
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 09 March 2021
Sec. Thoracic Surgery
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.649802
This article is part of the Research TopicReal-World Surgical Treatment of Thoracic Cancer in the Era of Precision MedicineView all 12 articles
 Boyan Wang1,2,3†
Boyan Wang1,2,3† Yongjie Zhou4†
Yongjie Zhou4† Min Jia1,2,3
Min Jia1,2,3 Zhiping Yan4
Zhiping Yan4 Jiayan Chen1,2,3
Jiayan Chen1,2,3 Xueguan Lu1,2
Xueguan Lu1,2 Ruiyan Wu1,2*‡
Ruiyan Wu1,2*‡ Junmiao Wen1,2,3*‡
Junmiao Wen1,2,3*‡Background: According to the lung cancer staging project, T2b (>5–7 cm) and T3 (>7 cm) non-small cell lung cancers (NSCLC) should be reclassified into T3 and T4 groups. The objective of this study was to evaluate the effect of surgery alone or surgery plus adjuvant radiation (SART) on survival of node-negative patients with NSCLC >5 cm.
Methods: We identified 4557 N0 patients with NSCLC >5 cm in the Surveillance, Epidemiology, and End Results database from 2004 to 2014. Overall survival (OS) and cancer–specific survival (CSS) were compared among patients who underwent surgery alone and SART. The proportional hazards model was applied to evaluate multiple prognostic factors.
Results: 1,042 and 525 patients who underwent surgery alone and SART, respectively were enrolled after propensity-score matching. OS and CSS favored surgery alone rather than SART. Multivariate analysis showed that the number of lymph nodes examined more than six was associated with better OS and CSS for NSCLC >5 cm, especially in patients treated with surgery alone. Lobectomy should be recommended as the primary option for NSCLC >5 to 7 cm, whereas its superiority was not significant over sublobectomy for NSCLC >7 cm.
Conclusion: Surgery alone should be recommended as the first choice for patients with NSCLC >5 cm. The number of examined lymph nodes should be more than six in patients with NSCLC >5 cm, especially for those who undergo surgery alone. For patients with NSCLC >7 cm who could not tolerate lobectomy, sublobectomy might be an alternative surgical procedure.
Lung cancer is the leading cause of cancer death and the second most prevalent cancer in both men and women in the United States (1), with ~222,500 estimated new cases in 2017 (1). Non-small cell lung cancer (NSCLC) constitute the most common type of lung cancer (2). Surgery with or without chemotherapy has been adopted as the main treatment offered for curative intent among patients presenting with early-stage disease, and multimodality consultation has become particularly important for curative-intent treatment of locally advanced NSCLC (3) (stage II-III disease).
The optimal treatment strategy for large pulmonary tumors remains uncertain. The International Association for the Study of Lung Cancer (IASLC) proposed a significant change on T descriptor in the eighth edition of the TNM classification for lung cancer in 2015 (4), in which tumors >5 cm to less than or equal to 7 cm were reclassified as T3, and those greater than 7cm as T4 (4). The proposal has been adopted in the 8th edition of the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) staging system. Notably, stage IIB disease includes T3 tumors > 5 cm with no lymph node extension (T3N0), while stage IIIA includes T4 tumors >7 cm without lymph node involvement (T4N0). However, there has not yet been specific study focusing on the optimal treatment modality for patients with NSCLC > 5 to 7 cm and > 7 cm based on the latest TNM staging system.
Surgery plus adjuvant radiotherapy has been considered an important treatment for locally advanced lung cancer (5). However, postoperative radiotherapy (PORT) was routinely not recommended for patients with pathologic stage N0 or N1 disease, at least when using older radiation techniques (3, 6). In addition, the Nation Comprehensive Cancer Network (NCCN) clinical practice guidelines on NSCLC has recommended a minimum number of six nodes removed during surgical resection, three from N1 and three from N2 stations (3). Due to the uncertainty in surgical practice, the resected nodes may not achieve the required number. Since large tumor size is considered as a risk factor of mediastinal lymph nodes involvement even in early clinical stage lung cancer (7, 8), insufficient mediastinal lymph nodes evaluation may lead to a false-negative N descriptor. The consequent imprecise staging can probably misguide the therapeutic strategies, especially PORT, and lead to higher risk of recurrence and metastasis (9, 10). However, the value of PORT for node-negative large tumors has been frequently buried among plenty of studies on the impact of adjuvant therapy for the various stages of disease. With the rapid advance in radiation techniques in the past two decades, the role of PORT should be reevaluated.
The purpose of this study was to evaluate the effect of postoperative radiotherapy on long-term survival of patients with node-negative solitary large NSCLC within a large national database.
