- 1Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, Academic Assembly, University of Toyama, Toyama, Japan
- 2Department of Diagnostic Pathology, Faculty of Medicine, Academic Assembly, University of Toyama, Toyama, Japan
Amyloidosis is a disorder of protein folding in which various proteins automatically aggregate into a highly abnormal fibrillar conformation. Amyloidosis is classified into systemic and localized forms depending on whether the abnormal proteins deposited in several different organs or only a single organ. In localized amyloidosis of the head and neck regions, laryngeal amyloidosis is common; however, localized amyloidosis of the nose is extremely rare. We herein report a case of localized amyloidosis of the nose and review the relevant literature on localized sinonasal amyloidosis. A 41-year-old man presented with a history of severe nasal obstruction, which had persisted for two decades. Nasal endoscopy and imaging studies showed extensive thickening of the bilateral nasal mucosa and diffuse submucosal deposition of calcification. After histopathological and systemic examinations, he was diagnosed with localized amyloidosis of the nasal mucosa. Septoplasty and bilateral inferior turbinoplasty, which consisted of mucosal resection using an ultrasonic bone curette, was performed and his symptoms markedly improved. Localized sinonasal amyloidosis has a good prognosis and surgical resection should be selected as a first-line treatment; however, clinicians should recognize the high probability of recurrence.
Introduction
Amyloidosis is a disorder of protein folding in which various proteins automatically aggregate into a highly abnormal fibrillar conformation (1). Amyloidosis is classified according to the type of protein deposited and can be either systemic (i.e., fibrils are deposited in various organs and tissues throughout the body) or localized (i.e., fibrils are produced only in one organ or site in the body) (2, 3). Presently, 18 proteins appearing as systemic amyloidosis and 22 as localized forms have been identified, and some proteins can appear as both systemic and localized amyloid deposits (4). The most common types are: AL (amyloid derived from the immunoglobulin light-chain) amyloidosis; AA (amyloid derived from serum amyloid A protein) amyloidosis, which is reactive amyloidosis due to chronic inflammatory diseases such as chronic infections and rheumatoid arthritis; ATTR (amyloid derived from the transport protein transthyretin) amyloidosis; and dialysis-associated amyloidosis (β2M type) (5, 6). Amyloidosis is a rare disease, with ~1,275–3,200 new cases per year occurring annually in the United States (1, 6). The most common subtype, AL amyloidosis has an incidence of 1 case per 100,000 person-years in Western countries (1, 6, 7).
Amyloid proteins can be deposited anywhere in the body (8). The deposition is extremely heterogeneous and the clinical presentation varies widely depending on which organs are involved (1). Amyloid can accumulate in the liver, spleen, kidney, heart, nerves, and blood vessels, causing different clinical syndromes, including cardiomyopathy, hepatomegaly, proteinuria, macroglossia, autonomic dysfunction, ecchymoses, neuropathy, renal failure, hypertension, and corneal and vitreous abnormalities (6). Systemic amyloidosis leads to serious signs and symptoms caused by progressive disease in organs and tissues (3). Localized amyloidosis is much rarer than systemic types, and as a result remains very poorly studied (9). Organ-specific localized amyloidosis can be found in Alzheimer's disease (β-protein in the plaques) and diabetes mellitus type 2 (amylin in the islands of Langerhans) (3). Nodular localized amyloid is an incidental finding and can be present in the skin, eyelid, conjunctiva, breast, larynx, bronchial tree, lungs, and genitourinary tract (3). Surgery is usually the treatment of choice in localized amyloidosis (3).
Localized amyloidosis in the head and neck region is a rare entity (10), and localized amyloidosis of the nose is extremely rare. Among localized amyloidosis foci in the head and neck region, the larynx is the most frequently involved site (61%), followed by the oropharynx (23%), trachea (9%), orbit (4%), and only 3% of cases are reported to involve the nasopharynx and sinonasal tract (11, 12). In 2019, only 19 cases of truly idiopathic primary localized sinonasal amyloidosis were documented (7).
We herein report a case of isolated localized AL amyloidosis of the bilateral nasal mucosa that was treated with debulking surgery using an ultrasound bone curette (UBC) and review the relevant literature on localized sinonasal amyloidosis.
Case Report
A 41-year- old man with a history of atopic dermatitis and allergic rhinitis visited our hospital with a complaint of severe nasal obstruction that had gradually worsened since childhood. He also suffered from hyposmia and watery nasal discharge. On nasal endoscopy, the nasal septum was deviated to the right, the nasal mucosa was smooth and pink with nodular thickening, and the common nasal meatus was so narrow that the bilateral middle turbinates or posterior part of the nasal cavity were not visible (Figures 1A,B). Plain computed tomography (CT) revealed the deviation of the nasal septum to the right, thickening of the bilateral nasal mucosa, and deposition of microcalcified material in the submucosa of the nasal cavity, including the bilateral nasal septum, middle, and inferior turbinates (Figures 1C,D). Non-enhanced MRI showed bilateral thickening of the nasal mucosa and numerous hypointense punctate signal areas in the submucosa of the entire nasal cavity on both T1- and T2-weighted images (Figures 1E,F). An excision biopsy of the left inferior turbinate was undertaken on the day of the first visit to our hospital. A histological examination revealed large deposits of amorphous eosinophilic substance with calcification (Figure 2A), which was stained black by von Kossa staining (Figure 2B), and the presence of amyloid on Congo-red staining with apple-green birefringence when viewed with polarized light (Figure 2C). Immunohistochemistry was positive for serum amyloid L protein and kappa immunoglobin light chain. The patient was referred to the department of internal medicine in our hospital, where he was assessed for systemic disease. A complete blood count (CBC), and liver and renal function tests revealed normal results, and serum and urine electrophoresis was negative for Bence Jones protein and M protein. Duodenal and rectal biopsy using digestive tract fiberscopes did not show the deposition of amyloid in the tissue, and a bone marrow biopsy from the pelvis was negative for multiple myeloma. Echocardiography and electrocardiography revealed a normal heart function. Based on these findings, the final diagnosis was localized amyloidosis (AL kappa type) of the nasal mucosa.
