
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 04 January 2023
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1003525
This article is part of the Research Topic Digital Revolution in Oncology: How Digital Tools Transform the Evaluation and Management of Cancer Patients View all 24 articles
 Hongming Cui1†
Hongming Cui1† Dawei Zhao1†
Dawei Zhao1† Jingren Jian2†
Jingren Jian2† Yifei Zhang1
Yifei Zhang1 Mi Jian1
Mi Jian1 Bin Yu1
Bin Yu1 Jinchen Hu1
Jinchen Hu1 Yanbao Li3
Yanbao Li3 Xiaoli Han3
Xiaoli Han3 Lixin Jiang1,3*
Lixin Jiang1,3* Xixun Wang1*
Xixun Wang1*
Purpose: To identify risk factors associated with short-term postoperative complications in patients with gastrointestinal cancer and develop and validate prediction models to predict the probability of complications.
Methods: A total of 335 patients enrolled in the primary cohort of this study were divided into training and validation sets in a chronological order. Using univariate and multivariate logistic regression analyses, the risk factors for postoperative complications were determined, and nomogram prediction models were constructed. The performance of the nomogram was assessed with respect to the receiver operator characteristic and calibration curves.
Results: Patients with complications had a stronger postoperative stress response and a longer duration of daily fluid intake/output ratio >1 after surgery. Logistic analysis revealed that body mass index (BMI), body temperature on POD4 (T.POD4), neutrophil percentage on POD4 (N.POD4), fasting blood glucose on POD4 (FBG.POD4), and the presence of fluid intake/output ratio <1 within POD4 were risk factors for POD7 complications, and that BMI, T.POD7, N.POD7, FBG.POD4, FBG.POD7, and the duration of daily fluid intake/output ratio >1 were risk factors for POD30 complications. The areas under the curve of Nomogram-A for POD7 complications were 0.867 and 0.833 and those of Nomogram-B for POD30 complications were 0.920 and 0.918 in the primary and validation cohorts, respectively. The calibration curves showed good consistency in both cohorts.
Conclusion: This study presented two nomogram models to predict short-term postoperative complications in patients with gastrointestinal cancer. The results could help clinicians identify patients at high risk of complications within POD7 or POD30.
Gastric and colorectal cancers are among the top five causes of morbidity and mortality among all cancer patients in China (1, 2). Surgical treatment of gastrointestinal cancers remains an essential but aggressive treatment option (3). Meanwhile, postoperative complications represent the greatest obstacle that hinders recovery (4). When complications are treated inappropriately, they may lead to a rapid decline in the quality of life of the patient as well as an increase in medical expenses and mortality (5, 6). Therefore, early detection of and intervention for complications can effectively reduce the duration of hospitalization and medical expenditure.
Most gastrointestinal surgical complications are not obvious and are difficult to detect in the early stages (7). Therefore, early recognition and intervention are critical for postoperative treatment. Physical symptoms or laboratory tests can reveal early signs of postoperative complications; however, only a handful of studies have efficiently integrated these clinical data to assist in clinical decision-making (8).
The occurrence of complications is intimately linked to the stress response after surgery, and their occurrence usually indicates a high level of stress in the body. The high stress response in the early postoperative period also indicates that postoperative complications are likely to occur, which provides a reference point for predicting postoperative complications (9, 10).
Minimally invasive surgery combined with enhanced recovery after surgery is an important aspect of standardized gastrointestinal tumor management. Clinical trials have shown that they can improve short-term outcomes and long-term survival. However, postoperative complications remain an important clinical problem (11–13). The aim of the current study was to develop and validate a nomogram to estimate the possibility of postoperative complications in patients undergoing gastrointestinal tract surgery by incorporating routine indicators monitored in the postoperative setting.
This study included patients with gastrointestinal tumors who underwent standard surgical treatment between June 2020 and July 2021 in the Gastrointestinal Unit of Yantai Yuhuangding Hospital, Qingdao University. The inclusion criteria were as follows: (i) age ≥18 years; (ii) no obvious contraindications in preoperative examination; (iii) preoperative pathology determined to be gastric or colorectal cancer; (iv) Eastern Cooperative Oncology Group performance status of ≤3; and (v) life expectancy of ≥6 months. The exclusion criteria were as follows: (i) without complete baseline examination; (ii) with the presence of secondary tumor or comorbidities on the preoperative examination that required emergency surgery; (iii) current or a history of malignancy in addition to gastrointestinal tumors; (iv) with other diseases that could either affect the study results or were uncontrollable.
