
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 06 January 2022
Sec. Thoracic Surgery
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.792709
This article is part of the Research Topic Non-Intubated Video-Assisted Thoracoscopic Surgery (NIVATS) View all 6 articles
Background: Non-intubated video-assisted thoracic surgery (NIVATS) can be safely performed in lung volume reduction surgery for patients with severe pulmonary dysfunction. However, there is still no cohort observation on the effects of NIVATS on patients with pulmonary dysfunction undergoing different types of thoracic procedures. This retrospective study aimed to observe the effects of NIVATS for this kind of patients.
Methods: Three hundred and twenty-eight patients with moderate to severe obstructive pulmonary dysfunction, who underwent video-assisted thoracic surgery (VATS), were retrospectively collected from June 1st, 2017 to September 30th, 2019. Patients in NIVATS were case-matched with those in intubated video-assisted thoracic surgery (IVATS) by a propensity score-matched analysis. The primary outcome was the comparison of perioperative values, the secondary outcome was the risk factors for postoperative clinical complications (PCP) which were identified by binary logistic regression analysis.
Results: After being matched, there were no differences in demographics and preoperative values of pulmonary function between NIVATS and IVATS groups. The duration of surgery and anesthesia had no difference (P = 0.091 and P = 0.467). As for the postoperative recovery, except for the mean intensive care unit (ICU) stay was longer in the IVATS group than in the NIVATS group (P = 0.015), the chest tube removal time and the postoperative hospital stay had no difference (P = 0.394 and P = 0.453), and the incidence of PCP also had no difference (P = 0.121). The binary logistic regression analysis revealed that the history of pulmonary disease, anesthesia method, and surgical location were risk factors of PCP.
Conclusion: For patients with pulmonary dysfunction when undergoing different types of thoracic procedures, the NIVATS can be performed as effectively and safely as the IVATS, and can reduce the ICU stay.
Lung volume reduction surgery (LVRS) has demonstrated significant improvements in respiratory function for patients with pulmonary dysfunction (1–3). However, LVRS may cause several complications, respectively leading to 5 and 59% mortality and morbidity rates (4).
Patients with pulmonary dysfunction usually underwent thoracic surgery with endotracheal intubation and general anesthesia, which can ensure intraoperative oxygenation and benefit airway management. However, patients need one-lung ventilation during operation, which may lead to pneumothorax on the ventilated side due to the fragile and inelastic lung tissue. In addition, many patients need postoperative ventilator support because of poor respiratory function compensation (5), and some patients are difficult to wean off the ventilator, resulting in lung infection, which increases complications and mortality (6).
A novel approach to this problem involves the increasing appliance of non-intubated video-assisted thoracic surgery (NIVATS). Perioperative morbidity may be reduced by avoiding positive pressure ventilation and employing a spontaneous respiration method (7), particularly in patients with poor cardiorespiratory performance (8). Small cases consisted of most early experience, which mainly dealt with patients with chronic respiratory failure or other comorbidities performed under epidural anesthesia (9).
However, a definitive conclusion on the efficacy of NIVATS on patients with pulmonary dysfunction cannot be drawn out on the small sample size (10). Besides, it was a lack of homology of the preoperative demographic (10, 11). Furthermore, many studies have just pointed out that NIVATS is feasible in patients with severe pulmonary dysfunction when undergoing LVRS, but there is no evidence for patients with moderate to severe pulmonary dysfunction when undergoing other types of thoracic procedures. Finally, there is scarce literature research on risk factors for postoperative clinical complications (PCP) after VATS for patients with pulmonary dysfunction. Identification of risk factors for PCP after VATS on a large cohort of patients is warranted. Therefore, the major outcome of this study is to make a retrospective comparison of NIVATS in patients with moderate to severe pulmonary dysfunction with similar patients of IVATS to undergo different types of thoracic procedures, and the secondary outcome was identified the risk factors for PCP for this kind of patients.