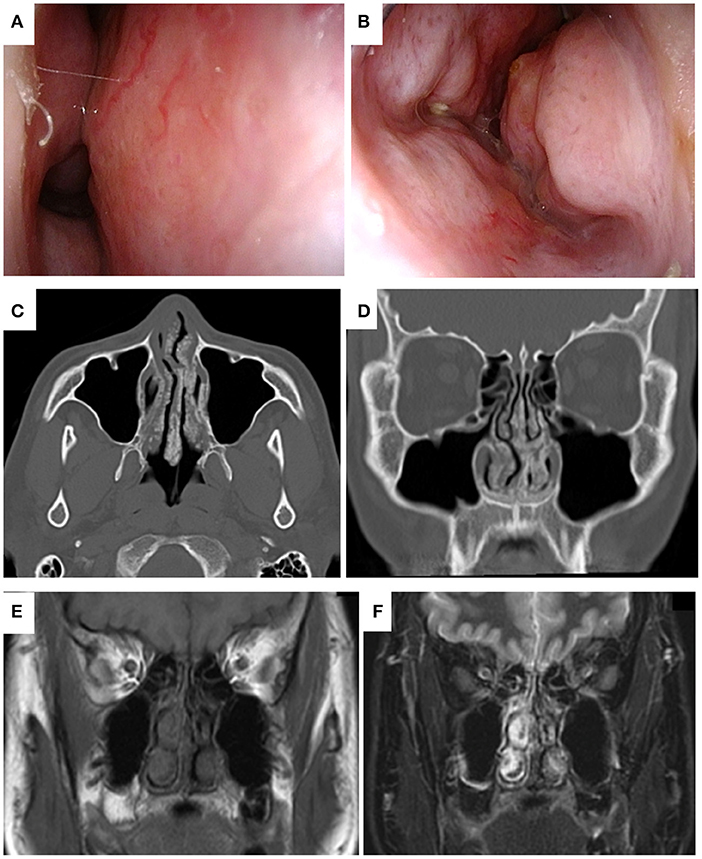
Figure 1. Endoscopic findings of localized sinonasal amyloidosis viewed from the right (A) and left (B) nasal cavities at the first visit to our hospital. The deviation of nasal septum to the right. The mucosa was smooth and pink, with nodular thickening, and bilateral narrowing of the common nasal meatus was observed. Plain computed tomography (CT) of the present case obtained at the previous hospital. Axial (C) and coronal (D) views of bone window CT of the head showed the deviation of the nasal septum to the right, thickening of the bilateral nasal mucosa, and deposition of microcalcified material in the submucosa of the nasal cavity, including the bilateral nasal septum, and middle and inferior turbinates. Magnetic resonance images of the present case obtained at the first visit to our hospital. Coronal views of plain T1-weighted (E) and T2-weighted imaging (F) of the head showed bilateral thickening of the nasal mucosa and numerous hypointense punctate signal areas in the submucosa of the entire nasal cavity.
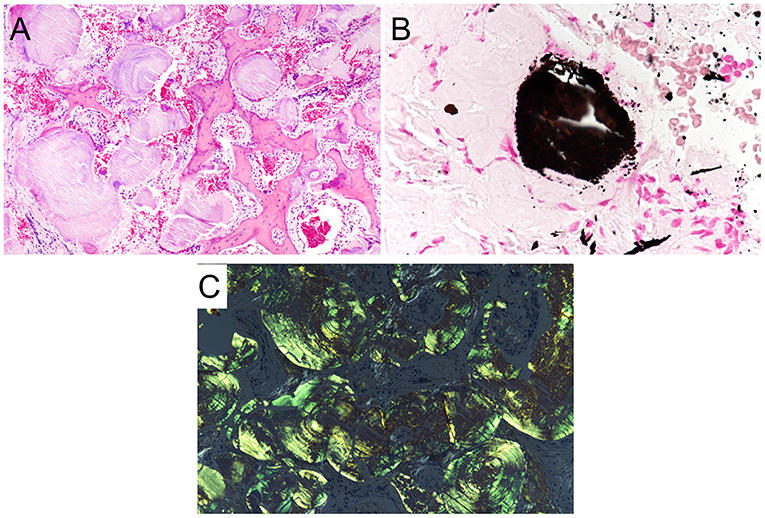
Figure 2. Histopathological features of localized amyloidosis of the nasal mucosa. (A) A histological examination revealed large deposits of amorphous eosinophilic with calcification (H&E staining, ×20). (B) The calcification was stained black (von Kossa staining, ×40). (C) Amyloid depositions with apple-green birefringence were found in the stroma when viewed with polarized light (Congo red staining, ×20).
In order to improve his main symptom of nasal obstruction, septoplasty and bilateral inferior turbinectomy were performed at 4 months after the patient's first visit to our department. In the inferior turbinectomy, we applied the submucosal resection with powered instrumentation to preserve the ciliary function of the mucosa and to prevent postoperative crusting and bleeding. A mucosal incision was made in the anterior head of the inferior turbinate, the mucosa on the sides of the common and middle nasal meatus was detached from the bone, and submucosal resection was performed to remove as much thickened submucosal tissue as possible using a microdebrider. The bone of the inferior turbinate was not removed in consideration of the possibility of reoperation for recurrence of the lesion in the future. In septoplasty, the Killian approach with cartilage preservation was used and the deviated perpendicular plate of ethmoid bone and vomer were resected. The thickened submucosal tissues of the bilateral septal mucosa were reduced in the same manner as in the inferior turbinectomy. Since the submucosal tissue with severe calcification was very hard and difficult to resect with the turbinate blade of a microdebrider (Figure 3A), an ultrasonic bone curette (UBC) (Sonopet®) was also used to partially resect the submucosal tissue by shaving it off (Figure 3B). The use of these two devices allowed us to enlarge the bilateral nasal meatus, albeit partially, while preserving some of the mucosal surface. No surgical manipulation of the external nose or nasal septum cartilage was performed based on the patient's wishes. There were no complications during surgery. To prevent postoperative mucosal adhesion and dryness, one 0.5-mm thick silicone plate was inserted into each nasal cavity, sutured to the mucosa of the nasal septum, and removed after 1 month. Subjective nasal obstruction was assessed using the Nasal Obstruction Symptom Evaluation (NOSE) scale (13) and a visual analog scale (VAS) pre and postoperatively. A VAS was a horizontal line, 100 mm in length, anchored by the word “No nasal obstruction at all” at the left end and by the word “Complete nasal obstruction” at the right end, and we asked the patient to mark the point that represented his perception of his current state on the line. The VAS score was determined by measuring in millimeters (mm) from the left end of the line to the point that the patient marks. At 6 months postoperatively, the symptoms of nasal obstruction had markedly improved and there were no significant post-operative symptoms. The NOSE scale improved from 8 points preoperatively to 3 points postoperatively on a 20-point scale and the VAS score improved from 72 mm preoperatively to 25 mm after surgery. The objective evaluation of nasal obstruction using rhinomanometry also revealed the improvement of nasal resistance in both nostrils (Figures 3C,D). CT revealed the enlargement of the bilateral common nasal meatus (Figure 3E). At 8 months after surgery, there was no recurrence or progression of symptoms. The patient is currently being followed up and is satisfied with the improvement of nasal obstruction and the absence of symptoms after surgery.