This retrospective study was approved by the ethics committee of Yantai Yuhuangding Hospital, Qingdao University, and the requirement for informed consent was waived due to the retrospective nature of the study.
Perioperative clinical data, such as baseline characteristics and laboratory results, were collected from each patient. The baseline characteristics included sex, age, body mass index (BMI), history of smoking and alcohol consumption, neoadjuvant chemoradiotherapy (nCR, and previous cardiovascular disease and diabetes. Laboratory data included preoperative white blood cell count, neutrophil percentage (N), fasting blood glucose (FBG), alanine aminotransferase, and aspartate aminotransferase levels on postoperative days 1 (POD1), 4 (POD4), and 7 (POD7). We also recorded the daily fluid intake and output per patient, including perioperative infusion, bleeding, drainage, and urine volume. We further calculated the daily fluid output and fluid difference using the following formula:
Note that VOL is short for the letter “volume”. Invisible water loss was set at 900 ml and increased with increasing body temperature; by 200 ml when body temperature was between 37.3 °C and 37.7 °C, 500 ml between 37.8 °C and 38.3 °C, and 800 ml above 38.3 °C. Then, we assessed whether each patient had a fluid intake/output ratio <1 within POD4 (It means two consecutive days of daily fluid intake/output ratio <1 within POD4) and the final duration of daily fluid intake/output ratio >1. The first day of the first two consecutive days with a ratio <1 was estimated as the final duration of fluid intake/output ratio >1 from POD1. If there were two consecutive days without a ratio <1 within POD7, the duration was estimated as 7 days when there was a ratio <1 on POD7 and 8 days when there was still a ratio >1 on POD7. To explore the stress status of the patients, we measured levels of stress indicators, including perioperative C-reactive protein (CRP), interleukin-6 (IL-6), and cortisol.
In addition, according to each of their postoperative monitored vital signs, laboratory test results, and postoperative treatment measures, the presence of postoperative complications was assessed and recorded within POD7 or POD30 based on the first day of its appearance. Each postoperative complication was graded according to the Clavien–Dindo classification (14).
Preoperative laboratory tests and examinations were performed in all patients to exclude clear contraindications to surgery. All surgeries were performed by five gastrointestinal specialists who performed more than 80 similar surgeries annually at Yantai Yuhuangding Hospital. At the end of the surgery, one or two drains were placed at the anastomosis and closed stump subcutaneously in all patients. All patients were routinely treated with prophylactic antibiotics, nutritional support, pain relief, and other symptomatic treatments after surgery. Postoperative routine blood tests and biochemistry were performed every 3 days. Gastrointestinal tract images was reviewed before discharge, and drains were removed if there were no signs of leakage. Simultaneously, patients were closely monitored for postoperative complications during the treatment process. Once they occurred, early intervention was provided.
We identified independent risk factors associated with postoperative complications by univariate and multifactorial logistic regression analyses, and the nomogram was built based on the independent risk factors in the multivariate analysis. First, we identified the independent risk factors associated with POD7 complications by using research variables and the presence or absence of a fluid intake/output ratio <1 within POD4 and then developed Nomogram A (Nomogram-A). Secondly, we identified the independent risk factors associated with POD30 complications by using research variables and the final duration of fluid intake/output ratio >1 within POD7 and then developed Nomogram B (Nomogram-B).
The recognition performance of Nomograms-A and -B were evaluated using receiver operator characteristic (ROC) curves in the training and validation sets (15). Comparisons between ROC curves were performed using the Delong test (16). The prediction accuracy of the nomogram was evaluated using calibration curves and the Hosmer–Lemeshow test (17).
Statistical analyses were performed using SPSS (version 26.0) and R (version 3.6.2) software. Exact variables were analyzed using Student's t-test, and categorical variables were analyzed using the χ2 test or Fisher's exact test in the baseline table. Correlation analysis was performed using Spearman's correlation test. The independent risk factors were determined using univariate and multifactorial logistic regression analyses. Nomograms and calibration curves were plotted using the “RMS” software package. The ROC curves were plotted using the “pROC” software package. For all tests, a two-sided P < 0.05 was considered statistically significant.
