
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Surg., 09 April 2021
Sec. Genitourinary Surgery and Interventions
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.647656
This article is part of the Research TopicSequelae of Prostate Cancer Therapy: Avoidance strategies and management optionsView all 13 articles
Prostate cancer is the second most common cancer in men worldwide. Radical prostatectomy and radiation beam therapy are the most common treatment options for localized prostate cancer and have different associated complications. The etiology of post prostatectomy incontinence is multifactorial. There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of post-prostatectomy incontinence. Among the many surgical and technical factors proposed in the literature, extensive dissection during surgery, damage to the neurovascular bundle and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates. Overactive bladder syndrome (OAB) is multifactorial and the exact role of prostate surgery in the development of OAB is still under debate. There are several variables that could contribute to detrusor overactivity. Detrusor overactivity in patients after radical prostatectomy has been mainly attributed to a partial denervation of the bladder during surgery. However, together with bladder denervation, other hypotheses, such as the urethrovesical mechanism, have been described. Although there is conflicting evidence regarding the importance of conservative treatment after post-prostatectomy urinary incontinence, pelvic floor muscle training (PFMT) is still considered as the first treatment choice. Duloxetin, either alone or in combination with PFMT, may hasten recovery of urinary incontinence but is often associated with severe gastrointestinal and central nervous side effects. However, neither PFMT nor duloxetine may cure male stress urinary incontinence. The therapeutic decision and the chosen treatment option must be individualized for each patient according to clinical and social factors. During the recent years, the development of new therapeutic choices such as male sling techniques provided a more acceptable management pathway for less severe forms of urinary incontinence related to radical prostatectomy. Following this perspective, technological improvements and the emergence of new dedicated devices currently create the premises for a continuously positive evolution of clinical outcomes in this particular category of patients.
Prostate cancer is the second most common cancer in men worldwide, affecting ~1.1 million men per year (1). Radical prostatectomy (rPR) and radiation beam therapy are comparable treatment options for localized prostate cancer (2) whereas treatment associated complications and incidences differ significantly.
Male stress urinary incontinence (SUI) has a predominantly iatrogenic cause after radical prostatectomy (3). It is defined by the complaint of involuntary leakage on effort or exertion or on sneezing, or coughing (4, 5). The mechanism for post-radical prostatectomy incontinence remains unclear (6), however, several hypothesis have been discussed. Despite direct injury to the internal sphincter itself, injury to the external rhabdosphincter or its shortened lengthwise (7), injuries to the supporting structures of the urethra (7), lesions to the nerve supply (6) or even detrusor underactivity (8) may impair continence.
The incidence of post-radical prostatectomy incontinence has become an increasingly common urological problem with a prevalence of 2.5–90% (9) depending on the definition for urinary continence. In a recent prospective non-randomized trial comparing open retropubic rPR and robotic assisted rPR including a total of 2,625 men, urinary incontinence defined by no change pad in 24 h after 12 month follow-up was 21.3 and 20.2% for robotic-assisted and open rPR, respectively (10). A meta-analysis did not identify a significant difference of urinary continence in comparison between open retropubic and robot assisted rPR (11, 12). A prospective randomized trial comparing laparoscopic and robotic-assisted rPR demonstrated significant better continence rates for robotic-assisted than laparoscopic rPR (95.0 vs. 83.3%) (13). A meta-analysis identified evidence for improved continence rates with robotic-assisted in comparison to laparoscopic rPR accordingly (14). Table 1 present the continence rates after radical prostatectomy reported by selected prospective trials.
Importantly, the impact of urinary incontinence to affected patients is substantial and include stigmatization and significant reduction of quality of life (20). In addition, the cost burden of urinary incontinence is currently estimated between $19 and $32 billion in the USA (9).
Overactive bladder (OAB), with or without urinary incontinence, can also occur after radical prostatectomy and is an underestimated cause for urinary incontinence after radical prostatectomy. However, so far there is a lack of robust data for its incidence.
In this non-systematic review, we provide an overview on pathophysiology and current treatment options of male stress urinary incontinence after radical prostatectomy.
There are different factors responsible for the post-rPR urinary incontinence. The most well-known factors include the changes that occur in the anatomy, the preoperative bladder function as well as the operation technique and the experience of the surgeon (21, 22). In addition, the anatomic support and the pelvic innervation have been identified as important contributors to post rPR continence (21). Among the many surgical and technical factors proposed in the literature as contributing to the development of urinary incontinence following rPR, extensive dissection during surgery, damage to the neurovascular bundle, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing rPR. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates (22).
Continence is generally facilitated by the combination of the action of the detrusor muscle, the proximal intrinsic sphincter, the rhabdosphincter (23), and the urethral suspensory mechanism composed of pubourethral ligaments (24). After rPR, the proximal urethral sphincter, the suspensory ligaments as well as parts of the proximal intrinsic sphincter are removed. As a consequence, post rPR urinary continence is largely dependent on the rhabdosphincter (25). In addition, the pudendal nerve fibers that innervate the rhabdosphincter are damaged during the operation which has functional implications. This has been studied by transurethral ultrasound, that has shown thinning or atrophy as well as impaired contractility of the rhabdosphincter (25). Moreover, the innervation of the detrusor muscle and trigonum are impair which leads to a decreased detrusor contractility and poor bladder compliance (26, 27). The most predominant finding in urodynamic measurements is the sphincteric incontinence (28). On the other hand, intrinsic detrusor dysfunction and overactivity or impaired detrusor contractility, and altered detrusor compliance play a role in the post rPR continence (29). Preoperative urodynamic abnormalities have been observed to be present in 41% of patients, with half of them having detrusor overactivity (28).
