- 1Department of Endocrinology, Beijing Jishuitan Hospital, Beijing, China
- 2Department of Endocrinology, Chinese People's Liberation Army (PLA) General Hospital, Beijing, China
- 3Department of Rheumatology, Beijing Jishuitan Hospital, Beijing, China
- 4Department of Emergency, Beijing Jishuitan Hospital, Beijing, China
Purpose: Currently, endoscopic transsphenoidal surgery (ETS) and microscopic transsphenoidal surgery (MTS) are commonly applied treatments for patients with pituitary adenomas. This meta-analysis was conducted to evaluate the efficacy and safety of ETS and MTS for these patients.
Methods: A computer search of Pubmed, Embase, Cochrane library, Web of Science, and Google Scholar databases was conducted for studies investigating ETS and MTS for patients with pituitary adenomas. The deadline is March 01, 2021. RevMan5.1 software was used to complete this meta-analysis after literature screening, data extraction, and literature quality evaluation.
Results: A total of 37 studies including 5,591 patients were included. There was no significant difference in gross tumor removal (GTR) and hormone-excess secretion remission (HES remission) between two groups [RR = 1.10, 95% CI (0.99–1.22), P = 0.07; RR = 1.09, 95% CI (1.00–1.20), P = 0.05]. ETS was associated with lower incidence of diabetes insipidus (DI) [RR = 0.71, 95% CI (0.58–0.87), P = 0.0008], hypothyroidism [RR = 0.64, 95% CI (0.47–0.89), P = 0.007], and septal perforation [RR = 0.32, 95% CI (0.13–0.79), P = 0.01] than those with MTS.
Conclusion: This meta-analysis indicated that ETS cannot significantly improve GTR and HES remission. However, ETS could reduce the incidence of DI, hypothyroidism, and septal perforation without increasing the rate of other complications.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/#myprospero, identifier: CRD42021241217.
Introduction
Rapid progressive pituitary adenomas is one of the most common intracranial tumors, constituting nearly 10% of intracranial tumors (1). It often leads to abnormal secretion of pituitary hormones, abnormal vision, and other nerve and brain damages, and ultimately endanger the life of patients. Medication, radiotherapy, and surgery are the main treatments. In addition to a prolactinoma, usually treated with dopamine agonists, surgery was considered the most effective method. However, due to the pivotal location of pituitary adenomas in the pituitary fossa and critical structures such as the hypothalamus, cavernous sinus, and internal carotid artery nearby, traditional surgery is difficult to remove tumors completely. In 1907, Schloffer first proposed transsphenoidal removal of pituitary adenomas (2). Since 1960, microscopic transsphenoidal surgery (MTS) is the preferred choice for pituitary adenoma because of its high cure rate and low incidence of complication. However, there are still some limitations, especially the poor vision above and to the side of the saddle area.
With the development of endoscopic technology, surgery has been developed rapidly. In 1992, Jankowski adopted endoscopic technology in the procedure of pituitary adenomas (3). In 2007, Laufer et al. found that endoscopic transsphenoidal surgery (ETS) is safer (4).
In recent years, some newly published controlled trials investigated the efficacy and safety between ETS and MTS for the treatment of pituitary adenomas. However, the results are inconclusive. This meta-analysis was conducted based on these inconclusive results.
Methods
Protocol and Registration
This meta-analysis was conducted based on the Preferred Reporting Items for Systematic reviews and meta-analyses (PRISMA) recommendations. Previously, we complete a protocol registration on PROSPERO with the number CRD42021241217.
Search Strategy
A search of Pubmed, Embase, Cochrane Controlled Center Register of Controlled Trials (CENTRAL), and Web of Science was performed (cutoff was set on March 01, 2021). Keywords include “microscopic,” “endoscopic,” “Cushing Syndrome,” “Cushing's Disease,” “pituitary adenoma,” “Pituitary Adenomas,” “Acromegaly,” “transsphenoidal.” References of included studies were screened for additional potential trials. No language restriction was applied. Endnote X9 (Thomson Reuters, New York, USA) was used to remove duplicate documents. Titles, abstracts, and full texts were screened respectively to select studies that met the inclusion criteria. We also supplemented it with Google Scholar and further searched the references of the selected articles.
Inclusion and Exclusion Criteria
Inclusion Criteria
(1) Research types: prospective cohort study (PCS), retrospective cohort study (RCS) or case-control study publicly published at home and abroad; (2) Research objects: patients with pituitary adenomas aged >18 years; (3) Intervention measures: experimental group using ETS and the control group was treated with MTS; (4) Main outcomes were: gross tumor removal (GTR), hormone-excess secretion remission (HES remission), treatment-related adverse events (TARE) including cerebrospinal fluid (CSF) leak, diabetes insipidus (DI), epistaxis, hypopituitarism, meningitis, overall complication, visual improvement, Visual loss, hyponatremia/(SIADH), and septal perforation.
Exclusion Criteria
Articles that do not meet the inclusion criteria, cannot obtain the main indicators in the article, and have not received a response through contacting the author and republished articles were excluded.
Data Extraction
General characteristics of the included studies were obtained through reading the full text, as well as the inclusion criteria, interventions, follow-up, main outcomes, etc. For unavailable data, we would try to contact the author through emails. Data extraction was performed by two authors independently. If there is any inconsistency or disagreement in the data, first discuss it. If it still cannot be resolved, the third author will resolve it.