A total of 335 patients (225 men and 110 women) were included in this study and were divided chronologically into training (n = 223) and validation (n = 112) sets. The patients were also divided into a group with (n = 233) and without (n = 102) complications. Baseline patient characteristics and outcomes of the training and validation sets are shown in Table 1. There were no significant differences in the baseline patient characteristics between the training and validation sets, indicating good consistency between the two cohorts.
Among all patients, 146 had gastric cancer and 189 had colorectal cancer. Additionally, 60 patients had defined borderline resectable tumors, and 60 patients received 2–4 cycles of nCRT. The median and average postoperative hospital stay were 7 and 8.7 days, respectively.
In the group with complications, 86 and 102 patients developed postoperative complications within POD7 and POD30 (including those within POD7), respectively (Table 2). According to the Clavien–Dindo classification, 20 patients had major complications (Clavien–Dindo grades III/IV/V). One patient with Clavien–Dindo grade V was a man who suffered respiratory failure after radical gastric cancer surgery and died after ineffective treatment in the ICU. The complications in the remaining patients were effectively controlled or cured after standard treatment.
We monitored the preoperative CRP, IL-6, and cortisol levels on POD1, POD4, and POD7 in partial patients from the training set (n = 168). Comparing the stress indicators between the groups with and without complications, the CRP, IL-6, and cortisol levels were significantly higher in the group with complications than in those without complications on POD1, POD4, and POD7 (P < 0.05); however, there was no significant difference between the groups before surgery (P > 0.05) (Table 3).
The results of this study further confirm that patients with postoperative complications generally have a stronger postoperative stress response than those without postoperative complications, and the presence of a strong stress response in the early postoperative period may reflect the occurrence of postoperative complications.
This study also analyzed perioperative fluid volumes between the groups of patients with and without postoperative complications. Intraoperative bleeding and infusion volumes in the group with complications were not significantly different from those in the group without complications (P > 0.05) (Table 4). In contrast, during postoperative fluid therapy, the daily fluid difference from POD2 in the group with complications was lower than that in the group without complications (P < 0.05), and the fluid gap increased significantly from POD4. This gap between the two groups persisted until the last day of statistical analysis. Short-term postoperative complications tended to occur 3–5 days after surgery; therefore, we considered this increase in variance to be related to the timing of postoperative complications. We also compared the presence or absence of fluid intake/output ratio <1 within POD4 and the final duration of fluid intake/output ratio >1 between both groups. Patients with complications were less likely to have a fluid intake/output ratio <1 within POD4 (10.5% vs. 36.5%, P < 0.05) and had a significantly longer duration of fluid intake/output ratio >1 postoperatively than those without complications (6.18 days vs. 3.86 days, P < 0.05).
Through our study, we considered that changes in postoperative fluid difference may be associated with the development of postoperative complications and further discuss whether they could be risk factors for the development of postoperative complications.
First, we used clinical data within POD4 and identified five risk factors associated with the occurrence of complications within POD7 by univariate and multifactorial logistic regression analyses (Table 5): BMI, body temperature on POD4 (T.POD4), neutrophil percentage on POD4 (N.POD4), FBG on POD4 (FBG.POD4), and fluid intake/output ratio <1 within POD4. Subsequently, we developed Nomogram-A (Figure 1A). In the training set, the nomogram yielded an area under the curve (AUC) of 0.867 (95% CI, 0.814–0.920) with a sensitivity of 0.764 and a specificity of 0.845 (Figure 1B). In the validation set, the nomogram exhibited an AUC of 0.833 (95% CI, 0.744–0.923) with a sensitivity of 0.821 and a specificity of 0.762 (Figure 1C).