About 50% of patients have preoperative impaired bladder compliance and impaired detrusor contractility and 47% de novo postoperative changes (30). Urodynamic studies carried out 1 year after rPR have shown sphincteric incontinence as the most common finding, which was responsible for about 88–100% urinary incontinence after rPR (26, 31, 32). About a third of the patients had an intrinsic sphincter deficiency as the single cause of their urinary incontinence (26, 32). Furthermore, detrusor overactivity and impaired bladder contractility were each found in up to a 30% of the cases (26, 32). However, in <9% of the cases, these findings were the only urodynamic finding (26, 32). In one out of five patients, bladder outlet obstruction was found, but this was the sole urodynamic finding in only 1% of the cases (31). Delayed first sensation (42%), mixed urgency-urge incontinence (48%), and decreased bladder capacity (< 300 mL) (41%) were the other findings on urodynamic measurements after rPR (26). It must also be stressed that, a highly well-established predictor of functional outcomes is the surgeon. It is well-known that patients treated in high volume centers and in experienced hands, are more likely to be dry. When reviewing different series, the absence of this variable could represent a limitation since, in some cases, an individual surgeon's outcomes may be much better, or worse, than any nomogram prediction. Better urinary continence recovery results can be expected by patients who undergo rPR performed by a surgeon with greater experience (33). An annual surgical case load of >50 cases/year results in improved continence recovery outcomes following rPR (33).
In the context of management of post-rPR OAB syndrome, it is important to understand its underlying pathophysiological mechanism (34). Since OAB is multifactorial (35), the exact role of prostate surgery in the development of OAB is still under debate as, after rPR, there are several variables that could contribute to detrusor overactivity.
Detrusor overactivity in patients after radical prostatectomy has been mainly attributed to a partial denervation of the bladder during surgery (30). However, together with bladder denervation, other hypotheses, such as the urethrovesical mechanism, have been described.
It has been demonstrated that urethral afferents are activated by urethral perfusion (36) and they could modulate the micturition reflex via pudendal and pelvic afferent and efferent signals, causing bladder contraction. This has been described as urethrovesical mechanism (37–39).
In a recent study, Mastukawa et al. identified that low maximum urethral closure pressure at baseline and its decrease postoperatively were strong predictors of de novo post-rPR OAB underlying the role of the intrinsic sphincter deficiency on the pathophysiology of OAB (40).
In contrast, detrusor underactivity may cause OAB syndrome as well, which seems contradictory at the first glance. Bladder underactivity may affect up to 40% of patients after radical prostatectomy mostly due to denervation (41).
Bladder outlet obstruction is a known cause of OAB. The obstruction after RP is mainly caused by bladder neck contracture and urethral stricture due to the anastomosis of the bladder neck with the urethra, which has an incidence up to 20% (42). BOO causes damage to the smooth muscle demonstrating histological changes in the bladder wall causing spontaneous myogenic contractions (43). Therefore, the presence of infravesical obstruction due to urethral stricture or bladder neck contracture must be excluded.
Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of urinary incontinence after rPR. In addition, certain biological factors and parameters known preoperatively, including older age, higher BMI, pre-existing LUTS, lower motor unit lesion, and functional bladder changes, have been identified to have a negative impact on continence rates after rPR (22).
Recently, a preoperative model was presented to predict incontinence before rPR (Figure 1) (44). According this nomogram, high risk for biochemical recurrence, adjuvant radiotherapy, lower results in the validated quality of life questionnaire EORCT QLQ-C30/QoL, higher sum score of the validated questionnaire International Consultation of Incontinence Questionnaire—Urinary Incontinence—Short form (ICIQ-UI-SF) and higher patient age, were associated with statistically significant higher sum scores of the 12-month ICIQ-UI-SF, thus, representing higher impact of urinary incontinence (Figure 1) (44). Together with the preoperative model a new, postoperative nomogram was introduced to inform patients about the probability of an additional surgery for incontinence or, on the other hand, about the importance of enduring with a strict pelvic floor muscle training protocol (Figure 2) (44).
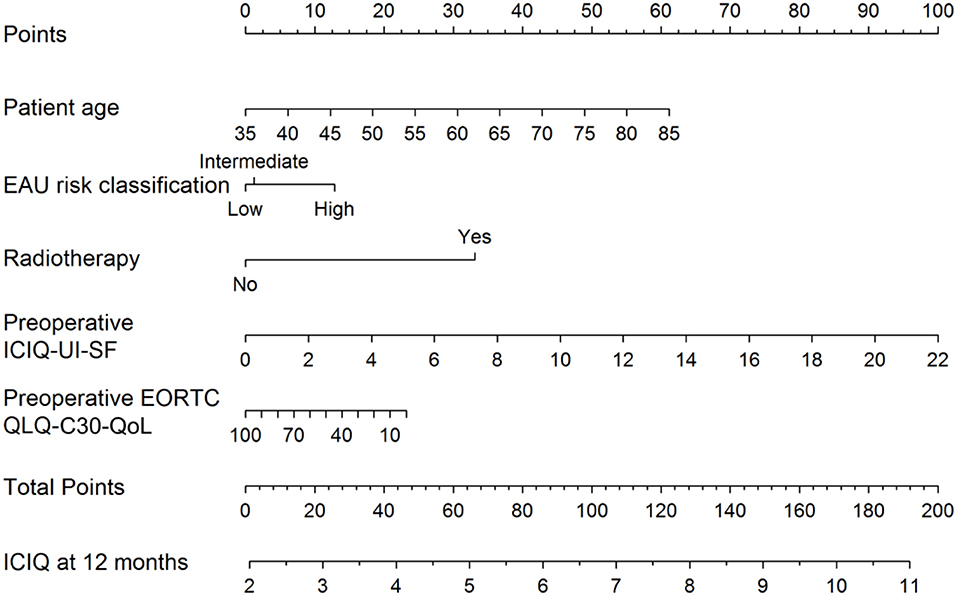
Figure 1. Nomogram for the preoperative prediction of the 12-month ICIQ-UI-SF score among patients diagnosed with prostate cancer and treated with robotic-assisted prostatectomy. Instructions: locate the patient's values for age, EAU risk classification, baseline EORCT QLQ-C30/QoL and baseline ICIQ-UI-SF on the corresponding axes. Draw a straight line up to the Points axis for each value to determine the number of points for that value. Calculate the sum of the values on the Points axis and locate this sum score on the Total Points axis. Draw a straight line down to find the patient's predicted ICIQ-UI-SF score at 12 months. From Tutolo et al. (44). EORCT QLQ-C30/QoL, European Organization for Research and Treatment for Cancer Quality of Life Questionnaire of Prostate Cancer; ICIQ-UI-SF, International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form; EAU, European Association of Urology.
