- 1Irvin S. Zubar Plastic Surgery Research Laboratory, Penn State College of Medicine, Hershey, PA, United States
- 2Department of Surgery, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, United States
Many pathologies, congenital defects, and traumatic injuries are untreatable by conventional pharmacologic or surgical interventions. Regenerative engineering represents an ever-growing interdisciplinary field aimed at creating biological replacements for injured tissues and dysfunctional organs. The need for bioengineered replacement parts is ubiquitous among all surgical disciplines. However, to date, clinical translation has been limited to thin, small, and/or acellular structures. Development of thicker tissues continues to be limited by vascularization and other impediments. Nevertheless, currently available materials, methods, and technologies serve as robust platforms for more complex tissue fabrication in the future. This review article highlights the current methodologies, clinical achievements, tenacious barriers, and future perspectives of regenerative engineering.
Key Points
- Regenerative engineering is an emerging field that combines tissue engineering with material science, stem cell biology, and developmental biology to create complex tissue constructs.
- A variety of stem cell sources are often used as the starting platform.
- An engineered three-dimensional microenvironment supports cellular differentiation into functional tissue.
- An assortment of manufacturing processes can facilitate tissue assembly.
- Bioengineered constructs often provide a superior model for preclinical disease and drug response modeling.
- While early, regenerative engineering is underway in the clinical domain, though its successes are predominantly limited to thin or avascular structures.
- Advances are rapidly being realized and subsequent clinical translatability will broaden.
- Surgeons should become acquainted with these emerging technologies.
Introduction
Tissue loss and organ dysfunction are commonplace in modern medical care. Over the past half century, the free transfer of tissue and organ transplantation has revolutionized our ability to care for these diverse entities. This spans the spectrum from breast reconstruction following mastectomy to solid organ transplants and most recently composite tissue allotransplantation. Despite the advances, these complex surgical strategies are not without many problems. All these approaches require a suitable donor source, which is often lacking at both the autologous and allogenic level. Autologous tissue transfers often have significant donor site morbidity and organ transplants require lifelong immunosuppression. Both approaches can suffer devastating effects from a thrombotic event. Furthermore, there still exist pathologies which are untreatable (1). Therefore, the need for engineered tissue and organ replacements permeates virtually every surgical specialty from reconstructive to transplant surgery (2).
Tissue engineering represents an ever-growing interdisciplinary field aimed at creating biological replacements which can mimic native tissue (3–5). Regenerative engineering has been described as the amalgamation of tissue engineering with material science, stem cell biology, and developmental biology (6). By combining cells, materials, and bioreactive molecules along with a proper manufacturing platform; regenerative engineering is slowly being realized. Ideally, these replacement tissues could be built on an autologous platform which would offer personalized treatment options. However, there are numerous hurdles to overcome until ubiquitous clinical translation. Nevertheless, there have been some successes in the field, both in the research and clinical arenas which provide a stable foundation for further advances (7–9). Here we review the basic aspects of the regenerative engineering platform along with some preclinical and clinical successes.
Stem Cells
There have been tremendous advancements in cell sourcing for regenerative engineering over the past two decades, varying in their derivation and differentiation ability. This includes both adult cells and stem cells. Since, stem cells are capable of both self-renewal and differentiation, they have found a distinctive niche role in tissue engineering applications (10). Stem cells can broadly be defined as being totipotent, pluripotent, and multi-/oligo-/unipotent (Figure 1, Table 1) (11).
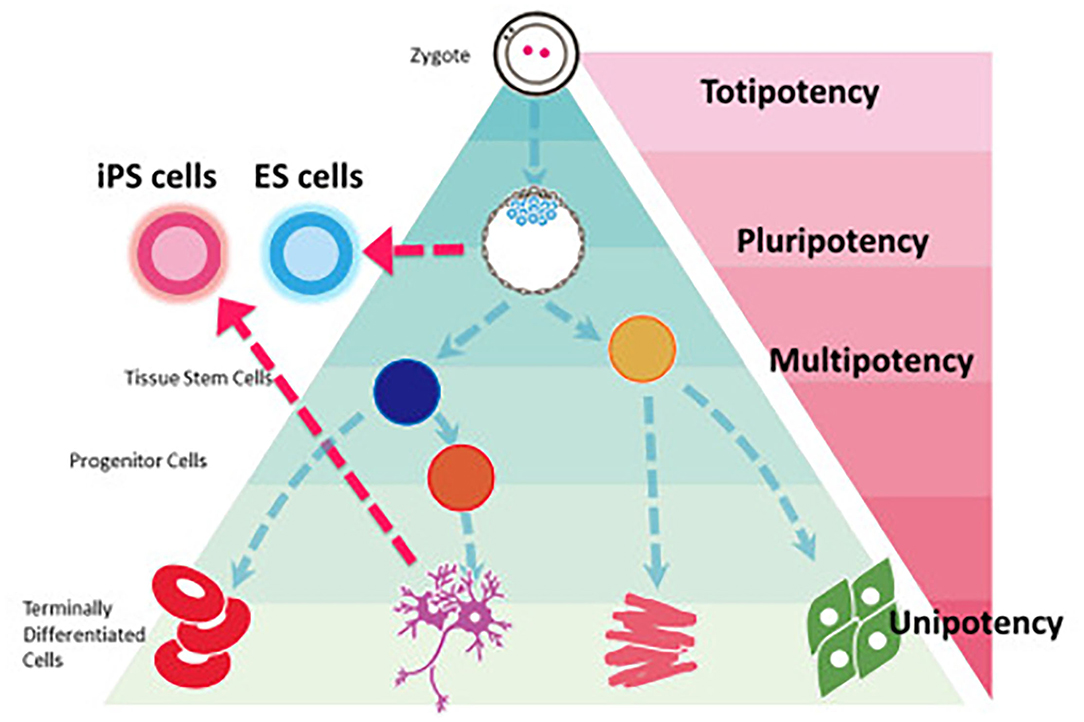
Figure 1. Stem cell classification based on potency. iPS cells can be reprogrammed from a fully differentiated cell to regain the pluripotency seen in embryonic stem cells. Most regenerative engineering applications focus on the use of either iPSCs or multipotent stem cells. Reprinted Sugawara et al. (11).
Totipotent Stem Cells
Totipotent stem cells can give rise to all three primary germ cell layers, as well as, extraembryonic tissues, such as the placenta. This means that they are only present in the first weeks of embryogenesis (12, 13). As such, they are inaccessible and unavailable for tissue engineering applications.
Pluripotent Stem Cells
Pluripotent stem cells are present in later embryogenesis, therefore while they similarly can differentiate into all three germ layers, they are unable to differentiate into the placenta (14). Widely recognized as embryonic stem cells (ESC), they represent the gold-standard in stem cell-based therapies because of limitless differentiation capability and self-renewal. Since being described over two decades ago (15), ESCs have significantly contributed toward the understanding of stem cell biology, developmental processes, and the translatability of regenerative endeavors. They have been used in various clinical trials such as macular degeneration, cardiac repair, and diabetes (16, 17). Additional experimental studies are investigating therapeutic utility for lung disease (18), and ischemic pathologies (19). Furthermore, with human ESCs, the ability to study disease specific mutations has expanded greatly. There have been over 100 human embryonic stem cell lines registered for this purpose (20), investigating wide-ranging congenital ailments. Despite tremendous scientific advancements, ESCs continue to be plagued by significant disadvantages. An autologous option is not available with ESCs and there exists the unwanted effects of possible immune rejection after allogenic grafting (21). Human ESCs have traditionally been grown utilizing a murine fibroblast feeder layer with potential exposure to mouse retroviruses (22), although this has been remedied. In vitro, ESCs spontaneously differentiate into embryonic bodies but can form teratomas during animal implantation. In addition, karyotype alterations can appear following prolonged culture with highly-passaged cells forming less mature tumors (23). The potential for tumor formation is a significant concern toward clinical translatability. Furthermore, long-term clinical sustainability of these efforts is uncertain secondary to ethical concerns, which continues to be the largest disadvantage of this type of therapy, as human embryo destruction is requisite to the generation of ESCs. These concerns have not diminished with time and there continues to be discordance between the protection of embryonic life vs. the potential to remedy postnatal ailments and alleviate suffering (16). Accordingly, this type of work is subject to significant regulations and even prohibited in some countries, while being threatened by frequent changes in governmental policies (24). In multicultural environments, such as the United States, the respect of various religious entities will need to be balanced to allow for the progress of human ESC research. This will only serve to complicate the discussion between physicians and patients regarding ESC-based treatments, while attempting to formulate a consensus on “how much manipulation can be allowed until it is considered playing God and morally unacceptable?” (25). Nonetheless, because of their differentiation ability, these pluripotent stem cells are extremely valuable to tissue engineering and regenerative medicine.
Fortunately, Shinya's Yamanaka's reprogramming strategy has permitted an alternative route for the widespread use of pluripotent stem cells (26, 27). Yamanaka has obviated the ethical concerns of ESCs by reprogramming fully differentiated fibroblasts into induced pluripotent stem cells (iPSCs) using transcription factors Oct3/4, Sox2, c-Myc, and Klf4 under embryonic stem cell culture conditions (28). It has also now been demonstrated that multiple differentiated adult cell varieties have the potential for reprogramming into a pluripotent state (29, 30). iPSCs seem to offer the benefit of pluripotency without the wide-ranging ethical quandaries associated with ESCs. Comparisons with the gold standard ESC has demonstrated significant similarities with regard to transcriptional profiles and differentiation potentials (31). This has generated much excitement since being first described in 2006 (26). iPSCs have been extensively researched and utilized in regenerative medicine and tissue engineering (10, 32–38). Additionally, iPSC clinical translation has recently been demonstrated in replacing eye tissue damaged by age related macular degeneration, suggesting safety (39). The advantages of programmed pluripotency are only heightened by the potential to use autologous cellular material which can mitigate against immune rejection.
Despite these recent advances there are still numerous disadvantages associated with iPSCs that need to be considered. For example, nearly $900,000 USD was needed to develop and test the iPSCs for the first clinical trial, mentioned above (40). Furthermore, no consistent protocols have been established that yields the induction of iPSCs with high efficiency. Likewise, the safety profile of iPSCs is still a concern, especially with numerous cellular starting sources and programming schemes. The development of new Next Generation Sequencing technologies has further demonstrated the potential for genomic instability in iPSCs (41) raising fear of tumor progression following implantation. Although ethical concerns seem less intense than those seen with ESCs, they are still somewhat prevalent. For example, some claim that iPSCs could, in theory, be used to create human embryos, and therefore may be as problematic as human ESCs (42). It would then need to be established if these embryos would be subject to the same ethical and legal implications regarding humanity and protection. Also, there are apprehensions with the ability to clone human beings and to produce germ cells (43). However, it is likely that guidelines can be formulated to address these concerns allowing for improved clinical translation of regenerative engineering innovations.
Multi-, Oligo-, or Unipotent Stem Cells
Adult stem cells (ASC) are typically described as unipotent, oligopotent, or multipotent cells that persist into the postnatal period (44). These stem cells have the capacity for self-renewal and to develop into individual or multiple cell types within a specific tissue or organ. Their persistence into adulthood offers them significant potential for clinical therapeutics. In fact, over the past four decades, patients afflicted by blood disorders have been treated with adult stem cells via bone marrow transplants (45, 46). While clinical use of these hematopoietic stem cells has become widespread, the true potential of adult stem cells has yet to be realized, but they also have found an enlarged role in regenerative engineering endeavors (47–58).
Adults stem cells have been identified in many tissue types including those of mesenchymal (59), intestinal (60), neural (61), and endothelial (62) origin. While they lack the diverse pluripotency seen with embryonic or induced pluripotent stem cells, their differentiation potential is still impressive and is continually being explored. For example, adipose derived stem cells (ADSCs) represent a type of mesenchymal stem cell that can easily be accessed and retrieved through a simple minimally invasive procedure (63). While their potential was initially identified for mesodermal replacement therapy (64), there has been some suggestion that they may have the ability to transdifferentiate into endodermal (65) and ectodermal (66) lineages, further expanding their therapeutic potential. With adipose excision (ADSCs) or other interventions, such as childbirth (umbilical cord stem cells); adult stem cell rich tissue could be utilized for the generation of biobanks (67). This represents a significant advantage, in that tissue that is typically discarded can yield large numbers of stem cells for either autologous or allogenic use. Thus, replicating what is currently done for packed red blood cells, platelets, and fresh frozen plasma. Specifically, it is believed that mesenchymal stem cells offer low immunogenicity (68) and are immunomodulators (69); thus allowing for allogenic transplant and pooled donors. Minimal immunogenicity has been observed across multiple mesenchymal stem cell types including umbilical cord, placenta, and dental pulp (70). While clinical translation is the desired endpoint, adult stem cells are ideal for human in vitro testing platforms such as disease modeling, drug screening, therapeutic efficacy, and organoid regeneration.
Disadvantages of adult stem cells are focused on the decrease in pluripotency along with the lack of continued self-renewal. Furthermore, the retrieval of ASCs can be plagued by problems with cellular cross-contamination. For example, the dissociation of adipose tissue to obtain the stromal vascular fraction and subsequent cell sorting does not guarantee a pure ADSC aliquot. In addition, the need for cellular expansion in long-term cultures may lead to bacterial contamination. However, the most profound benefit of adult stem cell sources is the ability to offer therapy that is not overshadowed by ethical disputes. While adult stem cells do not face the immense ethical dilemma of embryo destruction with associated legal and religious guidelines, there are still some noteworthy scenarios where principled practice must be developed. As ASCs are highly varied, widespread, and easily retrieved the potential for biobanking; associated abuse, has come to the forefront. Accordingly, issues of consent, control, and justice will need to be examined (71). In addition, with the potential for long-term self-renewal there is the troublesome concern of loss of patient privacy and identity. As biobanks are for-profit business endeavors stem cell donor compensation models may need to be established. However, these ethical dilemmas are more likely to achieve a consensus agreement than those which surround ESCs and iPSCs, leading many to believe that ASCs represent the most streamlined path toward large scale clinical translation. Engineered translation may become even more appealing as the true pluripotency of stem cells in adult tissues continues to be investigated and expanded (72).
While many researchers have a preference, there is currently no consensus for the best cell source for regenerative engineering, as each pose unique advantages and disadvantages. However, it is undeniable that our pool of cellular options has dramatically improved over the past two decades and represents a solid footing for further advances in tissue engineering and regenerative medicine.