Literature Quality Evaluation
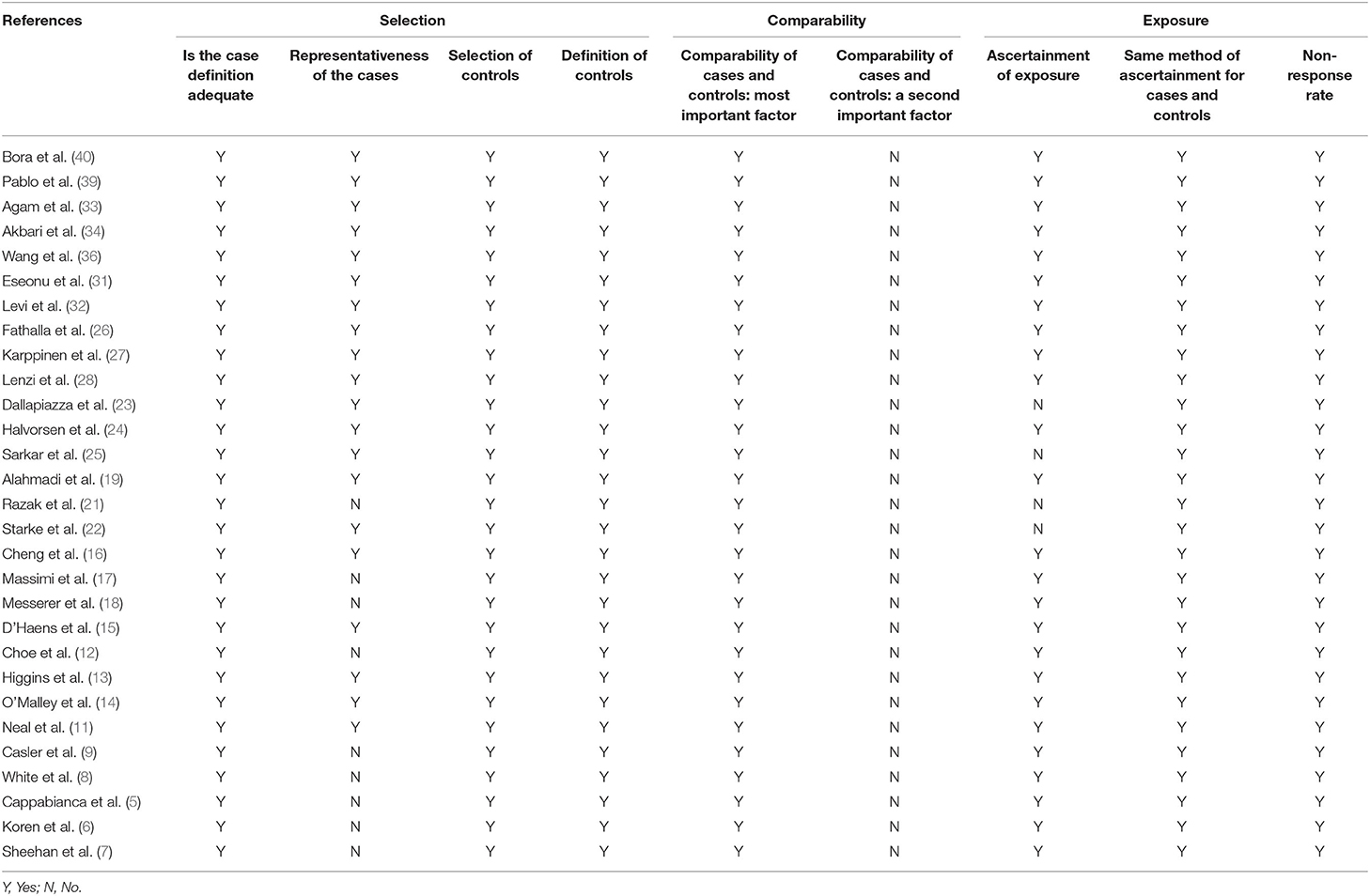
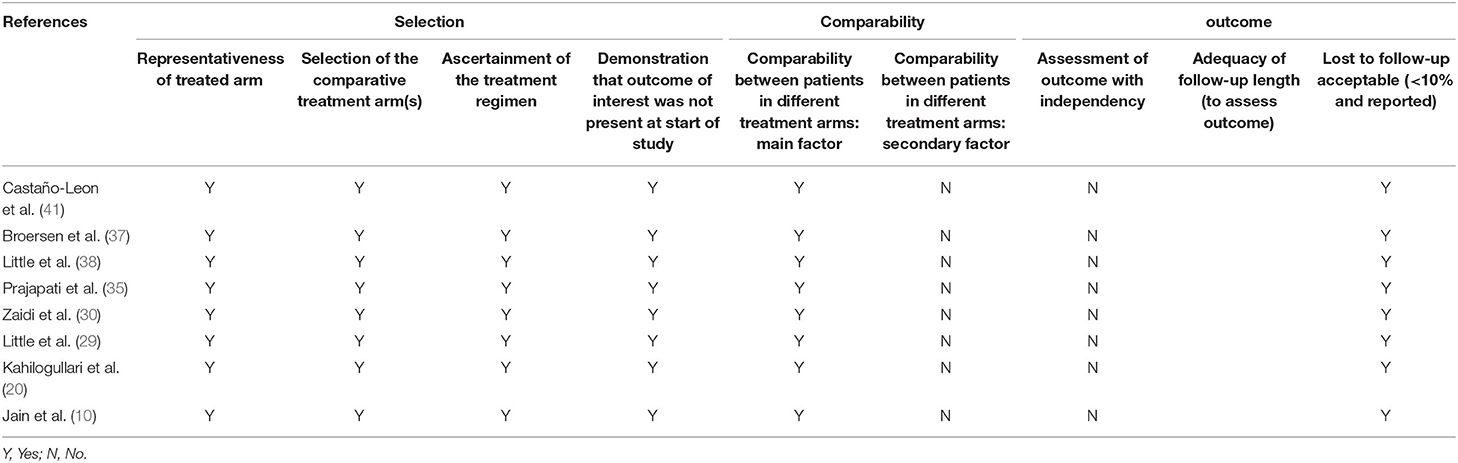
Two authors respectively assessed the included studies according to The Newcastle–Ottawa Scale, NOS. The quality evaluation contains three aspects with eight items: Selection, Comparability, and Exposure.
Statistical Analysis
RevMan5.1 software was used to complete this meta-analysis. The chi-square test and I2 test were used to analyze the heterogeneity among the studies. When low homogeneity among studies was observed (I2 <50%, p > 0.1), the fixed effects model would be adopted; otherwise, the random-effects model would be employed. The descriptive analysis would be performed once clinical data cannot be meta-analyzed.
Patient and Public Involvement
The design or conduct of this meta-analysis did not involve the patient or the public.
Results
Literature Screening and Characteristics of Included Studies
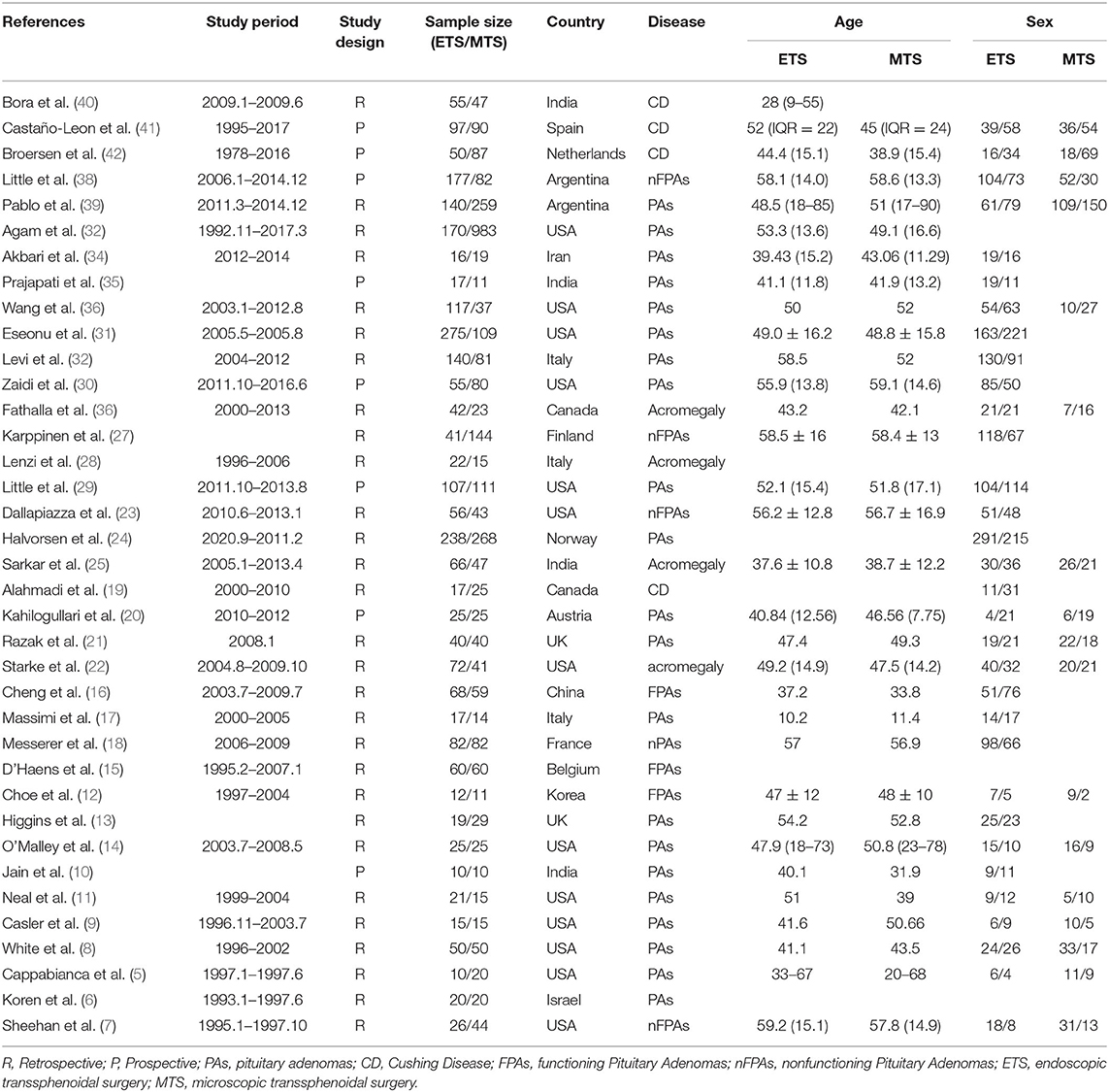
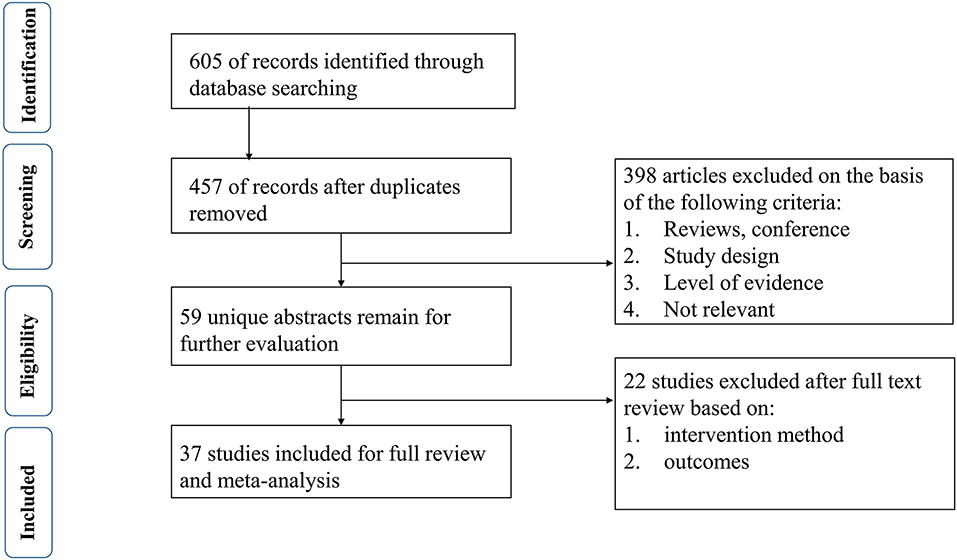
