- 1Department of Hepatology, First Bethune Hospital of Jilin University, Changchun, China
- 2General Laboratory of Human Anatomy, Changzhi Medical College, Changzhi, China
Variations of the hepatic artery are very common, but they greatly increase the difficulty of surgery and the risk of complications in perihepatic surgeries such as liver transplantation, liver segmentectomy, and gastroduodenal surgery. Thus, it is important to precisely define the type of hepatic artery variant before surgery. However, there are often rare variants that cannot be defined with existing classifications. For example, the type of hepatic artery variant in the current case could not be classified with conventional classifications, and no such variation has been reported to date, involving two accessory left hepatic arteries from the common hepatic and left inferior phrenic arteries, respectively. Based on the existing 3DCT technology and the CRL classification method, which is applicable to the most common hepatic artery variants, we reviewed many rare variant types and proposed a new classification method (ex-CRL classification) for hepatic artery variations that do not fit the classic scope. The ex-CRL classification can accurately classify the vast majority of rare cases in the literature, greatly compensates for the limitations of current hepatic artery classifications, improves the generalization and understanding of rare cases, and reduces surgical complications.
Introduction
The common hepatic artery (CHA) is one of the main arterial supplies of the liver, gallbladder, lesser omentum, and gastroduodenal region, and its branches and variations are complicated. Interestingly, normal patterns occur only in 42%–75.7% of cases (1, 2). Aberrant arteries may be accidentally injured during surgical procedures resulting in severe haemorrhage and other fatal complications (3–11). A thorough knowledge of possible variations in branching, courses, and distribution of the vessels supplying the liver and gallbladder is essential. Therefore, many variations of the hepatic artery have been described and classified, among which the classic classifications are Michel's 10 types and Hiatt's 6 types (1, 8). However, despite the many classifications, more than 10% of hepatic arteries still cannot be classified. In recent years, with the wide application of 3D visualization technology (3DVT) (9–11), Yan et al. (12) presented the CRL classification, which succinctly describes the hepatic artery and its branches and covers a wider range of arterial variants. However, some rare variants still cannot be categorized by the CRL classification, and these variations are more likely to be unidentified and injured. These rare types have been reported in the form of individual cases for many years, lacking systematic classification and generalization. The aim of this study was to report a variation of the hepatic artery not in any of the hepatic classifications. Thereafter, based on CRL classification the study puts forward an extended version (ex-CRL classification) specifically for some rare cases.
Case presentation
The variation of the hepatic artery was found during a routine dissection of an adult male cadaver for teaching purposes at Changzhi Medical College in 2020. In compliance with the confidentiality of the donor, we have not obtained the medical history and related information of the cadaver. The specimens were perfused with 5% formaldehyde and 10% alcohol, and fixed with 4% formaldehyde solution. The structure of the specimen was clear and intact.
CHA, originated from the celiac trunk (CT), which travelled behind the stomach and gave off three branches as follows: the accessory left hepatic artery (aLHA), proper hepatic artery (PHA) and gastroduodenal artery (GDA). The aLHA arose 22.0 mm distal to the origin of the CHA, travelling behind the PHA and finally entering into segment IV, posterior to the common hepatic duct (CHD), and its one bunch ran through the umbilical fissure. The PHA then travelled to the right about 11.4 mm and divided into the right gastric artery and cystic artery, and then ran superiorly about 34.2 mm and entered the liver through the porta hepatis (Figure 1).

Figure 1. Photograph and schematic diagram of hepatic artery variation. 1. common hepatic artery (CHA); 2. accessory left hepatic artery (aLHA); 3. gastroduodenal artery (GDA); 4. proper hepatic artery (PHA); 5. right gastric artery 6. cystic artery; 7. left gastric artery; 8. splenic artery; 9. left gastric vein; 10. phrenic artery; 11. another accessory left hepatic artery (aLHA); 12. portal vein; 13. common hepatic duct (CHD); 14. Gallbladder.
In addition, the abdominal aorta gave off the left inferior phrenic artery at about 23.0 mm above the CT and was associated with agenesis of the right inferior phrenic artery. The left inferior phrenic artery provided the second left accessory hepatic artery. The second left accessory hepatic artery partially joined the left interlobar artery of the left lobe through the venous ligament and partially supplied the left lobe of the liver, forming a small branch supplying the falcine ligament and partially supplying the right diaphragm.