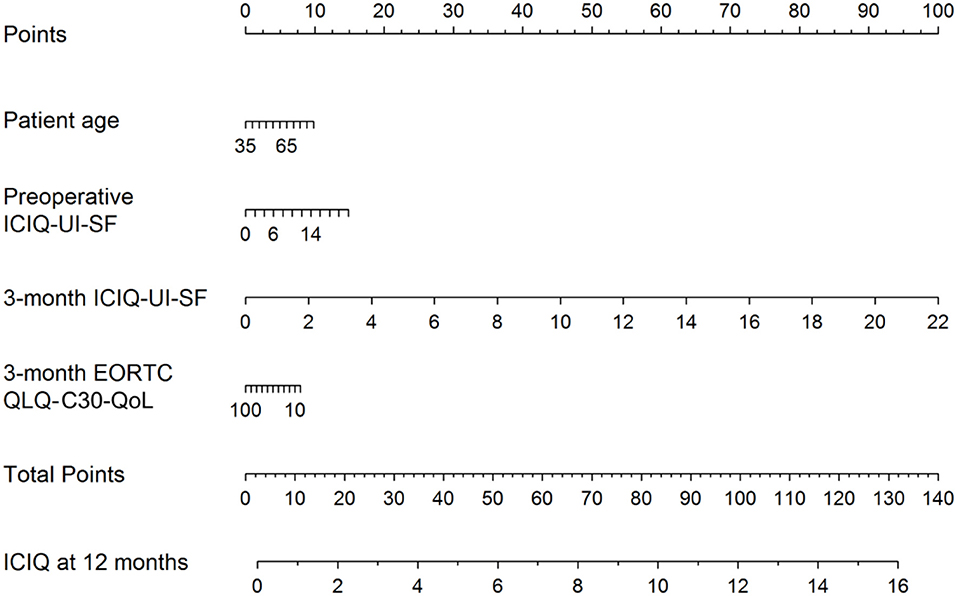
Figure 2. Nomogram for the postoperative prediction of the 12-month ICIQ-UI-SF score among patients diagnosed with prostate cancer and treated with robotic-assisted laparoscopic prostatectomy. Instructions: locate the patient's values for age, 3-month EORCT QLQ-C30/QoL, intraoperative complications, preoperative ICIQ-UI-SF and 3-month ICIQ-UI-SF on the corresponding axes. Draw a straight line up to the Points axis for each value to determine the number of points for that value. Calculate the sum of the values on the Points axis and locate this sum score on the Total Points axis. Draw a straight line down to find the patient's predicted ICIQ-UI-SF score at 12 months. Taken From Tutolo et al. (44). EORCT QLQ-C30/QoL, European Organization for Research and Treatment for Cancer Quality of Life Questionnaire of Prostate Cancer; ICIQ-UI-SF, International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form.
Interestingly, these results did not show any association with ICIQ-UI-SF, when including surgery volume (namely <50, 50–100, or >100 cases per year) (44).
R-squared (R2), the statistical measure that represents the proportion of the variance for a dependent variable, equalled 4% and 43% in the preoperative and postoperative models, respectively. This is mainly due to the retrospective nature of the study and to the intrinsic characteristics of the database (strict rules of the Belgian Cancer registry). The major drawback of this study, together with its retrospective nature, is that a single dataset has been used for development and validation of the model. Although a non-random splitting of the data is an acceptable design for evaluating model performance, external validation still has to be performed (42).
Although there is conflicting evidence regarding the importance of conservative treatment after post-prostatectomy urinary incontinence (45), pelvic floor muscle training (PFMT) is still considered as the first treatment choice (46). Duloxetin, a serotonin/norepinephrine reuptake inhibitor, either alone or in combination with PFMT, may hasten recovery of urinary incontinence but is often associated with severe gastrointestinal and central nervous side effects (47, 48). However, neither PFMT nor duloxetine may cure male stress urinary incontinence.
If conservative therapy fails, surgical treatment options should be offered to the patients. The artificial urinary sphincter (AUS) has been considered the gold standard for several decades. In a recent study urinary incontinence rates remained high, with no evidence of difference between male sling and AUS (49). The mode of function of AUS is a circumferential compression of the urethra based on a hydraulic mechanism. Nowadays, several alternative procedures with different operating principles compete against the AUS. These procedures are classified to bulking agents, male slings, and compressive devices. Table 2 presents success and complications rates of different treatment options of selected clinical trials and Figure 2 demonstrates the different surgical devices in situ. Table 2 presents success and complications rates of different treatment options of selected clinical trials and Figure 2 demonstrates the different surgical devices in situ.