Materials and Bioreactive Molecules
Although, cell sourcing is integral to tissue engineering, its organization and microenvironment determines ultimate success (73). Therefore, supporting materials and molecules are needed to create the proper microenvironment for cell proliferation and differentiation into functional tissue. This is often achieved by suitable scaffolding and enabling trophic biomolecules. These important factors provide durability to tissue-engineered constructs by facilitating growth, induction, and long-term maturation (5). Suitable scaffolds aim to mimic native tissue, providing a framework for cell attachment, migration, growth, and differentiation, while allowing cells for reorganization into a functional 3D network (5, 74).
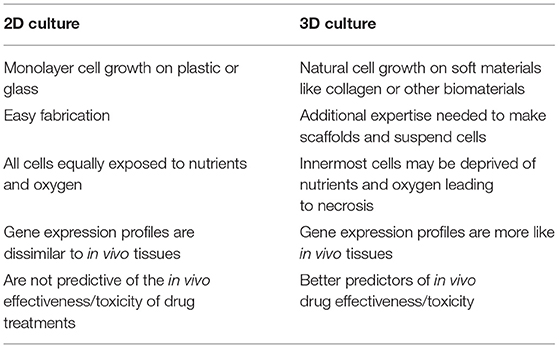
Until recently, most cell cultures had been performed in two-dimensions on flat and rigid materials. While useful, they fail to replicate the complex three-dimensional architecture found in living organisms. Therefore, the information obtained from them often does not translate to clinical success (75). This becomes most significant in regenerative medicine applications where the substitution part needs to function in three dimensions, such as a vascular graft or bone replacement. Therefore, it is not surprising that 3D cultures and scaffolds would be desired for these applications. 3D culture systems encompass cells grown on a matrix, within a matrix, or grown in suspension. With all these approaches, cells maintained in 3D culture differ both morphologically and physiologically compared to 2D (Table 2) (75).
Scaffolds/matrices used for 3D culture can be fabricated from a variety of techniques, being either biologically or synthetic based. When determining the suitability for regenerative engineering applications; biocompatibility, biodegradability, mechanical properties, manufacturing technology, and scaffold architecture need to be considered. Ideally, the scaffold can provide a framework and initial support for cell attachment, proliferation, and differentiation, ultimately forming an extracellular matrix (ECM) (76). The 3D environment facilitates complex cell-to-cell and cell-ECM interactions, mimicking what occurs in vivo; and cells tend to secrete more ECM in this setting. Accordingly, a wide range of cellular processes are impacted including proliferation, differentiation, morphology, gene expression, protein synthesis, and response to stimuli (75). As cells in 3D conditions aggregate, a diffusion gradient occurs where the innermost cells may proliferate under slightly different circumstances than those on the periphery (77). This will affect oxygen delivery and metabolite removal, both in vitro and in vivo. However, these conditions can be refined by controlling the porosity of the scaffold, which will also impact cell penetration and ingrowth, and may augment vascularization upon implantation (78). Scaffold porosity can also be used to impact the overall stiffness which may be beneficial as mechanical functionality will require a dense scaffold while other processes will benefit from a more porous one (79). Furthermore, scaffold stiffness has been found to have a substantial effect on stem cell differentiation potential (80).
In most native tissues, the ECM serves as this scaffold, providing structural integrity, functionality, and ideal conditions for facilitating cell growth (81, 82). Inspired by this natural, multi-tissue construct, various materials have been proposed for creating these engineered scaffold designs (2). Many of these designs utilize hydrogels which form a biochemically and mechanically appropriate environment for cell deposition and growth (2). The choice of material used is highly dependent on the mechanical and structural requirements of the construct, which differs based on the desired tissue product (83). However, to be functional for tissue engineering, they must be mechanically stable, non-toxic, and have an appropriate degradation rate (2). Organic polymers can be isolated from animal or human tissue and offer the advantage of inherent bioactivity and similarity to native ECM (83, 84). However, while they are more cell friendly, their mechanical properties are weak (85). Synthetic hydrogels have stronger mechanical and structural properties because of their ability to be tailored by chemical modification which may better suit them for these applications (2, 83). However, they have poorer biocompatibility, with increased chances of toxic degradation and loss of mechanical properties during tissue degradation in vivo (83). Both types of scaffolds are also able to be constructed either in vivo by implantation or in vitro in a bioreactor followed by implantation (5). Each scheme has benefits and detriments, and the better option depends on the specific tissue being constructed.
The extension of 3D cultured scaffolds has also been applied toward cell-sheet technologies, where multiple layers of cells can be grown, transferred en bloc, and combined with varying cells to develop thicker and more complex tissue grafts, without scaffold use (Figure 2) (86, 88). Cells grown in biologically derived scaffolds can be exposed to a variety of growth factors that are intrinsic to the material, such as seen with Matrigel (89). However, even with synthetic scaffolds, various cytokines, miRNAs, and other cues can be spatially loaded to affect cell growth (90). What is most interesting is that scaffolds can be designed to permit differential cell growth, with regards to both variability and density. All these properties of 3D cultures/scaffolds make them of particular use in regenerative medicine and confer a significant benefit over 2D cultures in the development of complex tissue and structures.
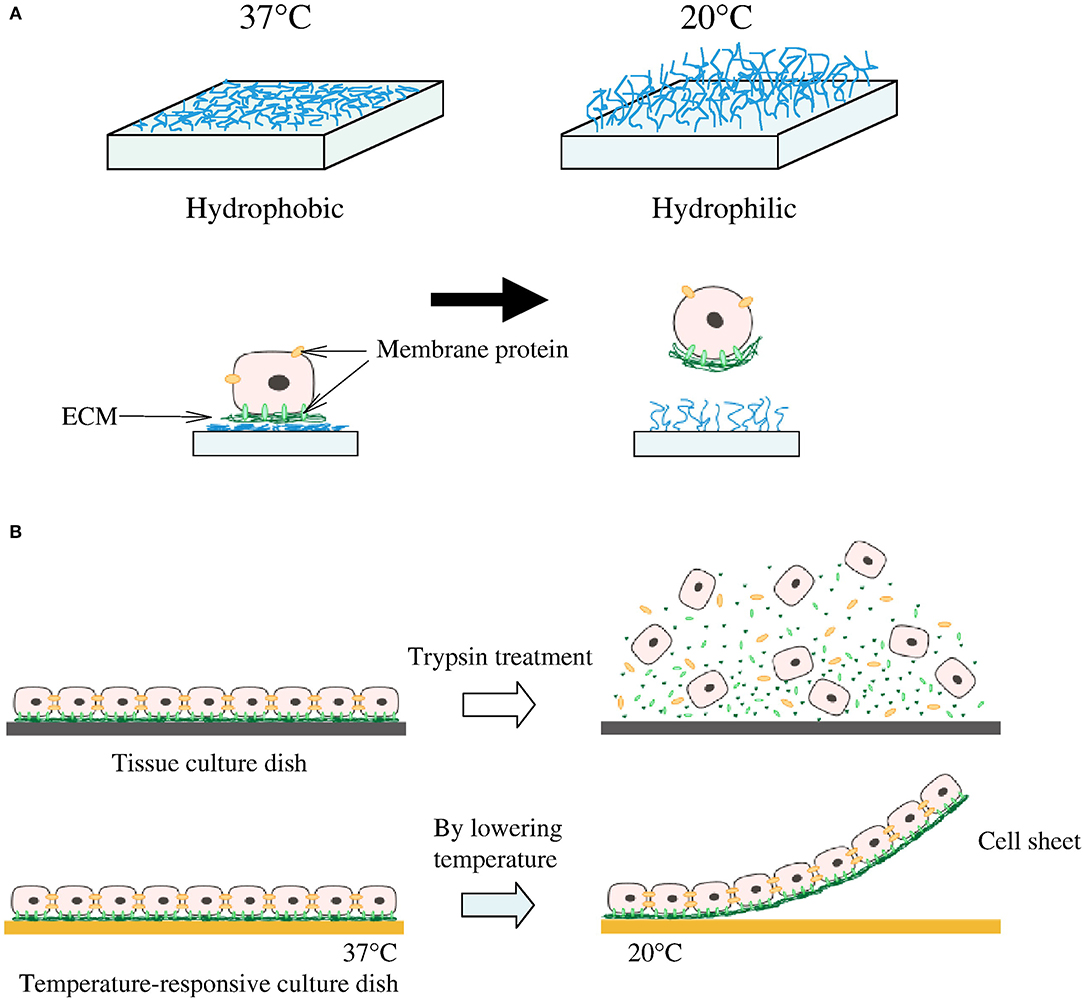
Figure 2. Cell sheeting. (A) At 37°C cells attach to the hydrophobic surface and at lower than 32°C detach from the hydrophilic surface. (B) Cells connect to each other by cell-to-cell junctions and ECM; when enzymes are introduced to remove cells from the surface, these junctions and ECM are disrupted. This is not the case for the temperature-responsive culture surface which is able to preserve the cell-to-cell junction and ECM. Reprinted from Moschouris et al. (87, 88).
But the scaffold is not the only environmental factor for bioengineered tissue; proper growth and maturation requires a unique combination of growth factors and differentiation signals provided by additional biomolecules (5). Over 300 ECM proteins, ECM-modifying enzymes and ECM-binding growth factors have been identified in mammalian cells as pivotal to growth, proliferation and regeneration processes (91). Of these, various collagens, proteoglycans and glycoproteins serve to provide strength, bind important growth factors, regulate protein complexes within tissues, promote cell adhesion, and participate in cellular signaling (83). There are further chemical signaling molecules, both autocrine and paracrine, utilized to promote proliferation and differentiation. The most critical for wound healing specifically are growth factors, namely fibroblast growth factors (FGF), epidermal growth factors (EGF), vascular endothelial growth factors (VEGF), transforming growth factor beta (TGF-beta), and platelet-derived growth factors (PDGF) (1, 92, 93). The combination of these proteins and growth factors initiates signaling cascades for hemostasis, inflammation, proliferation, angiogenesis, and wound healing (92, 93). Inclusion of these molecular and mechanical signals allow for engineered tissue cells to participate in multidirectional interactions within the tissue, as well as with the surrounding structures in vivo (94). Therefore, a proper microenvironment is crucial for clinically applicable bioengineering constructs. It is useful to note that many of these materials can be configured in a variety of ways utilizing a multitude of manufacturing approaches.
Manufacturing
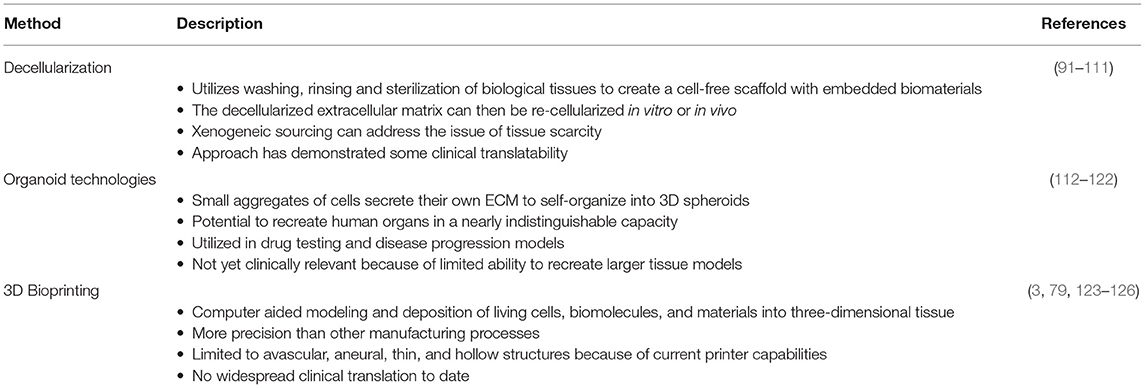
Within the field of regenerative engineering there are numerous manufacturing methods employed, most of which fall under three broad categories: decellularization, organoid technologies, and 3D bioprinting (Table 3). Each of the following methods have unique advantages for clinical application, as well as disadvantages for scale up capabilities.
Decellularization
One method for ensuring not only a proper, but an ideal environment for tissue engineering, is through the decellularization of biological tissues. This process utilizes detergents to strip nuclear and cellular components from natural tissue, leaving behind the native architecture, growth factors and proteins, to produce a biological scaffold already embedded with biomaterials (95). As a versatile scaffold and biomaterial, decellularized matrices serve as a compatible platform for stem cell proliferation and specialization (96, 97). This approach can be utilized for “simple” decellularization resulting in the fabrication of thin structured matrices or whole organ decellularization in order to engineer more complex structures (98). The process of decellularization includes three phases: wash, rinse, and sterilization (Figure 3) (99, 100). During the wash phase, a detergent, enzyme, or denaturing agent is applied to the natural tissue in order to destroy cellular material and breakdown the tissue (101). The tissue is then rinsed, removing any residual detergent from the decellularized extracellular matrix (dECM) (102). Remaining detergent is potentially cytotoxic by inhibiting cell proliferation during the recellularization process (102). The last stage is sterilization, in which residual antigenic components of the donor tissue are removed from the dECM in order to prevent immunogenic responses by recipients of the final bioengineered product (103). The final dECM product can then be re-cellularized, either in vitro or in vivo. A variety of such products have also been used clinically, in applications such as breast reconstruction (104–109) and hernia repair (110–115) where in situ cellularization is relied upon. While the decellularization process overcomes certain challenges in tissue engineering, the need for a donor, fails to resolve one of the major hurdles in transplant surgery, tissue scarcity. However, xenogenic sourcing is an attractive way to remedy this, at least with modest tissue fragments.