Discussion
To the best of our knowledge, there has been no case report of the hepatic artery arising from the inferior phrenic artery and replacing the right inferior phrenic artery. Only Covey et al. (13) reported the origin of the hepatic artery from the phrenic artery. They collected arterial anatomical data from 600 cases of visceral angiography from May 1996 to October 2000, and found that one case of rRHA and aRHA each originated from the right phrenic artery. Thereafter, Liang (14) searched the previous literature and analysed 21 studies, including that of Covey et al., with a total of 10,966 cases, and only 2 cases reported by Covey et al. noted that the hepatic artery originated from the phrenic artery. Jin (15) reviewed 10, 211 cases in 21 articles and found that only 2 cases originating from the phrenic artery were reported by Covey et al. Therefore, the origin of the accessory hepatic artery from the phrenic artery reported in this case is extremely rare and noteworthy.
In addition, another accessory hepatic artery in the current case originated from the common hepatic artery. Futara (16) dissected 110 cases and found that in 5 cases (4.5%), the left accessory hepatic artery originated from the common hepatic artery, while Pai (17) collected autopsy data from 72 cadavers between 2000 and 2005 and found only one (1.38%) variant of the accessory hepatic artery originating from CHA, which was much lower than the expected incidence (4.5%).
However, we think about reasons why this variant has not been previously described, except that it has an extremely low incidence and may also be associated with limited 2D CT techniques. 2D technologies very test the spatial imagination of surgeons (18), except for conventional variants such as left gastric artery and superior mesenteric artery, other rare variants are difficult to find. In this case, the accessory left hepatic artery originating from the inferior phrenic artery is short and concealed, and we only exposed its course completely after excising part of the left lobe, so it is easily overlooked.
In the current case, the hepatic artery that replaced the right inferior phrenic artery was thicker than the left inferior phrenic artery, and has a wider range of blood supply. This variation is likely to cause ischaemia and dysfunction of the right diaphragm during left hepatic lobectomy, lymph node dissection around a gastric cancer, infusion of chemotherapeutic drugs for hepatocellular carcinoma, and vascular embolisation. In addition, the accessory hepatic artery may not be fully visualised during selective hepatic arteriography, and such vascular variants interfere with effective tumour control by transcatheter arterial chemoembolisation (19). If a brain-dead donor of this variant type is encountered during liver transplantation, all blood vessels entering the liver should be preserved as long as possible. During vascular reconstruction, it can be flexibly determined according to the recipient's vascular condition, and blood vessels with an appropriate diameter and in good condition are selected for end-to-end anastomosis with the donor. When the recipient has poor vascular conditions such as the common hepatic artery and gastroduodenal artery due to the underlying liver disease, but the splenic artery is in good condition, an anastomosis with the easily separated splenic artery is selected; if the donor liver cannot obtain arterial blood supply in situ, The donor iliac vessels were selected to bypass the hepatic artery with the abdominal aorta (20). If this type of variant is a living donor, due to the presence of the double accessory left hepatic arteries, a right half liver can also be considered for living donor liver transplantation. Of course, it is still controversial as to whether multiple arteries need routine reconstruction, so full surgical protocol should also be made with adequate preoperative evaluation (21).
Although the variants of the two combinations in this case are extremely rare, these variants are complex and easy to ignore and could lead serious intraoperative complications. Therefore, the accumulation of knowledge regarding this type of variant has important anatomical significance for surgery and interventional therapy.