This study was based on the SEER-18 registry databases, which currently covers ~28% of the United States population and routinely collects data on demographics, tumor sites, stage at diagnosis, first course of treatment, and follow-up of vital status. We identified the patients diagnosed with lung cancer based on the value of primary site variable (C34.0-34.9). Non-small cell lung cancer (NSCLC) patients was identified using the ICD-O-3 codes, histologic subgroups were defined as squamous cell carcinomas (8050-8052, 8070-8078), adenocarcinomas (8140-8147, 8250-8255, 8260, 8310, 8430, 8480, 8481, 8571-8575) and other types such as large cell carcinoma (8012-8013). The eligible criteria included: (1) diagnosed between 2004 and 2014 and lung was the first primary site, (2) age older than 18 years, (3) underwent surgery to the primary site and with a survival time ≥3 months, (4) CS tumor size 2004+ >5 cm and pathological stage T2b-3, N0, and M0 (according to the 7th edition of the AJCC staging manual), (5) cases with death certificate or autopsy were excluded. Types of primary surgery included sublobar resection, lobectomy, and pneumonectomy.
The variables in our analysis included age at diagnosis, gender, race, marital status, characteristics of tumor (location, size, histologic grade and type) and treatment to the primary site (surgical type, sequence of radiation, number of lymph nodes examined) and months of survival and vital status. Patients were divided into two groups: (1) surgery group; (2) surgery plus adjuvant radiotherapy (SART) group, depending on whether they received PORT or not. In order to minimize selection bias under the analytic settings with observational data, we performed a propensity score matching (PSM) analysis between patients with and without PORT based on age, race, and marital status, characteristics of tumor and surgery types. Due to the significantly different number of patients in two groups, a one-to-two matching was conducted based on the nearest neighbor method. Student's t-test was employed for continuous data, and we evaluated categorical variables using the Chi-square test of Fisher's exact test. A log-rank test was used to compare Kaplan-Meier survival curves. We defined the Overall survival (OS) as the time from the date of initial treatment to the date of death or the last day of follow-up. Cancer-specific survival (CSS) was measured from the data of initial treatment to death from NSCLC. For multivariate analyses in the matched population, we used the Cox proportional hazards model adjusting all the variables included in the study with p-value <0.2 in the univariate analyses. Two-sided p-value < 0.05 was considered as statistically significant. Hazard ratios with 95% confidence intervals were employed to quantify the strength of the association between predictors and survival. All analyses were performed with the IBM SPSS Statistics 22.0 (IBM, NY, United States), and images of statistics were produced using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
Overall, the study cohort composed of 4,557 patients, of whom 526 patients (5.6%) underwent SART, as compared with 4,031 patients who underwent surgery alone (Table 1). The median follow-up time for the entire cohort was 29 (mean 39.6, range: 3–131). The mean age of the whole cohort was 67.1 years old (median, 68; range, 20–94 years old). Most patients were white in both groups (84.1 and 83.3%, respectively). Squamous cell carcinoma was the predominant histology type in the entire cohort, followed by adenocarcinoma. Notably, there were significant differences in patients' age, histology type, pathological grade, lobe distribution, and types of resection in both groups.
To eliminate selection biases caused by such confounding factors, a 1:2 PSM was conducted between the SART group and surgery group. 1,042 and 525 cases in surgery group and SART group were finally matched for analysis (Table 1). There was no significant difference in any patient characteristics between two groups after matching. Multivariate regression analysis identified gender, age, histology type, differentiation grade, tumor size, SART, and number of examined lymph nodes as risk factors for OS. These risk factors were also found to significantly impact CSS except for histology type (Supplementary Table 1).
Notably, the majority of patients underwent surgery alone in the entire PSM cohort (Table 1). Lobectomy predominated in the types of resection in both groups (Table 1). As shown in Figure 1, patients in the surgery group had significantly better OS (p < 0.001) and CSS (p < 0.001) than those in SART group. In other word, surgery alone remained the primary option in the treatment of patients with NSCLC larger than 5 cm without lymph nodes involvement.
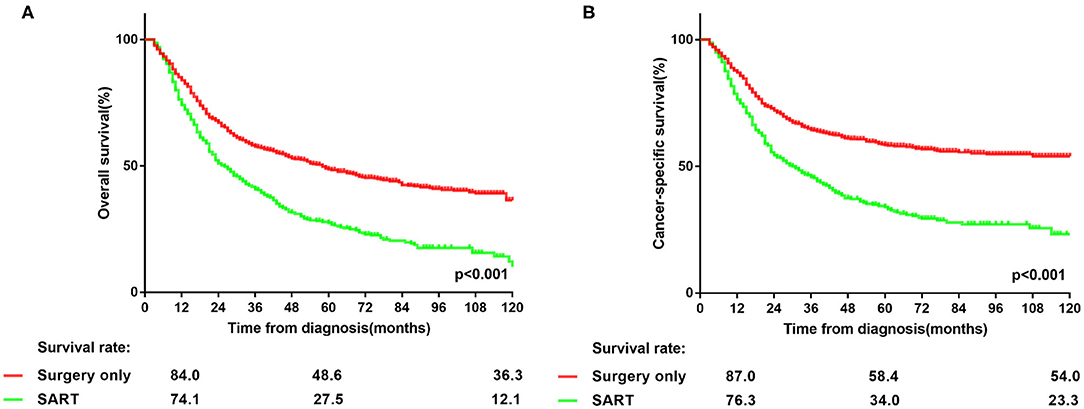
Figure 1. (A,B) Overall and lung cancer-specific survivals in patients with NSCLC >5 cm who underwent surgery alone or surgery plus adjuvant radiotherapy.