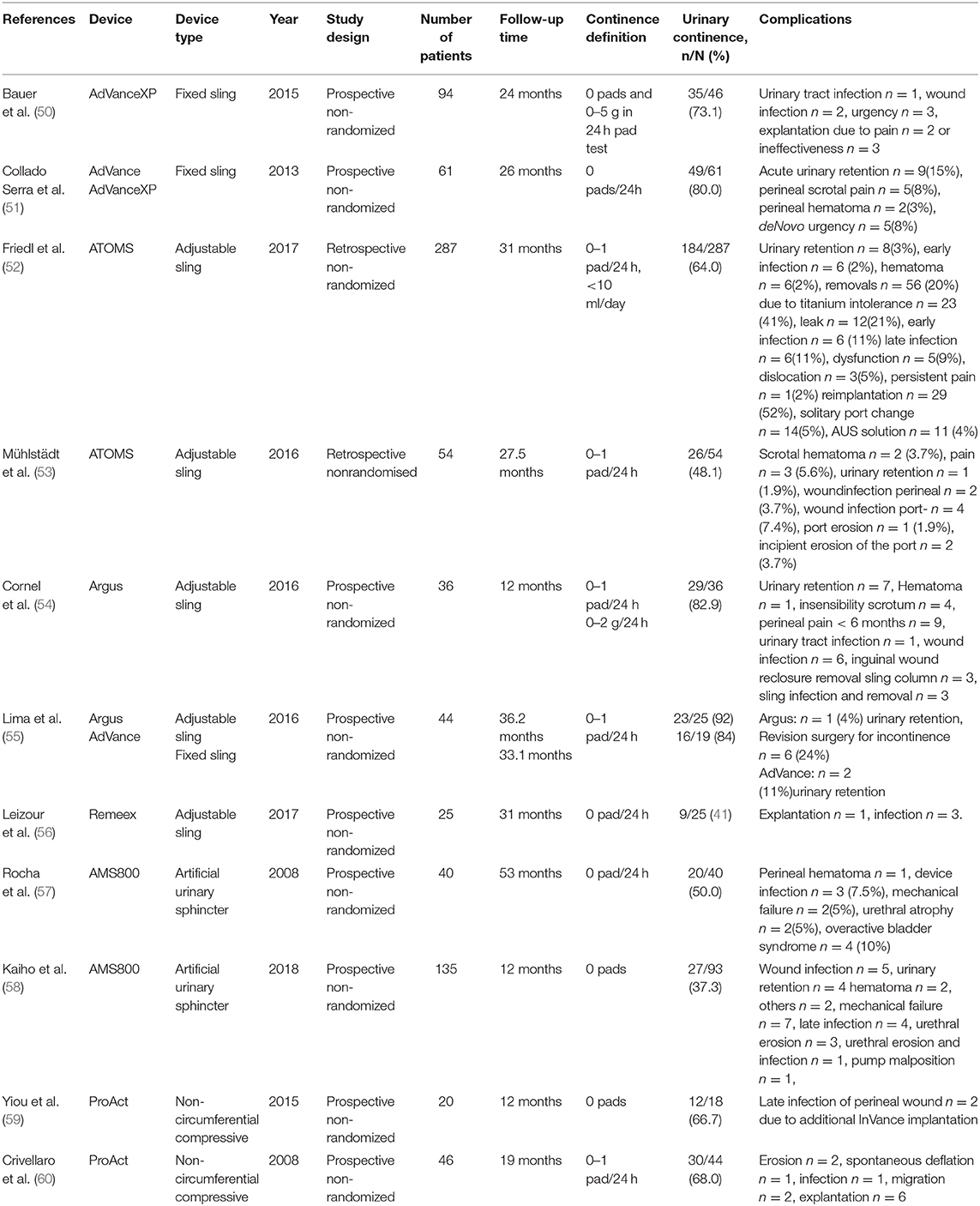
Table 2. Continence and complications rates after different treatment modalities of male stress urinary incontinence in selected clinical trials.
Theoretically, bulking agents may be an attractive treatment option for patients with limited amount of urine loss, unfit for surgery, or unwilling for surgery with implantable devices (61). However, bulking agents have been discredited due to various complications, such as embolization, migration, absorptions, allergic, and fibrotic reactions. Novel bulking agents are characterized by their non-migrating and non-absorbable properties (62). Although bulking agents are commonly used in female SUI, evidence regarding the treatment of male SUI is scarce. Moreover, there is no standardized surgical technique regarding amount and position of injection. In a recent systematic review of bulking agents utilized for male SUI including polydimethylsiloxane elastomer (Macroplastique), polyacrylate polyalcohol copolymer (Opsys), carbon coated zirconium (Durasphere), and vinyl dimethyl terminated poly-dimethylsiloxane polymer (Urolastic), no final conclusion could be drawn due to the high risk of bias, incoherent reporting of urinary incontinence and surgical technique and contradictory results (61). It can be concluded that, there is currently, no recommendation for the utilization of bulking agents for the treatment of male stress urinary incontinence outside of clinical trials (46).
Male slings are minimally invasive procedures where a sling is positioned under the bulbar urethra through a retropubic or transobturator approach (46). They are distinguished into fixed and adjustable slings.