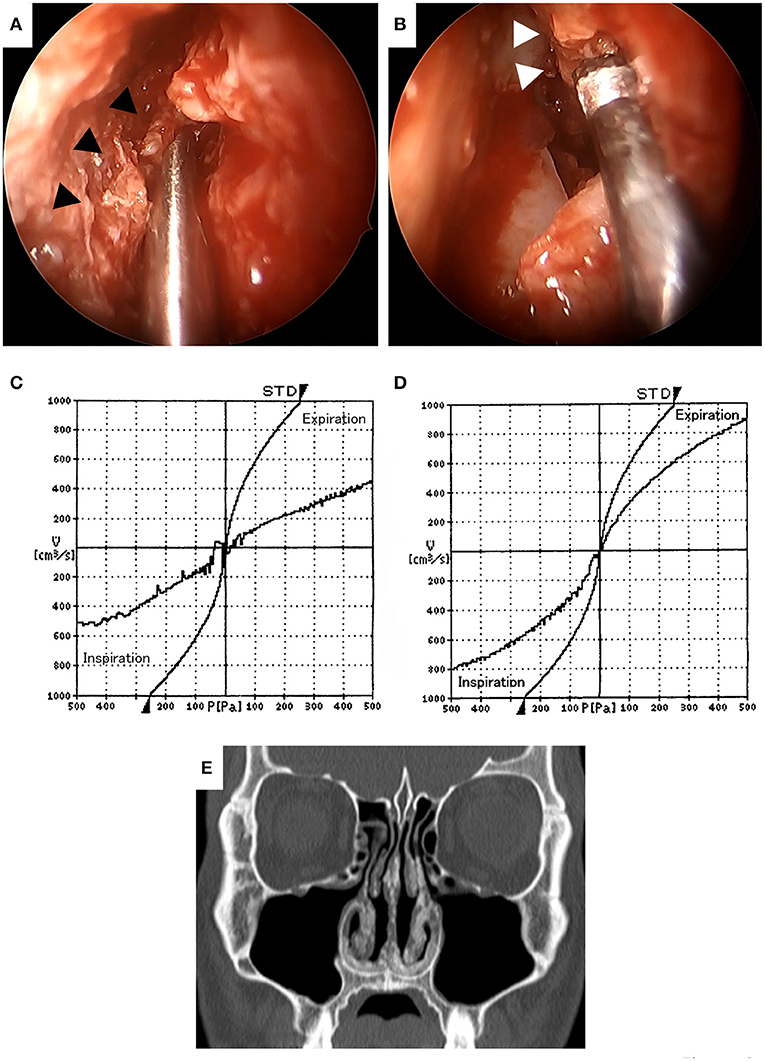
Figure 3. Intraoperative findings (A,B). The submucosal tissue with severe calcification (▲) was very hard and difficult to resect with the turbinate blade of a microdebrider (A). Hard submucosal tissue with calcification (Δ) was resected with an ultrasonic bone curette to preserve as much of the mucosal surface as possible (B). Comparison of preoperative (C) and postoperative (D) rhinomanometry results at 6 months. The nasal resistance in both nostrils was improved after surgery. Computed tomography at 6 months after surgery (E). Deviation of the nasal septum, mucosal thickening of the septum and bilateral inferior turbinates was improved and the bilateral common nasal meatuses were enlarged.
Review of the Relevant Literature on Localized Sinonasal Amyloidosis
We only identified 21 case reports of localized amyloidosis originating from the nasal cavity and paranasal sinuses that included detailed patient information in 20 English-language articles published from 1990 to date (7, 14–32). The clinical characteristics of the reported cases localized sinonasal amyloidosis, including our own, are shown in Table 1. Cases of localized amyloidosis originating from the nasopharynx were excluded from this review.
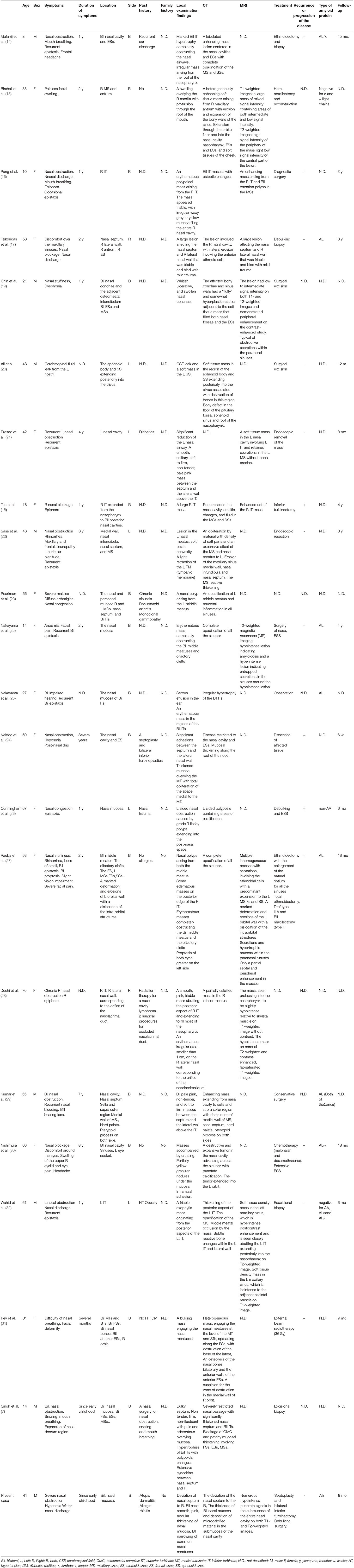
Table 1. Summary of clinical characteristics of previously reported localized sinonasal amyloidosis.
Clinical Characteristics of Localized Sinonasal Amyloidosis
In the 22 case reports included in our literature review (Table 1), the mean age of patients with localized sinonasal amyloidosis was 42.4 years (range: 8–81 years). Eight of the patients were men and 14 were women; thus, there is a female predominance in the incidence of localized sinonasal amyloidosis.