Since insufficient examined lymph nodes can result in a false-negative N stage, the prognosis of patients in two groups was compared to investigate whether PORT can benefit patients with solitary large tumors, based on the stratification of the number of dissected lymph nodes. The cut-off value was set as six according to the NCCN guidelines (3). As shown in Figure 2, the prognosis of patients in surgery group was better than that in the other group (p < 0.001), irrespective of the number of examined lymph nodes. Moreover, in surgery group, patients with more lymph nodes examined showed better prognosis than those with nodes examined less than six (p < 0.001) (Supplementary Table 2). In contrast, more examined lymph nodes provided no remarkably additional survival benefit for patients in SART group but only a trend of prolonged OS (p = 0.052) and CSS (p = 0.115) (Supplementary Table 2). Therefore, PORT should not be recommended for node-negative NSCLC patients with tumor size > 5 cm.
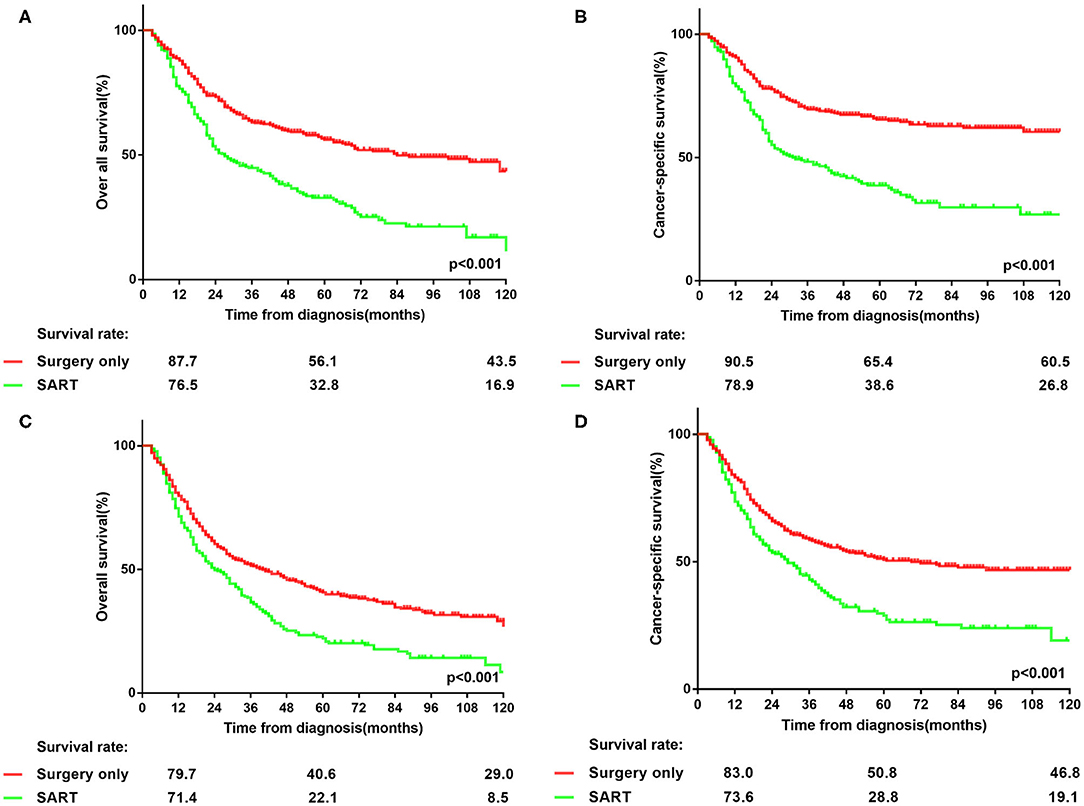
Figure 2. Stratification of overall survival and lung cancer-specific survival in patients with node-negative NSCLC >5 cm at the cut point of the number of harvested lymph nodes who underwent surgery or surgery plus adjuvant radiotherapy. (A,B) overall survival and lung cancer-specific survival in patients with node-negative NSCLC > 5 cm who had more than 6 lymph nodes dissected. (C,D) overall survival and lung cancer-specific survival in patients with node-negative NSCLC > 5 cm who had <6 lymph nodes examined.
Furthermore, a Cox proportional hazards regression model was applied to further study the potential risk factors in subgroups of NSCLC > 5 to 7 cm and > 7 cm (Table 2). In either subgroups, SART was associated with significantly decreased OS and CSS (OS with NSCLC > 5 to 7 cm: HR, 1.896; 95% CI: 1.573 to 2.285; p < 0.001; CSS with NSCLC > 5 to 7 cm: HR, 2.172; 95% CI: 1.755 to 2.689; p < 0.001; OS with NSCLC > 7 cm: HR, 1.635; 95% CI: 1.288 to 2.075; p < 0.001; CSS with NSCLC > 7 cm: HR, 1.751; 95% CI: 1.351 to 2.269; p < 0.001). Interestingly, number of lymph nodes dissected less than six was found to have a significantly adverse impact on OS (HR, 1.398; 95% CI: 1.162 to 1.68; p < 0.001) and CSS (HR, 1.462; 95% CI:1.18 to 1.811; p = 0.001) in patients with NSCLC > 5 to 7 cm, compared with more examined lymph nodes. Similar results for OS (HR, 0.748; 95% CI: 0.591 to 0.946; p = 0.015) and CSS (HR, 0.762; 95% CI: 0.589 to 0.986; p = 0.038) were observed in patients with NSCLC > 7 cm (Table 2).