This was a single-center retrospective cohort study. The perioperative clinical and radiological data have been stored according to a standardized protocol from June 1st, 2017 to September 30th, 2019. It was reviewed and approved by the Research Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China on February 9th, 2021, with the exemption from human subjects review and requirement of consent. Results of the NIVATS group were retrospectively compared with those of intubated video-assisted thoracic surgery group (IVATS group). Eligibility criteria were the same for both groups. The inclusion criteria were as follows: (1) adult patients over 18 years old underwent video-assisted thoracic surgery (VATS); (2) Patients with moderate-to-severe obstructive pulmonary dysfunction. According to the American Thoracic Society guideline, the preoperative forced expiratory volume in 1 second (FEV1) <60% predicted is defined as moderate to severe impairment of pulmonary function (12); (3) American Society of Anesthesiologists (ASA) score ≤ 3. Patients were excluded if they had a history of thoracic surgery. Patients were also excluded if they underwent overlapping operations besides lungs, bilateral lung surgery, mediastinal mass, thoracotomy, chest wall mass, bilateral sympathetic resection, infundibular thorax, pericardiectomy, pleural pathology, tracheal and esophageal surgery, or had an intraoperative conversion to endotracheal intubation due to bleeding, pleural adhesions, or other non-hypoxia factors. Patients with a lack of intraoperative values or incomplete postoperative medical records were also excluded (Figure 1).

Figure 1. Flow chart of data collection. VATS, video-assisted thoracic surgery; NIVATS, non-intubated video-assisted thoracic surgery; IVATS, intubated video-assisted thoracic surgery.
All patients received propofol target-controlled infusion (2–4 μg/ml), remifentanil (0.03–0.1 μg/kg/min), dexmedetomidine (0.5–1 μg/kg/h) for sedation and analgesic.
In the IVATS group, a Mallinckrodt double-lumen endobronchial tube was initiated under the assist of cisatracurium, and one-lung ventilation was commenced. A protective ventilation strategy was carried out to assure adequate oxygenation by low tidal volume 5–6 ml/kg and maximized exhalation time.
The technique for NIVATS anesthesia has been already described in detail (13). Briefly, the laryngeal mask airway (LMA) was inserted into the pharynx and combined with regional anesthesia with intercostal and vagus block. Spontaneous ventilation was maintained during the operation. Fraction of inspired O2 (FiO2) was increased to maintain SpO2 ≥90% (14), and the mean arterial pressure (MAP) >60 mmHg was maintained by Dopamine or Norepinephrine. Permissive hypercapnia was acceptable, and pH <7.2 was set up as a safety limit for correction of hypercapnia (11).
The thoracoscopic procedures were similar in NIVATS and IVATS groups, which followed the consensus guidelines of the American Association for Thoracic Surgery (AATS) (15). The patient was placed in a full lateral decubitus position. The surgical procedure for each patient was determined according to the stage and location of the lesion in computed tomography images. Anatomical resection includes radical resection of lung cancer and segmental resection; Non-anatomical resection includes lung wedge resection, bullae resection, and lung volume reduction surgery. All patients were transferred to the post-anesthesia care unit (PACU) and then was sent back to the ward or intensive care unit (ICU). The blood cells analysis and blood gas analysis were performed on the first day after the operation. The PCP was based on the Clavien-Dindo classification. No PCP and fever were classified in Grade I; Dyspnea and arrhythmia were classified in Grade II; Thoracentesis and air leakage required replacing chest-tube were classified in Grade III; Mechanical ventilation was classified in Grade IV. The postoperative chest radiography results on the 1st and 4th days were compared by radiologists. Atelectasis, pulmonary exudation, or pleural effusion, were regarded as abnormal results. The chest tube was removed when serous fluid loss <200 ml in 24 h and no air leak following 2 h of tube clamping. Criteria for discharge were stable clinical conditions, oxygen saturation of 90% or above at rest, and all chest tubes removed. Postoperative hospital stay was defined as the number of hospitalized days after surgery.