Six hundred and five studies containing 601 initially retrieved and 4 obtained through screening reference list of the included studies were identified. One hundred forty-eight duplicate articles were eliminated by EndNote software. Three hundred ninety-eight studies were abandoned after checking the title and abstract. Twenty-two studies were removed based on the full-text screen, and finally, 37 (5–41) studies with 5,591 participants were confirmed to meet the inclusion criteria. Patients mainly come from Europe, North America, and South America. More details are summarized in Table 1. The literature selection process is shown in Figure 1.
Methodological Quality
Tables 2, 3 showed the details of the quality evaluation of all included studies.
Outcomes
GTR
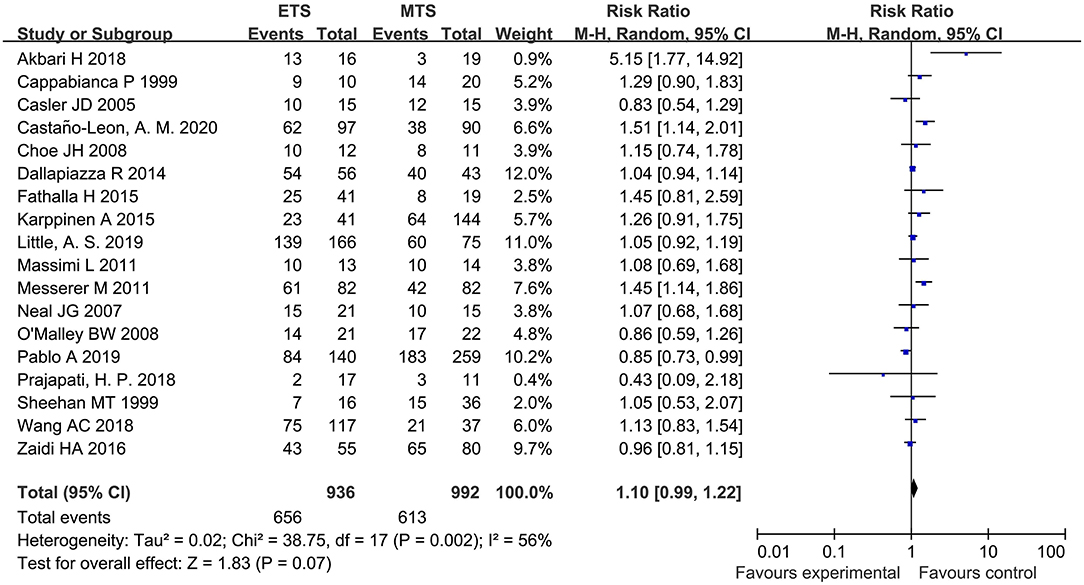
Eighteen studies analyzed the GTR between ETS and MTS for pituitary adenoma. No significant difference was observed in GTR between two groups [RR = 1.10, 95% CI (0.99–1.22), P = 0.07; Figure 2].
HES Remission
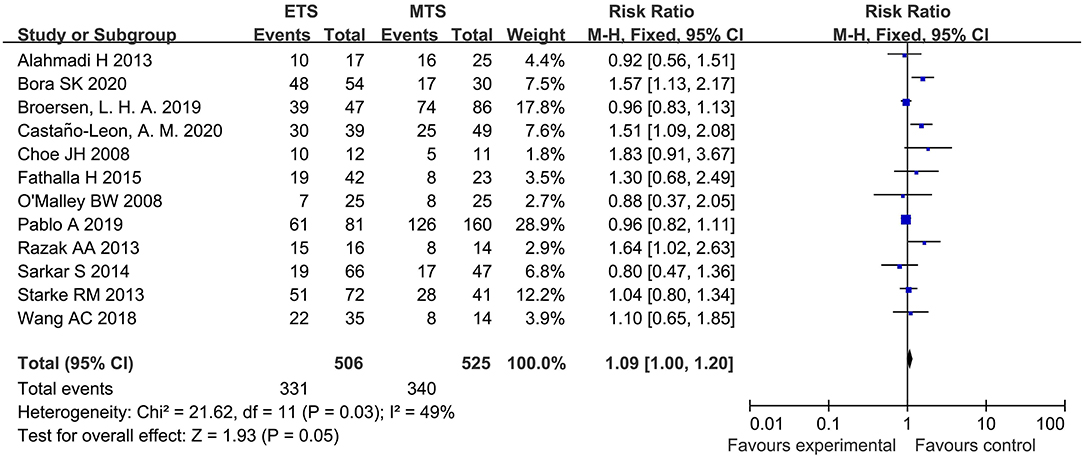
Twelve studies analyzed the HES remission between ETS and MTS for pituitary adenoma. Results indicated that there was no significant difference in HES remission between two groups [RR = 1.09, 95% CI: (1.00–1.20), P = 0.05; Figure 3].