The mode of function of fixed slings is the reposition of the urethra to a proximal position without affecting the sphincter mechanism directly. This mechanism bases on the hypothesis, that urinary incontinence with residual sphincter function is caused by a urethral or perineal descent which is associated with lacity, iatrogenic causes, or aging in the levator ani complex (63). The distal urethral sphincter may be supported indirectly by a hammock underneath the urethral bulb though increasing the coaptative zone within the sphincteric membranous urethra. During increased physical exercise the blood flow is accumulated within the supported corpus spongiosum and hereby increases the zone of coaptation which is enabled by the male sling (7).
However, the current considerations base on the existence of an at least partial or complete presence of the urethral sphincter. Therefore, fixed slings are indicated in patients with mild to moderate male SUI (46) whereas, higher degrees of urinary incontinence should be reserved to compressive devices.
The most investigated fixed male sling is the AdVance, and second generation AdVanceXP (Boston Scientific, Marlborough, Massachusetts, USA). In mid-term follow up of the AdVanceXP in a selected patient population, 68.8% and 22.8% of the patients were either cured or improved, respectively, with a mean urine loss decreased to 19.1 g. Importantly, no intraoperative or long-term complications occurred in either of these patients (64). In a recent meta-analysis, the objective cure rates for fixed slings were reported between 8.3 and 87%. Pain was the most common complication although chronic pain was only reported in 1.3%. The second most common complication is urinary retention but being mostly a temporary condition (65).
Adjustable slings offer the possibility of adjuvant adaptation of the sling tension or compression of the urethra by either tighten the sling arms or filling a cushion, which is localized beneath the urethra. The mode of function of adjustable slings are therefore complemented by the possibility of mechanical compression of the urethra (Figure 3).
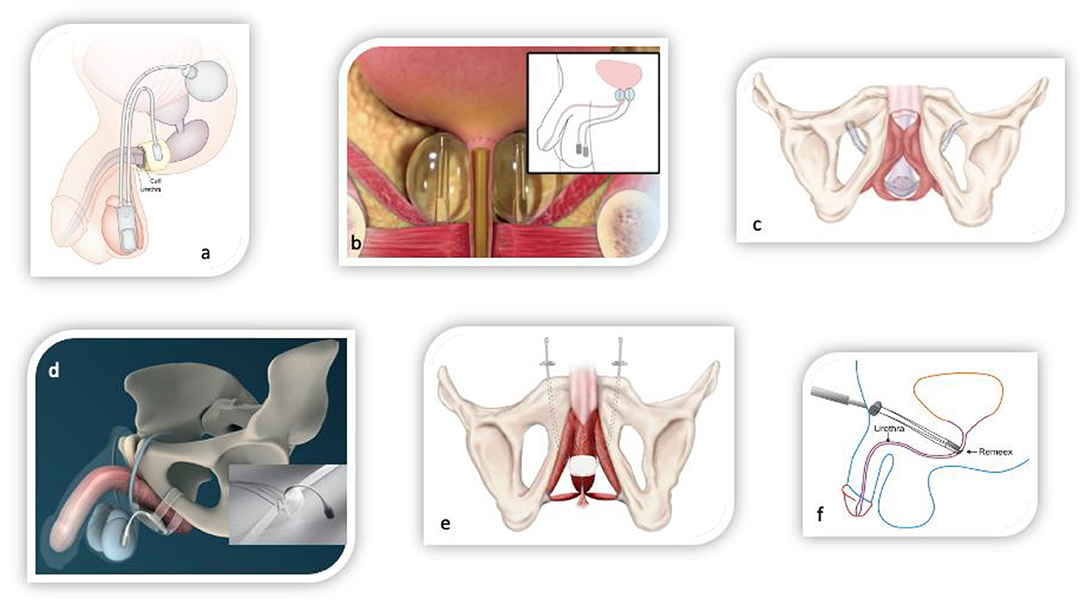
Figure 3. Surgical devices for the treatment of male stress urinary incontinence. (a) Circumferential compressive three-piece artificial urinary sphincter AMS800 (Boston scientific, USA). (b) Non-circumferential compressive device ProACT (UroMedica, USA). (c) Fixed male sling AdVanceXP (Boston Scientific, USA). (d) Adjustable male sling ATOMS (A.M.I., Austria). (e) Adjustable male sling Argus (Promedon, Argentina). (f) Adjustable male sling Remeex (Neomedic, Spain).
Currently, there are three commercialized adjustable sling available: Remeex (Neomedic, Madrid, Spain), Argus (Promedon, Cordoba, Argentina), and ATOMS (A.M.I., Feldkirch, Austria). The currently most investigated adjustable sling is the ATOMS. In a recent meta-analysis including a total of 1.393 patients with an ATOMS, the mean cure rate was 67% and improvement of urinary SUI was 90%. The complication rate was 16.5% although major complications occurred in only 3% (66). Including all adjustable slings, the cure rate is reported between 17 and 92% in a meta-analysis. Chronic painful condition was 1.5% and the most common complication is infection with subsequent explantation of the device (65). These results are accordance with a large cohort trial, reporting a significant higher infection rate of 2.3% and pain rate of 11.9%. The total explantation rate was 4.0% (67). Furthermore, it could be demonstrated that adjustable slings are more commonly utilized in patients with higher degree of SUI and risk factors, although functional outcomes remained comparable to fixed slings.
In conclusion, there might be beneficial cure rates in adjustable slings in comparison to fixed slings. However, complications rates might be higher in adjustable slings.
Compressive devices can be distinguished between circumferential and non-circumferential devices.