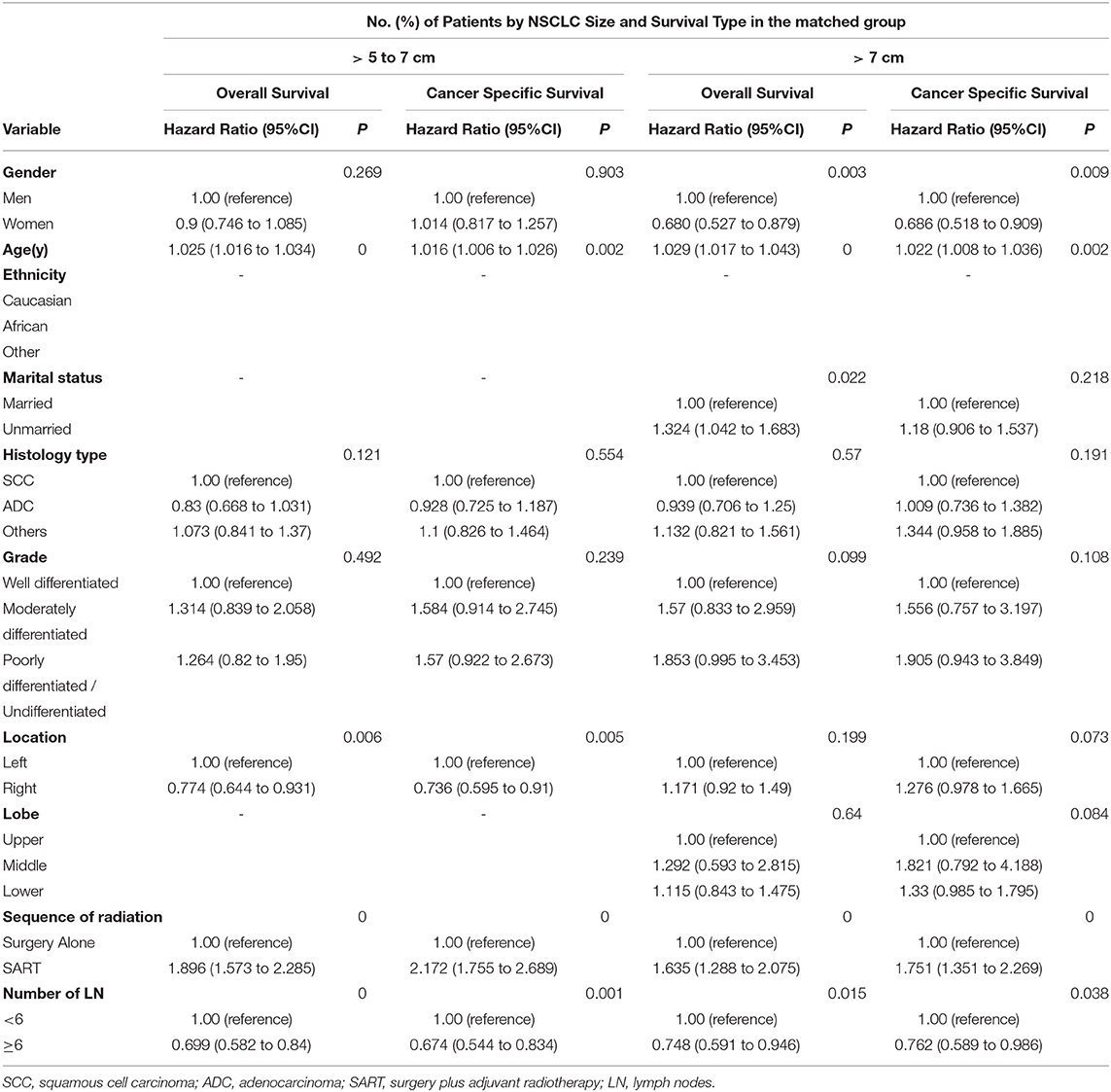
Table 2. Cox proportional hazards regression model for overall survival and lung cancer–specific survival in patients with non–small-cell lung cancer > 5 to 7 cm and > 7 cm.