Overall Complication
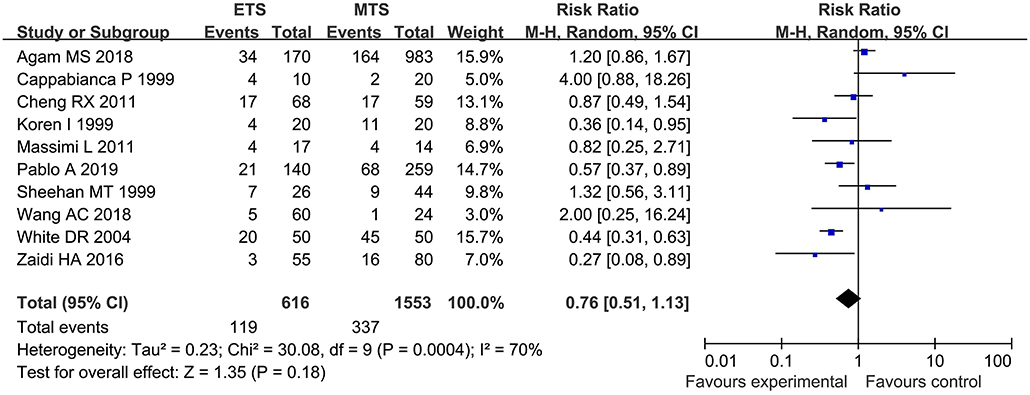
Ten studies investigated the overall complication between ETS and MTS for pituitary adenoma. Results demonstrated that there was no significant difference in overall complication between ETS group and MTS group [RR = 0.76, 95% CI: (0.51–1.33), P = 0.18, Figure 4].
Cerebrospinal Fluid Leak
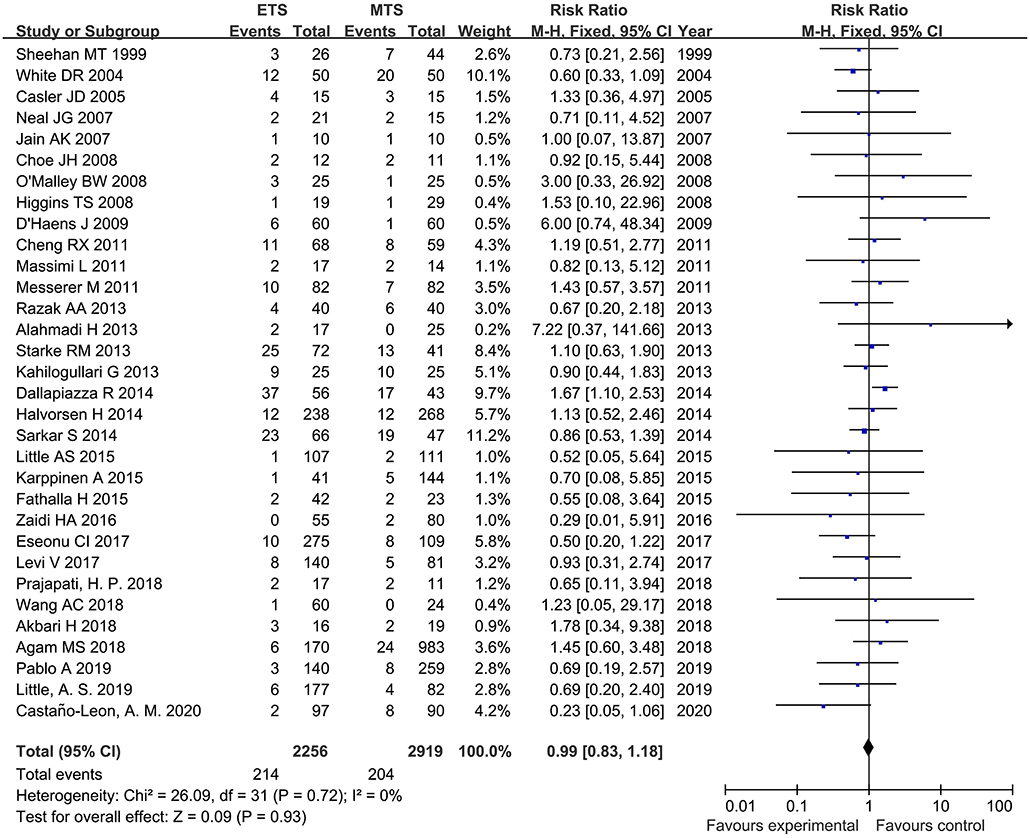
Thirty-two studies analyzed the CSF leak of ETS and MTS for pituitary adenoma. There was no significant difference was observed regarding on incidence of CSF leak between ETS group and MTS group [RR = 0.99, 95% CI: (0.83–1.18), P = 0.05] (Figure 5).
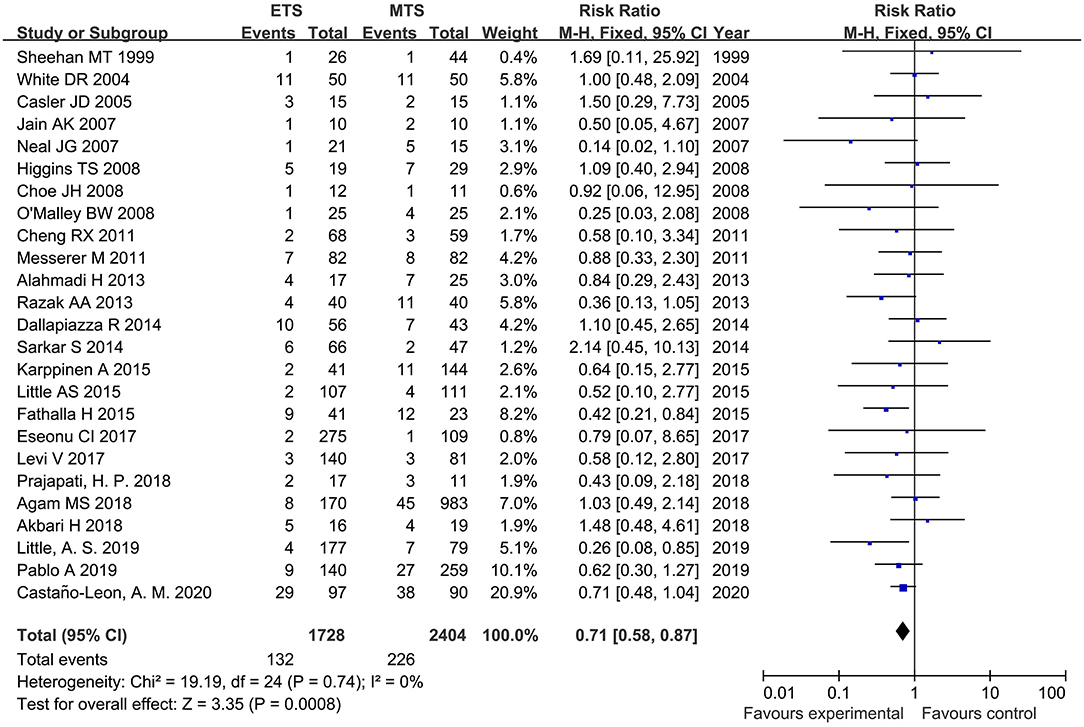
Diabetes Insipidus
Twenty-five studies analyzed the DI of ETS and MTS for pituitary adenoma. For DI, ETS group related to a significantly lower rate in comparison to MTS group [RR = 0.71, 95% CI: (0.58–0.87), P = 0.0008, Figure 6).