Regarding the past medical history of the patients, 4 cases (18.2%) had no history and 3 cases (13.6%) had a history of nasal surgery. In addition, diabetes, hypertension, obesity, radiotherapy for a nasal cavity lymphoma, nasal trauma, rheumatoid arthritis, monoclonal gammopathy, and ear discharge were reported in one case each (4.5%). The past medical history was not described in 9 cases (40.9%) reported in 8 studies. Three cases (13.6%), including our case, had no relevant family history, and the remaining case reports did not describe the family history.
The clinical symptoms of the patients included nasal obstruction (including nasal difficulty, nasal blockage, nasal congestion or stuffiness) (n = 18; 81.8%), bleeding (including recurrent epistaxis, n = 9; 40.9%), rhinorrhea or nasal discharge (n = 7; 31.8%). Facial swelling and olfactory impairment (including anosmia or hyposmia) were found in 4 cases each (18.2%). Mouth breathing, epiphora and hearing impairment [including aural fullness, described as “auricular plenitude” in the case report by Saas et al. (22)], were found in 3 cases each (13.6%). Headache, facial pain, facial discomfort, or recurrent sinusitis (including maxillary and frontal sinusopathy) were found in 2 cases each (9.1%). Snoring, eye pain, proptosis with visual impairment, cerebral fluid leak from the nostril, severe malaise and diffuse arthralgia were found in one case each (4.5%).
The duration of symptoms was 1 year in 6 cases (27.2%), 2 years in 4 cases (18.2%), and since early childhood in 2 cases (9.1%). One patient each (4.5%) reported that the duration of symptoms was several months, 3 years, 4 years, 7 years, 8 years, and several years. The duration of symptoms was not described in 4 reports.
The locations of lesions included nasal mucosa (including septum, floor, middle or inferior turbinate) (n = 20; 90.9%), maxillary sinus or antrum (n = 8; 36.4%), ethmoid sinus (n = 6; 27.3%), frontal sinus (n = 3; 13.6%) and sphenoid sinus (n = 2; 9.1%). Extra-nasal or extra-sinus extension to the sella, parasellar region, clivus, hard palate, pterygoid process and nasal bone were found in one case each (4.5%) in 4 case reports.
The laterality of regions included bilateral lesions in 12 cases (54.5%), and right- and left-side regions in 5 cases each (22.7%).
Regarding the findings of local examinations, a mass in the nose was found in 14 cases (63.6%). The mass was characterized as erythematous (n = 4), pale pink or pale (n = 3), friable (n = 2), smooth (n = 2), soft to firm (n = 2), and non-tender (n = 2). Local examinations revealed adhesion (or synaechie) and nasal polyp in 3 cases each. Thickening of the mucosa of the nasal cavity, especially affecting the nasal septum and nasal conchae was reported in 5 cases (22.7%) and localized lesions in the nasal mucosa were reported in 2 cases, which were described as a partially yellow granular nodule (n = 1) and an erythematous irregular area (n = 1).
Regarding the diagnostic imaging studies, X-ray, CT, and MRI were used in 2 (9.1%), 21 (95.5%), and 10 (45.5%) cases, respectively. Of the 21 patients who underwent CT, all showed increased lesions of soft tissue density located in the nose or sinuses; findings included opacification, mass or mucosal thickening of the nasal meatus or sinus, and masses. Among them, calcification in the lesion was observed in 5 cases (23.8%), osteitic, osteotic, or reactive bone change were observed in 3 cases (14.3%), and destructive changes of the bone or osteolysis were observed in 4 cases (19.0%). MRI signal intensity of the amyloid legion was documented on T1-weighted images in five cases and T2-weighted images in six cases. For T1-weighted images, three of the five cases (60.0%) were hypointense and two were hypointense to isointense (40.0%); for T2-weighted images, three of the six cases were hypointense (50.0%), one was hypointense to isointense (16.7%), one was dominantly hyperintense (16.7%), and one was hyperintense in the periphery and hypointense in the center (16.7%).
Regarding the type of amyloid protein, 11 cases in 10 studies were subjected to an immunohistochemical examination. Among them, serum amyloid light protein (AL) was positive in 8 cases, including kappa immunoglobin light chain in 2 cases, lambda immunoglobin light chain in one case and both kappa and lambda in one case. The remaining 3 case reports described negative studies for AL, serum amyloid A protein (AA) and both (n = 1 each).
Seventeen cases (81.8%) were treated with surgery. Among them, 4 cases (18.2%) were treated bv diagnostic surgery or excisional biopsy alone. The remaining 13 patients underwent resection or debulking of the lesion, with sinus surgery performed as needed. In one case, chemotherapy (four 28-day cycles of melphalan [8 mg] and dexamethasone [40 mg/day] on days 1–4) was administered prior to surgery, but no reduction was seen (30). External radiotherapy was selected for a large destructive amyloid lesion in one case and the progression of the amyloid lesion was halted (31). Observation was selected in one case and the details of treatment were not described in 2 cases. Recurrence or the progression of the disease was confirmed in 7 of the 15 cases in which it was described (46.7%).
The mean follow-up period was 20.4 months (range, 1.5 months [6 weeks] to 48 months [4 years]).
Discussion
In this latest review of the relevant literature, we aimed to clarify the clinical features of localized sinonasal amyloidosis. From an epidemiological point of view, our review of the literature revealed that localized sinonasal amyloidosis occurs in a wide age range (8–81 years), has a female predominance (8 males:14 females), and tends to not be associated with family history. Regarding past medical history, three of the cases had undergone nasal surgery prior to the diagnosis of amyloidosis, which suggests that patients with undiagnosed amyloidosis are mixed in among those undergoing nasal surgery to improve nasal symptoms. The most common clinical symptoms were nasal obstruction (81.8%), epistaxis (40.9%), and nasal discharge (31.8%), in that order, and there no symptoms were specific for localized sinonasal amyloidosis. However, there were some cases with symptoms suggestive of severe inflammation or malignancy, such as headache, facial pain, visual impairment or cerebral fluid leak from the nostril (15, 20, 29–31). The duration of symptoms up to the initial diagnosis was in the order of years for most patients, suggesting that the symptoms of localized sinonasal amyloidosis progress very slowly. There was no predominant laterality of the lesions.
On local examination, sinonasal amyloidosis tended to present as erythematous, pale pink or pink, friable, smooth-surfaced masses. Localized mucosal lesions showed a yellowish, granular, irregular mucosa. A certain number of non-operative or early postoperative cases showed mucosal adhesions, which were thought to be a feature of amyloidosis.