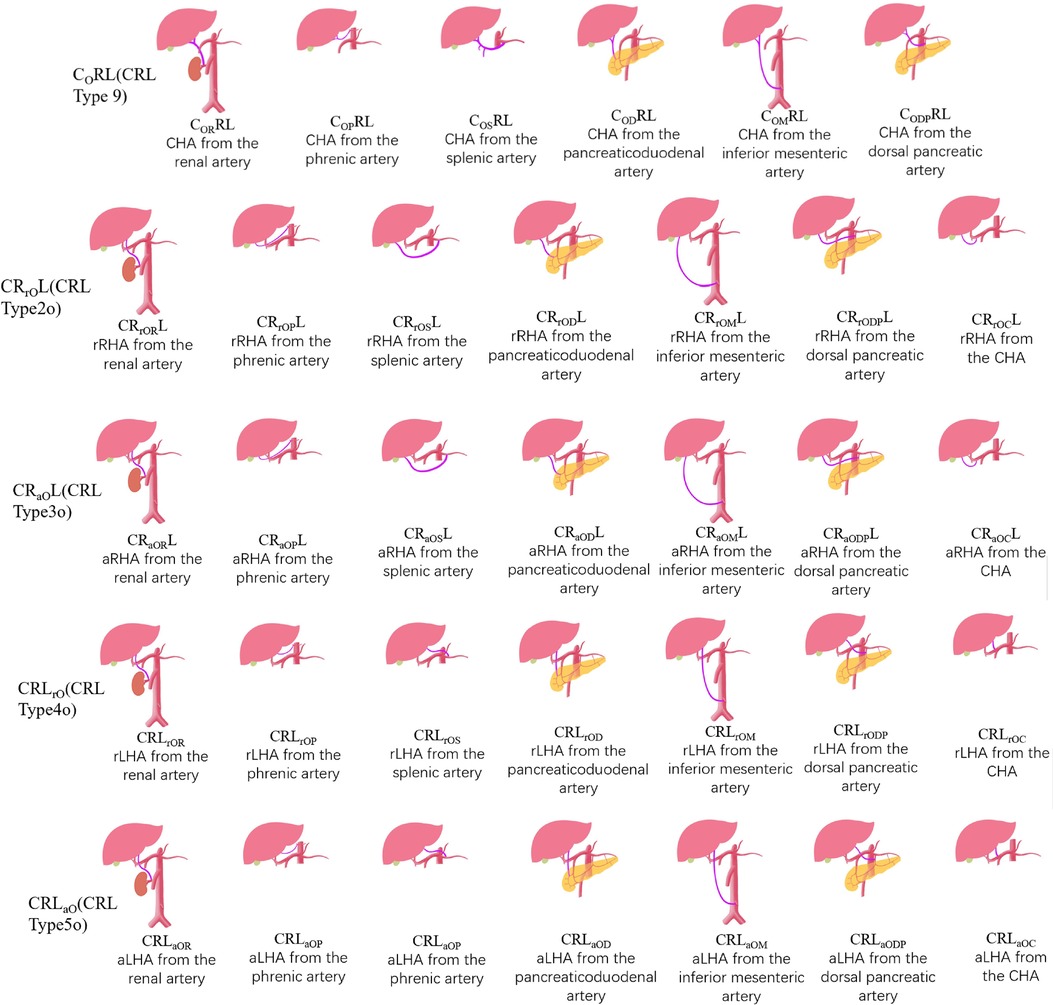
In recent years, with the development of 3DCT technology, 3DVT is clearer and more accurate in imaging blood vessels compared to 2D imaging (22). 3DVT is widely used in preoperative evaluation and provides a clearer understanding of the origin and course of CHA, LHA, and RHA. Therefore, there is also a need for a new type of classification that is more compatible with 3DCT to describe and classify hepatic arteries more concisely. The CRL classification is a new classification developed based on 3DCT technology, with each hepatic artery described in detail. It conforms to the current requirements for preoperative evaluation and is suitable for most routine variants. However, it is currently not specific for rare cases. Therefore, we propose a new classification, the ex-CRL classification (Figure 2), on the basis of the CRL classification to name and classify variants that cannot be classified by the CRL classification.
CRL classification rules
CRL describes the origin of CHA, RHA, and LHA, with CRL being the abbreviation of their initials. CRL describes normal origins; that is, CHA originates from CT, while RHA and LHA originate from PHA. Thereafter, the origins of other arteries are marked with capital letters corresponding to their arteries. For example, CSRL means that the origin of CHA has changed, not from CT but from SMA, while the origins of RHA and LHA remain changed. The replaced or accessory hepatic arteries need to be represented by lowercase letters “r” or “a”. For example, CRrGLaA means that CHA originates from CT, rRHA originates from GDA, and aLHA originates from the aorta. The CRL classification is detailed in Table 1.
