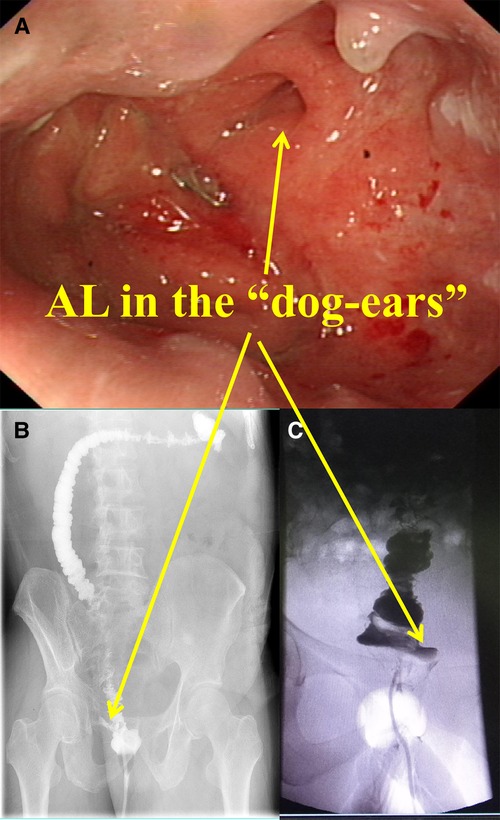
Figure 3. Diagram of AL. (A) The colonoscopy of the AL patinets. (B) and (C) The x-ray of the AL patinets.
In recent years, laparoscopic rectal surgery has become popular because of the its minimally invasiveness and advantage of its magnified view (2–5). This surgery requires a safe and precise technique for completing the total mesorectal excision within the narrow pelvis. However, many large-scale RCTs comparing the oncological outcomes and long-term outcomes between laparoscopic and open surgery did not reveal a reduced postoperative complication rate, including AL (2–5). The present study demonstrated that MDST could reduce the incidence of AL in the laparoscopic low anterior resection (22–26). Moreover, the location of AL was explored in the DST group, and the result demonstrated that it was located in the same place of the “dog-ears”. The above result indicated that the removal of the “dog-ears” could efficiently decrease AL in the laparoscopic high/mid anterior resection (22–26).
DST for low anterior resection was firstly reported by Knight et al., in 1980, and involves rectal transection with a linear stapler and the creation of an anastomosis with a circular stapler. This method commonly used in the past to perform open surgery contributed to the recent explosive spread of laparoscopic low anterior resection (17). Despite various studies explored the risk factors for AL in the open low anterior resection, few reports explored them in laparoscopic low anterior resection (22–26). The low anterior resection is often performed with a stoma creation to reduce AL or reoperation (15, 27–30). However, since stoma can cause stoma-related complications and cosmetic problems, it should not be performed in all patients with low anterior resection. Thus, to explore the risk factors for AL in patients without stoma can help surgeons to decide to create stoma or not.
The anastomosis is the key of the success and difficulty of the whole surgery, that is directly related to the postoperative functional recovery and complications of patients with middle and high rectal cancer (15, 17). Conventional DST significantly improved the anal preservation rate of rectal cancer patients, but the incidence of AL was not significantly improved (31), The occurrence of AL might be closely related to the following points, except for the anastomotic blood supply and anastomotic tension (32). First, when the distal rectum with linear cutting is closer to the transverse cutting, it gradually tapered off until becoming very thin. “Dog-ears” were produced on both sides when the two ends were closed using tubular anastomotic matches. The tissue of the dog-ear is relatively weak due to the elongation and thinning. This could be the anatomical and histological basis of AL and diverticulitis. Second, a “T” shaped junction of the suture line was formed after anastomosis, which is a recessive ischemia area and high-risk area of AL. Many scientists noticed its dangerous consequences and called it the “dangerous triangle” (21). Finally, at present a consensus was reached regarding the inevitable risk of anastomotic leakage after pre-rectal resection. The AL rate has been reported by various studies as very different. Thus, further studies are needed to establish a relationship with the surgical design and operation.