The AUS is a three-piece device including an urethral cuff, pump, and reservoir. The mode of function is a mechanical circumferential compression of the urethra and is based on a hydraulic mechanism. The most investigated device is the AMS800 (Boston Scientific, Marlborough, Massachusetts, USA). Its predecessor has been introduced in 1972 (68) and is available in the current shape since 1982 (69). The continence rate of the AUS are reported between 61 and 100% (70) and in a long-term analysis with a mean of 15 years, the continence rate was still 77.2%. Including any degree of urinary incontinence independently of the existence of the urethral sphincter. Therefore, the AUS is recommended for the treatment of moderate to severe male SUI and in particular in patients with a history of pelvic irradiation or urethral stricture disease.
Despite the favorable functional outcome, the AUS is associated with higher complications rates than male slings (71). The mean rate of infection and erosion in a pooled analysis was 8.5%, mechanical failure 6.2%, urethral atrophy 7.9%, and the mean rate of reintervention was 26%. Nevertheless, in particular patients with higher degree of urinary incontinence facing limited treatment options. If the AUS fails, the ultima ratio is urinary diversion.
Apart from the AMS800, which offer the largest amount of literature and follow-up time, there are several other commercialized AUS available. Victo (Promedon, Cordoba, Argentina) is three-piece device similar to the AMS800 but offers additional the possibility to adjust the device by increasing the intraluminal pressure through percutaneous fluid injection into a port which is located in the bottom of the pump. Zephyr (Zephyr Surgical Implants, Geneva, Switzerland) offers a two-piece device including only a pump and an urethral cuff. Furthermore, the device also offers the possibility of postoperative adjustability in a similar approach as described.
ProAct (Uromedica, Plymouth, USA) is a non-circumferential compressive device. The mechanism is based on two balloons which are positioned lateral to the proximal urethra. The balloons are filled in an ambulatory matter and results in a mechanical compression of the urethra. The success rates are reported between 62 and 68% accompanied by explantation rate of 12.3%. The most common complications are erosion (3.2–10.9 %) and dislocation (4–6.2 %) (72). Other prospective series even reported complication rates between 11 and 58% (46). There is currently no direct recommendation for the utilization of ProAct in mal SUI in the European guidelines. However, in the summary of very limited evidence, it is evaluated as effective in short term, although associated with a high risk of complications and should not be offered to patients with a history of pelvic irradiation (46).
Prostate cancer is one of the most problematic and frequently encountered malignancies in male patients. It often occurs when men are still in the active period of their lives. Consequently, there is a high demand for minimally invasive therapeutic approaches, susceptible of preserving urinary continence and sexual function. Unfortunately, stress urinary incontinence is a common adverse event in men with localized or locally advanced prostate cancer undergoing radical prostatectomy, but also secondary to radiotherapy (external beam radiotherapy as well as brachytherapy) and to cryosurgery (73).
Despite rehabilitative procedures such as pelvic floor muscle training, biofeedback, electrical stimulation, lifestyle changes, or a combination of these strategies, no fully efficient treatment alternative has yet been established for this pathology (74). On the other hand, it should be acknowledged that nursing care, including the understanding of the patient's needs, education, and psychosocial support remain essential features while aiming to improve the quality of life of prostate cancer patients.
Concerning the newest experimental treatments made available for urinary incontinence subsequent to prostate cancer surgery, there are studies that have shown a significant improvement of continence after ultrasound guided injection of fibroblasts and myoblasts into the sphincter (75). Other clinical trials also emphasized encouraging outcomes provided by stem-cells injection into the rhabdosphincter (76). Last but not least, promising outcomes have been outlined as a result of intravesical Onabotulinum toxin A injection (77).
Most importantly, the therapeutic decision and the chosen treatment option must be individualized for each patient according to clinical and social factors. During the recent years, the development of new therapeutic choices such as male sling techniques provided a more acceptable management pathway for less severe forms of urinary incontinence related to radical prostatectomy. Following this perspective, technological improvements and the emergence of new dedicated devices currently create the premises for a continuously positive evolution of clinical outcomes in this particular category of patients.
MR: data collection, coordination and drafting the manuscript, and supervising. TM and TH: data collection, drafting the manuscript, and supervising. BG and MT: data collection and drafting the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. (2018) 103:356–87. doi: 10.1016/j.ejca.2018.07.005
2. EAU-EANM-ESTRO-ESUR-SIOG. Guidelines on prostate cancer 2020v4 2020. Available online at: https://uroweb.org/wp-content/uploads/EAU-EANM-ESTRO-ESUR-SIOG-Guidelines-on-Prostate-Cancer-2020v4.pdf.
3. Kretschmer A, Nitti V. Surgical treatment of male postprostatectomy incontinence: current concepts. Eur Urol Focus. (2017) 3:364–76. doi: 10.1016/j.euf.2017.11.007
4. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. (2002) 21:167–78. doi: 10.1002/nau.10052
5. D'Ancona C, Haylen B, Oelke M, Abranches-Monteiro L, Arnold E, Goldman H, et al. The international continence society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol Urodyn. (2019) 38:433–77. doi: 10.1002/nau.23897
6. John H, Sullivan MP, Bangerter U, Hauri D, Yalla SV. Effect of radical prostatectomy on sensory threshold and pressure transmission. J Urol. (2000) 163:1761–6. doi: 10.1016/S0022-5347(05)67537-4
7. Rehder P, Staudacher NM, Schachtner J, Berger ME, Schillfahrt F, Hauser V, et al. Hypothesis that urethral bulb (Corpus Spongiosum) plays an active role in male urinary continence. Adv Urol. (2016) 2016:6054730. doi: 10.1155/2016/6054730
8. Sallami S. Predictive factors of urinary incontinence after radical prostatectomy: systematic review. Tunis Med. (2017) 95:229–35.