All statistical analyses were performed using SPSS software (version 26.0, Chicago, IL, USA). The missing data (<5% of total values) were replaced by series mean. The selection bias of the NIVATS and IVATS groups was minimized by a propensity score matching analysis. Caliper matching method of 1:1 without replacement was used with caliper set as 0.2 standard deviations of the propensity scores. Balance examination was conducted by using standardized differences. The propensity score model development was done by including age, BMI, gender, and surgery type in the logistic regression model to predict the IVATS group. For continuous variables, restricted cubic spline function with three knots was also included, and non-significant cubic splines were excluded. We also examined potential interactions between predictor variables by stepwise logistic regression (entry significance = 0.2 and stay significance = 0.05). Owing to a large number of potential interactions, only two-way interaction was considered. The final model was then used to estimate the propensity scores.
Continuous data were presented as the mean± standard deviations for normal distribution, or as median (lower, upper quartiles) for skewness distribution. Dichotomous data were presented as numbers (%). The independent samples t-test was analyzed for continuous data between two groups. Mann-Whitney U-test was used for dichotomous data and skewed distributed data. The preoperative and postoperative counts of leukocyte and neutrophil were analyzed by paired t-test. The comparisons of clinical complications between two groups were analyzed by a Binomial test. P < 0.05 was considered to be statistically significant.
Binary logistics regression was used to analyze the linear regression relationship between preoperative factors and PCP to find risk factors. The Chi-square test or independent sample t-test was used to compare the perioperative clinical data between the two groups. P < 0.05 was indicated statistically significant.
A total of 328 patients with moderate to severe pulmonary dysfunction who underwent VATS were included, 278 patients in the IVATS group and 50 patients in the NIVATS group. Thirty-seven cases were identified in each group by matching propensity scores (Table 1).
There were no statistically significant differences in age, gender, BMI, comorbidity, ThRCRI, types of thoracic procedure, surgical location, surgical site, and the values of pulmonary function test between IVATS and NIVATS groups after matching (P > 0.05) (Table 1).
The duration of surgery and anesthesia had no difference (P = 0.091 and P = 0.467). As for the postoperative recovery, the duration of chest-tube indwelling and the postoperative hospital stay had no difference (P = 0.394 and P = 0.453), except for the mean ICU stay was longer in the IVATS group than in the NIVATS group (P = 0.015). The PCP based on the Clavien-Dindo classification also had no difference (P = 0.121) (Table 2).
Logistic regression analysis showed that pulmonary disease, anesthesia method, surgery location in the upper lobe, were the risk factors for PCP (P < 0.05) (Table 3).
Preoperative and postoperative counts of leukocytes were higher in the IVATS group than those in the NIVATS group (P = 0.011 and P = 0.021), and the postoperative counts of leukocytes were higher than preoperative ones in the IVATS group (P < 0.001). The preoperative values of neutrophils were higher in the IVATS group (P = 0.007), but the values of preoperative and postoperative nurtrophils had no difference between the IVATS and NIVATS groups (P = 0.465 and P = 0.492) (Figure 2).
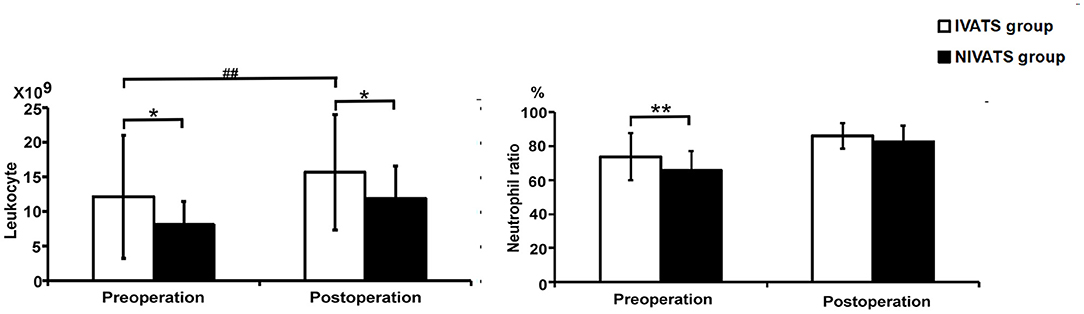
Figure 2. Blood cells analysis (the left is leukocyte and the right is neutrophil ratio) in the IVATS group and NIVATS group before and after the operation. *P-value presents the comparison of values between IVATS and NIVATS group (*presented P < 0.05 and **presented P < 0.01) #P-value presents the comparison of preoperative and postoperative values in the IVATS group (#presented P < 0.05 and ##presented P < 0.01).