Since the preferred role of surgery alone has been proved, the surgical procedures were compared to assess the optimal one in patients treated with surgery alone. Another Cox proportional hazards regression model was applied to confirm the impact on prognosis of different types of resection (Table 3). In patients with NSCLC > 5 to 7 cm, lobectomy and pneumonectomy, compared with sublobectomy, was associated with increased OS (OS with lobectomy vs. sublobectomy: HR, 0.596; 95% CI: 0.433 to 0.82; p = 0.002; OS with pneumonectomy vs. sublobectomy: HR, 1.023; 95% CI: 0.566 to 1.847; p = 0.093) and CSS (CSS with lobectomy vs. sublobectomy: HR, 0.525; 95% CI: 0.36 to 0.766; p = 0.001; CSS with pneumonectomy vs. sublobectomy: HR, 0.867; 95% CI: 0.427 to 1.759; p = 0.692). Meanwhile, lobectomy was associated with increased OS (HR, 0.583; 95% CI: 0.348 to 0.976, p = 0.040) and equal CSS (HR, 0.606; 95% CI: 0.325 to 1.131, p = 0.116) in patients with NSCLC > 5 to 7 cm. Therefore, lobectomy should be attempted as the optimal type of resection for patients with NSCLC > 5 to 7 cm. In terms of NSCLC > 7 cm, neither lobectomy nor pneumonectomy was associated with increased OS (OS with lobectomy vs. sublobectomy: HR, 0.842; 95% CI: 0.521 to 1.36; p = 0.482; OS with pneumonectomy vs. sublobectomy: HR, 0.921; 95% CI: 0.476 to 1.784; p = 0.807) and CSS (CSS with lobectomy vs. sublobectomy: HR, 0.922; 95% CI: 0.532 to 1.598; p = 0.773; CSS with pneumonectomy vs. sublobectomy: HR, 0.891; 95% CI: 0.411 to 1.931; p = 0.77) compared with sublobectomy. Thus sublobectomy might be considered as an alternative to lobectomy for patients with NSCLC > 7 cm who cannot tolerate lobectomy. In addition, for patients who underwent only surgery, multivariate regression analysis identified age and number of examined lymph nodes as significant prognostic factors (Table 3).
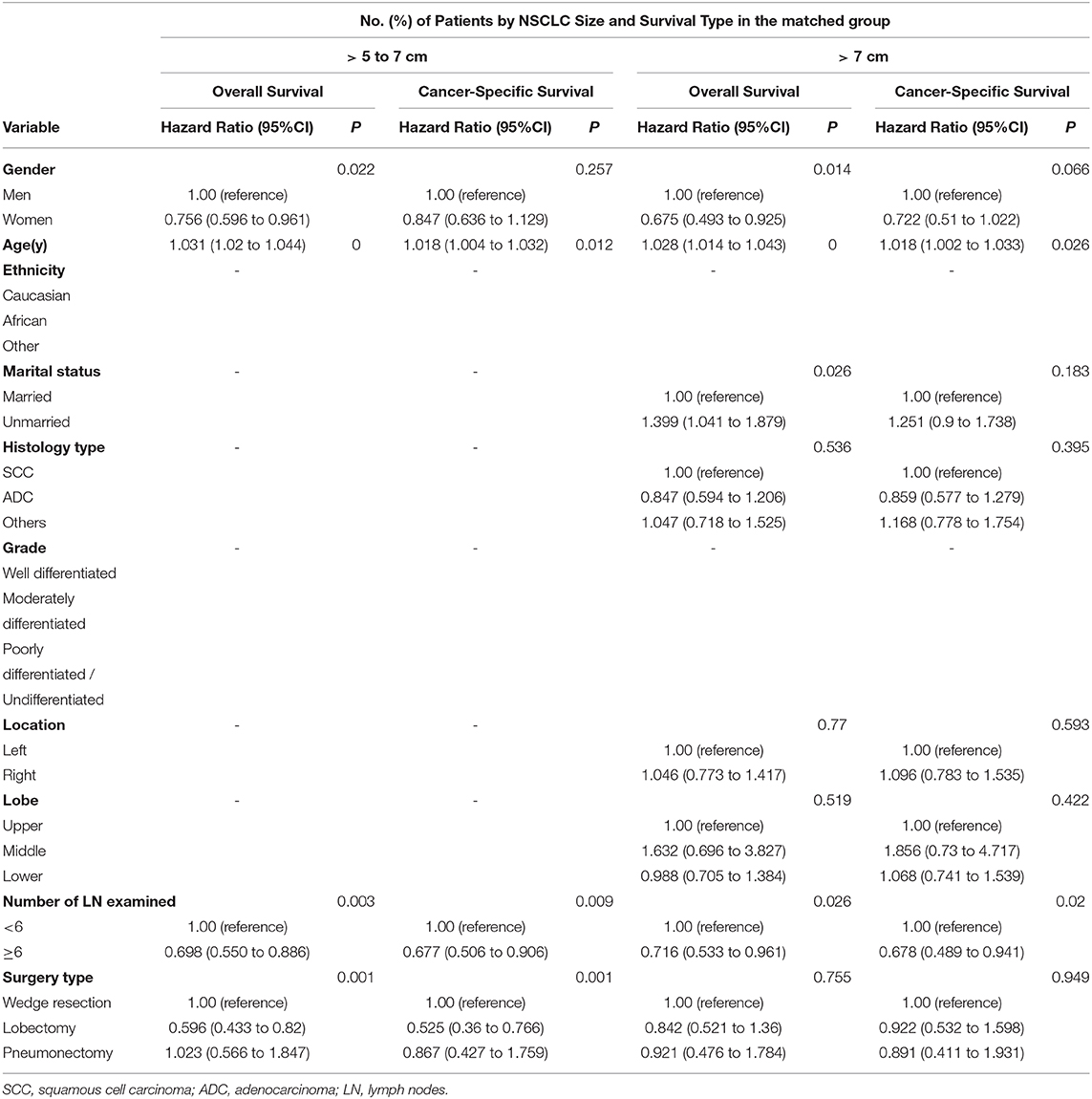
Table 3. Cox proportional hazards regression model for overall survival and lung cancer–specific survival in patients with non–small-cell lung cancer > 5 to 7 cm and > 7 cm who underwent surgery alone.