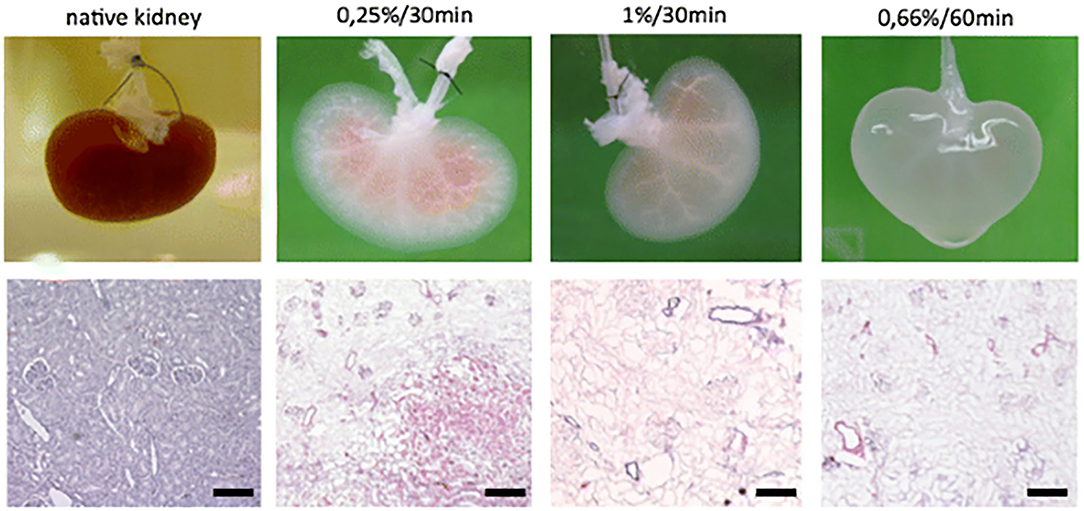
Figure 3. Macroscopic and histologic investigation of kidney decellularization efficacy of different SDS concentration/perfusion time combinations. Reprinted Schmitt et al. (100).
Organoid Technologies
Organoid technologies are 3D stem cell cultures able to reproduce human tissue both in structure and physiologic function, thereby, offering great potential for a personalized treatment strategy (116). As small aggregates of pluripotent or adult stem cells (117), organoids are able to self-organize into 3D spheroid structures, without the influence of foreign material (118). Like all in vitro models, these models confer a high degree of clinical and biological relevance (118), by mimicking tissue regeneration in a controlled environment (Figure 4) (119). However, what differentiates them from other in vitro models is their capability of recreating the histology and physiology of human organs in a nearly indistinguishable manner (120–126). This unique ability arises from stem cells secreting their own extracellular matrix and interacting with cells in their original microenvironment (118). The value of these small replicas of human tissue has increased quickly due to their function as drug testing and disease progression models, but they have numerous disadvantages when it comes to scale-up applications for bioengineering larger tissue and organs (118). As such, they have yet to find clinical relevance in surgical reconstructions (127).
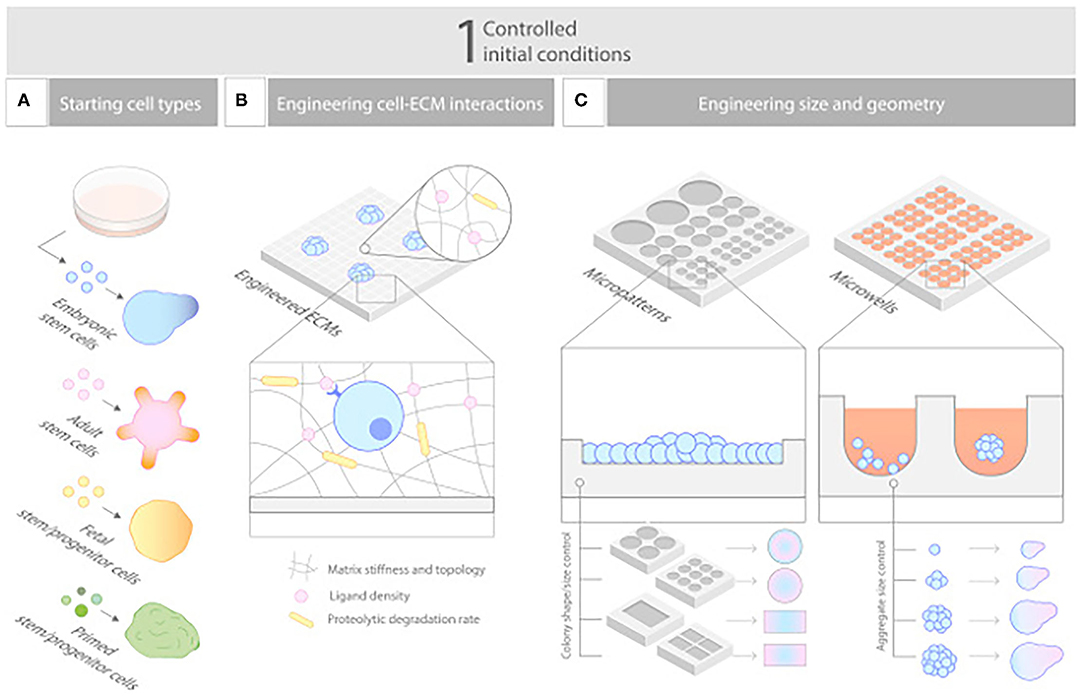
Figure 4. Controlling initial conditions. (A–C) Carefully designed starting conditions are critical for robust organoid formation, and these include (A) choosing the nature of the cells, (B) controlling their aggregation to a defined size and shape, and (C) engineering their environment to empower their ability to self-organize. Reprinted from Brassard and Lutolf (119).
3D Bioprinting
Bioprinting is the modeling of living cells, biomolecules, and biocompatible materials into three-dimensional functional tissue (83). These biologics are stacked and assembled in a computer-aided layer-by-layer seeding approach (128). 3D bioprinting is capable of a precision fabrication which was previously unavailable in traditional tissue engineering techniques. There exists the capability of exact positioning of various biologics and high digital control over speed, resolution, concentration, volume, and diameter of the cells being printed (3, 129). While this approach has numerous advantages, the method is limited by current bioprinter technology regarding cell concentration, drop volume, and diameter of printed cells; currently limiting its applicability to avascular, aneural, thin, and hollow tissues (3, 129). In recent years, several new methods have been developed to expand applicability of bioprinting, they can be classified into three major modalities based on their working mechanism: extrusion-based bioprinting (EBB), droplet-based, (DBB), and laser-based bioprinting (LBB) (130, 131). Each offers its own set of advantageous and disadvantages, signifying that widespread clinical translation is not yet on the immediate horizon. However, research is progressing rapidly across a multitude of diverse applications, such as breast reconstruction (132), craniomaxillofacial injuries (133), and cardiovascular repair (134).
Preclinical Regenerative Engineering
In the past, 2D cell culture systems or animal models were used for both pharmaceutical screening and disease modeling, but due to their inability to properly mimic human tissues they have largely been replaced by bioengineered models (135–137). While 2D cell culture systems utilize the same cellular components as human tissue, they are unable to reproduce the complex 3D microenvironment of in vivo tissue (136, 138, 139). Conversely, animal models properly replicate the 3D structure of tissues and organs but their distinct multicellularity (140) decreases their efficacy in predicting human toxicology and adverse reactions (141). Therefore, bioengineered constructs have revolutionized medical research by providing a physiologic indistinguishable in vitro model of human tissue along with the ability to provide more accurate data on the pathophysiology of disease, drug efficacy, and drug toxicities (142). These simplified platforms provide us the capacity for preclinical testing of both specific disease entities as well as engineered constructs on a small scale.
Drug Screening
Drug candidates' molecular interactions must be extensively studied with their associated biochemical target before market approval (3). Prior to the development of bioengineered tissue models, the use of in vitro toxicity and efficacy assays to accurately predict in vivo responses posed a major challenge to efficient and effective pharmacological advancement (3). However, organoid technologies have become a solution to this very challenge, by creating in vitro tissue and organ models that are structurally, cellularly, and physiologically indistinguishable from in vivo tissue (3). Skardal et al. proved this by testing a panel of medications that had passed studies using 2D in vitro cultures and animal models, only to be recalled once they were made available to the public secondary to significant toxicities and adverse reactions (143). These drugs were then retested using organoids, which were able to successfully demonstrate toxicity (143). Their study concluded that use of organoid models in drug testing could indicate both short and long-term toxicity. At present, organoids of all types of human tissues, including lung (144), heart (145), kidney, liver, and muscle tissue, have been utilized for this purpose (137).
Disease Modeling
Similarly, organoids developed from either engineered cells or patient biopsy samples have been used to model disease for the study of infectious agents, genetic disorders, and malignancies (120). Lancaster et al. became the first researchers to utilize bioengineered tissue in this way in their study of microcephaly development using brain organoids in 2013 (123). A few years later, during the 2016 Zika epidemic, numerous studies related ZIKV to microencephaly using similar models (146–149). It has become apparent, that human organoid models are ideal for the study of infectious pathogenesis specifically, due to their tendency for species, or even tissue, tropism (120). Some examples of successful infectious disease research using organoid technology include: intestinal organoids for norovirus (150) and rotavirus (151, 152), airway organoids for respiratory viruses including RSV (153) and animal born influenza strains (154–156), co-cultivated human epithelia organoids in the study of bacteria and parasites including Helicobacter pylori (157), Cryptosporidium (158–160) and most recently in SARS-CoV-2 research to determine which organs the virus can infect and propagate in (161–163). The list of pathogens grown in organoid models is increasing rapidly (120).
Human organoid technologies are also becoming an increasingly important asset for precision medicine, by being able to directly test pathogenic genes and mutations in models derived directly from the patient (120). While this approach is still new, the most prominent example is the use of rectal organoids, developed from small endoscopic biopsies of Cystic Fibrosis patients to predict their response to more individualized medications and combined treatments (164–166).
Organoid models have also revolutionized the study of cancer, allowing researchers to study the mechanisms of angiogenesis, carcinogenesis, and metastasis in histologically and physiologically accurate in vitro models (167, 168). Using these models, researchers can appreciate the biochemical interactions leading to malignancies during replication, morphogenesis, differentiation, and growth, with the added ability of being able to control and change variables and environmental conditions (167). For example, numerous studies have validated the driver pathway mutations in tumorigenesis using gene-edited organoid models (169–172). Furthermore, it is believed that failure of most conventional cancer therapies is mainly due to cancer heterogeneity, meaning that the ability to reconstruct gene-mutation specific, and even patient-specific organoid models, is crucial for producing personalized cancer treatments (168). Thus far, cancer organoid models have been developed using patient samples from colon (173–177), brain (178, 179), prostate (180), pancreas (181–183), liver (184), breast (185), bladder (186), stomach (187–189), esophageal (190), endometrial (191), and lung tissues (153, 192).
Clinical Applications
While large-scale clinical translation of bioengineered organs is years away, there have been several clinical applications that have improved patient care and become the standard of care, specifically in regenerative medicine (2). These successes are currently limited to thin and/or avascular tissues (130), including skin, cartilage, heart, and liver (193).
Skin
The earliest documented clinical application of bioengineered tissue, in 1980, used fibroblasts, keratinocytes, and a scaffold to create skin tissue for wound healing (1). Since then, the use of engineered skin has been extensively studied, with researchers attempting to fabricate tissue capable of mimicking the natural healing process of skin, to accelerate wound healing as well as recovering function (194). These skin substitutes can serve as an alternative to conventional skin grafts when standard autologous replacement options are limited (195–198). In recent years, these bioengineered skin substitutes have become viable, mainstream wound healing option for patients, with numerous types and brands approved for clinical use by the Food and Drug Administration (FDA) (2). Furthermore, some bioengineered options have successfully promoted skin restoration in previously untreatable injuries (194).
The many bioengineered skin substitutes are classified based on both material origin (194), whether they are autologous, allogenic, or xenogeneic (198, 199); along with their cellularity (200). Different classifications have greater clinical utility depending on wound depth and whether a temporary or permanent dressing is needed (194). Acellular skin substitutes, like Integra®, utilize collagen and a silicon membrane in place of a dermis and epidermis, respectively (199) (Figure 5). As such, they are most applicable for superficial wounds or burns (199), providing a protective barrier against contamination and fluid loss, while also delivering dermal matrix components, cytokines and growth factors to promote natural wound healing (201, 202). Cellular skin substitutes are more complex, being composed of one to two layers of scaffold that are seeded with either autologous or allogeneic cells (200, 203). They enhance the healing process much like their acellular counterparts but are also capable of long-term and complete restoration of the injured tissue (204, 205). Allogeneic cellular skin substitutes, such as Dermagraft, are widely used, because they are not limited by donor availability (194). However, these are only suitable as a temporary wound dressing, and often are regrafted or replaced by either autologous cellular skin substitutes or split thickness skin grafts (194, 199, 206–210). Commercially available autologous options include Epicil®, Epidex®, and Tiscover® (A-Skin). These are more difficult to create, as grafts must include the full epidermal and superficial dermis layers to provide qualified cells (194). While these cultured epidermal autografts may be useful for long-term wound coverage (211, 212) they fail to provide the stability and resilience of native skin. Therefore, they are primarily used when there is a paucity of donor skin or when just the keratinocyte layer needs to be replaced (194).
Cartilage
Unlike skin, cartilage lacks robust vasculature, nerves, and lymphatics. This often prevents natural healing in vivo (213). Furthermore, defects are difficult to repair due to insufficient chondrocyte supply (214). Until recently, repairing these common injuries required highly invasive, complicated, and imperfect solutions (215); leading the way for innovative bioengineered replacements (213). Within the field of bioengineered cartilage substitutes there are two main clinical applications being studied: joint and tracheal.
From the musculoskeletal perspective, successful bioengineered cartilage substitutes have currently only been developed with dimensions suitable for small joints (193). Clinically applicable examples include, Lee et al. generating vascularized, anatomically shaped tibial condyles (216). Scale-up for larger joints, like the hip and knee, has been limited due to vascular limitations and load bearing capabilities (193). Atala et al. developed a hybrid system using electrospinning and inkjet printing techniques to successfully produce scaffolds for cartilage tissue regeneration with greater mechanical and biologic properties (217). Still, while bioengineered cartilage repair was first introduced nearly three decades ago (218), current approaches are still unable to fabricate cartilage with zonal organization, ECM composition and mechanical properties that are indistinguishable from native tissue (219).
Bioengineered tracheal transplants were once considered the first large scale clinical application of tissue engineering; but ethical concerns and detrimental outcomes later caused much skepticism toward early successes (220). The three earliest reported cases of successful transplantation of tissue-engineered airways were published by the same research group, but with each utilizing a distinct approach (221). The first case produced a repopulated ex vivo decellularized tracheal allotransplant (222, 223). While initial analysis was promising, the transplant did not last long-term and the patient suffered numerous complications (224). In the second publication a synthetic tracheal prosthesis was repopulated using an in vivo tissue-engineering technique. However, this study was later retracted for unethical behavior in both clinical application and data reporting; after the patient in question developed serious complications and eventually died (225, 226). While the third case, utilizing a decellularized allotransplant with in vivo repopulation (227), was devoid of scandal, the researchers' association to the above cases caused skepticism within the medical community. Fortunately, in recent years, there have been several additional animal studies with encouraging results in tracheal bioengineering (228, 229).