The imaging features of localized amyloidosis in the head and neck are not specific (33). On CT, our review indicated thickening of the sinonasal mucosa or formation of a soft-density mass, occasionally including calcification or osteotic change of the adjacent bone. Chin et al. reported a “fluffy” appearance around the sinonasal bones adjacent to the amyloid deposits as a possible representative finding suggestive of this disorder, because fluffy bone changes were not seen in other calcifying sinonasal diseases, including inspissated secretion, fungal mycetoma, cartilaginous tumor, and olfactory neuroblastoma (19). They suggested that the osteoblastic reaction to submucosal amyloid deposition in the submucosa may induce fluffy bone changes (19). In our case, there were few fluffy bony changes, and the image showed a lot of punctuate calcification deposited under the extensive nasal mucosa, which seemed to depict calcification of the amyloid material itself. The MRI signal intensity of amyloidosis can be widely variable, appearing similar to skeletal muscle on T1- and T2-weighted imaging (19). A previous study indicated that the MRI signal intensity of amyloidoma, which is a solitary mass of amyloid protein, is low-to-intermediate on T1-weighted imaging and intermediate-to-high on T2-weighted imaging (8). Our review of the relevant literature showed that the sinonasal amyloidosis had a low to intermediate signal intensity on T1-weighted MRI imaging, and varying signal intensity, mainly low but sometimes moderate to high intensity, on T2-weighted MRI imaging. Lee et al. hypothesized that varying MRI signal intensity could be caused by the density of the amyloid protein deposition (34).
In order to make a diagnosis of amyloidosis, the first step is to obtain histological evidence of amyloid, followed by looking for evidence of systemic amyloid deposition in the patient. The next steps are the detection and (in the case of AA and AL) quantification of the precursor proteins in the blood to confidently determine the type of amyloid (3). The presence of amyloid is proved by a tissue specimen that is positive for Congo Red staining, with characteristic apple-green birefringence under polarized light (3). Fat tissue stained with Congo red has high sensitivity (up to 90%) and high specificity (almost 100%) for AL, AA, and hereditary ATTR. The sensitivity for these types is ~80% in rectal tissue and ~60% for bone marrow in AL (3). If amyloid is detected in sites specific for localized amyloidosis (genitourinary tract, eyelid, conjunctiva, larynx, and so forth), checking for amyloid in other sites—such as fat tissue, rectum, bone marrow, or salivary glands—is recommended before diagnosing localized amyloidosis (3). In general, the prognosis for localized and systemic amyloidosis is quite different. Localized amyloidosis has a high survival rate (about the same as the general population), while systemic dissemination has a dismal average survival time of 5–15 months (10). It is very rare for localized amyloidosis to become systemic in the natural course of the disease, with only 7 of 606 cases (1%) showing this change in a previous study (9). Thus, differentiating between localized and systemic amyloidosis is critical to its management. Systemic amyloidosis is diagnosed when amyloid is present in two different body parts (3). In our case, histological evidence of amyloid deposits was only observed in the nasal mucosa, no amyloid deposit was observed in the duodenum, rectum, or bone marrow, and no abnormalities were found in other vital tissues (heart, liver, kidney, and blood), which finally led to the diagnosis of localized amyloidosis of the nasal mucosa.
After the detection of amyloid, the type of amyloid should be identified by the immunohistochemical analysis of biopsy samples using specific antibodies (3). AL is the most common subtype of amyloid precursor proteins in most organs (35), and AL is associated with the clonal proliferation of plasma cells, producing a monoclonal protein that circulates in the blood and amyloid fibrils that are derived from the immunoglobulin light chain or its fragments (5). AL lambda chains represent the majority of cases in systemic amyloidosis (2–4 times more frequent than AL kappa type) (36), whereas kappa type AL amyloidosis is as common as that of lambda type in localized amyloidosis, suggesting that the pathogenesis of the localized form is different from that of the systemic form (2). Our review of the literature indicated that AL was the most common type of amyloid protein (8/11 cases, 72.7%) in localized sinonasal amyloidosis. Of these, the ratio of AL kappa to AL lambda was 3:2, suggesting that the frequency of AL kappa type is approximately equal to that of AL lambda type in localized sinonasal amyloidosis. Previous studies support our results.
For localized amyloidosis of the head and neck region, symptom-based management is recommended. If systemic amyloidosis is ruled out by appropriate testing, treatment may include observation in the absence of symptoms or conservative resection for symptom relief followed by annual follow-up for up to 10 years (10). However, the risk of recurrence after conservative resection for localized amyloidosis is high. In a previous study that examined the natural history and outcome of localized amyloidosis in 606 patients, approximately half of all patients required only a single surgical intervention and a fifth of all patients required repeated interventions to improve their symptoms (9). In our review of the literature on localized sinonasal amyloidosis, surgical intervention for symptom relief was performed in ~60% of all cases, and the recurrence or the progression of symptoms was reported in approximately half of the cases for which the clinical course was described, which was similar to the results of previous studies.
Inferior turbinoplasty is an effective adjunctive procedure to septoplasty for patients with inferior turbinate hypertrophy, and surgical procedures involve resection, ablation or crushing of part, or all, of the turbinate to increase the size of the nasal airway (37). Among these approaches, submucosal resection involves preservation of the mucosa and bone with reduction of the submucosal tissue (37). A common technique involves using a number 15 blade to make an incision in the anterior head of the inferior turbinate and then a powered instrument, such as a small microdebrider can be introduced to resect the submucosa. The theory behind submucosal resection is that it preserves the ciliary function and mucociliary clearance by protecting the mucosa, but removes the tissue that is hypertrophied and leads to nasal obstruction (37). In our case, fine calcified material was densely deposited in the submucosa of the nasal cavity over a wide area, making it difficult to completely resect the lesion; thus, we decided to perform debulking surgery to improve the symptoms. In the operation, we tried to resect and reduce the submucosal tissues of the nasal septum and inferior turbinate where the amyloid material was deposited using a small microdebrider; however, but the calcified material was too tightly deposited to be reduced. Therefore, we attempted to reduce the calcified submucosal tissue and preserve the surface of the mucosa as much as possible using a UBC. A UBC is a metal instrument with a vibrating tip that was developed to resect bone tissue (38). It has the following features: it can specifically resect hard tissues (e.g., bone) while preserving soft tissues (e.g., dura mater) using the difference in natural frequencies; and it has no rotating parts, so it does not penetrate into the surrounding vital structures or pull structures into the axis of rotation (38). The UBC permits precise graded removal of bone without damage to the surrounding nasal soft tissue or mucosa (39). Previous studies have reported the applications of the UBC in various otorhinolaryngological surgeries to prevent accidental injuries to the adjacent soft tissue or vital structures (40–48). The use of UBC in septoplasty and turbinoplasty, submucosal dissection of septal spur, bony septum, and bone of inferior turbinate, and was associated with a good prognosis and fewer surgical complications in comparison of the use of a microdebrider (40, 44, 48). In our case, we used a UBC to resect diffuse calcified submucosal deposits of amyloid protein instead of dissection of the septal or turbinate bone, preserving as much of the mucosal surface as possible. There were no major complications associated with the use of UBC, such as nasal septal perforation, bleeding, empty nose syndrome or severe atrophic rhinitis. The UBC could be a useful tool for debulking surgery for sinonasal amyloidosis, especially in patients with extensive diffuse submucosal calcified lesions, such as our case, and in patients with lesions adjacent to vital structures, such as the skull base, orbit, or optic nerve.