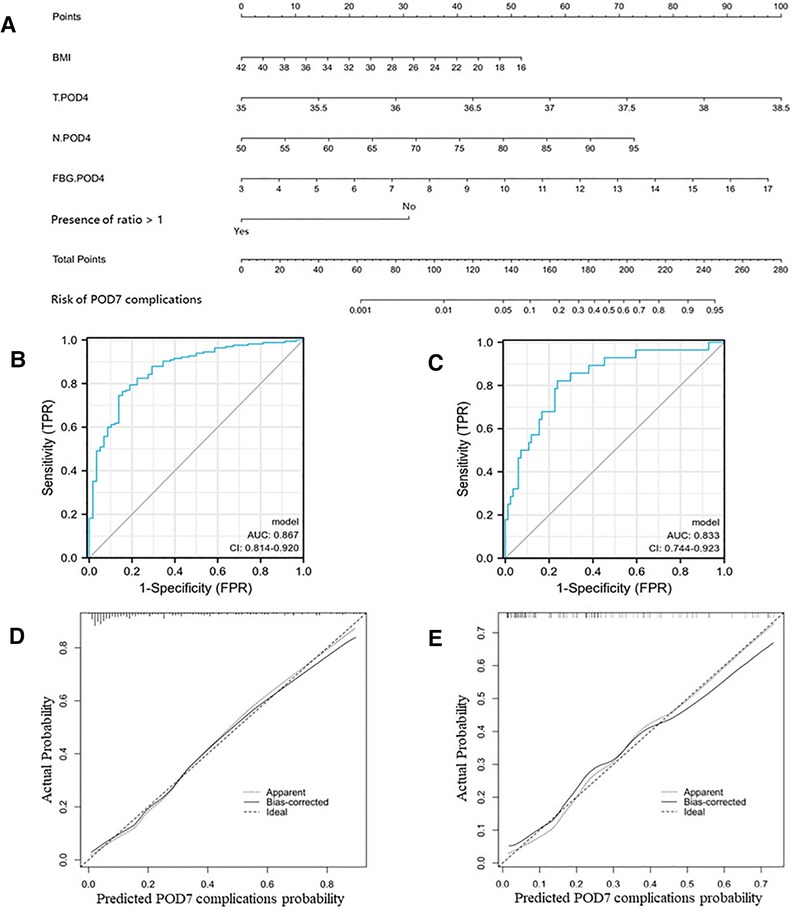
Figure 1. Nomogram-A and performance of the nomogram. (A) The possibility of POD7 complication was estimated by summing the scores corresponding to each risk factor. ROC and calibration curves of the nomogram for the probability of POD7 complication in the training set (B,D) and the validation set (C,E). In the calibration curve, the y-axis represents the probability of actual POD7 complication occurring and the x-axis represents the predicted probability. The wide dashed line represented a perfect prediction of the ideal model, and the solid line represented the actual performance of the Nomogram-A. The closer they were, the better the prediction performed.
Similarly, we identified six independent risk factors associated with the occurrence of complications within POD30 in Table 6, including BMI, body temperature on POD7 (T.POD7), neutrophil percentage on POD7 (N.POD7), FBG on POD4 and POD7 (FBG.POD4 and FBG.POD7), and duration of fluid intake/output ratio >1. To avoid data redundancy, we compared FBG.POD4 and FBG.POD7 and further defined the higher values as the maximum FBG (FBG.MAX). Further, we used BMI, T.POD7, N.POD7, FBG.MAX, and a fluid intake/output ratio of >1 to develop Nomogram-B (Figure 2A). In the training set, the nomogram yielded an AUC of 0.920 (95% CI, 0.884–0.955) with a sensitivity of 0.813 and specificity of 0.882 (Figure 2B). In the validation set, the nomogram exhibited an AUC of 0.918 (95% CI, 0.855–0.980) with a sensitivity of 0.971 and a specificity of 0.833 (Figure 2C).