Heart
Cardiovascular disease is a leading cause of worldwide morbidity and mortality affecting multiple anatomic sites. Although pharmacologic and surgical interventions have greatly improved therapeutic options; they are still incomplete. This can be appreciated across both congenital and acquired pathologies. For example, in disorders of the pediatric heart, such as septal defects, it would be optimal to have a replacement part which could grow with the child (230). However, in an adult who has suffered a myocardial infarction it is more beneficial to devise a thick patch that is contractile and able to propagate electrical currents (231). To fulfill these varied specifications, stem cells, and scaffolds have been used for cardiovascular repair over the past two decades and replacement parts have been generated. While the heart may house a limited amount of resident stem cells offering some innate regenerative capacity, other stem cell sources have proven more beneficial (232). This has led to mesenchymal (233), induced pluripotent, and hematopoietic stem cells being utilized for cardiovascular repair. For example, Matsumara described the usage of endothelial cells and bone marrow derived stem cells for scaffold seeding leading to the creation of tissue engineered vascular autografts (234). This approach has profound potential for the treatment of malfunctioning vessels, occluded arteries, and replacement hemodialysis access grafts. However, the in vitro generation of replacement parts, such as heart valves and blood vessels, requires cell isolation and expansion alongside scaffold fabrication followed by cell seeding and operative implantation (Figure 6) (235). This typically takes a substantial amount of time and effort, especially if autologous stem cells are utilized. Furthermore, the lack of an integrated vascular tree limits the potential of substantially thick tissues or whole organ generation (236). These limitations have led to the development of injectable therapeutics, where stem cells are introduced with or without the use of a carrier scaffold. A major advantage of an injectable is minimally invasive delivery, such as transcoronary infusions or transendocardial injections. This approach was first described in a rat model of myocardial infarction in which the survival of injectable myoblasts was significantly enhanced by a fibrin carrier (237). This may have been secondary to fibrin being a natural scaffold containing a variety of relevant growth factors. Since then, multiple innovative approaches have been described, such as alginate crosslinked with calcium delivered via a percutaneous catheter-based transcoronary infusion to reverse left ventricular remodeling following myocardial infarction in a swine model (238). Most of the scaffolds described have been hydrogel-based secondary to their structural similarities to native extracellular matrix and favorable environment for cell growth (235). Although hydrogel-based scaffolds may be better suited for injectable applications because of their malleability they can be modified and combined with other materials to provide the structural support required for in vitro tissue generation and surgical manipulation.
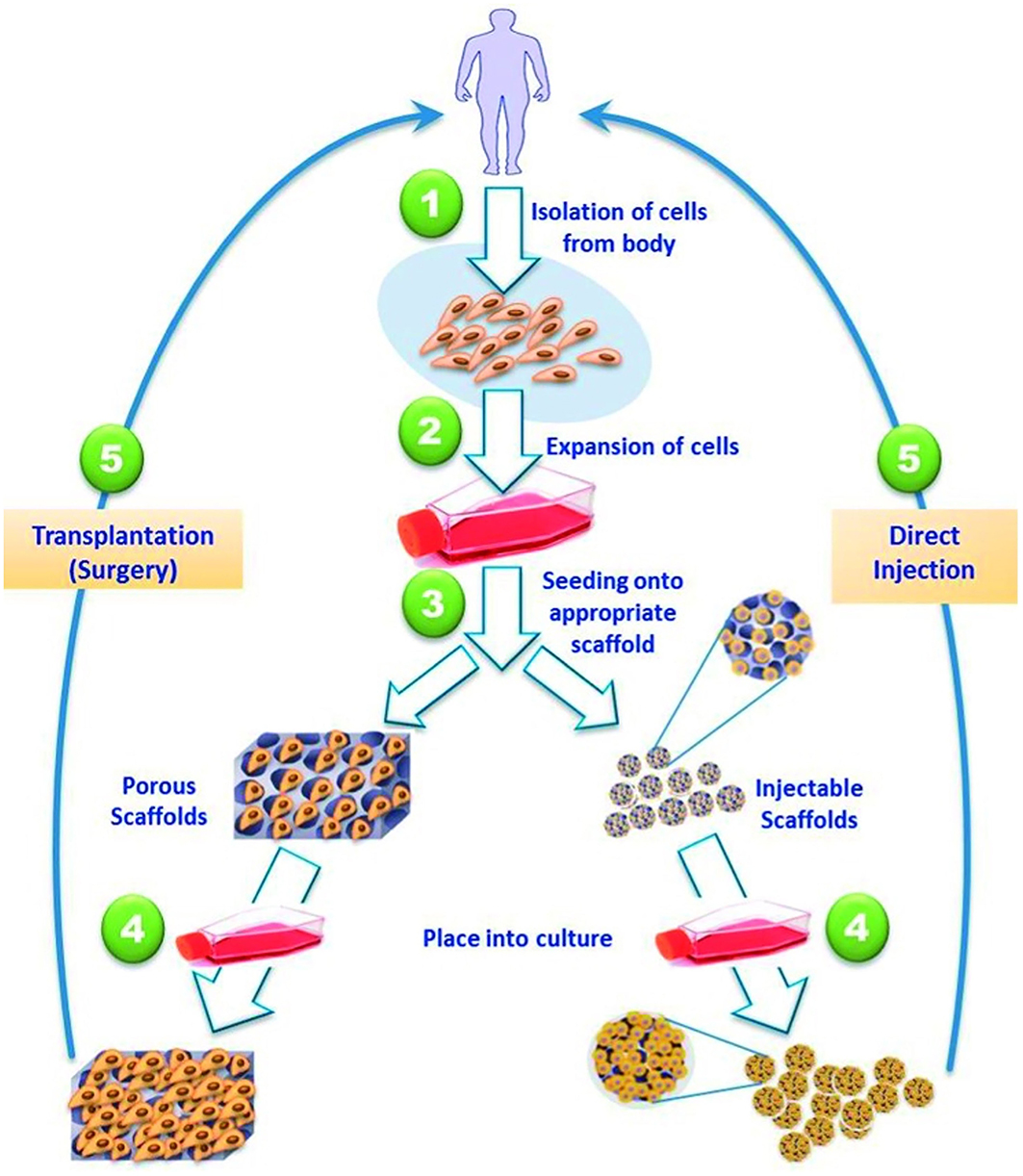
Figure 6. Strategies for tissue engineered cardiac replacement parts delivered either via surgical or percutaneous implantation. Reprinted from El-Sherbiny et al. (235).
Due to the complex structure and function of the human heart, 3D bioprinting has proven promising in some current applications, due to its ability to effectively replicate native structural, mechanical, and functional properties of target tissues (83, 138, 239, 240). Currently, application of 3D bioprinted cardiac tissue is limited to myocardial patches and valves (3). Numerous distinct subsets of 3D bioprinting have successfully printed myocardial patches capable of beating spontaneously and undergoing action potential and uniform conduction, much like native ventricles (241). Laser Induced-Forward Transfer (LIFT) bioprinting was the first method to develop functional cardiac patches for clinical use (3). Testing proved enhanced angiogenesis in the border zone of infarction and greater retention of cardiac function post MI (242). Soon after, Gaetani et al. used extrusion printing to fabricate pre-vascularized myocardial patches that stimulated vascularization, and prolonged cell survival and tissue remodeling, after implantation (3, 243, 244). Further innovation by Tijore et al. increased contractile capabilities of their patches by fabricating microchanneled gelatin hydrogels (245).
While several traditional mechanical and bio-prosthetic valves have been used surgically for years, recent research has begun exploring 3D bioprinted valves (3). Current valve replacement options are limited by their durability, anticoagulation requirements and lack of patient specificity (3). Early studies hypothesized that bioengineered scaffolds have the potential to achieve increased elasticity (10-fold), improve shape retention and allow for greater cell viability (3). However, more research needs to be done regarding physiologic functionality in clinical applications (3). The further development of materials science and stem cell biology alongside enabling technologies will continue to improve tissue engineered replacement parts for cardiovascular repair, leading to improved patient outcomes.
Bladder
Currently, the only complete human organ able to be bioengineered is the bladder, due to its thin, hollow structure. Yoo et al. produced the first successful canine example in 1998, by seeding a decellularized bladder submucosa with autologous cells (246). An equal number of subjects were implanted with either cell-seeded or acellular matrices, and it was discovered that the cell seeded group developed normal bladder tissue which was functionally and histologically indistinguishable from native tissue. Meanwhile, the acellular matrices developed into abnormal and functionally defective tissue (247). Within a year, Oberpenning et al. translated these results to humans using a biodegradable scaffold seeded with autologous cells (248). These were successfully implanted into patients, who continue to have moderately efficacious outcomes even 15 years later (247–249). This research is now in late stage human clinical trials using the same protocols and manufacturing steps as the original studies, with hopes of FDA approval in the near future (247).
Hurdles
Despite tremendous advancement in regenerative engineering, there has been only limited clinical translation and important hurdles for future expansions remain, including both ethical and biological aspects. For example, in vitro tissue generation continues to be limited by requirements for long-term bioreactor maturation (250, 251), lacks the structural stability necessary for surgical manipulation, and is prone to significant hypoxia (252) until inosculation is achieved. Furthermore, as it is still arduous to fabricate an uninterrupted microvasculature that lengthens to an anastomosable vessel segment, all engineered cellularized constructs suffer some hypoxia upon implantation. This profoundly impedes our ability to generate thicker tissues (253). Hence, rapid vascularization continues to be a significant limiting factor in regenerative engineering.
Integrating Vasculature Into Engineered Tissue
As isolated cells merged to form multicellular organisms, they were no longer able to obtain oxygen and remove waste via diffusion. Thus, the need for a circulatory system developed. Currently, tissue engineering is at the same crossroad. As mentioned above, current clinical application of bioengineered tissues and organs is limited to thin avascular tissues (2). The fundamental problem is the failure to manufacture microvascular networks able to both support tissue survival and withstand physiological pressures (4). While researchers have agreed for decades that a hierarchical vascular network is essential for successful scale-up of engineered tissues (254), it has persisted as a major limitation (255, 256).
Importance of Vasculature in Engineered Tissue
Unlike larger vasculature, which can be identified as distinct anatomical entities, microvasculature, namely arterioles, capillaries, and venules, are structurally and functionally incorporated into the tissue they supply (128). These vessels are crucial for tissue survival, ensuring delivery of oxygen and essential nutrients, as well as the removal of metabolic waste (257, 258). Given that the tissue diffusion limit is ~100–200 μm (257, 259, 260), thicker tissues than this must have an integrated microvascular network to keep up with their high volumetric oxygen-consumption (255). Accordingly, thick organs like the liver, kidney, lungs, spleen, and heart are highly vascularized, and as such require formation of new vasculature once they grow past the diffusion limit (213, 261–263).
Despite the immense progress made in regenerative engineering, current technologies and methods are not yet able to produce large scale, thick, vascularized tissues, and organs (2, 213). With bioprinting, the main hurdle is the incapability to print sufficiently small structures. As the name implies, the microvasculature is miniscule in size, with luminal diameters of arterioles and venules averaging about 30 microns (10–200 microns) and capillaries averaging 5–10 microns (128). Current printer heads are unable of printing at this size, despite development of increasingly smaller nozzle sizes (131). While decellularization platforms can retain vascular structures, including micro-vessels, it faces the challenge of maintaining appropriate functionality (99). Thus far, the solutions used during the wash phase are too harsh for the treated vasculature to retain normal physiology (101).
Current Approaches to Vascularize Engineered Tissues
Given that thick and complex organs are the most in demand for transplantation (>90%), namely liver, kidney and heart, overcoming the vascular limitation would revolutionize modern medicine (213). Currently, three different approaches have been largely studied to successfully vascularize engineered tissue for scaled-up applications: isolated 3D printing of the microvasculature, prevascularization, and the integration of oxygen generating particles.
There are several studies being done into the viability of separately bioprinting the microvasculature and tissue (255). Given that current 3D bioprinting technology is incapable of printing at the submicron scale, one proposed approach is to print vasculature at the smallest size possible, and allow for the natural progression of vascular anastomoses to create the desired capillary network (255). Two different methods have been explored: direct and indirect bioprinting. Direct bioprinting produces microscale neotissues through printing of organoids into an anatomical model and allowing the vasculature to self-assemble without the use of a scaffold (118, 255). Whereas, indirect printing uses a fugitive ink that is then removed by heat stimulated decrosslinking, leaving behind the vascular network (255).
Another proposed approach is the pre-vascularization of bioengineered tissue constructs, that allow an engineered tissue to rapidly integrate with host vasculature once implanted (254). The process of pre-vascularization requires encapsulating endothelial cells (ECs) or their progenitors, with other cell types in vitro to produce a ready-to-go microvasculature (254). In theory, once implanted this construct could quickly inosculate with the recipient and facilitate rapid perfusion (140, 258, 260). However, proper distribution of this vasculature, especially in thick tissue constructs, relies on in vitro cellular infiltration and self-organization (254), often resulting in slow and non-uniform vascular networks (264). Another limitation to this approach, is that for vascular self-assembly to occur, there must be a critical concentration of ECs seeded into the tissue construct, thereby limiting the co-culture number of other cells (264).
Given the capacity for angiogenesis, albeit slowly, another method to prevent initial hypoxia and cell death in implanted bioengineered tissues is the inclusion of oxygen generating particles. These particles can be designed to maintain a certain partial pressure of oxygen for a defined time period. With the goal of maintaining cell oxygenation and viability until vascularization has taken place from the recipient into the engineered graft. Oxygen generating particles can either be cerium oxide nanoparticles (CNPs), sometimes referred to as nanoceria, or silver nanoparticles (AgNPs) (265, 266). CNPs have been a successful therapeutic agent in tissue repair and regeneration because of its reactive oxygen species (ROS) properties, high angiogenic potential (265), and ability to induce stem cell differentiation (267). Similarly, AgNPs are also popular, due to their physiochemical properties generating ROS (266). However, they are less commonly used despite their therapeutic benefits, because of their potential toxicity to cells and tissues (266). However, it is well-appreciated that hypoxia is a profound angiogenic stimulator and it is possible that inclusion of these particles may actually delay vascularization of the implanted graft.