No significant difference in preoperative and postoperative values of PaO2 and PaCO2 between the two groups (P > 0.05) (Figure 3).
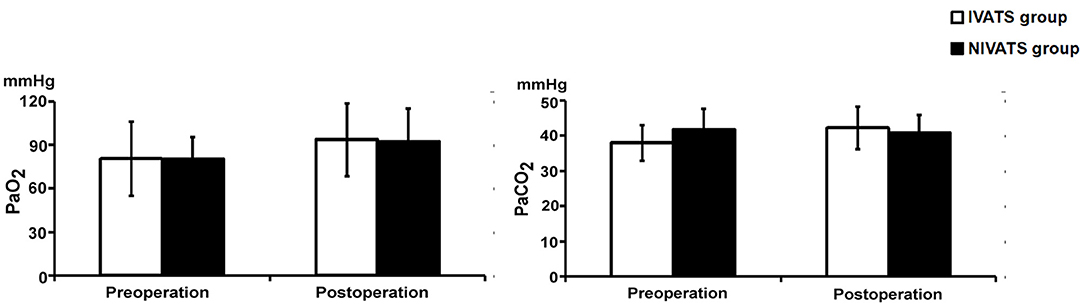
Figure 3. Blood gas values changed in the IVATS group and NIVATS group. Before and after operation, the PaO2 (Left) and PaCO2 (Right) of the two groups were compared. No significant difference of preoperative and postoperative values between the two groups.
The major finding of this retrospective study revealed that no patients of the NIVATS group were reintubated due to hypoxia. It was believed that, for patients with moderate to severe pulmonary dysfunction, the methods of NIVATS, did not affect the duration of surgical and anesthesia, and did not affect the incidence of PCP, when compared with IVATS, but the NIVATS decreased the length of ICU stay. Postoperative clinical complications occurred in 71 (21.6%) of 328 patients. The main risk factors for PCP were the pulmonary disease, anesthesia method, surgery location in the upper lobe.
With the improvement of surgical techniques and anesthesia methods, patients with pulmonary dysfunction, which was taboo in surgery decades ago, can also receive thoracic surgery. This population mostly is older, with multiple comorbidities, and poor pulmonary function, which makes the perioperative period full of challenges. Video-assisted thoracic surgery is becoming a standard of care (16), and can reduce the incidence of respiratory complications in patients with poor pulmonary function (17). At present, the NIVATS also has been performed in this kind of patients with good outcomes (10, 11).
Our study confirmed that patients with moderate to severe pulmonary dysfunction can tolerate different types of thoracic procedures when initiated NIVATS. Complex and time-consuming anatomical procedures were more than 40% in NIVATS groups. The duration of anesthesia and surgery were the same as those in IVATS. As a result, the ICU stay was shorter than that of the IVATS group. Finally, the postoperative hospital stay and chest-tube duration, and the incidence of PCP were improved, and these improvements did not differ from those achieved in the IVATS group. When VATS was performed for patients with pulmonary dysfunction, endotracheal intubation general anesthesia was initiated in the past. Indeed, the positive pressure ventilation may be harmful, when in contrast to spontaneous respiration which tidal volume is created by negative inspiratory pressure. Positive pressure ventilation can aggravate fragile lungs by irreversible barotrauma (18). Non-intubated thoracic surgery aims to do as least damage as possible.