Treatment for systemic AL amyloidosis includes chemotherapy to control clonal plasma cell dyscrasia and to decrease the synthesis of amyloidogenic light chains to reduce the progressive damage to amyloidotic organs (49). For decades, the treatment of systemic AL amyloidosis has centered on alkylating agent-based therapy with high-dose melphalan and autologous stem cell transplantation (ASCT). Within the framework of clinical trials, the disease has most likely been treated in accordance with the risk stratification of the standard Mayo Clinic staging system (6). Low-risk patients receive ASCT with melphalan (200 mg/m2) (6). Induction therapy with cyclophosphamide, bortezomib, and dexamethasone should be considered if bone marrow, plasma cell infiltration is >10% or if the patient refuses transplantation. Post-transplant bortezomib increases the complete response rate, and if a complete response is not achieved, combination therapy with bortezomib and dexamethasone should be administered (6). In intermediate-risk patients, combination therapy with melphalan and dexamethasone is preferred, especially if neuropathy or t(11,14) translocation is present (6). Cyclophosphamide combined with bortezomib and dexamethasone is a stem cell-sparing regimen and is suitable for patients with renal failure and increased 1q21 (6). The combination of bortezomib, melphalan, and dexamethasone is preferred if11 the noninvasive free light chain level is >180 mg/l (6). In high-risk patients, bortezomib—which has a faster onset of action—may be preferred; however, low-dose combination therapy may also be preferred (6). In contrast to systemic AL amyloidosis, both localized and systemic chemotherapy for localized amyloidosis have been shown to be ineffective (10). In our review of the literature, one case was treated with systemic chemotherapy with melphalan and dexamethasone, which was also ineffective (30), suggesting that chemotherapy may be also ineffective for localized sinonasal amyloidosis.
Radiotherapy targeting the presumed clonal proliferation seems to be a useful treatment method for localized amyloidosis and is effective for stopping the progression of disease and improving symptoms caused by localized amyloidosis (9). In localized laryngeal or tracheobronchial amyloidosis, radiotherapy with or without debulking surgery has been performed for local control of progressive legions and was associated with good results (50–55). Among the cases identified in our review of the literature on localized sinonasal amyloidosis, only one case was treated with radiotherapy (36 Gy), which achieved good local control and good symptomatic improvement (31). In this case, the amyloid lesions had destructive extension to the bilateral frontal and anterior ethmoid sinuses, nasal cavity, and nasal bones, and were judged to be inoperable. Thus, radiotherapy seems to be an effective treatment for cases of sinonasal amyloidosis with inoperable extension in order to prevent the further progression of lesions. The appropriate dose of radiotherapy to treat localized amyloidosis has not yet been established (56). In the case of airway amyloidosis, doses of 20–45 Gy have been reported (50–55). Further studies are required to determine the adequate dose or scheduling of radiation therapy for localized sinonasal amyloidosis.
Conclusion
We presented an extremely rare case of localized, idiopathic, primary nasal AL amyloidosis. Based on our review of the relevant literature, localized sinonasal amyloidosis shows a slow progression of nonspecific symptoms, such as nasal obstruction, epistaxis, and rhinorrhea, over a period of several years, and non-specific findings, such as mass formation in the nose or thickening of the mucosa; thus, clinicians should always suspect the presence of amyloidosis. The presence of adhesion of the nasal mucosa in the absence of previous surgery is a clue to the suspicion of amyloidosis. The only way to diagnose amyloidosis is to demonstrate amyloid deposits in the tissue by biopsy. CT often shows a fluffy appearance, which is an osteogenic reaction adjacent to sinonasal bone, or a large degree of punctuate calcification in soft tissue. MRI shows low to moderate signal intensity on T1-weighted images and variable signal intensity, ranging from low to high, on T2-weighted images. Chemotherapy is not effective for localized sinonasal amyloidosis, and the first-line treatment is resection or debulking of the lesion without impairing the sinonasal function; however, long-term follow-up is necessary because recurrence is frequently observed after treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee, Toyama University Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HTak, HTac, KT, and HS: conception and design. HTak, HTac, KT, and JI: literature search and obtaining of images. HTac, JI, and HS: writing the article. All authors critical revision and final approval of the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AA, amyloid derived from serum amyloid A protein; AL, amyloid derived from the immunoglobulin light-chain; ASCT, autologous stem cell transplantation; ATTR, amyloid derived from the transport protein transthyretin; CBC, Complete blood count; CT, computed tomography; MRI, magnetic resonance imaging; UBC, ultrasonic bone curette.