Future Perspectives
Regenerative engineering is expanding briskly, with continued advances being made on the tangible platform that has developed over the past-quarter century. Most current approaches often rely heavily on in vitro manufacturing followed by implantation. However, newer technologies may allow for direct surgical repair. For example, intraoperative bioprinting would revolutionize surgical care (255). In theory, this technology would enable immediate regeneration of complex, large tissues in situ (255, 268, 269). Along with the technique itself, in situ bioprinting requires the development of simplified, portable bioprinters as well as scalable automated biofabrication production lines, which will need to be adequately explored prior to any attempt at clinical translation (130, 270, 271). In situ bioprinting, nevertheless, represents a promising future (130).
Beyond manufacturing, scale-up for tissue engineering is inhibited by the sheer multitude of cells comprising each organ, and the associated time constraints of developing organs on a patient-by-patient basis. It is estimated that each organ in the human body is made up of several billion cells (272). Meeting this need requires not only accelerating cell cycle speeds for increasing the quantity of stem cells produced and acquired, but also development of new materials or mechanisms capable of supporting differentiation of engineered tissue cells into specific phenotypes (131). Moreover, while current tissue engineering methods have proven successful in smaller, thin tissues and organs, the complex multicellularity of large, thick organs will require precise cell and biomaterial placement to ensure proper 3D structure, which has yet to be achieved on this large a scale (213). Additionally, further breakthroughs, such as cell encapsulation technologies (273), miRNA therapies and the clustered regularly interspaced short palindromic repeats (CRISPR) system of gene editing, may significantly alter the regenerative surgical landscape in years to come (Figure 7) (274–276).
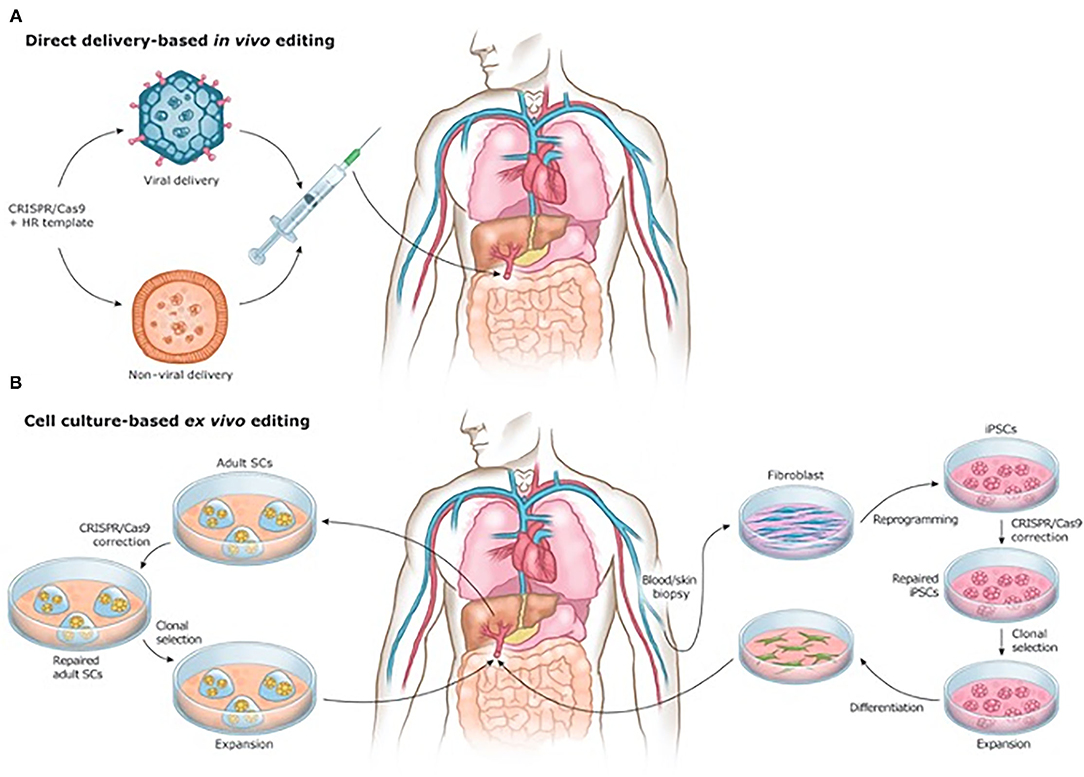
Figure 7. (A) In vivo and (B) ex vivo strategies for CRISPR/Cas9-based gene therapies. Reprinted from Savić et al. (276).
Conclusion
Regenerative engineering is a rapidly evolving field that combines the advances of tissue engineering with materials science, stem cell biology, and developmental biology. It has already produced clinically relevant technologies capable of improving patient outcomes and promises to continue revolutionizing the fields of medicine and surgery. Surgeons should become acquainted with some of its core components, as it will likely permeate into patient care, to a certain degree, within the foreseeable future.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This research was supported by the Pink Zone at the Pennsylvania State University (DJR), the American Association of Plastic Surgeons/Plastic Surgery Foundation Combined Grant (DJR) and the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R56HL 157190 (DJR).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ikada Y. Challenges in tissue engineering. J R Soc Interface. (2006) 3:589–601. doi: 10.1098/rsif.2006.0124
2. Ravnic DJ, Leberfinger AN, Koduru SV, Hospodiuk M, Moncal KK, Datta P, et al. Transplantation of bioprinted tissues and organs: technical and clinical challenges and future perspectives. Ann Surg. (2017) 266:48–58. doi: 10.1097/SLA.0000000000002141
3. Cui H, Miao S, Esworthy T, Zhou X, Lee SJ, Liu C, et al. 3D bioprinting for cardiovascular regeneration and pharmacology. Adv Drug Deliv Rev. (2018) 132:252–69. doi: 10.1016/j.addr.2018.07.014
4. Nichols JE, La Francesca S, Niles JA, Vega SP, Argueta LB, Frank L, et al. Production and transplantation of bioengineered lung into a large-animal model. Sci Transl Med. (2018) 10:eaao3926. doi: 10.1126/scitranslmed.aao3926
5. Al-Himdani S, Jessop ZM, Al-Sabah A, Combellack E, Ibrahim A, Doak SH, et al. Tissue-engineered solutions in plastic and reconstructive surgery: principles and practice. Front Surg. (2017) 4:4. doi: 10.3389/fsurg.2017.00004
6. Laurencin CT, Khan Y. Regenerative engineering. Sci Transl Med. (2012) 4:160ed9. doi: 10.1126/scitranslmed.3004467
7. Croce S, Peloso A, Zoro T, Avanzini MA, Cobianchi L. A hepatic scaffold from decellularized liver tissue: food for thought. Biomolecules. (2019) 9:813. doi: 10.3390/biom9120813
8. Peloso A. Editorial: alternative regenerative medicine for diabetes: beyond the stem cell approach. Front Endocrinol. (2021) 12:648763. doi: 10.3389/fendo.2021.648763
9. Bellofatto K, Moeckli B, Wassmer CH, Laurent M, Oldani G, Andres A, et al. Bioengineered islet cell transplantation. Curr Transplant Rep. (2021) 8:57–66. doi: 10.1007/s40472-021-00318-1
10. Kwon SG, Kwon YW, Lee TW, Park GT, Kim JH. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater Res. (2018) 22:36. doi: 10.1186/s40824-018-0148-4
11. Sugawara T, Nishino K, Umezawa A, Akutsu H. Investigating cellular identity and manipulating cell fate using induced pluripotent stem cells. Stem Cell Res Ther. (2012) 3:8. doi: 10.1186/scrt99
12. Thomson JA, Marshall VS. Primate embryonic stem cells. Curr Top Dev Biol. (1998) 38:133–65. doi: 10.1016/S0070-2153(08)60246-X
13. Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. (1998) 95:13726–31. doi: 10.1073/pnas.95.23.13726
14. Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. (2001) 17:435–62. doi: 10.1146/annurev.cellbio.17.1.435
15. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. (1998) 282:1145–7. doi: 10.1126/science.282.5391.1145
16. Ilic D, Devito L, Miere C, Codognotto S. Human embryonic and induced pluripotent stem cells in clinical trials. Br Med Bull. (2015) 116:19–27. doi: 10.1093/bmb/ldv045
17. Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of functional human pancreatic beta cells in vitro. Cell. (2014) 159:428–39. doi: 10.1016/j.cell.2014.09.040
18. Conese M, Beccia E, Castellani S, Di Gioia S, Colombo C, Angiolillo A, et al. The long and winding road: stem cells for cystic fibrosis. Expert Opin Biol Ther. (2017) 18:281–92. doi: 10.1080/14712598.2018.1413087
19. Staudacher DL, Sela Y, Itskovitz-Eldor J, Flugelman MY. Intra-arterial injection of human embryonic stem cells in athymic rat hind limb ischemia model leads to arteriogenesis. Cardiovasc Revasc Med. (2011) 12:228–34. doi: 10.1016/j.carrev.2010.11.004
20. Ilic D, Ogilvie C. Concise review: human embryonic stem cells-what have we done? What Are We Doing? Where Are We Going? Stem Cells. (2017) 35:17–25. doi: 10.1002/stem.2450
21. Rong Z, Wang M, Hu Z, Stradner M, Zhu S, Kong H, et al. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell. (2014) 14:121–30. doi: 10.1016/j.stem.2013.11.014
22. Amit M, Margulets V, Segev H, Shariki K, Laevsky I, Coleman R, et al. Human feeder layers for human embryonic stem cells. Biol Reprod. (2003) 68:2150–6. doi: 10.1095/biolreprod.102.012583
23. Clement F, Grockowiak E, Zylbersztejn F, Fossard G, Gobert S, Maguer-Satta V. Stem cell manipulation, gene therapy and the risk of cancer stem cell emergence. Stem Cell Investig. (2017) 4:67. doi: 10.21037/sci.2017.07.03
24. Servick K. Embryonic stem cells and fetal tissue research—will Trump intervene?. Science. (2016). doi: 10.1126/science.aal0375
25. Yap KK. Stem cells and religion. Stem Cells Transl Med. (2014) 3:977. doi: 10.5966/sctm.2014-0092
26. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. (2006) 126:663–76. doi: 10.1016/j.cell.2006.07.024
27. Hayden EC. Stem cells: The growing pains of pluripotency. Nature. (2011) 473:272–4. doi: 10.1038/473272a
28. Yamanaka S, Takahashi K. [Induction of pluripotent stem cells from mouse fibroblast cultures]. Tanpakushitsu Kakusan Koso. (2006) 51:2346–51. doi: 10.1111/j.1365-2184.2008.00493.x
29. Salas S, Ng N, Gerami-Naini B, Anchan RM. Induced pluripotent stem cells from ovarian tissue. Curr Protoc Hum Genet. (2017) 95:21 10 1–21 10 22. doi: 10.1002/cphg.47
30. Revilla A, Gonzalez C, Iriondo A, Fernandez B, Prieto C, Marin C, et al. Current advances in the generation of human iPS cells: implications in cell-based regenerative medicine. J Tissue Eng Regen Med. (2016) 10:893–907. doi: 10.1002/term.2021
31. Li M, Izpisua Belmonte JC. Izpisua Belmonte, Looking to the future following 10 years of induced pluripotent stem cell technologies. Nat Protoc. (2016) 11:1579–85. doi: 10.1038/nprot.2016.108
32. Ardeshirylajimi A. Applied induced pluripotent stem cells in combination with biomaterials in bone tissue engineering. J Cell Biochem. (2017) 118:3034–42. doi: 10.1002/jcb.25996
33. Bastami F, Nazeman P, Moslemi H, Rezai Rad M, Sharifi K, Khojasteh A. Induced pluripotent stem cells as a new getaway for bone tissue engineering: a systematic review. Cell Prolif. (2017) 50:e12321. doi: 10.1111/cpr.12321
34. Du C, Narayanan K, Leong MF, Wan AC. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials. (2014) 35:6006–14. doi: 10.1016/j.biomaterials.2014.04.011
35. Gao L, Kupfer ME, Jung JP, Yang L, Zhang P, Da Sie Y, et al. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ Res. (2017) 120:1318–25. doi: 10.1161/CIRCRESAHA.116.310277
36. Huang Z, Powell R, Phillips JB, Haastert-Talini K. Perspective on schwann cells derived from induced pluripotent stem cells in peripheral nerve tissue engineering. Cells-Basel. (2020) 9:2497. doi: 10.3390/cells9112497
37. Luo J, Lin Y, Shi X, Li G, Kural MH, Anderson CW, et al. Xenogeneic-free generation of vascular smooth muscle cells from human induced pluripotent stem cells for vascular tissue engineering. Acta Biomater. (2021) 119:155–68. doi: 10.1016/j.actbio.2020.10.042
38. Luo J, Shi X, Lin Y, Yuan Y, Kural MH, Wang J, et al. Efficient differentiation of human induced pluripotent stem cells into endothelial cells under xenogeneic-free conditions for vascular tissue engineering. Acta Biomater. (2021) 119:184–96. doi: 10.1016/j.actbio.2020.11.007
39. Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. (2017) 376:1038–46. doi: 10.1056/NEJMoa1608368
40. Normile D. Cutting-edge stem cell therapy proves safe, but will it ever be effective? Science. (2017). doi: 10.1126/science.aal0914
41. Yoshihara M, Hayashizaki Y, Murakawa Y. Genomic instability of iPSCs: challenges towards their clinical applications. Stem Cell Rev. (2017) 13:7–16. doi: 10.1007/s12015-016-9680-6
42. Lehrman S. Undifferentiated ethics. Sci Am. (2010) 303:18, 20. doi: 10.1038/scientificamerican0910-18
43. Zheng YL. Some ethical concerns about human induced pluripotent stem cells. Sci Eng Ethics. (2016) 22:1277–84. doi: 10.1007/s11948-015-9693-6
44. Dulak J, Szade K, Szade A, Nowak W, Jozkowicz A. Adult stem cells: hopes and hypes of regenerative medicine. Acta Biochim Pol. (2015) 62:329–37. doi: 10.18388/abp.2015_1023
45. Fefer A, Thomas ED, Buckner CD, Cheever MA, Einstein AB, Neiman PE, et al. Marrow transplantation for acute leukemia in man. Ann N Y Acad Sci. (1976) 277:52–9. doi: 10.1111/j.1749-6632.1976.tb41691.x
46. Storb R, Thomas ED, Buckner CD, Clift RA, Fefer A, Fernando LP, et al. Allogeneic marrow grafting for treatment of aplastic anemia: a follow-up on long-term survivors. Blood. (1976) 48:485–90. doi: 10.1182/blood.V48.4.485.bloodjournal484485
47. Bauge C, Boumediene K. Use of adult stem cells for cartilage tissue engineering: current status and future developments. Stem Cells Int. (2015) 2015:438026. doi: 10.1155/2015/438026
48. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. (2007) 213:341–7. doi: 10.1002/jcp.21200
49. Hodgkinson T, Yuan XF, Bayat A. Adult stem cells in tissue engineering. Expert Rev Med Devices. (2009) 6:621–40. doi: 10.1586/erd.09.48
50. Kalpakci KN, Brown WE, Hu JC, Athanasiou KA. Cartilage tissue engineering using dermis isolated adult stem cells: the use of hypoxia during expansion versus chondrogenic differentiation. PLoS ONE. (2014) 9:e98570. doi: 10.1371/journal.pone.0098570
51. Karam JP, Muscari C, Montero-Menei CN. Combining adult stem cells and polymeric devices for tissue engineering in infarcted myocardium. Biomaterials. (2012) 33:5683–95. doi: 10.1016/j.biomaterials.2012.04.028
52. Kwon H, Haudenschild AK, Brown WE, Vapniarsky N, Paschos NK, Arzi B, et al. Tissue engineering potential of human dermis-isolated adult stem cells from multiple anatomical locations. PLoS ONE. (2017) 12:e0182531. doi: 10.1371/journal.pone.0182531
53. Martinez EC, Kofidis T. Adult stem cells for cardiac tissue engineering. J Mol Cell Cardiol. (2011) 50:312–9. doi: 10.1016/j.yjmcc.2010.08.009
54. Moradi SL, Golchin A, Hajishafieeha Z, Khani MM, Ardeshirylajimi A. Bone tissue engineering: adult stem cells in combination with electrospun nanofibrous scaffolds. J Cell Physiol. (2018) 233:6509–22. doi: 10.1002/jcp.26606