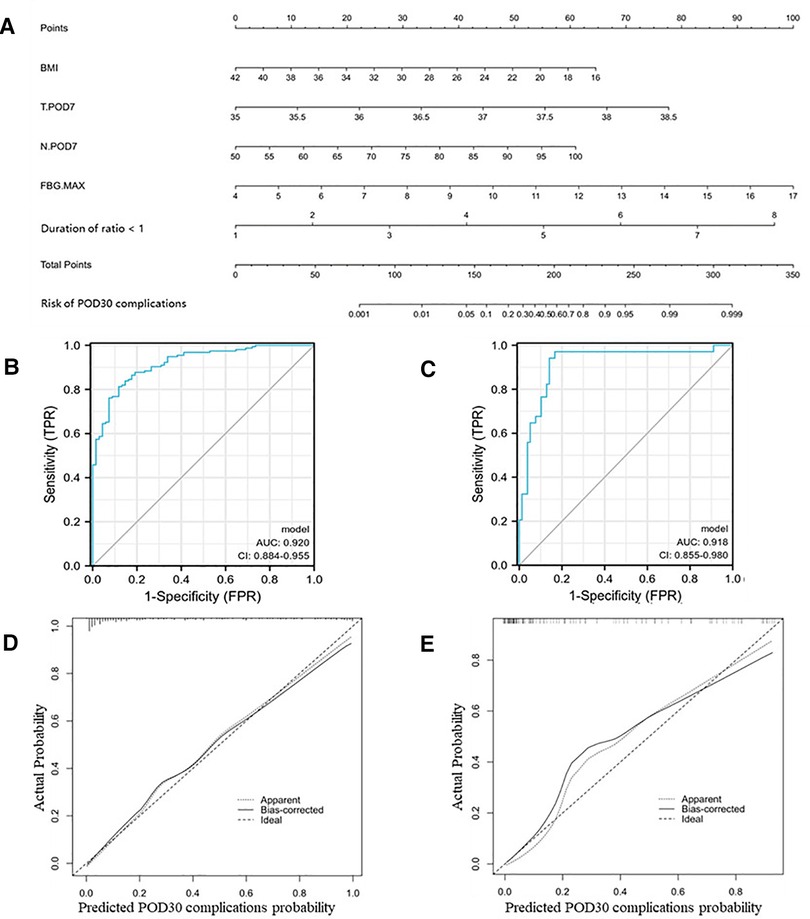
Figure 2. Nomogram-B and performance of the nomogram. (A) The possibility of POD30 complication was estimated by summing the scores corresponding to each risk factor. ROC and calibration curves of the nomogram for the probability of POD30 complication in the training set (B,D) and the validation set (C,E). In the calibration curve, the y-axis represents the probability of actual POD30 complication occurring and the x-axis represents the predicted probability. The wide dashed line represented a perfect prediction of the ideal model, and the solid line represented the actual performance of the Nomogram-B. The closer they were, the better the prediction performed.
The calibration curves and Hosmer–Lemeshow test showed good agreement between the predicted and actual probabilities of the two nomograms in the training and validation sets (PA = 0.755 vs. 0.738 (Figures 1D,E), PB = 0.768 vs. 0.125 (Figures 2D,E)).
In the present study, we successfully developed and validated nomograms for predicting postoperative complications within POD7 and POD30 in patients with gastrointestinal tumors. Both nomogram models were developed based on routine clinical indicators, including body temperature, neutrophil percentage, fasting glucose, and fluid volume within POD7. The AUCs of Nomogram-A and Nomogram-B were both greater than 0.8 in the training and validation sets, which may achieve the best performance in predicting the likelihood of postoperative complications. Comprehensively, this model is used to identify people at high risk of postoperative complications, thereby reducing its impact on postoperative recovery. The goal of both models is to improve short-term outcomes and quality of life after surgery and to reduce healthcare costs.
It has been documented that the occurrence of postoperative complications is closely associated with stress, and their occurrence usually indicates a strong stress response in the patient that induces hypermetabolism (9, 18). The main reparative cells and leukocytes increase in activity and number to satisfy the glucose supply for body recovery during this period (19). Meanwhile, with the upregulation of pro-inflammatory cytokines and acute-phase proteins and activation of the hypothalamic-pituitary-adrenal axis, there is an increase or decrease in the levels of some hormones, including cortisol, catecholamines, insulin, and glucagon, which promote glycogen catabolism and gluconeogenesis in muscle tissue that induces hyperglycemia to compensate for the “concentration gradient” needed for tissue repair (20). In addition, with the influence of hormones, capillary permeability and urine concentration increase (21). A large amount of fluid is stored in the interstitial space, resulting in tissue edema and is known as “water and sodium retention” When the stress response is reduced due to intervention and self-healing, excessive fluid is reabsorbed into the blood and excreted in the urine (22).