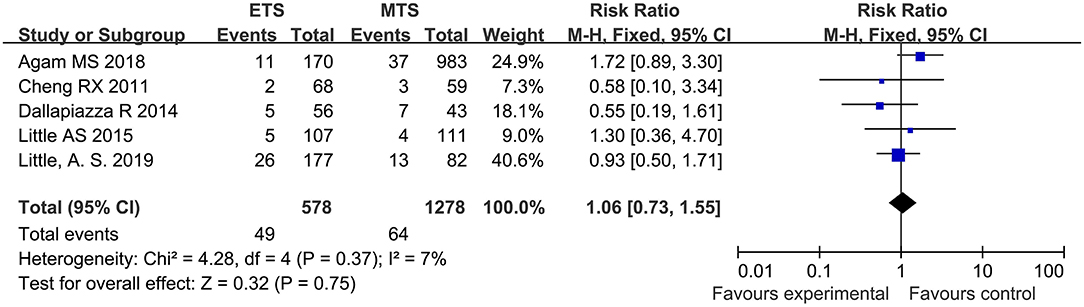
Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIAHD)
Five studies analyzed the SIAHD of ETS and MTS for pituitary adenoma. No significant difference was observed in SIAHD between two groups [RR = 106, 95% CI: (0.73–1.55), P = 0.75; Figure 7].
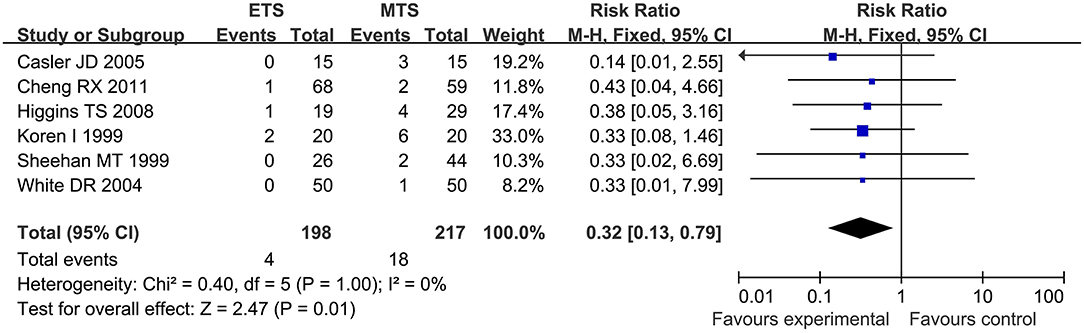
Septal Perforation
Six studies analyzed the septal perforation of ETS and MTS for pituitary adenoma. ETS group showed a significantly lower rate in comparison to MTS group [RR = 0.32, 95% CI: (0.13–0.79), P = 0.01; Figure 8].
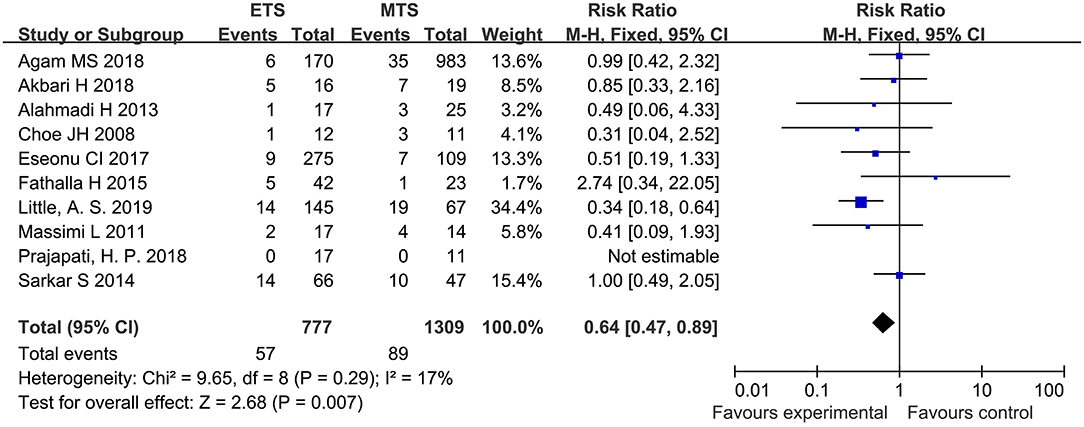
Hypothyroidism
Ten studies analyzed the hypothyroidism of ETS and MTS for the treatment of pituitary adenoma. The results showed that the hypothyroidism in the ETS group was significantly lower than that in the MTS group [RR = 0.64, 95% CI: (0.47–0.89), P = 0.007; Figure 9].
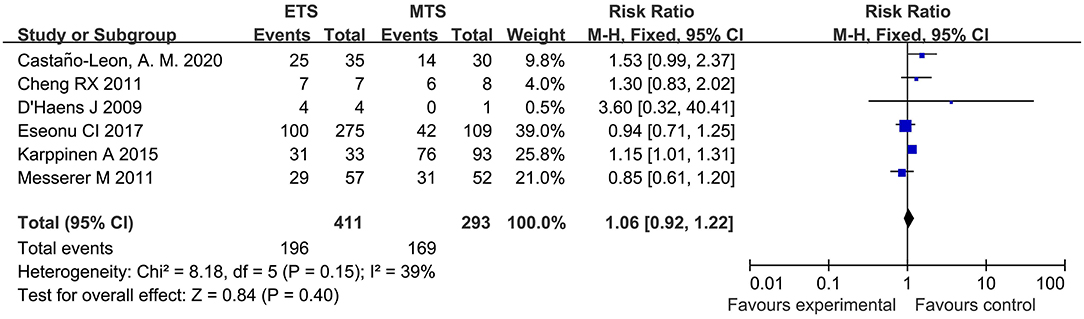
Visual Improvement
Six studies investigated the visual improvement of ETS and MTS for the treatment of pituitary adenoma. No significant difference was observed in visual improvement between two groups [RR = 1.06, 95% CI: (0.92–1.22), P = 0.40, Figure 10].
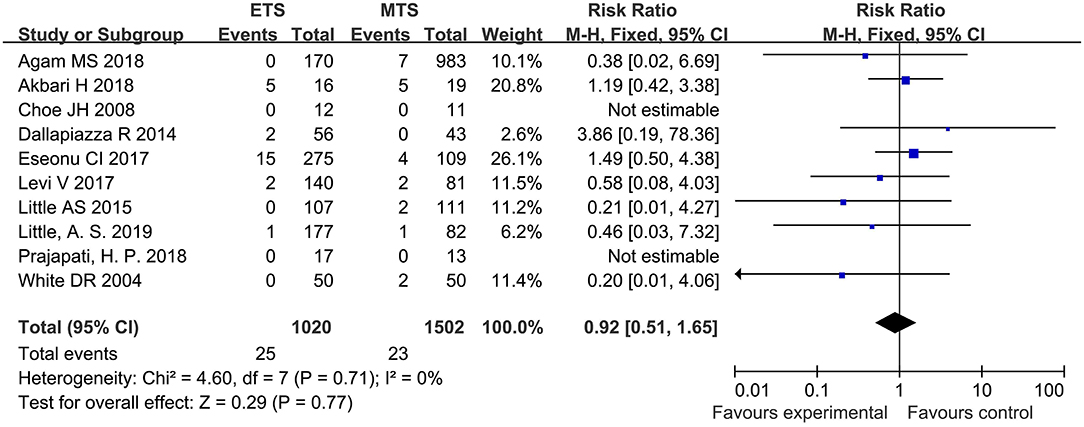
Visual Loss
Ten studies analyzed the visual loss of ETS and MTS for the treatment of pituitary adenoma. No significant difference was observed in visual loss between two groups [RR = 0.92, 95% CI: (0.51–1.65), P = 0.77; Figure 11].