References
1. Pinney JH, Hawkins PN. Amyloidosis. Ann Clin Biochem. (2012) 49:229–41. doi: 10.1258/acb.2011.011225
2. Westermark P. Localized AL amyloidosis: a suicidal neoplasm? Ups. J Med Sci. (2012) 117:244–50. doi: 10.3109/03009734.2012.654861
3. Hazenberg BPC. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. (2013) 39:323–45. doi: 10.1016/j.rdc.2013.02.012
4. Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJM, Sekijima Y, et al. Amyloid nomenclature 2020: update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. (2020) 27:217–22. doi: 10.1080/13506129.2020.1835263
5. Picken MM. The pathology of amyloidosis in classification: a review. Acta Haematol. (2020) 143:322–34. doi: 10.1159/000506696
7. Singh A, Handa KK, Kumar A. Idiopathic isolated nasal amyloidosis: report of a rare case with review of literature. Indian J Otolaryngol Head Neck Surg. (2019) 71:2106–9. doi: 10.1007/s12070-018-1528-8
8. Desai SS, Rizzo MG, Rush AJ III, Rosenberg AE, Al Maaieh M. Amyloidoma: a review and case report. Skeletal Radiol. (2021) 50:437–44. doi: 10.1007/s00256-020-03560-3
9. Mahmood S, Bridoux F, Venner CP, Sachchithanantham S, Gilbertson JA, Rowczenio D, et al. Natural history and outcomes in localised immunoglobulin light-chain amyloidosis: a long-term observational study. Lancet Haematol. (2015) 2:e241–50. doi: 10.1016/S2352-3026(15)00068-X
10. Singh A, Haq M, Gautam P, Gautam D, Handa AC, Handa KK. Clinical profile of patients with head and neck amyloidosis: a single-institution retrospective chart review. Int Arch Otorhinolaryngol. (2020) 24:e450–6. doi: 10.1055/s-0039-3402494
11. Glenner GG. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). N Engl J Med. (1980) 302:1333–43. doi: 10.1056/NEJM198006123022403
12. Scott PP, Scott WW Jr, Siegelman SS. Amyloidosis: an overview. Semin Roentgenol. (1986) 21:103–12. doi: 10.1016/0037-198X(86)90027-1
13. Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. (2004) 130:157–63. doi: 10.1016/j.otohns.2003.09.016
14. Mufarrij AA, Busaba NY, Zaytoun GM, Gallo GR, Feiner HD. Primary localized amyloidosis of the nose and paranasal sinuses. a case report with immunohistochemical observations and a review of the literature. Am J Surg Pathol. (1990) 14:379–83. doi: 10.1097/00000478-199004000-00011
15. Birchall D, Fields JM, Poon CL. Case report: focal amyloidosis of the maxillary antrum: Plain film, CT and MR appearances. Clin Radiol. (1997) 52:392–4. doi: 10.1016/S0009-9260(97)80137-7
16. Pang KP, Chee LW, Busmanis I. Amyloidoma of the nose in a pediatric patient: a case report. Am J Otolaryngol. (2001) 22:138–41. doi: 10.1053/ajot.2001.22576
17. Tsikoudas A, Martin-Hirsch DP, Woodhead CJ. Primary sinonasal amyloidosis. J Laryngol Otol. (2001) 115:55–6. doi: 10.1258/0022215011906803
18. Teo DT, Lau DP, Sethi DS. Recurrent localized sinonasal amyloidosis: a case report. Otolaryngol Head Neck Surg. (2003) 129:270. doi: 10.1016/S0194-5998(03)01273-7
19. Chin SC, Fatterpeckar G, Kao CH, Chen CY, Som PM. Amyloidosis concurrently involving the sinonasal cavities and larynx. AJNR Am J Neuroradiol. (2004) 25:636–8.
20. Ali EA, Philip R, Prepageran N, Peh SC. Cerebrospinal fluid rhinorrhoea secondary to amyloidosis of the sphenoid sinus. Med J Malaysia. (2008) 63:341–2.
21. Prasad D, Somayaji G, Aroor R, Abdulla M. Primary nasal amyloidosis. Internet J Otorhinolaryngol. (2009) 9:1–5. doi: 10.5580/1d37
22. Saas SMG, Pinto MC, Campos DS, Maeda CAS, Mello PF. Localized nasopharyngeal amyloidosis. Intl Arch Otorhinolaryngol. (2009) 13:207–10.
23. Pearlman AN, Jeffe JS, Zynger DL, Yeldandi AV, Conley DB. Localized amyloidosis of the nasal and paranasal mucosa: a rare pathology. Am J Otolaryngol. (2010) 31:130–1. doi: 10.1016/j.amjoto.2008.11.003
24. Naidoo YS, Gupta R, Sacks R. A retrospective case review of isolated sinonasal amyloidosis. J Laryngol Otol. (2012) 126:633–7. doi: 10.1017/S0022215112000503
25. Nakayama T, Otori N, Komori M, Takayanagi H, Moriyama H. Primary localized amyloidosis of the nose. Auris Nasus Larynx. (2012) 39:107–9. doi: 10.1016/j.anl.2011.01.022
26. Cunningham A, Kalwani S, Alsanjari N, Fayad G. Rare subtype of localised nasal amyloidosis. Otorhinolaryngol. (2013) 6:60–3.
27. Rauba D, Lesinskas E, Petrulionis M, Sukyte D, Valeviciene N, Palionis D, et al. Isolated nasal amyloidosis: a case report. Medicina. (2013) 49:497–503. doi: 10.3390/medicina49110078
28. Doshi PH, Roman B, Lim J, Shatzkes DR. A rare sinonasal entity. sinonasal amyloidosis. JAMA Otolaryngol Head Neck Surg. (2014) 140:477–8. doi: 10.1001/jamaoto.2014.334
29. Kumar B, Pant B, Kumar V, Negi M. Sinonasal globular amyloidosis simulating malignancy: a rare presentation. Head Neck Pathol. (2016) 10:379–83. doi: 10.1007/s12105-016-0681-1
30. Nishimura K, Tanaka S, Takahashi Y, Uchida Y, Tanigawa T, Ueda H, et al. Huge localized amyloidosis of the sinonasal cavity: a rare case report. J Clin Case Rep. (2016) 6:797. doi: 10.4172/2165-7920.1000797
31. Iliev G, Ivanova P, Kerimov K, Petya G, Kalchev K. Localised, isolated amyloidosis of the nose and paranasal sinuses. Otolaryngol Head Neck Surg. (2019) 5:1–4. doi: 10.24966/OHNS-010X/100025
32. Wahid NW, Abed T, Meghji S, Gilbertson J, Barnes M. Localized sinonasal amyloidosis. Allergy Rhinol. (2019) 10:2152656719860821. doi: 10.1177/2152656719860821
33. Parmar H, Rath T, Castillo M, Gandhi D. Imaging of focal amyloid depositions in the head, neck, spine: amyloidoma. AJNR Am J Neuroradiol. (2010) 31:1165–70. doi: 10.3174/ajnr.A1977
34. Lee J, Krol G, Rosenblum M. Primary amyloidoma of the brain: CT and MR presentation. AJNR Am J Neuroradiol. (1995) 16:712–4.