In our study, we also found a higher level of the stress response in patients with postoperative complications. In line with the study by Espiner EA, the alteration of stress results in increased water and sodium retention and further leads to more liquid intake than output, ultimately resulting in a higher daily fluid intake/output ratio (23). And we further observed that the presence of fluid intake/output ratio <1 within POD4 was related to the occurrence of complications within POD7, and that patients who developed complications within POD30 had a longer duration of fluid intake/output ratio >1. Under theoretical frameworks of perioperative stress response, we investigated the relationship between postoperative complications and fluid intake and output. The results demonstrated that the fluid intake/output ratio could be a convincing risk factor and predictor for the occurrence of complications.
Our study also validated the correlation between postoperative complications and routine monitoring indicators, such as body temperature, neutrophil percentage, and FBG (24). Slight changes in one or more these indicators were often overlooked by clinicians, leading to a failure of early identification of complications. Numerous studies have shown that changes in routine monitoring indicators may be affected by the regulation of inflammatory cytokines and hormones during the hypermetabolic period of the stress response (25, 26). In our analysis, stress level was positively correlated with certain routine monitoring indicators, such as body temperature, neutrophil percentage and FBG levels. Considering the correlation between acute stress pathophysiological and postoperative complications, these routine monitoring indicators may be potential risk factors and candidate predictors for postoperative complications (27–30). In addition, it is generally believed that perioperative routine monitoring indicators also reflect inflammation level. Elevated level of these indicators indicated a higher inflammatory level in patients with complications (31). In this study, we use acute stress-associated indicators to reflect inflammation level, thereby provide an early warning tool for complications. For patients with gastrointestinal tumor undergoing radical surgery, surgery- and tumor-associated factors, such as loss of blood, tissue injury, infection, impaired nutritional status and immunocompromised status, may place the patients in a high risk of complications (10, 32, 33). Timely identification of postoperative complication will urge us to recognize the problems in patient management, and timely intervention may reduce likelihood of severe advent events (33, 34). Mechanically, timely intervention for complication will reduce overconsumption of protein and duration of immune dysregulation, and further decrease the possibility of malnutrition, infection, thrombosis, etc. The final objective is to improve outcomes for patients and save medical resources (35, 36). Similarly, evidence by previous researches showed that preoperative glucocorticoid therapy combined with fast-track surgery can attenuate the inflammatory response, avoid organ dysfunction, and prevent the occurrence of complications, resulting in improved short-term outcome (37–39).
Our study had some limitations. First, this study was a retrospective study that might have led to bias. Further prospective data are needed to validate the accuracy of our model. Second, a small number of patients were not included in this study owing to missing data, which resulted in a higher population of the group with complications. This may also lead to selection bias. Third, because of the high cost of measuring stress indicators, we only collected partial data from training set. And we will further consider ending the limitations by future prospective studies needed.
This study presents two nomogram models for predicting short-term postoperative complications in patients with gastrointestinal tumors. Our results can help clinicians identify patients at high risk of complications within POD7 or POD30. In addition, we explored the correlation between postoperative complications and fluid intake and output under the framework of pathophysiology and stress response and successfully identified new risk factors for complications. This study also provides a novel idea for predicting postoperative complications.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee at Yantai Yuhuangding Hospital Affiliated to Medical College of Qingdao University. The patients/participants provided their written informed consent to participate in this study.