Contrary to the intubated method, NIVATS offers an option to avoid more damage on pathological lungs by preserving the negative inspiratory pressure instead of positive inspiratory pressure and maybe currently the best option for fragile patients. Furthermore, general anesthesia applied on patients with pulmonary dysfunction would have carried the risk of it being difficult to wean off these patients from ventilators (5, 6). Therefore, NIVATS without muscle relaxants and maintaining spontaneous ventilation may avoid this concern. Non-intubated video-assisted thoracoscopy resulted in a significantly shorter ICU stay, which offered the double advantage of less risk of iatrogenic infection, reducing medical costs because of quicker discharge, makes more space on the patient's waiting list and also saves human resources because ICU staff can deal with patients staying less time in the intermediate care or the ICU. More and more samples have confirmed that fragile patients can benefit from NIVATS with good outcomes from thoracic surgery (19, 20).
Spontaneous respiration inevitably leads to hypercapnia resulting from intraoperative CO2 accumulation. In this case, the benefit of NIVATS outweighs the risk of intraoperative hypercapnia due to its fast recovery, and on the other hand, these patients have poor pulmonary function, the basic level of CO2 is high, so they can tolerate high levels of hypercapnia. Some of the patients included in this study were ASA grade 3, which implied that NIVATS could also be extended to patients higher than ASA II. But this requires that, firstly, Anesthesiologists need to have much experience of NIVATS, for the reason that it may have to convert to endotracheal intubation due to unpredictable conditions, such as bleeding or pleural adhesion (21); secondly, the surgeon should have rich experience in thoracoscopy, preferably more than 5 years, and can complete various types of thoracoscopic procedures (22); thirdly, the operation time should not be too long and the surgical procedure is not too difficult. Since long-term spontaneous breathing during NIVATS may lead to hypoxia and hypercapnia. Previous unilateral lobectomy and contralateral wedge resection (23), as well as lung volume reduction surgery in patients with COPD (7, 24–26), these patients with severe pulmonary dysfunction, are more suitable for NIVATS.
Licker showed that an FEV1 <60% was associated with an increase in respiratory complications (27). Awake non-resectional LVRS has been proved feasible and safe for patients with severe pulmonary dysfunction with a faster recovery (11, 28, 29) and satisfactory 6-month outcome (23), and with less prolonged air leak (30). As for patients with moderate pulmonary dysfunction, NIVATS can also be proved to work (10). Our anesthesia method is similar to Wang's (10), but Wang's sample size is small and lack of the control group. The current analysis results add to the previous findings that, as for patients with moderate to severe pulmonary dysfunction, the NIVATS can offer similar clinical outcomes with IVATS when performed different types of thoracic procedures, especially with a reduced duration of ICU stay.
The anesthesia method was one of the main risk factors for PCP, which can be preluded that the complications of intubated general anesthesia, such as residual neuromuscular blockade, ventilator-induced lung injury, and airway trauma, can be avoided in NIVATS (31–34). Furthermore, refraining from general anesthesia in patients combined with pulmonary diseases, such as COPD, is associated with lower incidences of pulmonary infection, long-term ventilator dependence, and postoperative reintubation (35). Surgery location in the upper lobe was also a risk factor for PCP. The possible reason was that, under the effect of intrathoracic pressure, the alveolar expansion of the lower lobe is inferior to that of the upper lobe. When the upper lobe was removed, the blood flow of the lower lobe increased, but the ventilation did not improve immediately after the operation, which easily caused the imbalance of ventilation to perfusion, thus increased the complications such as postoperative hypoxia. In addition, upper lobe surgery accounted for more than 60% of all operations in this study. Accordingly, the probability of postoperative complications was also increased.
The elements of fast-track surgery—regional analgesia, smaller incision, minimizing opiate use, rapid metabolism of anesthetics, and a goal-directed fluid therapy—are not novelties to thoracic surgery (36). And the NIVATS contains all these elements. Non-intubated video-assisted thoracic surgery can accelerate postoperative rehabilitation, and our results also suggested that NIVATS can provide postoperative clinical results similar to IVATS. But in our study, patients with higher preoperative values of leukocyte and neutrophil mostly initiated IVATS, indicating that patients with severe preoperative infection are still recommended to choose intubated general anesthesia, which is owing to its effectiveness draining sputum during operation, and avoiding intraoperative hypoxia.