9. Lee R, Te AE, Kaplan SA, Sandhu JS. Temporal trends in adoption of and indications for the artificial urinary sphincter. J Urol. (2009) 181:2622–7. doi: 10.1016/j.juro.2009.01.113
10. Haglind E, Carlsson S, Stranne J, Wallerstedt A, Wilderang U, Thorsteinsdottir T, et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol. (2015) 68:216–25. doi: 10.1016/j.eururo.2015.02.029
11. Tang K, Jiang K, Chen H, Chen Z, Xu H, Ye Z. Robotic vs. Retropubic radical prostatectomy in prostate cancer: a systematic review and an meta-analysis update. Oncotarget. (2017) 8:32237–57. doi: 10.18632/oncotarget.13332
12. Coughlin GD, Yaxley JW, Chambers SK, Occhipinti S, Samaratunga H, Zajdlewicz L, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. (2018) 19:1051–60. doi: 10.1016/S1470-2045(18)30357-7
13. Porpiglia F, Morra I, Lucci Chiarissi M, Manfredi M, Mele F, Grande S, et al. Randomised controlled trial comparing laparoscopic and robot-assisted radical prostatectomy. Eur Urol. (2013) 63:606–14. doi: 10.1016/j.eururo.2012.07.007
14. Basiri A, de la Rosette JJ, Tabatabaei S, Woo HH, Laguna MP, Shemshaki H. Comparison of retropubic, laparoscopic and robotic radical prostatectomy: who is the winner? World J Urol. (2018) 36:609–21. doi: 10.1007/s00345-018-2174-1
15. Choo MS, Choi WS, Cho SY, Ku JH, Kim HH, Kwak C. Impact of prostate volume on oncological and functional outcomes after radical prostatectomy: robot-assisted laparoscopic versus open retropubic. Korean J Urol. (2013) 54:15–21. doi: 10.4111/kju.2013.54.1.15
16. Rocco B, Matei DV, Melegari S, Ospina JC, Mazzoleni F, Errico G, et al. Robotic vs open prostatectomy in a laparoscopically naive centre: a matched-pair analysis. BJU Int. (2009) 104:991–5. doi: 10.1111/j.1464-410X.2009.08532.x
17. Son SJ, Lee SC, Jeong CW, Jeong SJ, Byun SS, Lee SE. Comparison of continence recovery between robot-assisted laparoscopic prostatectomy and open radical retropubic prostatectomy: a single surgeon experience. Korean J Urol. (2013) 54:598–602. doi: 10.4111/kju.2013.54.9.598
18. Kim M, Park M, Pak S, Choi SK, Shim M, Song C, et al. Integrity of the urethral sphincter complex, nerve-sparing, and long-term continence status after robotic-assisted radical prostatectomy. Eur Urol Focus. (2019) 5:823–30. doi: 10.1016/j.euf.2018.04.021
19. Olsson LE, Salomon L, Nadu A, Hoznek A, Cicco A, Saint F, et al. Prospective patient-reported continence after laparoscopic radical prostatectomy. Urology. (2001) 58:570–2. doi: 10.1016/S0090-4295(01)01261-4
20. Sumarsono B, Jong JJ, Wang JY, Liao L, Lee KS, Yoo TK, et al. The prevalence of urinary incontinence in men and women aged 40 years or over in China, Taiwan and South Korea: a cross-sectional, prevalence-based study. Low Urin Tract Symptoms. (2020) 12:223–34. doi: 10.1111/luts.12308
21. Averbeck MA, Marcelissen T, Anding R, Rahnama'i MS, Sahai A, Tubaro A. How can we prevent postprostatectomy urinary incontinence by patient selection, and by preoperative, peroperative, and postoperative measures? International Consultation on Incontinence-Research Society 2018. Neurourol Urodyn. (2019) 38(Suppl 5):S119–26. doi: 10.1002/nau.23972
22. Heesakkers J, Farag F, Bauer RM, Sandhu J, De Ridder D, Stenzl A. Pathophysiology and contributing factors in postprostatectomy incontinence: a review. Eur Urol. (2017) 71:936–44. doi: 10.1016/j.eururo.2016.09.031
23. Oelrich TM. The urethral sphincter muscle in the male. Am J Anat. (1980) 158:229–46. doi: 10.1002/aja.1001580211
24. Steiner MS. The puboprostatic ligament and the male urethral suspensory mechanism: an anatomic study. Urology. (1994) 44:530–4. doi: 10.1016/S0090-4295(94)80052-9
25. Strasser H, Frauscher F, Helweg G, Colleselli K, Reissigl A, Bartsch G. Transurethral ultrasound: evaluation of anatomy and function of the rhabdosphincter of the male urethra. J Urol. (1998) 159:100–4; discussion 4–5. doi: 10.1016/S0022-5347(01)64025-4
26. Gomha MA, Boone TB. Voiding patterns in patients with post-prostatectomy incontinence: urodynamic and demographic analysis. J Urol. (2003) 169:1766–9. doi: 10.1097/01.ju.0000059700.21764.83
27. John H. Bulbourethral composite suspension: a new operative technique for post-prostatectomy incontinence. J Urol. (2004) 171:1866–70; discussion 9–70. doi: 10.1097/01.ju.0000121413.62521.cc
28. Majoros A, Bach D, Keszthelyi A, Hamvas A, Romics I. Urinary incontinence and voiding dysfunction after radical retropubic prostatectomy (prospective urodynamic study). Neurourol Urodyn. (2006) 25:2–7. doi: 10.1002/nau.20190
29. Matsukawa Y, Hattori R, Komatsu T, Funahashi Y, Sassa N, Gotoh M. De novo detrusor underactivity after laparoscopic radical prostatectomy. Int J Urol. (2010) 17:643–8. doi: 10.1111/j.1442-2042.2010.02529.x