ex-CRL classification rules
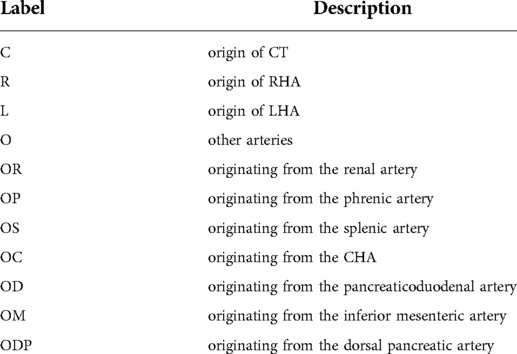
The ex-CRL classification is suitable for a small number of variants outside the CRL classification. Other arteries in the CRL classification are uniformly represented by “O”. For example, CRaOL, indicates that aRHA originates from other arteries other than AA, CT, PHA, LGA, GDA, or SMA, while ex-CRL subdivides other arteries that have appeared in the past, such as “OR”, which indicates origin from the renal artery; “ OP”, from the phrenic artery; “OS”, from the splenic artery; “OC”, from CHA; “OD”, from the pancreaticoduodenal artery; “OM”, from the inferior mesenteric artery; and “ODP” from the dorsal pancreatic artery. Detailed rules for the ex-CRL classification are shown in Tables 2, 3.
In addition, ex-CRL complements multiple replaced or accessory arteries, as well as in the case of MHA. It uses “a1”, “a2”, “r1”, and “r2” to represent multiple replaced or accessory hepatic arteries. As reported in this case, aLHA1 originates from CHA, and aLHA2 originates from the phrenic artery, and is represented by CRLa1OC, a2OP, which succinctly and clearly illustrates the variation of the two aLHAs. In addition, MHA is not uncommon in variants. When MHA originates from PHA, RHA, or LHA, it does not need special notation, because it is close to the hepatic portal and bifurcates in advance before entering the liver to supply the fourth segment of liver. However, when MHA originates from other arteries and is difficult to be classified as RHA or LHA, special labeling is required. For example, Gündoğdu et al. (23) described MHA originating from the pancreaticoduodenal artery, and aRHA originating from the dorsal pancreatic artery in 2021, which can be represented by CRaODPMOPL.
Finally, cases with variations in CHA, RHA, and LHA have been supplemented. For example, CHA from AA, aLHA from LGA, aRHA from SMA, were reported by Gruttadauria et al. (24) in 2001. However, the current case cannot be classified with the CRL classification, but can be expressed as CARaSLaL based on the ex-CRL classification.
Evaluation of the ex-CRL classification
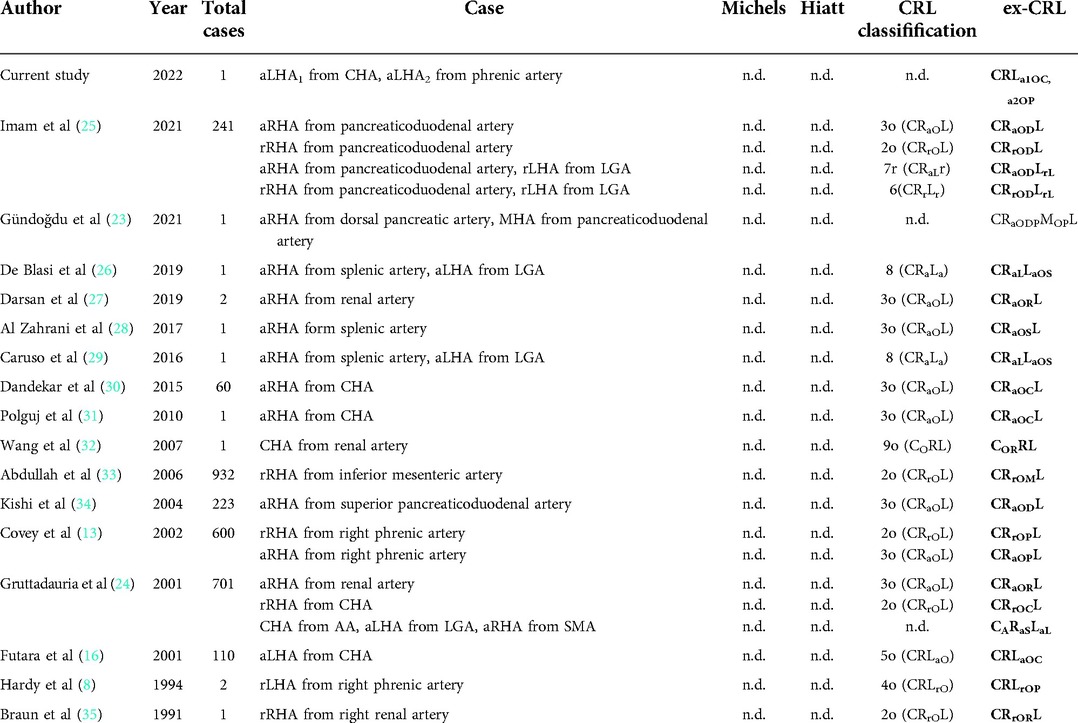
Different classifications were used to describe rare cases in the previous literature (Table 4). We reviewed the literature on hepatic artery variants from 1994 to 2021, and finally retained 16 studies (2878 cases) describing rare variants, including 17 rare hepatic arterial variants that cannot be described by common classification methods. We found that the classic Hiatt and Michel classifications were unsuitable for these rare and complex classifications, and their coverage by the CRL classification was relatively improved, but the specificity for these rare cases was not high. For example, rRHA described by Abdullah et al. (33) in 2006 originated from the inferior mesenteric artery, which is classified as CRrOML by ex-CRL, and rRHA described by Imam et al. (25) in 2021 was derived from the pancreaticoduodenal artery, which was classified as CRrODL by ex-CRL, but both cases are represented by CRL classification as “2o (CRrOL)”. The CRL classification does not clearly describe this part of “other arteries”, making it necessary to use ex-CRL for reclassification.