HC, DZ, and JJ: contributed equally to this study, performed the experiments, analyzed the data, and wrote the manuscript. YZ, MJ and JH: designed the experiments. BY and YL: collected the data. XH: checked and revised the manuscript. LJ: confirmed all the data in the manuscript. XW and LJ: firstly modified the language of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the National Natural Science Foundation of China (No. 82102785), the Natural Science Foundation of Shandong Province (ZR2021QH314) and the Natural Science Foundation of Yantai Yuhuangding Hospital (No. 2020-14, No. 2021-24, No. 2021-36). The funding bodies did not have any role in the design of the study, data collection, and analysis, nor on the interpretation and dissemination of the results.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun. (2021) 41:1037–48. doi: 10.1002/cac2.12197
2. Wei W, Zeng H, Zheng R, Zhang S, An L, Chen R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. (2020) 21:e342–9. doi: 10.1016/S1470-2045(20)30073-5
3. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese society of clinical oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. (2021) 41:747–95. doi: 10.1002/cac2.12193
4. Slankamenac K, Slankamenac M, Schlegel A, Nocito A, Rickenbacher A, Clavien P, et al. Impact of postoperative complications on readmission and long-term survival in patients following surgery for colorectal cancer. Int J Colorectal Dis. (2017) 32:805–11. doi: 10.1007/s00384-017-2811-y
5. van Kooten RT, Bahadoer RR, Peeters KCMJ, Hoeksema JHL, Steyerberg EW, Hartgrink HH, et al. Preoperative risk factors for major postoperative complications after complex gastrointestinal cancer surgery: a systematic review. Eur J Surg Oncol. (2021) 47:3049–58. doi: 10.1016/j.ejso.2021.07.021
6. Selby LV, Gennarelli RL, Schnorr GC, Solomon SB, Schattner MA, Elkin EB, et al. Association of hospital costs with complications following total gastrectomy for gastric adenocarcinoma. JAMA Surg. (2017) 152:953. doi: 10.1001/jamasurg.2017.1718
7. Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. (2009) 361:1368–75. doi: 10.1056/NEJMsa0903048
8. Watt DG, McSorley ST, Park JH, Horgan PG, McMillan DC. A postoperative systemic inflammation score predicts short- and long-term outcomes in patients undergoing surgery for colorectal cancer. Ann Surg Oncol. (2017) 24:1100–9. doi: 10.1245/s10434-016-5659-4
9. Crippa J, Mari GM, Miranda A, Costanzi ATM, Maggioni D. Surgical stress response and enhanced recovery after laparoscopic surgery—a systematic review. Chirurgia. (2018) 113:455. doi: 10.21614/chirurgia.113.4.455
10. Wilmore DW. From cuthbertson to fast-track surgery: 70 years of progress in reducing stress in surgical patients. Ann Surg. (2002) 236:643–8. doi: 10.1097/00000658-200211000-00015
11. Liu K, Wang Y, Wang J, Luo N, Gong J, Wang J, et al. Application of accelerated rehabilitation surgery in gastrointestinal surgery. Comput Math Method M. (2021) 2021:1–9. doi: 10.1155/2021/2968347
12. Pędziwiatr M, Mavrikis J, Witowski J, Adamos A, Major P, Nowakowski M, et al. Current status of enhanced recovery after surgery (ERAS) protocol in gastrointestinal surgery. Med Oncol. (2018) 35(6):95. doi: 10.1007/s12032-018-1153-0
13. Ramshaw BJ. Laparoscopic surgery for cancer patients. CA Cancer J Clin. (1997) 47:327–50. doi: 10.3322/canjclin.47.6.327
14. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
15. Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. (2003) 21:1232–7. doi: 10.1200/JCO.2003.06.100
16. Delong ER, Delong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45. doi: 10.2307/2531595
17. Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the hosmer-lemeshow test revisited*. Crit Care Med. (2007) 35:2052–6. doi: 10.1097/01.CCM.0000275267.64078.B0
18. Oka Y, Nishijima J, Oku K, Azuma T, Inada K, Miyazaki S, et al. Usefulness of an estimation of physiologic ability and surgical stress (E-PASS) scoring system to predict the incidence of postoperative complications in gastrointestinal surgery. World J Surg. (2005) 29:1029–33. doi: 10.1007/s00268-005-7719-y
19. Monk DN, Plank LD, Franch-Arcas G, Finn PJ, Streat SJ, Hill GL. Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Ann Surg. (1996) 223:395–405. doi: 10.1097/00000658-199604000-00008
20. Carli F. Physiologic considerations of enhanced recovery after surgery (ERAS) programs: implications of the stress response. Can J Anesth. (2015) 62:110–9. doi: 10.1007/s12630-014-0264-0