Previous studies reported that dog-ears retained by the traditional DST are an important risk factor for AL (33, 34). In addition, the anastomotic stenosis rate of traditional DST with retained “dog-ears” were higher than that in the single stapling technique (35). Many surgeons made efforts in removing the dog-ears in order to reduce AL. Marecik et al. (36). described a reliable single-stapled double purse-string anastomosis, which could also remove the dog-ears. Only one case of class C AL occurred in this study (0.6%). Sileri et al. (37). used the KOLTM circular stapler for the removal of the dog-ears. They used two straight and long needles and four 2-0 prolene sutures to pull the staple line on the rectal stump into the rectal lumen through the circular anal dilator placed in the anus. Kim et al. (21) reported that the single stapled technique without dog-ears could be intracorporeally performed with the application of robotic surgery. The AL rate was not significantly different between the single stapled technique group and DST group in this study. Kang et al. (32) reported a modified MDST to eliminate the dog-ears in open surgery by pulling out the distal bowel. The AL rate was clearly reduced in MDST. Kang's procedure was not performed entirely using laparoscopy, and the procedure was mainly studied on sigmoid colon cancer, but it was not effective when applied to middle and high rectal cancer.
At present, many reports are available regarding the use of DST for rectal cancer, but only few reports are available on the radical cure, safety and short-term efficacy of DST and MDST. In this study, these two surgical techniques were compared, with the aim of providing an individualized treatment and more reasonable surgical strategies for patients with middle and high rectal cancer. A prospective randomized controlled study was performed, and the distal rectum insufflate was removed using Johnson EC60 linear cutting anastomat, which is 5 cm above the anal edge. The two horns of the distal intestinal canal were dragged into the Johnson GF-33 mm tubular anastomat nail “warehouse” within “end-to-end” anastomosis with stitches during the laparoscopic surgery. No “dog-ears” and “dangerous triangle” remained after the anastomosis as long as the two-cut end anastomosis ring was complete, all without prophylactic enterostomy. As regard the rectal stump, which is less than 5 cm below the anal margin, it is difficult to suture and embed the two “dog-ears” under the laparoscope, while it is easy to stretch and split the rectal stump with the Johnson GF-33 mm tubular staplers. Therefore, this study only discussed the application of this method for middle and high rectal cancer, which could significantly reduce AL rate and anterior resection syndrome without increasing the difficulty and cost of the surgery. In addition, it avoids the pain caused by prophylactic enterostomy and produces economic and social benefits.
Several limitations of our study should be mentioned. First, the present randomized controlled trial was performed on patients from one center. Thus, the results obtained should be verified by a multi-center randomized controlled trial involving different countries and races. Second, a higher number of patients was not used in the present study due to the limitation of the study time. Thirdly, we did not explore the rate od th AL in the locally advanced rectal patients who accepted the neo-chemoradiotherapy, which has the high rates of the AL. Despite these limitations, our study increases our understanding of the impact of MDST in the low anterior resection.
This study revealed that the AL rate in the MDST patients was lower than that in the DST patients, and the postoperative defecation time was reduced. Thus, MDST was the protective factor for AL in the laparoscopic low anterior resection. Moreover, the location of the leakage was associated with the residue “dog-ears”.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by This study was carried out in accordance with the committee of Fujian Medical University Union Hospital with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by committee of the Fujian Medical University Union Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YF, FD, TBX, XL and GXG: designed the experiments, performed the experiments, analyzed the data, and wrote the paper. ZP and JFZ: performed the experiments. All authors contributed to the article and approved the submitted version.