Our study has several weaknesses. Firstly, it was a single-center study that is partly retrospective and the number of patients was limited. Secondly, the surgical procedures were variable, rather than a single procedure. But this can further show that NIVATS is feasible for different types of thoracic procedures. Thirdly, although previous literature has reported the effectiveness of NIVATS in patients with severe pulmonary dysfunction, the results of NIVATS in patients with moderate to severe pulmonary dysfunction is a useful supplement to previous studies. Fourth, patients, who had an intraoperative conversion to endotracheal intubation due to surgical factors, not non-hypoxia factors, were excluded from the study. A small number of patients may be excluded, but this allowed a more homogeneous sample. In addition, the effects of the two anesthesia methods on the postoperative recovery were mainly observed. Thus, only the successful completion of anesthesia can be included in the study. Patients excluded due to surgical factors would not affect the risk factor analysis.
Our study results have shown that patients with moderate and severe obstructive ventilation dysfunction when undergoing different types of thoracoscopic surgery, NIVATS show similar outcomes with IVATS during postoperative hospitalization, except for the decreased the length of ICU stay. In other words, for patients with moderate to severe obstructive ventilation dysfunction, the NIVATS or IVATS does not affect the short-term postoperative rehabilitation, both NIVATS and IVATS are feasible and safe. In the future, we need more randomized controlled trials to confirm this conclusion, and we need to observe the comparison of long-term rehabilitation.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the First Affiliated Hospital Guangzhou Medical University. The ethics committee waived the requirement of written informed consent for participation.
SYD collected the clinical data and writing the manuscript. LL participated in the drafting and critically revised the manuscript. YYC and LJ supervised the study and conducting statistical analyses. All authors substantially contributed to the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to everyone involved in carrying out the study, analyzing the data, and producing the manuscript.
VATS, video-assisted thoracic surgery; NIVATS, non-intubated video-assisted thoracic surgery; IVATS, intubated VATS; ICU, intensive care unit; LVRS, lung volume reduction surgery; PCP, postoperative clinical complications; FEV1, forced expiratory volume in 1 second; LMA, laryngeal mask airway; FiO2, fraction of inspired O2; MAP: mean arterial pressure; AATS, American Association for Thoracic Surgery; PACU, post-anesthesia care unit.
1. Naunheim KS, Wood DE, Moshenifar Z, Sternberg AL, Criner GJ, DeCamp MM, et al. Long term followup of patients receiving lung-volume reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg. (2006) 82:431–4. doi: 10.1016/j.athoracsur.2006.05.069
2. Ramsey SD, Berry K, Etzioni R, Kaplan RM, Sullivan SD, Wood DE. Cost effectiveness of lung-volume-reduction surgery for patients with severe emphysema. New Eng J Med. (2003) 348:2092–102. doi: 10.1056/NEJMsa030448
3. Naunheim KS, Wood DE, Krasna MJ, DeCamp MM, Ginsburg ME, McKenna RJ, et al. Predictors of operative morbidity and mortality in the National Emphysema Treatment Trial. J Thorac Cardiovasc Surg. (2006) 131:43–53. doi: 10.1016/j.jtcvs.2005.09.006
4. Criner GJ, Cordova F, Sternberg AL, Martinez FJ. The National Emphysema Treatment Trial (NETT) Part II: lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med. (2011) 184:881–93. doi: 10.1164/rccm.201103-0455CI