21. Scott MJ, Miller TE. Pathophysiology of Major surgery and the role of enhanced recovery pathways and the anesthesiologist to improve outcomes. Anesthesiol Clin. (2015) 33:79–91. doi: 10.1016/j.anclin.2014.11.006
22. Feldheiser A, Aziz O, Baldini G, Cox BPBW, Fearon KCH, Feldman LS, et al. Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesth Scand. (2016) 60:289–334. doi: 10.1111/aas.12651
23. Espiner EA. 7—the Effects of stress on salt and water balance. Baillière's Clin Endocrinol Metab. (1987) 1:375–90. doi: 10.1016/S0950-351X(87)80068-X
24. Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery. (2015) 157:362–80. doi: 10.1016/j.surg.2014.09.009
25. Romain B, Chemaly R, Meyer N, Chilintseva N, Triki E, Brigand C, et al. Diagnostic markers of postoperative morbidity after laparoscopic roux-en-Y gastric bypass for obesity. Langenbeck's Arch Surg. (2014) 399:503–8. doi: 10.1007/s00423-014-1180-z
26. Albanopoulos K, Alevizos L, Natoudi M, Dardamanis D, Menenakos E, Stamou K, et al. C-reactive protein, white blood cells, and neutrophils as early predictors of postoperative complications in patients undergoing laparoscopic sleeve gastrectomy. Surg Endosc. (2013) 27:864–71. doi: 10.1007/s00464-012-2526-7
27. Margraf A, Ludwig N, Zarbock A, Rossaint J. Systemic inflammatory response syndrome after surgery: mechanisms and protection. Anesth Analg. (2020) 131:1693–707. doi: 10.1213/ANE.0000000000005175
28. McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol. (2016) 23:2832–40. doi: 10.1245/s10434-016-5204-5
29. Podgoreanu MV, Michelotti GA, Sato Y, Smith MP, Lin S, Morris RW, et al. Differential cardiac gene expression during cardiopulmonary bypass: ischemia-independent upregulation of proinflammatory genes. J Thorac Cardiovasc Surg. (2005) 130:330–9. doi: 10.1016/j.jtcvs.2004.11.052
30. Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil. (2004) 16(Suppl 2):54–60. doi: 10.1111/j.1743-3150.2004.00558.x
31. Cuthbertson D, Tilstone WJ. Metabolism during the postinjury period. Adv Clin Chem. (1969) 12:1–55. doi: 10.1016/S0065-2423(08)60257-7
32. Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr. (2013) 37:21S–9S. doi: 10.1177/0148607113496117
33. Kohl BA, Deutschman CS. The inflammatory response to surgery and trauma. Curr Opin Crit Care. (2006) 12:325–32. doi: 10.1097/01.ccx.0000235210.85073.fc
34. Van den Berghe GH. Acute and prolonged critical illness are two distinct neuroendocrine paradigms. Verh K Acad Geneeskd Belg. (1998) 60:487–518, 518–520.10230322
35. Shirakawa Y, Noma K, Maeda N, Tanabe S, Sakurama K, Sonoyama-Hanaoka A, et al. Early intervention of the perioperative multidisciplinary team approach decreases the adverse events during neoadjuvant chemotherapy for esophageal cancer patients. Esophagus. (2021) 18:797–805. doi: 10.1007/s10388-021-00844-y
36. Jhanji S, Pearse RM. The use of early intervention to prevent postoperative complications. Curr Opin Crit Care. (2009) 15:349–54. doi: 10.1097/MCC.0b013e32832c4a7e
37. Prete A, Yan Q, Al-Tarrah K, Akturk HK, Prokop LJ, Alahdab F, et al. The cortisol stress response induced by surgery: a systematic review and meta-analysis. Clin Endocrinol. (2018) 89:554–67. doi: 10.1111/cen.13820
38. Hall K, Shahrokhi S, Jeschke M. Enteral nutrition support in burn care: a review of current recommendations as instituted in the ross tilley burn centre. Nutrients. (2012) 4:1554–65. doi: 10.3390/nu4111554
Keywords: gastrointestinal tumor, postoperative complications, stress response, fluid intake and output, clinicopathological characteristics, nomogram
Citation: Cui H, Zhao D, Jian J, Zhang Y, Jian M, Yu B, Hu J, Li Y, Han X, Jiang L and Wang X (2023) Risk factor analysis and construction of prediction models for short-term postoperative complications in patients undergoing gastrointestinal tract surgery. Front. Surg. 9:1003525. doi: 10.3389/fsurg.2022.1003525
Received: 26 July 2022; Accepted: 8 December 2022;
Published: 4 January 2023.
Edited by:
Mengling Feng, National University of Singapore, Singapore© 2023 Cui, Zhao, Jian, Zhang, Jian, Yu, Hu, Li, Han, Jiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixin Jiang amlhbmdsaXhpbjE5NjlAaG90bWFpbC5jb20= Xixun Wang dzcwMDYzQHNpbmEuY29t
†These authors share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.