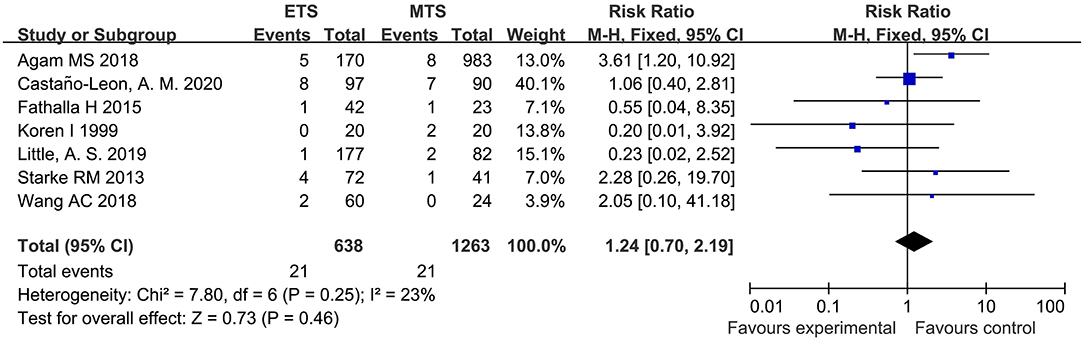
Epistaxis
Seven studies analyzed the epistaxis of ETS and MTS for the treatment of pituitary adenoma. Results indicated that no significant difference was observed in epistaxis between two groups [RR = 1.24, 95% CI: (0.70–2.19), P = 0.46; Figure 12].
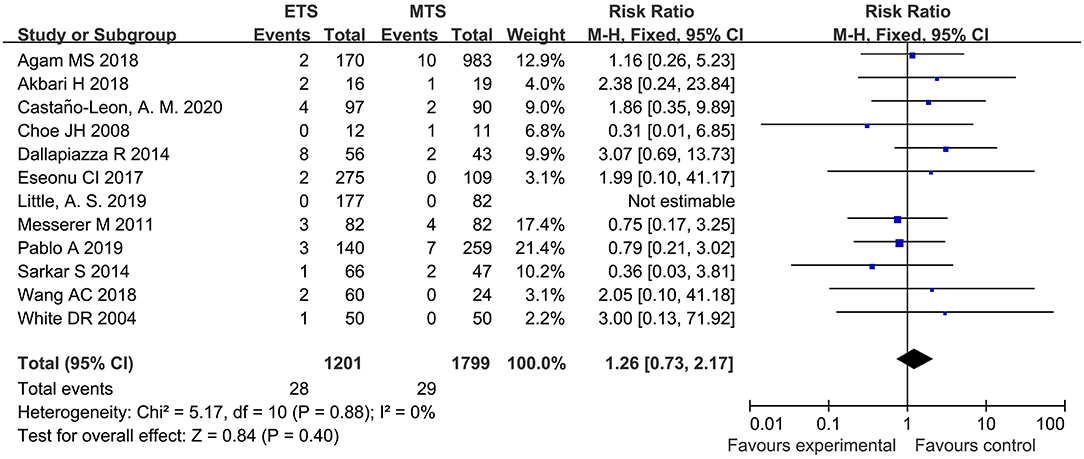
Meningitis
Twelve studies analyzed the meningitis of ETS and MTS for the treatment of pituitary adenoma. Results indicated that no significant difference was observed in meningitis between two groups [RR = 1.26, 95% CI: (0.73–2.17), P = 0.40; Figure 13].
Publication Bias
The funnel plot results showed there is no publication bias (Supplementary Materials).
Discussion
At present, the standard surgical method for pituitary adenomas is ETS or MTS. However, it is still controversy concerning the short-term effects of these two surgical methods.
This meta-analysis included a total of 37 controlled studies to study the efficacy and safety of ETS and MTS for the treatment of pituitary adenomas. Main outcomes included: 1. clinical efficacy: no significant difference was observed concerning GTR, HES remission, and visual improvement between two surgical treatments; 2. clinical safety: ETS could decrease the incidence of diabetes DI, hypothyroidism as well as septal perforation. However, no significant difference was observed regarding CSF leak, epistaxis, meningitis, overall complication, visual loss, and SIADH between the two methods.
Although the two methods do not show significant differences in GTR, ETS has its unique advantages in relatively tricky operations. The application of angled endoscopy, with a large range of movement, could ensure the removal of tumors which cannot be realized through the traditional transsphenoidal approach (4, 42). Secondly, due to the flexibility, the ETS can be inserted into the resected tumor cavity to explore residual tumors, which means that intraoperative MRI would be unnecessary in these cases (43). Besides, for large tumors possibly accompanied by CSF leakage, the panoramic field of the endoscope has its advantage (44–46).
Various factors could influence postoperative vision recovery, such as the age of onset, the degree of preoperative visual field defect, and the size of the tumor. The vision of most patients could be improved after surgery. However, no evidence demonstrated that the selection of surgical methods can affect the recovery of patients' postoperative vision. Our outcomes also indicated a similar conclusion.
For most patients, the postoperative DI is transient. Only a few patients will progress to permanent DI. Besides, the surgical precision has an impact on the occurrence of DI (14, 47). The reduced incidence of DI of ETS may benefit from the fact that endoscopy can ensure relatively good vision during the operation.
CSF leak is considered a common postoperative complication. The lower rate of postoperative CSF leak in patients treated by ETS has been reported (48, 49) which is possibly associated with the following facts: First, endoscopy can detect the lesion tissue and its surrounding structures. Second, the blind corners under microscopy could be observed through different angles of the endoscope. However, our results indicated that no significant difference was observed concerning the rate of postoperative CSF leak between two surgical treatments. Three potential reasons were: 1. the included studies are all observational studies with a relatively low level of evidence-based. 2. The sample size is not large. 3. The rate of CSF leak is not significantly influenced by surgical methods. A large number of high-quality randomized controlled studies were necessary for further confirmation.
Septal perforation is a common postoperative complication for patients treated by MTS due to the use of a retractor during the intraoperative procedure. ETS hardly damages septum nasi because it is unnecessary to adopt a retractor and the approach is the natural passage of the human body (50).
The present analysis has several limitations. First, there were no RCTs in the meta-analysis. Second, most of the included studies did not describe the evaluation method of the GTR in detail. Third, it is impossible to evaluate the relationship of the postoperative results and the classification of pituitary adenomas because no subgroup analysis was conducted according to the classification of pituitary adenomas in major studies included.
Conclusion
This meta-analysis found that endoscopic transsphenoidal surgery cannot significantly decrease GTR and HES remission, but it could decrease the rate of DI, hypothyroidism, and septal perforation without increasing the rate of other complications.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
WD designed the research and had primary responsibility for the final content. JC and HL conducted the research. SM, GL, and QL analyzed the data. JC, QZ, and LH wrote the manuscript. WL revised the manuscript. All authors approved the final manuscript.