5. Rossi A, Butorac-Petanjek B, Chilosi M, Cosío BG, Flezar M, Koulouris N, et al. Chronic obstructive pulmonary disease with mild airflow limitation: current knowledge and proposal for future research - a consensus document from six scientific societies. Int J Chron Obstruct Pulmon Dis. (2017) 12:2593–610. doi: 10.2147/COPD.S132236
6. O'Donnell DE, Laveneziana P, Webb K, Neder JA. Chronic obstructive pulmonary disease: clinical integrative physiology. Clin Chest Med. (2014) 35:51–69. doi: 10.1016/j.ccm.2013.09.008
7. Pompeo E, Rogliani P, Palombi L, Orlandi A, Cristino B, Dauri M. The complex care of severe emphysema: role of awake lung volume reduction surgery. Ann Transl Med. (2015) 3:108. doi: 10.3978/j.issn.2305-5839.2015.04.17
8. Armenta-Flores R, Sanchez-Quiroz J, Castillo-Delgado S, Camarena-Arredondo V. Non-intubated video-assisted thoracic surgery: breaking down paradigms. Eur J Cardiothorac Surg. (2017) 51:197. doi: 10.1093/ejcts/ezw231
9. Tacconi F, Pompeo E. Nonintubated video-assisted thoracic surgery: where does evidence stand? J Thorac Dis. (2016) 8(Suppl 4):S364–75. doi: 10.21037/jtd.2016.04.39
10. Wang ML, Hung MH, Hsu HH, Chan KCH, Cheng YJ, Chen JS. Non-intubated thoracoscopic surgery for lung cancer in patients with impaired pulmonary function. Ann Transl Med. (2019) 7:40. doi: 10.21037/atm.2018.11.58
11. Pompeo E, Tacconi F, Mineo TC. Comparative results of non-resectional lung volume reduction performed by awake or non-awake anesthesia. Eur J Card Thorac Surg. (2011) 39:e51–8. doi: 10.1016/j.ejcts.2010.11.071
12. O'Brien C, Guest PJ, Hill SL, Stockley R A. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. (2000) 55:635–42. doi: 10.1136/thorax.55.8.635
13. He J, Liang H, Wang W, Akopov A, Aiolfi A, Ang KL, et al. Tubeless video-assisted thoracic surgery for pulmonary ground-glass nodules: expert consensus and protocol. Trans Lung Cancer Res. (2021) 10:3503–19. doi: 10.21037/tlcr-21-663
14. Woldt P, Kruse P, Ellger B. Anesthesiological considerations in emphysema surgery. Ann Transl Med. (2020) 8:1470. doi: 10.21037/atm-2019-le-06
15. Svensson LG, Gillinov AM, Weisel RD, Keshavjee S, Bacha EA, Moon MR, et al. The American Association for thoracic surgery consensus guidelines: reasons and purpose. J Thorac Cardiovasc Surg. (2016) 151:935.e1–9.e1. doi: 10.1016/j.jtcvs.2015.09.138
16. Vannucci F, Gonzalez-Rivas D. Is vats lobectomy standard of care for operable non-small cell lung cancer? Lung Cancer. (2016) 100:114–9. doi: 10.1016/j.lungcan.2016.08.004
17. Ceppa DP, Kosinski AS, Berry MF, Tong BC, Harpole DH, Mitchell JD, et al. Thoracoscopic lobectomy has increasing benfit in patients with poor pulmonary function: a society of thoracic surgeons database analysis. Ann Surg. (2012) 256:487–93. doi: 10.1097/SLA.0b013e318265819c
18. Thachuthara-George J. Pneumothorax in patients with respiratory failure in ICU. J Thorac Dis. (2021) 13:5195–204. doi: 10.21037/jtd-19-3752
19. Kiss G, Claret A, Desbordes J, Porte H. Thoracic epidural anaesthesia for awake thoracic surgery in severely dyspnoeic patients excluded from general anaesthesia. Interact Cardiovasc Thorac Surg. (2014) 19:816–23. doi: 10.1093/icvts/ivu230
20. Mineo TC, Pompeo E, Mineo D, Tacconi F, Marino M, Sabato AF. Awake nonresectional lung volume reduction surgery. Ann Surg. (2006) 243:131–6. doi: 10.1097/01.sla.0000182917.39534.2c
21. Chen JS, Cheng YJ, Hung MH, Tseng YD, Chen KC, Lee YC, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg. (2011) 254:1038–43. doi: 10.1097/SLA.0b013e31822ed19b