Despite the increasing detection rate of early-stage NSCLC present as small pulmonary nodules, locally advanced NSCLC remain a complicated and thorny problem in clinical practice. For very large tumors, most clinicians would consider that the optimal treatment modality is still undefined. Part of the confusion arises from the reclassification of T2b tumors > 5 cm to T3 tumors and subsequent changes to stage groupings involving T3 tumors > 5 cm from stage IIA to IIB if node-negative (4, 11). Complete resection is still considered the optimal treatment for locally advanced disease with or without adjuvant chemotherapy to reduce the risk of distant recurrence (3, 12). Furthermore, treatment of stage IIIA disease including T4N0 may include determination of resectability as part of a multidisciplinary consultation (3).
Radiotherapy has been defined a role before or after surgery for locally advanced NSCLC (3), especially for microscopic residual disease (13). However, the latest NCCN guidelines has also pointed out that PORT is not recommended for patients with pathologic stage N0-1 disease at least when using older radiation techniques (3, 6), because it has been associated with increased mortality. Although the cited clinical evidence ranked the highest level, the source itself was a meta-analysis published in 2005. However, the radiotherapy planning underwent major changes during the past decades (14). The radiation techniques has also stridden forward from the era of two- dimension (2D) to three-dimension (3D) with 3D-conformal radiotherapy (3D-CRT), intensity modulated radiotherapy (IMRT) (14, 15), stereotactic body radiation therapy (SBRT) and the latest proton radiotherapy widely applied within 2004-2014. Therefore, whether the updated radiation techniques can additionally benefit postoperative patients awaits a definite answer. Hitherto, there have been neither radiotherapists nor surgeons focusing on the role of PORT with the new generation of radiation techniques for N0 advanced-stage disease. In our study, it has been well demonstrated that the survival advantages favor surgery alone rather than SART for NSCLC > 5 cm to 7 cm and > 7 cm. The results further validated the prior role of surgery for treating large pulmonary malignancy without nodal involvement.
A larger tumor size indicated potentially higher risk of occult lymph nodes metastasis (16–18) and micrometastasis (19, 20) in clinical N0 disease. Recent researches also indicated the residual malignant cells in lymph nodes plays a role in recurrence and distant metastasis (21, 22). Therefore, it is a reasonable assumption that PORT might benefit postoperative patients to some extent. However, evidence from our study conflicts with that logic and suggests the undoubted position of complete surgical resection. Another retrospective study published in 2006 using the SEER database drew a similar conclusion that PORT is associated with a decrease in survival in patients with N1 and N0 nodal disease. Additionally, due to the recommendation proposed by the AJCC, UICC, and IASLC that at least six nodes should be removed during surgical resection (three from N1 and three from N2 stations), great interest has been raised about whether there could be any difference in the prognosis of patients with NSCLC > 5 to 7 cm and NSCLC > 7 cm based on the suggested number of examined lymph nodes. Our data revealed that the superiority of examining more than six lymph nodes extends to both subgroups. Although more examined lymph nodes led to non-significant improvement on the prognosis of patients who underwent SART, it could be possibly attributed to the local control on residual lymph nodes by PORT. Actually, the long-term survival benefit of more examined lymph nodes on patients has already been reported by Liang et al. (10), who recommended 16 lymph nodes as the cut point for evaluating the quality of lymph nodes examination or prognostic stratification postoperatively for patients with declared node-negative disease. Therefore, our data kept consistent with their findings and supported the value of a thorough lymph nodes examination in NSCLC > 5 cm.
Tumor size has been recognized as a significant prognostic factor of survival outcomes, particularly in patients with early-stage NSCLC (4, 12). Morgensztern et al. (23) previously demonstrated that tumor size is an independent predictor of overall and lung cancer-specific survival in patients with locally advanced disease as well. In our study, tumor size was also associated with a higher risk of decreased OS and CSS upon multivariate analysis. Nowadays, lobectomy has been recommended as the standard surgical procedure for operable NSCLC (3, 24), especially for tumors larger than 2 cm (25–27). Based on our data, lobectomy should be considered as the first choice for NSCLC > 5 to 7 cm which was congruent with the current guidelines. However, lobectomy may be not suitable for NSCLC > 7 cm, at least not superior over sublobectomy in our study. It seemed that for patients who could not tolerate lobectomy with NSCLC > 7 cm, sublobectomy should be recommended as an optimal alternative surgical procedure. In fact, large NSCLC sometimes invade neighboring structures and possibly result in R1 or R2 resections even with lobectomy. Therefore, increasing tumor size could partly account for the non-significant difference in OS and CSS between patients who underwent lobectomy and sublobectomy in patients with NSCLC > 7 cm. A study by Dziedzic et al. (9) identified risk factors for recurrence including tumor size of 5-7 cm and > 7 cm, which partially supported our results. However, both sublobar resection and pneumonectomy were proved to associate with local and distant recurrence (9) which conflicted our data. The disparity may be attributed to the evaluation of appropriate surgical procedures based on stratification of tumor size in our study. To be cautious, we believe that high-quality evidence from ongoing randomized controlled trials are needed to verify our results.