Funding
This article was funded by the National Key Research and Development Program of China (No. 2020YFC2004900).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.806855/full#supplementary-material
References
1. Gittleman H, Ostrom QT, Farah PD, Ondracek A, Chen Y, Wolinsky Y, et al. Descriptive epidemiology of pituitary tumors in the United States, 2004–2009. J Neurosurg. (2014) 121:527–35. doi: 10.3171/2014.5.JNS131819
2. Wang AJ, Zaidi HA, Laws ED. History of endonasal skull base surgery. J Neurosurg Sci. (2016) 60:441–53.
3. Jankowski R, Auque J, Simon C, Marchal JC, Hepner H, Wayoff M. Endoscopic pituitary tumor surgery. Laryngoscope. (1992) 102:198–202. doi: 10.1288/00005537-199202000-00016
4. Laufer I, Anand VK, Schwartz TH. Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg. (2007) 106:400–6. doi: 10.3171/jns.2007.106.3.400
5. Cappabianca P, Alfieri A, Colao A, Ferone D, Lombardi G, de Divitiis E. Endoscopic endonasal transsphenoidal approach: an additional reason in support of surgery in the management of pituitary lesions. Skull Base Surg. (1999) 9:109–17. doi: 10.1055/s-2008-1058157
6. Koren I, Hadar T, Rappaport ZH, Yaniv E. Endoscopic transnasal transsphenoidal microsurgery versus the sublabial approach for the treatment of pituitary tumors: endonasal complications. Laryngoscope. (1999) 109:1838–40. doi: 10.1097/00005537-199911000-00022
7. Sheehan MT, Atkinson JL, Kasperbauer JL, Erickson BJ, Nippoldt TB. Preliminary comparison of the endoscopic transnasal vs the sublabial transseptal approach for clinically nonfunctioning pituitary macroadenomas. Mayo Clinic Proc. (1999) 74:661–70. doi: 10.4065/74.7.661
8. White DR, Sonnenburg RE, Ewend MG, Senior BA. Safety of minimally invasive pituitary surgery (MIPS) compared with a traditional approach. Laryngoscope. (2004) 114:1945–8. doi: 10.1097/01.mlg.0000147925.04605.cc
9. Casler JD, Doolittle AM, Mair EA. Endoscopic surgery of the anterior skull base. Laryngoscope. (2005) 115:16–24. doi: 10.1097/01.mlg.0000150681.68355.85
10. Jain AK, Gupta AK, Pathak A, Bhansali A, Bapuraj JR. Excision of pituitary adenomas: randomized comparison of surgical modalities. Br J Neurosurg. (2007) 21:328–31. doi: 10.1080/02688690701395447
11. Neal JG, Patel SJ, Kulbersh JS, Osguthorpe JD, Schlosser RJ. Comparison of techniques for transsphenoidal pituitary surgery. Am J Rhinol. (2007) 21:203–6. doi: 10.2500/ajr.2007.21.2981
12. Choe JH, Lee KS, Jeun SS, Cho JH, Hong YK. Endocrine outcome of endoscopic endonasal transsphenoidal surgery in functioning pituitary adenomas. J Korean Neurosurg Soc. (2008) 44:151–5. doi: 10.3340/jkns.2008.44.3.151
13. Higgins TS, Courtemanche C, Karakla D, Strasnick B, Singh RV, Koen JL, et al. Analysis of transnasal endoscopic vs. transseptal microscopic approach for excision of pituitary tumors. Am J Rhinol. (2008) 22:649–52. doi: 10.2500/ajr.2008.22.3246
14. O'Malley BW, Grady MS, Gabel BC, Cohen MA, Heuer GG, Pisapia J, et al. Comparison of endoscopic and microscopic removal of pituitary adenomas: single-surgeon experience and the learning curve. Neurosurg Focus. (2008) 25:E10. doi: 10.3171/FOC.2008.25.12.E10
15. D'Haens J, Van Rompaey K, Stadnik T, Haentjens P, Poppe K, Velkeniers B. Fully endoscopic transsphenoidal surgery for functioning pituitary adenomas: a retrospective comparison with traditional transsphenoidal microsurgery in the same institution. Surg Neurol. (2009) 72:336–40. doi: 10.1016/j.surneu.2009.04.012
16. Cheng RX, Tian HL, Gao WW, Li ZQ. A comparison between endoscopic trans-sphenoidal surgery and traditional trans-sphenoidal microsurgery for functioning pituitary adenomas. J Int Med Res. (2011) 39:1985–93. doi: 10.1177/147323001103900545
17. Massimi L, Rigante M, D'Angelo L, Paternoster G, Leonardi P, Paludetti G, et al. Quality of postoperative course in children: endoscopic endonasal surgery versus sublabial microsurgery. Acta Neurochir. (2011) 153:843–9. doi: 10.1007/s00701-010-0929-6
18. Messerer M, De Battista JC, Raverot G, Kassis S, Dubourg J, Lapras V, et al. Evidence of improved surgical outcome following endoscopy for nonfunctioning pituitary adenoma removal. Neurosurg Focus. (2011) 30:E11. doi: 10.3171/2011.1.FOCUS10308
19. Alahmadi H, Cusimano MD, Woo K, Mohammed AA, Goguen J, Smyth HS, et al. Impact of technique on cushing disease outcome using strict remission criteria. Can J Neurol Sci. (2013) 40:334–41. doi: 10.1017/S031716710001427X
20. Kahilogullari G, Beton S, Al-Beyati ES, Kantarcioglu O, Bozkurt M, Kantarcioglu E, et al. Olfactory functions after transsphenoidal pituitary surgery: endoscopic versus microscopic approach. Laryngoscope. (2013) 123:2112–9. doi: 10.1002/lary.24037
21. Razak AA, Horridge M, Connolly DJ, Warren DJ, Mirza S, Muraleedharan V, et al. Comparison of endoscopic and microscopic trans-sphenoidal pituitary surgery: early results in a single centre. Br J Neurosurg. (2013) 27:40–3. doi: 10.3109/02688697.2012.703353
22. Starke RM, Raper DM, Payne SC, Vance ML, Oldfield EH, Jane JA. Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab. (2013) 98:3190–8. doi: 10.1210/jc.2013-1036
23. Dallapiazza R, Bond AE, Grober Y, Louis RG, Payne SC, Oldfield EH, et al. Retrospective analysis of a concurrent series of microscopic versus endoscopic transsphenoidal surgeries for Knosp Grades 0-2 nonfunctioning pituitary macroadenomas at a single institution. J Neurosurg. (2014) 121:511–7. doi: 10.3171/2014.6.JNS131321
24. Halvorsen H, Ramm-Pettersen J, Josefsen R, Rønning P, Reinlie S, Meling T, et al. Surgical complications after transsphenoidal microscopic and endoscopic surgery for pituitary adenoma: a consecutive series of 506 procedures. Acta Neurochir. (2014) 156:441–9. doi: 10.1007/s00701-013-1959-7
25. Sarkar S, Rajaratnam S, Chacko G, Chacko AG. Endocrinological outcomes following endoscopic and microscopic transsphenoidal surgery in 113 patients with acromegaly. Clin Neurol Neurosurg. (2014) 126:190–5. doi: 10.1016/j.clineuro.2014.09.004
26. Fathalla H, Cusimano MD, Di Ieva A, Lee J, Alsharif O, Goguen J, et al. Endoscopic versus microscopic approach for surgical treatment of acromegaly. Neurosurg Rev. (2015) 38:541–8. doi: 10.1007/s10143-015-0613-7
27. Karppinen A, Kivipelto L, Vehkavaara S, Ritvonen E, Tikkanen E, Kivisaari R, et al. Transition from microscopic to endoscopic transsphenoidal surgery for nonfunctional pituitary adenomas. World Neurosurg. (2015) 84:48–57. doi: 10.1016/j.wneu.2015.02.024
28. Lenzi J, Lapadula G, D'Amico T, Delfinis CP, Iuorio R, Caporlingua F, et al. Evaluation of trans-sphenoidal surgery in pituitary GH-secreting micro- and macroadenomas: a comparison between microsurgical and endoscopic approach. J Neurosurg Sci. (2015) 59:11–8.