55. Travnickova M, Bacakova L. Application of adult mesenchymal stem cells in bone and vascular tissue engineering. Physiol Res. (2018) 67:831–50. doi: 10.33549/physiolres.933820
56. Vapniarsky N, Kwon H, Paschos NK, Haudenschild AK, Brown WE, DuRaine GD, et al. Adult dermal stem cells for scaffold-free cartilage tissue engineering: exploration of strategies. Tissue Eng Part C Methods. (2020) 26:598–607. doi: 10.1089/ten.tec.2020.0207
57. Wang W, Cao B, Cui L, Cai J, Yin J. Adipose tissue engineering with human adipose tissue-derived adult stem cells and a novel porous scaffold. J Biomed Mater Res B Appl Biomater. (2013) 101:68–75. doi: 10.1002/jbm.b.32816
58. Yoo C, Vines JB, Alexander G, Murdock K, Hwang P, Jun HW. Adult stem cells and tissue engineering strategies for salivary gland regeneration: a review. Biomater Res. (2014) 18:9. doi: 10.1186/2055-7124-18-9
59. Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. (2015) 35:e00191. doi: 10.1042/BSR20150025
60. Henning SJ, von Furstenberg RJ. GI stem cells - new insights into roles in physiology and pathophysiology. J Physiol. (2016) 594:4769–79. doi: 10.1113/JP271663
61. Andreopoulou E, Arampatzis A, Patsoni M, Kazanis I. Being a neural stem cell: a matter of character but defined by the microenvironment. Adv Exp Med Biol. (2017) 1041:81–118. doi: 10.1007/978-3-319-69194-7_6
62. Peters EB. Endothelial progenitor cells for the vascularization of engineered tissues. Tissue Eng Part B Rev. (2017) 24:1–24. doi: 10.1089/ten.TEB.2017.0127
63. Banyard DA, Salibian AA, Widgerow AD, Evans GR. Implications for human adipose-derived stem cells in plastic surgery. J Cell Mol Med. (2015) 19:21–30. doi: 10.1111/jcmm.12425
64. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. (2001) 7:211–28. doi: 10.1089/107632701300062859
65. Kim SC, Han DJ, Lee JY. Adipose tissue derived stem cells for regeneration and differentiation into insulin-producing cells. Curr Stem Cell Res Ther. (2010) 5:190–4. doi: 10.2174/157488810791268717
66. Zack-Williams SD, Butler PE, Kalaskar DM. Current progress in use of adipose derived stem cells in peripheral nerve regeneration. World J Stem Cells. (2015) 7:51–64. doi: 10.4252/wjsc.v7.i1.51
67. Pavon A, Beloqui I, Salcedo JM, Martin AG. Cryobanking mesenchymal stem cells. Methods Mol Biol. (2017) 1590:191–6. doi: 10.1007/978-1-4939-6921-0_14
68. Qi K, Li N, Zhang Z, Melino G. Tissue regeneration: the crosstalk between mesenchymal stem cells and immune response. Cell Immunol. (2017) 326:86–93. doi: 10.1016/j.cellimm.2017.11.010
69. Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. (2014) 15:1009–16. doi: 10.1038/ni.3002
70. Macrin D, Joseph JP, Pillai AA, Devi A. Eminent sources of adult mesenchymal stem cells and their therapeutic imminence. Stem Cell Rev. (2017) 13:741–56. doi: 10.1007/s12015-017-9759-8
71. King NM, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther. (2014) 5:85. doi: 10.1186/scrt474
72. Bhartiya D. Pluripotent stem cells in adult tissues: struggling to be acknowledged over two decades. Stem Cell Rev. (2017) 13:713–24. doi: 10.1007/s12015-017-9756-y
73. Pisani S, Croce S, Chiesa E, Dorati R, Lenta E, Genta I, et al. Tissue engineered esophageal patch by mesenchymal stromal cells: optimization of electrospun patch engineering. Int J Mol Sci. (2020) 21:1764. doi: 10.3390/ijms21051764
74. Lanza RP, Langer RS, Vacanti J. Principles of Tissue Engineering. Amsterdam: Academic Press, an imprint of Elsevier (2014).
75. Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. (2014) 12:207–18. doi: 10.1089/adt.2014.573
76. Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. (2001) 55:141–50.3. doi: 10.1002/1097-4636(200105)55:2<141::AID-JBM1000>3.0.CO;2-J
77. Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol. (2005) 15:365–77. doi: 10.1016/j.semcancer.2005.05.002
78. Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. (2013) 19:485–502. doi: 10.1089/ten.teb.2012.0437
79. Hollister SJ, Maddox RD, Taboas JM. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials. (2002) 23:4095–103. doi: 10.1016/S0142-9612(02)00148-5
80. Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. (2011) 32:3921–30. doi: 10.1016/j.biomaterials.2011.02.019
81. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. (2006) 126:677–89. doi: 10.1016/j.cell.2006.06.044
82. Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, et al. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. (2006) 27:1452–61. doi: 10.1016/j.biomaterials.2005.08.004
83. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. (2014) 32:773–85. doi: 10.1038/nbt.2958
84. Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev. (2011) 17:281–99. doi: 10.1089/ten.teb.2011.0077
85. Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. (2009) 103:655–63. doi: 10.1002/bit.22361
86. Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. (2003) 24:2309–16. doi: 10.1016/S0142-9612(03)00110-8
87. Masuda S, Shimizu T, Yamato M, Okano T. Cell sheet engineering for heart tissue repair. Adv Drug Deli Rev. (2008) 60:277–285.
88. Moschouris K, Firoozi N, Kang Y. The application of cell sheet engineering in the vascularization of tissue regeneration. Regen Med. (2016) 11:559–70. doi: 10.2217/rme-2016-0059
89. Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. (2011) 8:607–26. doi: 10.1586/erd.11.27
90. Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. (2005) 23:47–55. doi: 10.1038/nbt1055
91. Hynes RO, Naba A. Overview of the matrisome–an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. (2012) 4:a004903. doi: 10.1101/cshperspect.a004903
92. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. (2008) 16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x
93. Koria P. Delivery of growth factors for tissue regeneration and wound healing. BioDrugs. (2012) 26:163–75. doi: 10.2165/11631850-000000000-00000
94. Griffin MF, Butler PE, Seifalian AM, Kalaskar DM. Control of stem cell fate by engineering their micro and nanoenvironment. World J Stem Cells. (2015) 7:37–50. doi: 10.4252/wjsc.v7.i1.37
95. Xing H, Lee H, Luo L, Kyriakides TR. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol Adv. (2020) 42:107421. doi: 10.1016/j.biotechadv.2019.107421
96. Badylak SF. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann Biomed Eng. (2014) 42:1517–27. doi: 10.1007/s10439-013-0963-7
97. Badylak SF. Regenerative medicine and developmental biology: the role of the extracellular matrix. Anat Rec B New Anat. (2005) 287:36–41. doi: 10.1002/ar.b.20081
98. Arenas-Herrera JE, Ko IK, Atala A, Yoo JJ. Decellularization for whole organ bioengineering. Biomed Mater. (2013) 8:014106. doi: 10.1088/1748-6041/8/1/014106
99. Choudhury D, Yee M, Sheng ZLJ, Amirul A, Naing MW. Decellularization systems and devices: state-of-the-art. Acta Biomater. (2020) 115:51–9. doi: 10.1016/j.actbio.2020.07.060
100. Schmitt A, Csiki R, Tron A, Saldamli B, Tubel J, Florian K, et al. Optimized protocol for whole organ decellularization. Eur J Med Res. (2017) 22:31. doi: 10.1186/s40001-017-0272-y
101. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. (2011) 32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057
102. White LJ, Taylor AJ, Faulk DM, Keane TJ, Saldin LT, Reing JE, et al. The impact of detergents on the tissue decellularization process: a ToF-SIMS study. Acta Biomater. (2017) 50:207–19. doi: 10.1016/j.actbio.2016.12.033
103. Kim BS, Kim H, Gao G, Jang J, Cho DW. Decellularized extracellular matrix: a step towards the next generation source for bioink manufacturing. Biofabrication. (2017) 9:034104. doi: 10.1088/1758-5090/aa7e98
104. Broyles JM, Liao EC, Kim J, Heistein J, Sisco M, Karp N, et al. Acellular dermal matrix-associated complications in implant-based breast reconstruction: a multicenter, prospective, randomized controlled clinical trial comparing two human tissues. Plast Reconstr Surg. (2021) 148:493–500. doi: 10.1097/PRS.0000000000008194
105. Kim JYS, Mlodinow AS. What's new in acellular dermal matrix and soft-tissue support for prosthetic breast reconstruction. Plast Reconstr Surg. (2017) 140:30S−43S. doi: 10.1097/PRS.0000000000003950
106. Mura S, Caputo GG, Miotti G, Contessi Negrini F, Fin A, Rampino Cordaro E, et al. Direct-to-implant, prepectoral breast reconstruction with Braxon((R)) dermal matrix: a single-center experience with 111 cases. Breast J. (2021) 27:412–4. doi: 10.1111/tbj.14193
107. Oh C, Winocour S, Lemaine V. Acellular dermal matrix in submuscular implant-based breast reconstruction: a novel technique to improve symmetry. Plast Reconstr Surg. (2017) 140:641e−3e. doi: 10.1097/PRS.0000000000003738
108. Park KC, Park ES, Cha HG, Kim SY. Comparative analysis of sterile freeze-dried versus sterile pre-hydration acellular dermal matrix in implant-based breast reconstruction. Aesthetic Plast Surg. (2021) doi: 10.1007/s00266-021-02291-7
109. Sobti N, Ji E, Brown RL, Cetrulo CL Jr, Colwell AS, Liao EC. Evaluation of acellular dermal matrix efficacy in prosthesis-based breast reconstruction. Plast Reconstr Surg. (2018) 141:541–9. doi: 10.1097/PRS.0000000000004109
110. Roth JS, Brathwaite C, Hacker K, Fisher K, King J. Complex ventral hernia repair with a human acellular dermal matrix. Hernia. (2015) 19:247–52. doi: 10.1007/s10029-014-1245-5
111. Garcia A, Baldoni A. Complex ventral hernia repair with a human acellular dermal matrix and component separation: a case series. Ann Med Surg. (2015) 4:271–8. doi: 10.1016/j.amsu.2015.07.002
112. Kissane NA, Itani KMF. A decade of ventral incisional hernia repairs with biologic acellular dermal matrix: what have we learned? Plast Reconstr Surg. (2012) 130:194S−202S. doi: 10.1097/PRS.0b013e318265a5ec
113. Roth JS, Dexter DD, Lumpkins K, Bochicchio GV. Hydrated vs. freeze-dried human acellular dermal matrix for hernia repair: a comparison in a rabbit model. Hernia. (2009) 13:201–7. doi: 10.1007/s10029-008-0453-2
114. Diaz JJ Jr, Conquest AM, Ferzoco SJ, Vargo D, Miller P, Wu YC, et al. Multi-institutional experience using human acellular dermal matrix for ventral hernia repair in a compromised surgical field. Arch Surg. (2009) 144:209–15. doi: 10.1001/archsurg.2009.12
115. Candage R, Jones K, Luchette FA, Sinacore JM, Vandevender D, Reed R2nd L. Use of human acellular dermal matrix for hernia repair: friend or foe? Surgery. (2008) 144:703–9; discussion 709-11. doi: 10.1016/j.surg.2008.06.018
116. Cobianchi L, Moeckli B, Croce S. Commentary: insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Front Endocrinol. (2020) 11:546114. doi: 10.3389/fendo.2020.546114
117. Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development. (2015) 142:3113–25. doi: 10.1242/dev.118570
118. Fennema E, Rivron N, J. Rouwkema J, van Blitterswijk C, de Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. (2013) 31:108–15. doi: 10.1016/j.tibtech.2012.12.003
119. Brassard JA, Lutolf MP. Engineering stem cell self-organization to build better organoids. Cell Stem Cell. (2019) 24:860–76. doi: 10.1016/j.stem.2019.05.005
120. Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. (2020) 21:571–84. doi: 10.1038/s41580-020-0259-3
121. Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, Barrett's epithelium. Gastroenterology. (2011) 141:1762–72. doi: 10.1053/j.gastro.2011.07.050
122. Fujii M, Matano M, Toshimitsu K, Takano A, Mikami Y, Nishikori S, et al. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell. (2018) 23:787–93 e6. doi: 10.1016/j.stem.2018.11.016
123. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. (2013) 501:373–9. doi: 10.1038/nature12517
124. Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. (2015) 526:564–8. doi: 10.1038/nature15695
125. Hu H, Gehart H, Artegiani B, LÖpez-Iglesias C, Dekkers F, Basak O, et al. Long-Term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. (2018) 175:1591–606 e19. doi: 10.1016/j.cell.2018.11.013
126. Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. (2017) 19:568–77. doi: 10.1038/ncb3516
127. Hofer M, Lutolf MP. Engineering organoids. U. Bacterial longevity. Nat Rev Microbiol. (2021) 19:681. doi: 10.1038/s41579-021-00628-2
128. Datta P, Ayan B, Ozbolat IT. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater. (2017) 51:1–20. doi: 10.1016/j.actbio.2017.01.035
129. Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. (2013) 60:691–9. doi: 10.1109/TBME.2013.2243912
130. Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. (2016) 76:321–43. doi: 10.1016/j.biomaterials.2015.10.076
131. Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials. (2016) 102:20–42. doi: 10.1016/j.biomaterials.2016.06.012
132. Mu X, Zhang J, Jiang Y. 3D printing in breast reconstruction: from bench to bed. Front Surg. (2021) 8:641370. doi: 10.3389/fsurg.2021.641370
133. Moncal KK, Gudapati H, Godzik KP, Heo DN, Kang Y, Rizk E, et al. Intra-operative bioprinting of hard, soft, and hard/soft composite tissues for craniomaxillofacial reconstruction. Adv Funct Mater. (2021) 31:2010858. doi: 10.1002/adfm.202010858