We must acknowledge some limitations of this study. First, potential biases were inevitable because of the retrospective nature of this study. Though some advanced statistical methods were applied to balance the covariates among the arms, there were still some latent biases that could not be adjusted. For example, there was no information on anatomical location and pulmonary function which can affect the types of resection. Furthermore, the information absence of resection margin also poses an insurmountable obstacle for our study, since R1 and R2 resection often led to subsequent PORT and probably resulted in a worse prognosis. Meanwhile, there were potential biases on the prognostic impact of the number of examined lymph nodes because of the lack of definite lymph nodes stations and whether en-bloc resection was performed. In the SEER database there is no ability to discern which patients with tumors > 5 cm received adjuvant chemotherapy, therefore either group invariably included this subset of patients. Notably, information regarding the administration of chemotherapy, either as neoadjuvant or adjuvant therapy, is unavailable in the SEER database as well. Therefore, we could not comprehensively analyze the influence of neoadjuvant chemotherapy alone or adjuvant chemotherapy when used concurrently with radiotherapy on long-term survival of patients with NSCLC > 5 cm. Additionally, no information regarding radiation techniques, including total dose, fraction size, and beam energy, was available, and therefore was not accounted in our analysis. Variations in adjuvant chemotherapy and radiotherapy regimens are likely to be confounded in our study population and may have influenced the lack of significant PORT benefit on survival over pulmonary resection alone.
In conclusion, surgery alone should be recommended as the first choice for patients with NSCLC > 5 cm. The number of examined lymph nodes should be more than six in patients with NSCLC > 5 cm, especially for those who undergo surgery alone. For patients with NSCLC > 7 cm who could not tolerate lobectomy, sublobectomy might be an optimal alternative surgical procedure.
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/seerstat/.
The SEER database is available to the public and all patient identities are protected. Our study was therefore exempted from institutional review board at our hospital.
BW, YZ, MJ, and JW are lead authors who participated in data collection, manuscript drafting, table/figure creation, and manuscript revision. BW and YZ aided in data collection. JW and RW is the corresponding author who initially developed the concept and drafted and revised the manuscript. All authors read and approved the final manuscript.
This research was supported by the Clinical Research Plan of SHDC (No. SHDC2020CR3025B) and Shanghai Anticancer Association SOAR PROJECT (SACA-AX107). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The permission to access the SEER database was received from the National Cancer Institute (the private SEER ID 10425-Nov2018).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.649802/full#supplementary-material
NSCLC, non-small cell lung cancer; OS, overall survival; CSS, cancer–specific survival; IASLC, the International Association for the Study of Lung Cancer; AJCC, the American Joint Committee on Cancer; UICC, the Union for International Cancer Control; PORT, postoperative radiotherapy; NCCN, the Nation Comprehensive Cancer Network; SART, surgery plus adjuvant radiotherapy; PSM, propensity score matching.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. (2017) 67:7–30. doi: 10.3322/caac.21387
2. Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. (2017) 376:2109–21. doi: 10.1056/NEJMoa1616288
3. Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY, et al. NCCN guidelines insights: non-small cell lung cancer, Version 5.2018. J Natl Compr Canc Netw. (2018) 16:807–21. doi: 10.6004/jnccn.2018.0062
4. Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, et al. The IASLC Lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. (2015) 10:990–1003. doi: 10.1097/JTO.0000000000000559
5. Nestle U, De Ruysscher D, Ricardi U, Geets X, Belderbos J, Pöttgen C, et al. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother Oncol. (2018) 127:1–5. doi: 10.1016/j.radonc.2018.02.023
6. Burdett S, Stewart L. Postoperative radiotherapy in non-small-cell lung cancer: update of an individual patient data meta-analysis. Lung Cancer. (2005) 47:81–3. doi: 10.1016/j.lungcan.2004.09.010
7. Koike T, Koike T, Yamato Y, Yoshiya K, Toyabe S. Predictive risk factors for mediastinal lymph node metastasis in clinical stage IA non-small-cell lung cancer patients. J Thorac Oncol. (2012) 7:1246–51. doi: 10.1097/JTO.0b013e31825871de
8. Zhao F, Zhou Y, Ge PF, Huang CJ, Yu Y, Li J, et al. A prediction model for lymph node metastases using pathologic features in patients intraoperatively diagnosed as stage I non-small cell lung cancer. BMC cancer. (2017) 17:267. doi: 10.1186/s12885-017-3273-x