The CRL classification can classify some rare cases, but it lacks specificity and cannot describe them accurately. When the case base is large, the number of rare cases will increase; thus, attention is required for these rare cases. The application of ex-CRL, as an extension of the CRL classification, is conducive to improving the understanding and classification of rare variants and reducing the occurrence of surgical complications.
However, all classification methods, including ex-CRL classification, describe the origin of the variant artery, but lack the description of the path of the variant artery. Even if they originate from the same artery, their bifurcation planes and paths may be different. For example, two cases of aRHA originating from the renal artery reported by Darsan (27) in 2019 originated from the proximal end of the renal artery near the aorta and the distal end of the renal artery near the renal hilum, and their ascending paths were also different. This requires careful observation while performing an analysis of the detailed path of the artery to ensure that rapid and precise judgements are made in a limited surgical field of view.
Variations of the hepatic artery and surrounding blood vessels are associated with increased surgical difficulty and complications (36). For example, the accessory hepatic artery varies in liver transplant donors. If the accessory hepatic artery is ligated, it will lead to local arterial ischaemia, which may compromise liver function. If the accessory hepatic artery is preserved, it will lead to an increase in the number of arterial reconstructions and anastomosis, and a longer warm ischaemia time, which has been proven to be a risk factor for hepatic artery thrombosis (37). However, if the recipient has a complex hepatic artery vascular variation, due to the small and complex blood vessels of the hepatic artery, it will increase the incidence of hepatic artery thrombosis, hepatic artery stenosis, hepatic artery haemorrhage, or other nonvascular complications, including bile duct stenosis after liver transplantation as well as liver abscesses (38). Additionally, in pancreaticoduodenectomy, perihepatic arterial variation increases the risk of compromised hepatic arterial supply, which may lead to unintended bleeding or ischaemia, as well as an increased risk of biliary anastomotic leakage, transient liver function disorders, and, in some patients, liver failure (39).
In addition to invasive angiographic techniques, and noninvasive vascular imaging techniques, 3D reconstruction techniques such as volume rendering technique (VRT) and curved planar reformation (CPR) have rapidly evolved, they allow stereoscopic imaging of splanchnic arteriovenous and other conduits, reflecting the complete vessel diameter, length, course, and positional relationships of surrounding soft tissues and organs (40, 41). We expect that 3D visualization technology and CRL classification will become more widespread and routinely used for preoperative evaluation and surgical simulation of perihepatic surgery. Improved understanding of vascular anatomy, imaging, and classification of hepatic arteries will facilitate the development of individualized surgical protocol and avoid complications, while also expecting that more rare types of new hepatic artery variants will be discovered.