This study was supported by the National Foundation of China (grant no. 82172800), Science Foundation of the Fujian Province, (grant no. 2019J01161), Special Financial Foundation of Fujian Provincial (grant no. 2015-1297; 2020B019), the Joint Funds for the innovation of science and Technology, Fujian province (grant no. 2020Y9125), the Funds for the innovation of medical, Fujian province (grant no. 2021CXB009), and Talent programs granted from The First Affiliated Hospital of Fujian Medical University (grant no. YJRC3600).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. (2015) 150:17–22. doi: 10.1001/jamasurg.2014.1756
2. Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. (2005) 365:1718–26. doi: 10.1016/S0140-6736(05)66545-2
3. van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. (2013) 14:210–8. doi: 10.1016/S1470-2045(13)70016-0
4. Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. (2014) 15:767–74. doi: 10.1016/S1470-2045(14)70205-0
5. Acuna SA, Chesney TR, Ramjist JK, Shah PS, Kennedy ED, Baxter NN. Laparoscopic versus open resection for rectal cancer: a noninferiority meta-analysis of quality of surgical resection outcomes. Ann Surg. (2019) 269:849–55. doi: 10.1097/SLA.0000000000003072
6. Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. (2015) 314:1356–63. doi: 10.1001/jama.2015.12009
7. Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. (2015) 314:1346–55. doi: 10.1001/jama.2015.10529
8. Ha GW, Kim JH, Lee MR. Oncologic impact of anastomotic leakage following colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg Oncol. (2017) 24:3289–99. doi: 10.1245/s10434-017-5881-8
9. Hüttner FJ, Warschkow R, Schmied BM, Diener MK, Tarantino I, Ulrich A. Prognostic impact of anastomotic leakage after elective colon resection for cancer - A propensity score matched analysis of 628 patients. Eur J Surg Oncol. (2018) 44:456–62. doi: 10.1016/j.ejso.2018.01.079
10. Ramphal W, Boeding J, Gobardhan PD, Rutten H, de Winter L, Crolla R, et al. Oncologic outcome and recurrence rate following anastomotic leakage after curative resection for colorectal cancer. Surg Oncol. (2018) 27:730–6. doi: 10.1016/j.suronc.2018.10.003
11. Park JS, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, et al. Risk factors of anastomotic leakage and long-term survival after colorectal surgery. Medicine. (2016) 95:e2890. doi: 10.1097/MD.0000000000002890
12. Braga M, Frasson M, Vignali A, Zuliani W, Capretti G, Di Carlo V. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum. (2007) 50:464–71. doi: 10.1007/s10350-006-0798-5
13. Liang X, Hou S, Liu H, Li Y, Jiang B, Bai W, et al. Effectiveness and safety of laparoscopic resection versus open surgery in patients with rectal cancer: a randomized, controlled trial from China. J Laparoendosc Adv Surg Tech A. (2011) 21:381–5. doi: 10.1089/lap.2010.0059
14. Bernasconi M, Metzger J. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer (Br J Surg 2009; 96: 982-989). Br J Surg. (2010) 97:619–20; author reply 620. doi: 10.1002/bjs.7058
15. Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, et al. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery. (2009) 146:483–9. doi: 10.1016/j.surg.2009.03.030
16. Kim HJ, Choi GS, Park JS, Park SY. Comparison of intracorporeal single-stapled and double-stapled anastomosis in laparoscopic low anterior resection for rectal cancer: a case-control study. Int J Colorectal Dis. (2013) 28:149–56. doi: 10.1007/s00384-012-1582-8
17. Knight CD, Griffen FD. An improved technique for low anterior resection of the rectum using the EEA stapler. Surgery. (1980) 88:710–4. PMID: 74342117434211
18. Kang J, Lee HB, Cha JH, Hur H, Min BS, Baik SH, et al. Feasibility and impact on surgical outcomes of modified double-stapling technique for patients undergoing laparoscopic anterior resection. J Gastrointest Surg. (2013) 17:771–5. doi: 10.1007/s11605-012-2122-0
19. Chen ZF, Liu X, Jiang WZ, Guan GX. Laparoscopic double-stapled colorectal anastomosis without "dog-ears". Tech Coloproctol. (2016) 20:243–7. doi: 10.1007/s10151-016-1437-3
20. Kim JS, Cho SY, Min BS, Kim NK. Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg. (2009) 209:694–701. doi: 10.1016/j.jamcollsurg.2009.09.021
21. Kang J, Baek SE, Kim T, Hur H, Min BS, Lim JS, et al. Impact of fat obesity on laparoscopic total mesorectal excision: more reliable indicator than body mass index. Int J Colorectal Dis. (2012) 27:497–505. doi: 10.1007/s00384-011-1333-2
22. Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi T, et al. Incidence of and risk factors for anastomotic leakage after laparoscopic anterior resection with intracorporeal rectal transection and double-stapling technique anastomosis for rectal cancer. Am J Surg. (2011) 202:259–64. doi: 10.1016/j.amjsurg.2010.11.014
23. Yamamoto S, Fujita S, Akasu T, Inada R, Moriya Y, Yamamoto S. Risk factors for anastomotic leakage after laparoscopic surgery for rectal cancer using a stapling technique. Surg Laparosc Endosc Percutan Tech. (2012) 22:239–43. doi: 10.1097/SLE.0b013e31824fbb56
24. Kayano H, Okuda J, Tanaka K, Kondo K, Tanigawa N. Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc. (2011) 25:2972–9. doi: 10.1007/s00464-011-1655-8
25. Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, et al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. (2013) 257:665–71. doi: 10.1097/SLA.0b013e31827b8ed9
26. Katsuno H, Shiomi A, Ito M, Koide Y, Maeda K, Yatsuoka T, et al. Comparison of symptomatic anastomotic leakage following laparoscopic and open low anterior resection for rectal cancer: a propensity score matching analysis of 1014 consecutive patients. Surg Endosc. (2016) 30:2848–56. doi: 10.1007/s00464-015-4566-2
27. Veenhof AA, van der Peet DL, Meijerink WJ, Cuesta MA. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. (2008) 247:718–9; author reply 719-20. doi: 10.1097/SLA.0b013e31816a7493
28. Chude GG, Rayate NV, Patris V, Koshariya M, Jagad R, Kawamoto J, et al. Defunctioning loop ileostomy with low anterior resection for distal rectal cancer: should we make an ileostomy as a routine procedure? A prospective randomized study. Hepatogastroenterology. (2008) 55:1562–7. PMID: 1910234319102343
29. Ulrich AB, Seiler C, Rahbari N, Weitz J, Büchler MW. Diverting stoma after low anterior resection: more arguments in favor. Dis Colon Rectum. (2009) 52:412–8. doi: 10.1007/DCR.0b013e318197e1b1
30. Thoker M, Wani I, Parray FQ, Khan N, Mir SA, Thoker P. Role of diversion ileostomy in low rectal cancer: a randomized controlled trial. Int J Surg. (2014) 12:945–51. doi: 10.1016/j.ijsu.2014.07.012
31. Reilly F, Burke JP, Appelmans E, Manzoor T, Deasy J, McNamara DA. Incidence, risks and outcome of radiological leak following early contrast enema after anterior resection. Int J Colorectal Dis. (2014) 29:453–8. doi: 10.1007/s00384-013-1820-8
32. Yang L, Huang XE, Zhou JN. Risk assessment on anastomotic leakage after rectal cancer surgery: an analysis of 753 patients. Asian Pac J Cancer Prev. (2013) 14:4447–53. doi: 10.7314/apjcp.2013.14.7.4447
33. Roumen RM, Rahusen FT, Wijnen MH, van Uchelen FA C. “Dog ear” formation after double-stapled low anterior resection as a risk factor for anastomotic disruption. Dis Colon Rectum. (2000) 43:522–5. doi: 10.1007/BF02237198
34. Asao T, Kuwano H, Nakamura J, Hirayama I, Ide M, Moringa N, et al. Use of a mattress suture to eliminate dog ears in double-stapled and triple-stapled anastomoses. Dis Colon Rectum. (2002) 45:137–9. doi: 10.1007/s10350-004-6129-9
35. Sadahiro S, Kameya T, Iwase H, Ishikawa K, Suzuki T, Tokunaga N, et al. Which technique, circular stapled anastomosis or double stapling anastomosis, provides the optimal size and shape of rectal anastomotic opening. J Surg Res. (1999) 86:162–6. doi: 10.1006/jsre.1999.5701
36. Marecik SJ, Chaudhry V, Pearl R, Park JJ, Prasad LM. Single-stapled double-pursestring anastomosis after anterior resection of the rectum. Am J Surg. (2007) 193:395–9. doi: 10.1016/j.amjsurg.2006.12.008
Keywords: middle and high rectal cancer, anastomotic leakage, modified double-stapling technique, laparoscopic, anterior resection
Citation: Yang Y, Ding F, Xu T, Pan Z, Zhuang J, Liu X and Guan G (2023) Double-stapled anastomosis without “dog-ears” reduces the anastomotic leakage in laparoscopic anterior resection of rectal cancer: A prospective, randomized, controlled study. Front. Surg. 9:1003854. doi: 10.3389/fsurg.2022.1003854
Received: 26 July 2022; Accepted: 31 October 2022;
Published: 6 January 2023.
Edited by:
Cesare Ruffolo, University Hospital of Padua, ItalyReviewed by:
Willem Bemelman, Academic Medical Center, Netherlands© 2023 Yang, Ding, Xu, Pan, Zhuang, Liu and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoxian Guan eGh5eWdneEBmam11LmVkdS5jbg== Xing Liu ZmptdWZ5bHhAMTYzLmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.