22. Lan L, Cen Y, Jiang L, Miao H, Lu W. Risk factors for the development of intraoperative hypoxia in patients undergoing nonintubated video-assisted thoracic surgery: a retrospective study from a single center. Med Sci Monit. (2021) 27:e928965. doi: 10.12659/MSM.928965
23. Lan L, Qiu Y, Zhang C, Ma T, Cen Y. Comparison of single-stage and two-stage bilateral video-assisted thoracic surgery. J Int Med Res. (2020) 48:300060520967558. doi: 10.1177/0300060520967558
24. Deslee G, Klooster K, Hetzel M, Stanzel F, Kessler R, Marquette CH, et al. Lung volume reduction coil treatment for patients with severe emphysema: a European multicentre trial. Thorax. (2014) 69:980–6. doi: 10.1136/thoraxjnl-2014-205221
25. Deslee G, Mal H, Dutau H, Bourdin A, Vergnon JM, Pison C, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the revolens randomized clinical trial. JAMA. (2016) 315:175–84. doi: 10.1001/jama.2015.17821
26. Pertl D, Eisenmann A, Holzer U, Renner AT, Valipour A. Effectiveness and efficacy of minimally invasive lung volume reduction surgery for emphysema. GMS Health Technol Assess. (2014) 10:Doc01. doi: 10.3205/hta000117
27. Licker MJ, Widikker l, Robert J, Frey JG, Spiliopoulos A, Ellenberger C, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg. (2006) 81:1830–7. doi: 10.1016/j.athoracsur.2005.11.048
28. Pompeo E, Rogliani P, Tacconi F, Dauri M, Saltini C, Novelli G, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg. (2012) 143:47.e1–54.e1. doi: 10.1016/j.jtcvs.2011.09.050
29. Pompeo E, Mineo TC. Two-year improvement in multidimensional body mass index, airflow obstruction, dyspnoea and exercise capacity index after nonresectional lung volume reduction surgery in awake patients. Ann Thorac Surg. (2007) 84:1862–9. doi: 10.1016/j.athoracsur.2007.07.007
30. Tacconi F, Pompeo E, Mineo TC. Duration of air leak is reduced after awake nonresectional lung volume reduction surgery. Eur J Cardiothorac Surg. (2009) 35:822–8. doi: 10.1016/j.ejcts.2009.01.010
31. Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear T, Vender JS, et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth Analg. (2013) 117:133–41. doi: 10.1213/ANE.0b013e3182742e75
32. Della Rocca G, Coccia C. Acute lung injury in thoracic surgery. Curr Opin Anaesthesiol. (2013) 26:40–6. doi: 10.1097/ACO.0b013e32835c4ea2
33. Fitzmaurice BG, Brodsky JB. Airway rupture from double-lumen tubes. J Cardiothorac Vasc Anesth. (1999) 13:322–9. doi: 10.1016/s1053-0770(99)90273-2
34. Hung MH, Hsu HH, Cheng YJ, Chen JS. Nonintubated thoracoscopic surgery: state of the art and future directions. J Thorac Dis. (2014) 6:2–9. doi: 10.3978/j.issn.2072-1439.2014.01.16
35. Hausman MS Jr, Jewell ES, Engoren M. Regional versus general anesthesia in surgical patients with chronic obstructive pulmonary disease: does avoiding general anesthesia reduce the risk of postoperative complications? Anesth Analg. (2015) 120:1405–12. doi: 10.1213/ANE.0000000000000574
Keywords: NIVATS, IVATS, pulmonary dysfunction, spontaneous respiration, PCP
Citation: Deng SY, Cen YY, Jiang L and Lan L (2022) Effects of Non-intubated Video-Assisted Thoracic Surgery on Patients With Pulmonary Dysfunction. Front. Surg. 8:792709. doi: 10.3389/fsurg.2021.792709
Received: 11 October 2021; Accepted: 03 December 2021;
Published: 06 January 2022.
Edited by:
Marco Scarci, San Gerardo Hospital, ItalyReviewed by:
Jon Andri Lutz, Fribourg Cantonal Hospital, SwitzerlandCopyright © 2022 Deng, Cen, Jiang and Lan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Lan, bGFubGFuQGd6aG11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.