Conclusion
Comprehensive knowledge, accumulation, and classification of hepatic arterial variant types can greatly reduce surgical complications, and the ex-CRL classification can be used for hepatic arterial variants that cannot be classified with conventional methods.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Changzhi Medical College. The patients/participants provided their written informed consent to participate in this study.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XW conceptualized the topic and wrote the manuscript. JK and YL consulted the literature.JN and YS conceptualized the topic and supervised and facilitated the conduct of the study. GS modified the edited and made significant revisions to the manuscript. All authors read and approved the fnal manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors wish to sincerely thank those who donated their bodies. Thanks to Changzhi Medical College and the First Affiliated Hospital of Jilin University, and thanks to professors Niu Junqi and Shi Ying for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. (1966) 112(3):337–47. doi: 10.1016/0002-9610(66)90201-7
2. Németh K, Deshpande R, Máthé Z, Szuák A, Kiss M, Korom C, et al. Extrahepatic arteries of the human liver - anatomical variants and surgical relevancies. Transpl Int. (2015) 28(10):1216–26. doi: 10.1111/tri.12630
3. Takagi K, Domagala P, Polak WG, Ijzermans JNM, Boehnert MU. Right posterior segment graft for living donor liver transplantation: a systematic review. Transplant Rev (Orlando). (2020) 34(1):100510. doi: 10.1016/j.trre.2019.100510
4. Takeda M, Sakamoto S, Uchida H, Yoshimura S, Shimizu S, Hirata Y, et al. Technical considerations in liver transplantation for biliary atresia with situs inversus. Liver Transpl. (2019) 25(9):1333–41. doi: 10.1002/lt.25484.31063622
5. Albers BK, Khanna G. Vascular anomalies of the pediatric liver. Radiographics. (2019) 39(3):842–56. doi: 10.1148/rg.2019180146
6. Baker TB, Zimmerman MA, Goodrich NP, Samstein B, Pomfret EA, Pomposelli JJ, et al. Biliary reconstructive techniques and associated anatomic variants in adult living donor liver transplantations: the adult-to-adult living donor liver transplantation cohort study experience. Liver Transpl. (2017) 23(12):1519–30. doi: 10.1002/lt.24872
7. Schroering JR, Kubal CA, Fridell JA, Hathaway TJ, Robinson RC, Mangus RS. Impact of variant donor hepatic arterial anatomy on clinical graft outcomes in liver transplantation. Liver Transpl. (2018) 24(10):1481–4. doi: 10.1002/lt.25316
8. Hardy K, Jones R. Hepatic artery anatomy in relation to reconstruction in liver transplantation: some unusual variations. Aust N Z J Surg. (1994) 64(6):437–40. doi: 10.1111/j.1445-2197.1994.tb02248.x
9. Bogomolova K, Hierck BP, Looijen AEM, Pilon JNM, Putter H, Wainman B, et al. Stereoscopic three-dimensional visualisation technology in anatomy learning: a meta-analysis. Med Educ. (2021) 55(3):317–27. doi: 10.1111/medu.14352
10. Nation H, Kaliski D, Ortiz A. Narrated dissection videos and peer-mentoring to enhance anatomy performance of underrepresented minority students in physical therapy education. Anat Sci Educ. (2020) 13(6):794–9. doi: 10.1002/ase.1971
11. Wainman B, Wolak L, Pukas G, Zheng E, Norman GR. The superiority of three-dimensional physical models to two-dimensional computer presentations in anatomy learning. Med Educ. (2018) 52(11):1138–46. doi: 10.1111/medu.13683
12. Yan J, Feng H, Wang H, Yuan F, Yang C, Liang X, et al. Hepatic artery classification based on three-dimensional ct. Br J Surg. (2020) 107(7):906–16. doi: 10.1002/bjs.11458