134. Leberfinger AN, Dinda S, Wu Y, Koduru SV, Ozbolat V, Ravnic DJ, et al. Bioprinting functional tissues. Acta Biomater. (2019) 95:32–49. doi: 10.1016/j.actbio.2019.01.009
135. Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. (2009) 30:2164–74. doi: 10.1016/j.biomaterials.2008.12.084
136. Wust S, Muller R, Hofmann S. Controlled positioning of cells in biomaterials-approaches towards 3D tissue printing. J Funct Biomater. (2011) 2:119–54. doi: 10.3390/jfb2030119
137. Mohammadi MH, Obregon R, Ahadian S, Ramon-Azcon J, Radisic M. Engineered muscle tissues for disease modeling and drug screening applications. Curr Pharm Des. (2017) 23:2991–3004. doi: 10.2174/1381612823666170215115445
138. Melchels FPW, Domingos MAN, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW, et al. Additive manufacturing of tissues and organs. Prog Polym Sci. (2012) 37:1079–104. doi: 10.1016/j.progpolymsci.2011.11.007
139. Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. (2012) 33:6020–41. doi: 10.1016/j.biomaterials.2012.04.050
140. Alajati A, Laib AM, Weber H, Boos AM, Bartol A, Ikenberg K, et al. Spheroid-based engineering of a human vasculature in mice. Nat Methods. (2008) 5:439–45. doi: 10.1038/nmeth.1198
141. Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philos Ethics Humanit Med. (2009) 4:2. doi: 10.1186/1747-5341-4-2
142. Soman SS, Vijayavenkataraman S. Applications of 3D bioprinted-induced pluripotent stem cells in healthcare. Int J Bioprint. (2020) 6:280. doi: 10.18063/ijb.v6i4.280
143. Skardal A, Aleman J, Forsythe S, Rajan S, Murphy S, Devarasetty M, et al. Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication. (2020) 12:025017. doi: 10.1088/1758-5090/ab6d36
144. Vaidya M. Startups tout commercially 3D-printed tissue for drug screening. Nat Med. (2015) 21:2. doi: 10.1038/nm0115-2
145. Kurokawa YK, George SC. Tissue engineering the cardiac microenvironment: multicellular microphysiological systems for drug screening. Adv Drug Deliv Rev. (2016) 96:225–33. doi: 10.1016/j.addr.2015.07.004
146. Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. (2012) 135:1348–69. doi: 10.1093/brain/aws019
147. Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet. (2016) 387:719–21. doi: 10.1016/S0140-6736(16)00320-2
148. Calvet G, Aguiar RS, Melo ASO, Sampaio SA, de Filippis I, Fabri A, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. (2016) 16:653–60. doi: 10.1016/S1473-3099(16)00095-5
149. Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. N Engl J Med. (2016) 374:951–8. doi: 10.1056/NEJMoa1600651
150. Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, et al. Replication of human noroviruses in stem cell-derived human enteroids. Science. (2016) 353:1387–93. doi: 10.1126/science.aaf5211
151. Saxena K, Blutt SE, Ettayebi K, Zeng XL, Broughman JR, Crawford SE, et al. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J Virol. (2016) 90:43–56. doi: 10.1128/JVI.01930-15
152. Yin Y, Bijvelds M, Dang W, Xu L, van der Eijk AA, Knipping K, et al. Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antiviral Res. (2015) 123:120–31. doi: 10.1016/j.antiviral.2015.09.010
153. Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, Heo I, Bottinger L, Klay D, et al. Long-term expanding human airway organoids for disease modeling. EMBO J. (2019) 38:e100300. doi: 10.15252/embj.2018100300
154. To KK, Chan JF, Chen H, Li L, Yuen KY. The emergence of influenza A H7N9 in human beings 16 years after influenza A H5N1: a tale of two cities. Lancet Infect Dis. (2013) 13:809–21. doi: 10.1016/S1473-3099(13)70167-1
155. Zhou J, Li C, Sachs N, Chiu MC, Wong BH, Chu H, et al. Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc Natl Acad Sci USA. (2018) 115:6822–7. doi: 10.1073/pnas.1806308115
156. Klenk HD. Influenza viruses en route from birds to man. Cell Host Microbe. (2014) 15:653–4. doi: 10.1016/j.chom.2014.05.019
157. Bartfeld S, Bayram T, van de Wetering M Huch M, Begthel H, Kujala P, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. (2015) 148:126–36 e6. doi: 10.1053/j.gastro.2014.09.042
158. Bartfeld S. Modeling infectious diseases and host-microbe interactions in gastrointestinal organoids. Dev Biol. (2016) 420:262–70. doi: 10.1016/j.ydbio.2016.09.014
159. Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, et al. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect Immun. (2015) 83:138–45. doi: 10.1128/IAI.02561-14
160. Heo I, Dutta D, Schaefer DA, Iakobachvili N, Artegiani B, Sachs N, et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat Microbiol. (2018) 3:814–23. doi: 10.1038/s41564-018-0177-8
161. Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, et al. SARS-CoV-2 productively infects human gut enterocytes. Science. (2020) 369:50–4. doi: 10.1126/science.abc1669
162. Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. (2020) 181:905–13 e7. doi: 10.1016/j.cell.2020.04.004
163. Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. (2020) 11:771–5. doi: 10.1007/s13238-020-00718-6
164. Berkers G, van Mourik P, Vonk AM, Kruisselbrink E, Dekkers JF, de Winter-de Groot KM, et al. Rectal organoids enable personalized treatment of cystic fibrosis. Cell Rep. (2019) 26:1701–8 e3. doi: 10.1016/j.celrep.2019.01.068
165. Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. (2013) 19:939–45. doi: 10.1038/nm.3201
166. Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med. (2016) 8:344ra84. doi: 10.1126/scitranslmed.aad8278
167. Horch RE, Boos AM, Quan Y, Bleiziffer O, Detsch R, Boccaccini AR, et al. Cancer research by means of tissue engineering–is there a rationale? J Cell Mol Med. (2013) 17:1197–206. doi: 10.1111/jcmm.12130
168. Fan H, Demirci U, Chen P. Emerging organoid models: leaping forward in cancer research. J Hematol Oncol. (2019) 12:142. doi: 10.1186/s13045-019-0832-4
169. Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med. (2015) 21:256–62. doi: 10.1038/nm.3802
170. Nakayama M, Sakai E, Echizen K, Yamada Y, Oshima H, Han TS, et al. Intestinal cancer progression by mutant p53 through the acquisition of invasiveness associated with complex glandular formation. Oncogene. (2017) 36:5885–96. doi: 10.1038/onc.2017.194
171. Schell MJ, Yang M, Teer JK, Lo FY, Madan A, Coppola D, et al. A multigene mutation classification of 468 colorectal cancers reveals a prognostic role for APC. Nat Commun. (2016) 7:11743. doi: 10.1038/ncomms11743
172. Li X, Nadauld L, Ootani A, Corney DC, Pai RK, Gevaert O, et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med. (2014) 20:769–77. doi: 10.1038/nm.3585
173. Engel RM, Chan WH, Nickless D, Hlavca S, Richards E, Kerr G, et al. Patient-derived colorectal cancer organoids upregulate revival stem cell marker genes following chemotherapeutic treatment. J Clin Med. (2020) 9:128. doi: 10.3390/jcm9010128
174. Ooft SN, Weeber F, Dijkstra KK, McLean CM, Kaing S, van Werkhoven E, et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med. (2019) 11:eaay2574. doi: 10.1126/scitranslmed.aay2574
175. Weeber F, van de Wetering M, Hoogstraat M, Dijkstra KK, Krijgsman O, Kuilman T, et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci USA. (2015) 112:13308–11. doi: 10.1073/pnas.1516689112
176. Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. (2016) 18:827–38. doi: 10.1016/j.stem.2016.04.003
177. van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. (2015) 161:933–45. doi: 10.1016/j.cell.2015.03.053
178. Jacob F, Salinas RD, Zhang DY, Nguyen PTT, Schnoll JG, Wong SZH, et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. (2020) 180:188–204 e22. doi: 10.1016/j.cell.2019.11.036
179. Fusco P, Parisatto B, Rampazzo E, Persano L, Frasson C, Di Meglio A, et al. Patient-derived organoids (PDOs) as a novel in vitro model for neuroblastoma tumours. BMC Cancer. (2019) 19:970. doi: 10.1186/s12885-019-6149-4
180. Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. (2014) 159:176–87. doi: 10.1016/j.cell.2014.08.016
181. Boj SF, Hwang CI, Baker LA, Chio, II, Engle DD, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. (2015) 160:324–38. doi: 10.1016/j.cell.2014.12.021
182. Driehuis E, van Hoeck A, Moore K, Kolders S, Francies HE, Gulersonmez MC, et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc Natl Acad Sci USA. (2019) 116:26580–90. doi: 10.1073/pnas.1911273116
183. Seino T, Kawasaki S, Shimokawa M, Tamagawa H, Toshimitsu K, Fujii M, et al. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell. (2018) 22:454–67 e6. doi: 10.1016/j.stem.2017.12.009
184. Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarro LM, Bradshaw CR, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. (2017) 23:1424–35. doi: 10.1038/nm.4438
185. Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. (2018) 172:373–86 e10. doi: 10.1016/j.cell.2017.11.010
186. Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. (2018) 173:515–28 e17. doi: 10.1016/j.cell.2018.03.017
187. Seidlitz T, Merker SR, Rothe A, Zakrzewski F, von Neubeck C, Grutzmann K, et al. Human gastric cancer modelling using organoids. Gut. (2019) 68:207–17. doi: 10.1136/gutjnl-2017-314549
188. Yan HHN, Siu HC, Law S, Ho SL, Yue SSK, Tsui WY, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. (2018) 23:882–97 e11. doi: 10.1016/j.stem.2018.09.016
189. Nanki K, Toshimitsu K, Takano A, Fujii M, Shimokawa M, Ohta Y, et al. Divergent routes toward wnt and R-spondin niche independency during human gastric carcinogenesis. Cell. (2018) 174:856–69 e17. doi: 10.1016/j.cell.2018.07.027
190. Li X, Francies HE, Secrier M, Perner J, Miremadi A, Galeano-Dalmau N, et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat Commun. (2018) 9:2983. doi: 10.1038/s41467-018-05190-9
191. Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. (2019) 21:1041–51. doi: 10.1038/s41556-019-0360-z
192. Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. (2019) 10:3991. doi: 10.1038/s41467-019-11867-6
193. Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. (2020) 226:119536. doi: 10.1016/j.biomaterials.2019.119536
194. Goodarzi P, Falahzadeh K, Nematizadeh M, Farazandeh P, Payab M, Larijani B, et al. Tissue engineered skin substitutes. Adv Exp Med Biol. (2018) 1107:143–18. doi: 10.1007/5584_2018_226
195. Alonso L, Fuchs E. Stem cells of the skin epithelium. Proc Natl Acad Sci USA. (2003) 100(Suppl. 1):11830–5. doi: 10.1073/pnas.1734203100
196. Clark RA, Lin F, Greiling D, An J, Couchman JR. Fibroblast invasive migration into fibronectin/fibrin gels requires a previously uncharacterized dermatan sulfate-CD44 proteoglycan. J Invest Dermatol. (2004) 122:266–77. doi: 10.1046/j.0022-202X.2004.22205.x
197. de Mel A, Seifalian AM, Birchall MA. Orchestrating cell/material interactions for tissue engineering of surgical implants. Macromol Biosci. (2012) 12:1010–21. doi: 10.1002/mabi.201200039
198. Dixit S, Baganizi DR, Sahu R, Dosunmu E, Chaudhari A, Vig K, et al. Immunological challenges associated with artificial skin grafts: available solutions and stem cells in future design of synthetic skin. J Biol Eng. (2017) 11:49. doi: 10.1186/s13036-017-0089-9
199. Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, et al. Advances in skin regeneration using tissue engineering. Int J Mol Sci. (2017) 18:789. doi: 10.3390/ijms18040789
200. Biedermann T, Boettcher-Haberzeth S, Reichmann E. Tissue engineering of skin for wound coverage. Eur J Pediatr Surg. (2013) 23:375–82. doi: 10.1055/s-0033-1352529
201. Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K. Skin tissue engineering–in vivo and in vitro applications. Adv Drug Deliv Rev. (2011) 63:352–66. doi: 10.1016/j.addr.2011.01.005
202. Catalano E, Cochis A, Varoni E, Rimondini L, Azzimonti B. Tissue-engineered skin substitutes: an overview. J Artif Organs. (2013) 16:397–403. doi: 10.1007/s10047-013-0734-0
203. Varkey M, Ding J, Tredget EE. Advances in skin substitutes-potential of tissue engineered skin for facilitating anti-fibrotic healing. J Funct Biomater. (2015) 6:547–63. doi: 10.3390/jfb6030547
204. Supp DM, Boyce ST. Engineered skin substitutes: practices and potentials. Clin Dermatol. (2005) 23:403–12. doi: 10.1016/j.clindermatol.2004.07.023
205. Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. (2010) 7:229–58. doi: 10.1098/rsif.2009.0403
206. Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. current status in wound healing. Am J Clin Dermatol. (2001) 2:305–13. doi: 10.2165/00128071-200102050-00005
207. Rockwell WB, Daane S, Zakhireh M, Carroll KL. Human skin allograft used to treat open wounds after club foot release. Ann Plast Surg. (2003) 51:593–7. doi: 10.1097/01.sap.0000095657.75750.4f
208. Janeway TPC, Walport M, Shlomchik M. Immunobiology: The Immune System in Health and Disease. New York, NY: Garland Science (2005).