35. Abe R, Katoh N, Takahashi Y, Takasone K, Yoshinaga T, Yazaki M, et al. Distribution of amyloidosis subtypes based on tissue biopsy site - Consecutive analysis of 729 patients at a single amyloidosis center in Japan. Pathol Int. (2021) 71:70–9. doi: 10.1111/pin.13041
36. Desport E, Bridoux F, Sirac C, Delbes S, Bender S, Fernandez B, et al. Al amyloidosis. Orphanet J Rare Dis. (2012) 7:54. doi: 10.1186/1750-1172-7-54
37. Bergmark RW, Gray ST. Surgical management of turbinate hypertrophy. Otolaryngol Clin North Am. (2018) 51:919–28. doi: 10.1016/j.otc.2018.05.008
38. Chang HS, Joko M, Song JS, Ito K, Inoue T, Nakagawa H. Ultrasonic bone curettage for optic canal unroofing and anterior clinoidectomy. technical note. J Neurosurg. (2006) 104:621–4. doi: 10.3171/jns.2006.104.4.621
39. Greywoode JD, Pribitkin EA. Sonic rhinoplasty: histologic correlates and technical refinements using the ultrasonic bone aspirator. Arch Facial Plast Surg. (2011) 13:316–21. doi: 10.1001/archfaci.2011.52
40. Greywoode JD, Van Abel K, Pribitkin EA. Ultrasonic bone aspirator turbinoplasty: a novel approach for management of inferior turbinate hypertrophy. Laryngoscope. (2010) 120(Suppl. 4):S239. doi: 10.1002/lary.21706
41. Ehieli E, Chu J, Gordin E, Pribitkin EA. Frontal sinus osteoma removal with the ultrasonic bone aspirator. Laryngoscope. (2012) 122:736–7. doi: 10.1002/lary.23202
42. Bolzoni Villaret A, Schreiber A, Esposito I, Nicolai P. Endoscopic ultrasonic curette-assisted removal of frontal osteomas. Acta Otorhinolaryngol Ital. (2014) 34:205–8.
43. Kakehata S, Watanabe T, Ito T, Kubota T, Furukawa T. Extension of indications for transcanal endoscopic ear surgery using an ultrasonic bone curette for cholesteatomas. Otol Neurotol. (2014) 35:101–7. doi: 10.1097/MAO.0b013e3182a446bc
44. Kim JY, Choi G, Kwon JH. The application of an ultrasonic bone aspirator for septoturbinoplasty. J Craniofac Surg. (2015) 26:893–6. doi: 10.1097/SCS.0000000000001426
45. Massey CJ, Bury S, Diamond J, Singh A. Novel use of an ultrasonic bone aspirator for extended endoscopic frontal sinusotomy: a feasibility study. Am J Rhinol Allergy. (2016) 30:443–7. doi: 10.2500/ajra.2016.30.4358
46. Yawn RJ, Daniero JJ, Gelbard A, Wootten CT. Novel application of the Sonopet for endoscopic posterior split and cartilage graft laryngoplasty. Laryngoscope. (2016) 126:941–4. doi: 10.1002/lary.25596
47. Modest MC, Carlson ML, Link MJ, Driscoll CL. Ultrasonic bone aspirator (Sonopet) for meatal bone removal during retrosigmoid craniotomy for vestibular schwannoma. Laryngoscope. (2017) 127:805–8. doi: 10.1002/lary.26219
48. Kim J, Choi G, Kwon JH. Novel application of ultrasonic bone aspirator for hump nose. J Craniofac Surg. (2018) 29:1291–3. doi: 10.1097/SCS.0000000000004528
49. D'aguanno V, Ralli M, Artico M, Russo FY, Scarpa A, Fiore M, et al. Systemic amyloidosis: a contemporary overview. Clin Rev Allergy Immunol. (2020) 59:304–22. doi: 10.1007/s12016-019-08759-4
50. Poovaneswaran S, Razak AR, Lockman H, Bone M, Pollard K, Mazdai G. Tracheobronchial amyloidosis: utilization of radiotherapy as a treatment modality. Medscape J Med. (2008) 10:42.
51. Neuner GA, Badros AA, Meyer TK, Nanaji NM, Regine WF. Complete resolution of laryngeal amyloidosis with radiation treatment. Head Neck. (2012) 34:748–52. doi: 10.1002/hed.21626
52. Truong MT, Kachnic LA, Grillone GA, Bohrs HK, Lee R, Sakai O, et al. Long-term results of conformal radiotherapy for progressive airway amyloidosis. Int J Radiat Oncol Biol Phys. (2012) 83:734–9. doi: 10.1016/j.ijrobp.2011.07.036
53. Firlinger I, Setinek U, Koller H, Feurstein P, Prosch H, Burghuber OC, et al. A case of tracheobronchial amyloidosis treated with endoscopic debulking and external beam radiation therapy. Pneumologie. (2013) 67:398–400. doi: 10.1055/s-0033-1344186
54. Almadana Pacheco V, Luque Crespo E, Wals Zurita AJ, Montemayor Rubio T. External beam radiation therapy: a treatment modality in diffuse tracheobronchial amyloidosis. Arch Bronconeumol. (2015) 51:607–8. doi: 10.1016/j.arbr.2015.09.003
55. Bertelsen C, Chadwick K, Holland J, Flint P, Schindler JS. Long-term follow-up after radiation therapy for laryngeal amyloidosis. Laryngoscope. (2021) 131:1810–5. doi: 10.1002/lary.29061
Keywords: localized sinonasal amyloidosis, calcification, ultrasonic bone curette, clinical features, debulking surgery
Citation: Takakura H, Tachino H, Takii K, Imura J and Shojaku H (2021) Localized Amyloidosis of the Nasal Mucosa: A Case Report and Review of the Literature. Front. Surg. 8:774469. doi: 10.3389/fsurg.2021.774469
Received: 12 September 2021; Accepted: 15 October 2021;
Published: 05 November 2021.
Edited by:
Małgorzata Wierzbicka, Poznan University of Medical Sciences, PolandReviewed by:
Jeremy Hornibrook, University of Canterbury, New ZealandJiang Yan, The Affiliated Hospital of Qingdao University, China
Copyright © 2021 Takakura, Tachino, Takii, Imura and Shojaku. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hideo Shojaku, aHNob2pha3VAbWVkLnUtdG95YW1hLmFjLmpw
 Hiromasa Takakura
Hiromasa Takakura Hirohiko Tachino
Hirohiko Tachino Kouji Takii1
Kouji Takii1 Johji Imura
Johji Imura Hideo Shojaku
Hideo Shojaku