9. Dziedzic DA, Rudzinski P, Langfort R, Orlowski T, Polish Lung Canc Study Grp P. Risk factors for local and distant recurrence after surgical treatment in patients with non-small-cell lung cancer. Clinical Lung Cancer. (2016) 17:E157–67. doi: 10.1016/j.cllc.2015.12.013
10. Liang W, He J, Shen Y, Shen J, He Q, Zhang J, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: a population study of the US SEER database and a chinese multi-institutional registry. J Clin Oncol. (2017) 35:1162–70. doi: 10.1200/JCO.2016.67.5140
11. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
12. Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of non-small cell lung cancer stage I and stage II - ACCP evidence-based clinical practice guidelines (2nd edition). Chest. (2007) 132:234S−42S. doi: 10.1378/chest.07-1378
13. Hancock JG, Rosen JE, Antonicelli A, Moreno A, Kim AW, Detterbeck FC, et al. Impact of adjuvant treatment for microscopic residual disease after non-small cell lung cancer surgery. Ann Thor Surg. (2015) 99:406–13. doi: 10.1016/j.athoracsur.2014.09.033
14. Fleckenstein J, Eschler A, Kremp K, Kremp S, Rube C. Dose distribution and tumor control probability in out-of-field lymph node stations in intensity modulated radiotherapy (IMRT) vs 3D-conformal radiotherapy (3D-CRT) of non-small-cell lung cancer: an in silico analysis. Rad Oncol. (2015) 10:178. doi: 10.1186/s13014-015-0485-6
15. Chang JY. Intensity-modulated radiotherapy, not 3 dimensional conformal, is the preferred technique for treating locally advanced lung cancer. Sem Rad Oncol. (2015) 25:110–16. doi: 10.1016/j.semradonc.2014.11.002
16. Moon Y, Kim KS, Lee KY, Sung SW, Kim YK, Park JK. Clinicopathologic factors associated with occult lymph node metastasis in patients with clinically diagnosed N0 lung adenocarcinoma. Ann Thor Surg. (2016) 101:1928–35. doi: 10.1016/j.athoracsur.2015.11.056
17. Ye B, Cheng M, Li W, Ge XX, Geng JF, Feng J, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thor Surg. (2014) 98:217–23. doi: 10.1016/j.athoracsur.2014.03.005
18. Moreno AC, Morgensztern D, Yu JB, Boffa DJ, Decker RH, Detterbeck FC, et al. Impact of preoperative radiation on survival of patients with T3N0 >7-cm non-small cell lung cancers treated with anatomic resection using the Surveillance, Epidemiology, and End Results database. J Surg Res. (2013) 184:10–8. doi: 10.1016/j.jss.2013.03.053
19. Dai CH, Li J, Yu LC, Li XQ, Shi SB, Wu JR. Molecular diagnosis and prognostic significance of lymph node micrometastasis in patients with histologically node-negative non-small cell lung cancer. Tumour Biol. (2013) 34:1245–53. doi: 10.1007/s13277-013-0667-5
20. Dai C, Xie H, Kadeer X, Su H, Xie D, Ren Y, et al. Relationship of lymph node micrometastasis and micropapillary component and their joint influence on prognosis of patients with stage i lung adenocarcinoma. Am J Surg Pathol. (2017) 41:1212–20. doi: 10.1097/PAS.0000000000000901
21. Pereira ER, Kedrin D, Seano G, Gautier O, Meijer EFJ, Jones D, et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science. (2018) 359:1403–7. doi: 10.1126/science.aal3622
22. Brown M, Assen FP, Leithner A, Abe J, Schachner H, Asfour G, et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. (2018) 359:1408–11. doi: 10.1126/science.aal3662
23. Morgensztern D, Waqar S, Subramanian J, Gao F, Trinkaus K, Govindan R. Prognostic significance of tumor size in patients with stage III non-small-cell lung cancer a surveillance, epidemiology, and end results (SEER) Survey from 1998 to 2003. J Thor Oncol. (2012) 7:1479–84. doi: 10.1097/JTO.0b013e318267d032
24. Hristov B, Eguchi T, Bains S, Dycoco J, Tan KS, Isbell JM, et al. Minimally invasive lobectomy is associated with lower noncancer-specific mortality in elderly patients: a propensity score matched competing risks analysis. Ann Surg. (2019) 270:1161–9. doi: 10.1097/SLA.0000000000002772
25. Bao F, Ye P, Yang Y, Wang L, Zhang C, Lv X, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardio-Thor Surg. (2014) 46:1–7. doi: 10.1093/ejcts/ezt554
26. D'Andrilli A, Rendina EA. POINT: should segmentectomy rather than lobectomy be the operation of choice for early-stage non-small cell lung cancer? Yes. Chest. (2018) 153:590–2. doi: 10.1016/j.chest.2017.10.038
Keywords: NSCLC, surgery, postoperative radiotherapy, node-negative, T-stage
Citation: Wang B, Zhou Y, Jia M, Yan Z, Chen J, Lu X, Wu R and Wen J (2021) Choice of Treatment for Patients With Non–small-cell Lung Cancer >5 cm Between Surgery Alone and Surgery Plus Adjuvant Radiotherapy. Front. Surg. 8:649802. doi: 10.3389/fsurg.2021.649802
Received: 05 January 2021; Accepted: 08 February 2021;
Published: 09 March 2021.
Edited by:
Yongbing Chen, Second Affiliated Hospital of Soochow University, ChinaCopyright © 2021 Wang, Zhou, Jia, Yan, Chen, Lu, Wu and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junmiao Wen, ZHJqaW1taWV3ZW5AMTI2LmNvbQ==; Ruiyan Wu, MTM1NzA1OTQyMjBAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.