209. Cardinal M, Eisenbud DE, Armstrong DG, Zelen C, Driver V, Attinger C, et al. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair Regen. (2009) 17:306–11. doi: 10.1111/j.1524-475X.2009.00485.x
210. Halim AS, Khoo TL, Mohd Yussof SJ. Biologic and synthetic skin substitutes: an overview. Indian J Plast Surg. (2010) 43:S23–8. doi: 10.1055/s-0039-1699458
211. Rheinwald JG, Green H. Seria cultivation of strains of human epidemal keratinocytes: the formation keratinizin colonies from single cell is. Cell. (1975) 5:331–43. doi: 10.1016/0092-8674(75)90052-5
212. Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. (1997) 265:421–4. doi: 10.1038/265421a0
213. Cui X, Boland T, D'Lima DD, Lotz MK. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul. (2012) 6:149–55. doi: 10.2174/187221112800672949
214. Sharma SGC, Dinda AK, Mishra NC. Cartilage tissue engineering: current scenario and challenges. Adv Mater Lett. (2011) 2:90–9. doi: 10.5185/amlett.2011.1211
215. Rasanen P, Paavolainen P, Sintonen H, Koivisto AM, Blom M, Ryynanen OP, et al. Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthop. (2007) 78:108–15. doi: 10.1080/17453670610013501
216. Lee CH, Marion NW, Hollister S, Mao JJ. Tissue formation and vascularization in anatomically shaped human joint condyle ectopically in vivo. Tissue Eng Part A. (2009) 15:3923–30. doi: 10.1089/ten.tea.2008.0653
217. Atala A, Kasper FK, Mikos AG. Engineering complex tissues. Sci Transl Med. (2012) 4:160rv12. doi: 10.1126/scitranslmed.3004890
218. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. (1994) 331:889–95. doi: 10.1056/NEJM199410063311401
219. Hunziker EB. Articular cartilage repair: basic science and clinical progress. a review of the current status and prospects. Osteoarthritis Cartilage. (2002) 10:432–63. doi: 10.1053/joca.2002.0801
220. Etienne H, Fabre D, Gomez Caro A, Kolb F, Mussot S, Mercier O, et al. Tracheal replacement. Eur Respir J. (2018) 51:1702211. doi: 10.1183/13993003.02211-2017
221. Delaere PR. Stem-cell-based, tissue-engineered tracheal replacement in a child. Lancet. (2013) 381:113. doi: 10.1016/S0140-6736(13)60043-4
222. Macchiarini P, Walles T, Biancosino C, Mertsching H. First human transplantation of a bioengineered airway tissue. J Thorac Cardiovasc Surg. (2004) 128:638–41. doi: 10.1016/j.jtcvs.2004.02.042
223. Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. (2008) 372:2023–30. doi: 10.1016/S0140-6736(08)61598-6
224. Vogel G. Trachea transplants test the limits. Science. (2013) 340:266–8. doi: 10.1126/science.340.6130.266
225. The Lancet Editors. Retraction-Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. (2018) 392:11. doi: 10.1016/S0140-6736(18)31558-7
226. Claesson-Welsh L, Hansson GK, Royal Swedish Academy of Sciences. Tracheobronchial transplantation: The Royal Swedish Academy of Sciences' concerns. Lancet. (2016) 387:942. doi: 10.1016/S0140-6736(16)00520-1
227. Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. (2012) 380:994–1000. doi: 10.1016/S0140-6736(12)60737-5
228. Taniguchi D, Matsumoto K, Tsuchiya T, Machino R, Takeoka Y, Elgalad A, et al. Scaffold-free trachea regeneration by tissue engineering with bio-3D printing. Interact Cardiovasc Thorac Surg. (2018) 26:745–52. doi: 10.1093/icvts/ivx444
229. Ke D, Yi H, Est-Witte S, George S, Kengla C, Lee SJ, et al. Bioprinted trachea constructs with patient-matched design, mechanical and biological properties. Biofabrication. (2019) 12:015022. doi: 10.1088/1758-5090/ab5354
230. Zimmermann WH, Cesnjevar R. Cardiac tissue engineering: implications for pediatric heart surgery. Pediatr Cardiol. (2009) 30:716–23. doi: 10.1007/s00246-009-9405-6
231. Radisic M, Christman KL. Materials science and tissue engineering: repairing the heart. Mayo Clin Proc. (2013) 88:884–98. doi: 10.1016/j.mayocp.2013.05.003
232. Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. (2003) 114:763–76. doi: 10.1016/S0092-8674(03)00687-1
233. Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. (2008) 26:2201–10. doi: 10.1634/stemcells.2008-0428
234. Matsumura G, Hibino N, Ikada Y, Kurosawa H, Shin'oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. (2003) 24:2303–8. doi: 10.1016/S0142-9612(03)00043-7
235. El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: progress and challenges. Glob Cardiol Sci Pract. (2013) 2013:316–42. doi: 10.5339/gcsp.2013.38
236. Vunjak-Novakovic G. Tissue engineering of the heart: an evolving paradigm. J Thorac Cardiovasc Surg. (2017) 153:593–5. doi: 10.1016/j.jtcvs.2016.08.057
237. Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. (2004) 44:654–60. doi: 10.1016/j.jacc.2004.04.040
238. Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. (2009) 54:1014–23. doi: 10.1016/j.jacc.2009.06.010
239. Cui H, Nowicki M, Fisher JP, Zhang LG. 3D bioprinting for organ regeneration. Adv Healthc Mater. (2017) 6:1601118. doi: 10.1002/adhm.201601118
240. Derby B. Printing and prototyping of tissues and scaffolds. Science. (2012) 338:921–6. doi: 10.1126/science.1226340
241. Ong CS, Fukunishi T, Zhang H, Huang CY, Nashed A, Blazeski A, et al. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci Rep. (2017) 7:4566. doi: 10.1038/s41598-017-05018-4
242. Gaebel R, Ma N, Liu J, Guan J, Koch L, Klopsch C, et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. (2011) 32:9218–30. doi: 10.1016/j.biomaterials.2011.08.071
243. Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, et al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. (2012) 33:1782–90. doi: 10.1016/j.biomaterials.2011.11.003
244. Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL, et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. (2015) 61:339–48. doi: 10.1016/j.biomaterials.2015.05.005
245. Tijore A, Irvine SA, Sarig U, Mhaisalkar P, Baisane V, Venkatraman S. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication. (2018) 10:025003. doi: 10.1088/1758-5090/aaa15d
246. Yoo JJ, Meng J, Oberpenning F, Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology. (1998) 51:221–5. doi: 10.1016/S0090-4295(97)00644-4
247. Atala A. Bladder tissue engineering: the past and the future. Urology. (2020) 145:337–8. doi: 10.1016/j.urology.2020.04.020
248. Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. (1999) 17:149–55. doi: 10.1038/6146
249. Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. (2006) 367:1241–6. doi: 10.1016/S0140-6736(06)68438-9
250. Rouwkema J, Gibbs S, Lutolf MP, Martin I, Vunjak-Novakovic G, Malda J. In vitro platforms for tissue engineering: implications for basic research and clinical translation. J Tissue Eng Regen Med. (2011) 5:e164–7. doi: 10.1002/term.414
251. Radisic M, Marsano A, Maidhof R, Wang Y, Vunjak-Novakovic G. Cardiac tissue engineering using perfusion bioreactor systems. Nat Protoc. (2008) 3:719–38. doi: 10.1038/nprot.2008.40
252. Rademakers T, Horvath JM, van Blitterswijk CA, LaPointe VLS. Oxygen and nutrient delivery in tissue engineering: approaches to graft vascularization. J Tissue Eng Regen Med. (2019) 13:1815–29. doi: 10.1002/term.2932
253. Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. (1973) 138:745–53. doi: 10.1084/jem.138.4.745
254. Leong MF, Toh JK, Du C, Narayanan K, Lu HF, Lim TC, et al. Patterned prevascularised tissue constructs by assembly of polyelectrolyte hydrogel fibres. Nat Commun. (2013) 4:2353. doi: 10.1038/ncomms3353
255. Ozbolat IT. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. (2015) 33:395–400. doi: 10.1016/j.tibtech.2015.04.005
256. Lee VK, Lanzi AM, Haygan N, Yoo SS, Vincent PA, Dai G. Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell Mol Bioeng. (2014) 7:460–72. doi: 10.1007/s12195-014-0340-0
257. Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. (2005) 23:821–3. doi: 10.1038/nbt0705-821
258. Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. (2005) 23:879–84. doi: 10.1038/nbt1109
259. Laschke MW, Vollmar B, Menger MD. Inosculation: connecting the life-sustaining pipelines. Tissue Eng Part B Rev. (2009) 15:455–65. doi: 10.1089/ten.teb.2009.0252
260. Asakawa N, Shimizu T, Tsuda Y, Sekiya S, Sasagawa T, Yamato M, et al. Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering. Biomaterials. (2010) 31:3903–9. doi: 10.1016/j.biomaterials.2010.01.105
261. Richards D, Jia J, Yost M, Markwald R, Mei Y. 3D bioprinting for vascularized tissue fabrication. Ann Biomed Eng. (2017) 45:132–47. doi: 10.1007/s10439-016-1653-z
262. Santos MI, Reis RL. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci. (2010) 10:12–27. doi: 10.1002/mabi.200900107
263. Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vascularization–the conduit to viable engineered tissues. Tissue Eng Part B Rev. (2009) 15:159–69. doi: 10.1089/ten.teb.2008.0193
264. Schechner JS, Nath AK, Zheng L, Kluger MS, Hughes CC, Sierra-Honigmann MR, et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci USA. (2000) 97:9191–6. doi: 10.1073/pnas.150242297
265. Kargozar S, Baino F, Hoseini SJ, Hamzehlou S, Darroudi M, Verdi J, et al. Biomedical applications of nanoceria: new roles for an old player. Nanomedicine. (2018) 13:3051–69. doi: 10.2217/nnm-2018-0189
266. Xu ML, Gao Y, Jin J, Xiong JF, Han XX, Zhao B. Role of 2(13)C isotopic glyphosate adsorption on silver nanoparticles based on ninhydrin reaction: a study based on surface-enhanced raman spectroscopy. Nanomaterials). (2020) 10:2539. doi: 10.3390/nano10122539
267. Chigurupati S, Mughal MR, Okun E, Das S, Kumar A, McCaffery M, et al. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials. (2013) 34:2194–201. doi: 10.1016/j.biomaterials.2012.11.061
268. Skardal A, Atala A. Biomaterials for integration with 3-D bioprinting. Ann Biomed Eng. (2015) 43:730–46. doi: 10.1007/s10439-014-1207-1
269. Xu T, Zhao W, Zhu JM, Albanna MZ, Yoo JJ, Atala A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. (2013) 34:130–9. doi: 10.1016/j.biomaterials.2012.09.035
270. Yu Y, Moncal KK, Li J, Peng W, Rivero I, Martin JA, et al. Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Sci Rep. (2016) 6:28714. doi: 10.1038/srep28714
271. Mironov V, Kasyanov V, Markwald RR. Organ printing: from bioprinter to organ biofabrication line. Curr Opin Biotechnol. (2011) 22:667–73. doi: 10.1016/j.copbio.2011.02.006
272. Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, et al. An estimation of the number of cells in the human body. Ann Hum Biol. (2013) 40:463–71. doi: 10.3109/03014460.2013.807878
273. Peloso A, Citro A, Zoro T, Cobianchi L, Kahler-Quesada A, Bianchi CM, et al. Regenerative medicine and diabetes: targeting the extracellular matrix beyond the stem cell approach and encapsulation technology. Front Endocrinol. (2018) 9:445. doi: 10.3389/fendo.2018.00445
274. Roh DS, Li EB, Liao EC. CRISPR craft: DNA editing the reconstructive ladder. Plast Reconstr Surg. (2018) 142:1355–64. doi: 10.1097/PRS.0000000000004863
275. Yue J, Gou X, Li Y, Wicksteed B, Wu X. Engineered epidermal progenitor cells can correct diet-induced obesity and diabetes. Cell Stem Cell. (2017) 21:256–63 e4. doi: 10.1016/j.stem.2017.06.016
Keywords: regeneration, engineering, surgery, tissue dysfunction, transplantation
Citation: Goldenberg D, McLaughlin C, Koduru SV and Ravnic DJ (2021) Regenerative Engineering: Current Applications and Future Perspectives. Front. Surg. 8:731031. doi: 10.3389/fsurg.2021.731031
Received: 25 June 2021; Accepted: 13 October 2021;
Published: 03 November 2021.
Edited by:
Lorenzo Cobianchi, University of Pavia, ItalyReviewed by:
Peter Dziewulski, St. Andrews Centre for Plastic Surgery and Burns, United KingdomFrancesca Dal Mas, University of Lincoln, United Kingdom
Copyright © 2021 Goldenberg, McLaughlin, Koduru and Ravnic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dino J. Ravnic, ZHJhdm5pY0BwZW5uc3RhdGVoZWFsdGgucHN1LmVkdQ== orcid.org/0000-0003-2261-5180
 Dana Goldenberg
Dana Goldenberg Caroline McLaughlin1,2
Caroline McLaughlin1,2 Dino J. Ravnic
Dino J. Ravnic