13. Covey A, Brody L, Maluccio M, Getrajdman G, Brown K. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. (2002) 224(2):542–7. doi: 10.1148/radiol.2242011283
14. Liang Y, Li E, Min J, Gong C, Wu L. Rare anatomic variation of the right hepatic artery and accessory right hepatic artery supplying hepatocellular carcinoma: a case report and literature review. Medicine (Baltimore). (2017) 96(39):e8144. doi: 10.1097/md.0000000000008144
15. Jin W, Dong M, Pan J, Zhang Q, Li M, Guo D, et al. Rare combined variations of accessory left hepatic artery and accessory right hepatic artery: a case report and literature review. Surg Radiol Anat. (2020) 42(4):443–7. doi: 10.1007/s00276-019-02396-4
16. Futara G, Ali A, Kinfu Y. Variations of the hepatic and cystic arteries among Ethiopians. Ethiop Med J. (2001) 39(2):133–42.11501290
17. Pai R, Hunnargi A, Srinivasan M. Accessory left hepatic artery arising from common hepatic artery. Indian J Surg. (2008) 70(2):80–2. doi: 10.1007/s12262-008-0021-0
18. Bijlstra OD, Broersen A, Oosterveer TTM, Faber RA, Achterberg FB, Hurks R, et al. Integration of three-dimensional liver models in a multimodal image-guided robotic liver surgery cockpit. Life (Basel). (2022) 12(5):667. doi: 10.3390/life12050667
19. Kim H-C, Chung JW, Lee W, Jae HJ, Park JH. Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. Radiographics. (2005) 25(Suppl 1):S25–39. doi: 10.1148/rg.25si055508
20. Li P, Thorat A, Jeng L, Yang H, Li M, Yeh C, et al. Hepatic artery reconstruction in living donor liver transplantation using surgical loupes: achieving low rate of hepatic arterial thrombosis in 741 consecutive recipients-tips and tricks to overcome the poor hepatic arterial flow. Liver Transpl. (2017) 23(7):887–98. doi: 10.1002/lt.24775
21. Thenappan A, Jha RC, Fishbein T, Matsumoto C, Melancon JK, Girlanda R, et al. Liver allograft outcomes after laparoscopic-assisted and minimal access live donor hepatectomy for transplantation. Am J Surg. (2011) 201(4):450–5. doi: 10.1016/j.amjsurg.2010.10.007
22. Fang C, Tao H, Yang J, Fang Z, Cai W, Liu J, et al. Impact of three-dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma. J Am Coll Surg. (2015) 220(1):28–37. doi: 10.1016/j.jamcollsurg.2014.09.023
23. Gündoğdu E, Kebapçı M. Two novel hepatic arterial variations in a living liver donor detected by multidetector computed tomography angiography. Surg Radiol Anat. (2021) 43(8):1385–9. doi: 10.1007/s00276-021-02730-9
24. Gruttadauria S, Foglieni C, Doria C, Luca A, Lauro A, Marino I. The hepatic artery in liver transplantation and surgery: vascular anomalies in 701 cases. Clin Transplant. (2001) 15(5):359–63. doi: 10.1034/j.1399-0012.2001.150510.x
25. Imam A, Karatas C, Mecit N KA, Yildirimoglu T, Kalayoglu M, et al. Anatomical variations of the hepatic artery: a closer view of rare unclassified variants. Folia Morphol (Praha). (2022) 81(2):359–64 doi: 10.5603/FM.a2021.0024
26. De Blasi V, Makkai-Popa S, Arru L, Pessaux P, Azagra J. Rare anatomic variation of the hepatic arterial blood supply: case report and literature review. Surg Radiol Anat. (2019) 41(3):343–5. doi: 10.1007/s00276-018-2163-5
27. Darsan L, Vishal V, Cardoza F. Accessory right hepatic artery originating from proximal and distal right renal artery in two subjects. Indian J Urol. (2019) 35(4):305–6. doi: 10.4103/iju.IJU_86_19
28. Al Zahrani Y, AlMat’hami A, Alobaidi H, Wiseman D, Mujoomdar A. Accessory right hepatic artery arising from splenic artery supplying hepatocellular carcinoma identified by computed tomography scan and conventional angiography: a rare anatomic variant. Ann Vasc Surg. (2017) 38(316):e1–e5. doi: 10.1016/j.avsg.2016.05.096