29. Little AS, Kelly DF, Milligan J, Griffiths C, Prevedello DM, Carrau RL, et al. Comparison of sinonasal quality of life and health status in patients undergoing microscopic and endoscopic transsphenoidal surgery for pituitary lesions: a prospective cohort study. J Neurosurg. (2015) 123:799–807. doi: 10.3171/2014.10.JNS14921
30. Zaidi HA, Awad AW, Bohl MA, Chapple K, Knecht L, Jahnke H, et al. Comparison of outcomes between a less experienced surgeon using a fully endoscopic technique and a very experienced surgeon using a microscopic transsphenoidal technique for pituitary adenoma. J Neurosurg. (2016) 124:596–604. doi: 10.3171/2015.4.JNS15102
31. Eseonu CI, ReFaey K, Rincon-Torroella J, Garcia O, Wand GS, Salvatori R, et al. Endoscopic vs. microscopic transsphenoidal approach for pituitary adenomas: comparison of outcomes during the transition of methods of a single surgeon. World Neurosurg. (2017) 97:317–25. doi: 10.1016/j.wneu.2016.09.120
32. Levi V, Bertani GA, Guastella C, Pignataro L, Zavanone ML, Rampini PM, et al. Microscopic versus endoscopic transsphenoidal surgery for pituitary adenoma: analysis of surgical safety in 221 consecutive patients. Clin Otolaryngol. (2017) 42:466–9. doi: 10.1111/coa.12631
33. Agam MS, Wedemeyer MA, Wrobel B, Weiss MH, Carmichael JD, Zada G. Complications associated with microscopic and endoscopic transsphenoidal pituitary surgery: experience of 1153 consecutive cases treated at a single tertiary care pituitary center. J Neurosurg. (2018) 2018:1–8. doi: 10.3171/2017.12.JNS172318
34. Akbari H, Malek M, Ghorbani M, Ramak Hashemi SM, Khamseh ME, Zare Mehrjardi A, et al. Clinical outcomes of endoscopic versus microscopic trans-sphenoidal surgery for large pituitary adenoma. Br J Neurosurg. (2018) 32:206–9. doi: 10.1080/02688697.2018.1429569
35. Prajapati HP, Jain SK, Sinha VD. Endoscopic versus microscopic pituitary adenoma surgery: an institutional experience. Asian J Neurosurg. (2018) 13:217–21. doi: 10.4103/ajns.AJNS_160_16
36. Wang AC, Shah AH, Sidani C, Gaynor BG, Dockrell S, Burks SS, et al. Volumetry in the assessment of pituitary adenoma resection: endoscopy versus microscopy. J Neurol Surg B Skull Base. (2018) 79:538–44. doi: 10.1055/s-0038-1639618
37. Broersen LHA, van Haalen FM, Biermasz NR, Lobatto DJ, Verstegen MJT, van Furth WR, et al. Microscopic versus endoscopic transsphenoidal surgery in the Leiden cohort treated for Cushing's disease: surgical outcome, mortality, and complications. Orphanet J Rare Dis. (2019) 14:64. doi: 10.1186/s13023-019-1038-0
38. Little AS, Kelly DF, White WL, Gardner PA, Fernandez-Miranda JC, Chicoine MR, et al. Results of a prospective multicenter controlled study comparing surgical outcomes of microscopic versus fully endoscopic transsphenoidal surgery for nonfunctioning pituitary adenomas: the Transsphenoidal Extent of Resection (TRANSSPHER) Study. J Neurosurg. (2019) 2019:1–11. doi: 10.3171/2018.11.Jns181238
39. Pablo A, Sofia B, Maximiliano T, Patricia FD, Alvaro C, Claudio Y, et al. Endoscopic versus microscopic pituitary adenoma surgery: a single-center study. Neurol India. (2019) 67:1015–21. doi: 10.4103/0028-3886.266241
40. Bora SK, Suri A, Khadgawat R, Tandon N, Suri V, Chand Sharma M, et al. Management of Cushing's disease: changing trend from microscopic to endoscopic surgery. World Neurosurg. (2020) 134:e46–54. doi: 10.1016/j.wneu.2019.08.165
41. Castaño-Leon AM, Paredes I, Munarriz PM, Jiménez-Roldán L, Hilario A, Calatayud M, et al. Endoscopic transnasal trans-sphenoidal approach for pituitary adenomas: a comparison to the microscopic approach cohort by propensity score analysis. Neurosurgery. (2020) 86:348–56. doi: 10.1093/neuros/nyz201
42. Bobeff EJ, Sánchez-Viguera C, Arráez-Manrique C, Arráez-Sánchez M. Suprasellar epidermoid cyst: case report of extended endoscopic transsphenoidal resection and systematic review of the literature. World Neurosurg. (2019) 128:514–26. doi: 10.1016/j.wneu.2019.05.100
43. Nimsky C, von Keller B, Ganslandt O, Fahlbusch R. Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery. (2006) 59:105–14. doi: 10.1227/01.NEU.0000219198.38423.1E
44. Al-Mefty O, Pravdenkova S, Gragnaniello C. A technical note on endonasal combined microscopic endoscopic with free head navigation technique of removal of pituitary adenomas. Neurosurg Rev. (2010) 33:243–8. doi: 10.1007/s10143-010-0241-1
45. McLaughlin N, Eisenberg AA, Cohan P, Chaloner CB, Kelly DF. Value of endoscopy for maximizing tumor removal in endonasal transsphenoidal pituitary adenoma surgery. J Neurosurg. (2013) 118:613–20. doi: 10.3171/2012.11.JNS112020
46. Broersen LHA, Biermasz NR, van Furth WR, de Vries F, Verstegen MJT, Dekkers OM, et al. Endoscopic vs. microscopic transsphenoidal surgery for Cushing's disease: a systematic review and meta-analysis. Pituitary. (2018) 21:524–34. doi: 10.1007/s11102-018-0893-3
47. Little AS, Chapple K, Jahnke H, White WL. Comparative inpatient resource utilization for patients undergoing endoscopic or microscopic transsphenoidal surgery for pituitary lesions. J Neurosurg. (2014) 121:84–90. doi: 10.3171/2014.2.JNS132095
48. Netea-Maier RT, van Lindert EJ, den Heijer M, van der Eerden A, Pieters GF, Sweep CG, et al. Transsphenoidal pituitary surgery via the endoscopic technique: results in 35 consecutive patients with Cushing's disease. Eur J Endocrinol. (2006) 154:675–84. doi: 10.1530/eje.1.02133
49. Cho DY, Liau WR. Comparison of endonasal endoscopic surgery and sublabial microsurgery for prolactinomas. Surg Neurol. (2002) 58:371–5. doi: 10.1016/S0090-3019(02)00892-3
Keywords: pituitary adenoma, endoscopic, meta-analysis, microscopic, transsphenoidal surgery
Citation: Chen J, Liu H, Man S, Liu G, Li Q, Zuo Q, Huo L, Li W and Deng W (2022) Endoscopic vs. Microscopic Transsphenoidal Surgery for the Treatment of Pituitary Adenoma: A Meta-Analysis. Front. Surg. 8:806855. doi: 10.3389/fsurg.2021.806855
Received: 01 November 2021; Accepted: 20 December 2021;
Published: 02 February 2022.
Edited by:
Lukas Rasulić, University of Belgrade, SerbiaReviewed by:
Giacomo Fiacchini, University of Pisa, ItalyPetar Vulekovic, University of Novi Sad, Serbia
Copyright © 2022 Chen, Liu, Man, Liu, Li, Zuo, Huo, Li and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Deng, ZGVuZ3dlaUAxNjMuY29t
†These authors have contributed equally to this work
 Jia Chen1†
Jia Chen1† Hongyan Liu
Hongyan Liu