29. Caruso F, Dondossola D, Fornoni G, Caccamo L, Rossi G. Right hepatic artery from splenic artery: the four-leaf clover of hepatic surgery. Surg Radiol Anat. (2016) 38(7):867–71. doi: 10.1007/s00276-016-1617-x
30. Dandekar U, Dandekar K, Chavan S. Right hepatic artery: a cadaver investigation and its clinical significance. Anat Res Int. (2015):412595. doi: 10.1155/2015/412595
31. Polguj M, Gabryniak T, Topol M. The right accessory hepatic artery; a case report and review of the literature. Surg Radiol Anat. (2010) 32(2):175–9. doi: 10.1007/s00276-009-0536-5
32. Wang K, Hu S, Jiang X, Zhu M, Jin B. Liver transplantation for patient with variant hepatic artery arising from right renal artery: a case report. Transplant Proc. (2007) 39(5):1716–7. doi: 10.1016/j.transproceed.2006.11.008
33. Abdullah S, Mabrut J, Garbit V, De La Roche E, Olagne E, Rode A, et al. Anatomical variations of the hepatic artery: study of 932 cases in liver transplantation. Surg Radiol Anat. (2006) 28(5):468–73. doi: 10.1007/s00276-006-0121-0
34. Kishi Y, Sugawara Y, Kaneko J, Akamatsu N, Imamura H, Asato H, et al. Hepatic arterial anatomy for right liver procurement from living donors. Liver Transpl. (2004) 10(1):129–33. doi: 10.1002/lt.20010
35. Braun M, Collins M, Wright P. An aberrant right hepatic artery from the right renal artery: anatomical vignette. Cardiovasc Intervent Radiol. (1991) 14(6):349–51. doi: 10.1007/bf02577895
36. Nakata K, Higuchi R, Ikenaga N, Sakuma L, Ban D, Nagakawa Y, et al. Precision anatomy for safe approach to pancreatoduodenectomy for both open and minimally invasive procedure: a systematic review. J Hepatobiliary Pancreat Sci. (2022) 29(1):99–113. doi: 10.1002/jhbp.901
37. Montalti R, Benedetti Cacciaguerra A, Nicolini D, Ahmed EA, Coletta M, De Pietri L, et al. Impact of aberrant left hepatic artery ligation on the outcome of liver transplantation. Liver Transpl. (2018) 24(2):204–13. doi: 10.1002/lt.24992
38. Ishigami K, Zhang Y, Rayhill S, Katz D, Stolpen A. Does variant hepatic artery anatomy in a liver transplant recipient increase the risk of hepatic artery complications after transplantation? AJR Am J Roentgenol. (2004) 183(6):1577–84. doi: 10.2214/ajr.183.6.01831577
39. Shukla PJ, Barreto SG, Kulkarni A, Nagarajan G, Fingerhut A. Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol. (2010) 17(1):186–93. doi: 10.1245/s10434-009-0757-1
40. Radtke A, Sotiropoulos GC, Molmenti EP, Schroeder T, Peitgen HO, Frilling A, et al. Computer-assisted surgery planning for complex liver resections: when is it helpful? A single-center experience over an 8-year period. Ann Surg. (2010) 252(5):876–83. doi: 10.1097/SLA.0b013e3181fdd012
Keywords: accessory hepatic artery, vascular variants, phrenic artery, classification, case report
Citation: Wu X, Kang J, Liu Y, Sun G, Shi Y and Niu J (2022) A rare hepatic artery variant reporting and a new classification. Front. Surg. 9:1003350. doi: 10.3389/fsurg.2022.1003350
Received: 26 July 2022; Accepted: 8 August 2022;
Published: 29 August 2022.
Edited by:
Juan José Segura-Sampedro, Hospital Universitario Son Espases, SpainReviewed by:
Victor Lopez-Lopez, Murcia BioHealth Research Institute, University of Murcia, SpainMiguel Angel Gomez Bravo, University Hospital Virgen del Rocío of Seville, Spain
© 2022 Wu, Kang, Liu, Sun, Shi and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guodong Sun c2dkMzA1OTEyNjEzQDE2My5jb20=Ying Shi c2hpeTcwN0BqbHUuZWR1LmNu Junqi Niu anVucWluaXVAamx1LmVkdS5jbg==
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Xiaojing Wu
Xiaojing Wu Jianxiong Kang
Jianxiong Kang Yuwei Liu
Yuwei Liu Guodong Sun
Guodong Sun Ying Shi
Ying Shi Junqi Niu
Junqi Niu