30. Porena M, Mearini E, Mearini L, Vianello A, Giannantoni A. Voiding dysfunction after radical retropubic prostatectomy: more than external urethral sphincter deficiency. Eur Urol. (2007) 52:38–45. doi: 10.1016/j.eururo.2007.03.051
31. Groutz A, Blaivas JG, Chaikin DC, Weiss JP, Verhaaren M. The pathophysiology of post-radical prostatectomy incontinence: a clinical and video urodynamic study. J Urol. (2000) 163:1767–70. doi: 10.1016/S0022-5347(05)67538-6
32. Kielb SJ, Clemens JQ. Comprehensive urodynamics evaluation of 146 men with incontinence after radical prostatectomy. Urology. (2005) 66:392–6. doi: 10.1016/j.urology.2005.03.026
33. Trieu D, Ju IE, Chang SB, Mungovan SF, Patel MI. Surgeon case volume and continence recovery following radical prostatectomy: a systematic review. ANZ J Surg. (2020). doi: 10.1111/ans.16491
34. Peyronnet B, Brucker BM. Management of overactive bladder symptoms after radical prostatectomy. Curr Urol Rep. (2018) 19:95. doi: 10.1007/s11934-018-0847-3
35. Chapple C. Chapter 2: Pathophysiology of neurogenic detrusor overactivity and the symptom complex of “overactive bladder”. Neurourol Urodyn. (2014) 33(Suppl 3):S6–13. doi: 10.1002/nau.22635
36. Jung SY, Fraser MO, Ozawa H, Yokoyama O, Yoshiyama M, De Groat WC, et al. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J Urol. (1999) 162:204–12. doi: 10.1097/00005392-199907000-00069
37. Shafik A, Shafik AA, El-Sibai O, Ahmed I. Role of positive urethrovesical feedback in vesical evacuation. The concept of a second micturition reflex: the urethrovesical reflex. World J Urol. (2003) 21:167–70. doi: 10.1007/s00345-003-0340-5
38. Shafik A, el-Sibai O, Ahmed I. Effect of urethral dilation on vesical motor activity: identification of the urethrovesical reflex and its role in voiding. J Urol. (2003) 169:1017–9. doi: 10.1097/01.ju.0000046384.71563.51
39. Hubeaux K, Deffieux X, Desseaux K, Verollet D, Damphousse M, Amarenco G. Stand up urgency: is this symptom related to a urethral mechanism? Prog Urol. (2012) 22:475–81. doi: 10.1016/j.purol.2012.04.011
40. Matsukawa Y, Yoshino Y, Ishida S, Fujita T, Majima T, Funahashi Y, et al. De novo overactive bladder after robot-assisted laparoscopic radical prostatectomy. Neurourol Urodyn. (2018) 37:2008–14. doi: 10.1002/nau.23556
41. Chung DE, Dillon B, Kurta J, Maschino A, Cronin A, Sandhu JS. Detrusor underactivity is prevalent after radical prostatectomy: a urodynamic study including risk factors. Can Urol Assoc J. (2013) 7:E33–7. doi: 10.5489/cuaj.192
42. Jarosek SL, Virnig BA, Chu H, Elliott SP. Propensity-weighted long-term risk of urinary adverse events after prostate cancer surgery, radiation, or both. Eur Urol. (2015) 67:273–80. doi: 10.1016/j.eururo.2014.08.061
43. Drake MJ, Kanai A, Bijos DA, Ikeda Y, Zabbarova I, Vahabi B, et al. The potential role of unregulated autonomous bladder micromotions in urinary storage and voiding dysfunction; overactive bladder and detrusor underactivity. BJU Int. (2017) 119:22–9. doi: 10.1111/bju.13598
44. Tutolo M, Bruyneel L, Van der Aa F, Van Damme N, Van Cleynenbreugel B, Joniau S, et al. A novel tool to predict functional outcomes after robot-assisted radical prostatectomy and the value of additional surgery for incontinence. BJU Int. (2020). doi: 10.1111/bju.15242
45. Anderson CA, Omar MI, Campbell SE, Hunter KF, Cody JD, Glazener CM. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev. (2015) 1:CD001843. doi: 10.1002/14651858.CD001843.pub5
46. Burkhard FC, Bosch RJL, Cruz F, Lemack GE, Nambiar AK, Thiruchelvam N, et al. EAU Guidelines on Urinary Incontinence (2020).
47. Alan C, Eren AE, Ersay AR, Kocoglu H, Basturk G, Demirci E. Efficacy of duloxetine in the early management of urinary continence after radical prostatectomy. Curr Urol. (2015) 8:43–8. doi: 10.1159/000365688
48. Filocamo MT, Li Marzi V, Del Popolo G, Cecconi F, Villari D, Marzocco M, et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. Eur Urol. (2007) 51:1559–64. doi: 10.1016/j.eururo.2006.08.005
49. Abrams P, Constable LD, Cooper D, MacLennan G, Drake MJ, Harding C, et al. Outcomes of a noninferiority randomised controlled trial of surgery for men with urodynamic stress incontinence after prostate surgery (MASTER). Eur Urol. (2021). doi: 10.1016/j.eururo.2021.01.024
50. Bauer RM, Gozzi C, Klehr B, Kretschmer A, Grabbert M, Rehder P, et al. AdVanceXP male sling: 2-year results of a multicentre study. World J Urol. (2015) 34:1025–30. doi: 10.1007/s00345-015-1731-0
51. Collado Serra A, Resel Folkersma L, Dominguez-Escrig JL, Gomez-Ferrer A, Rubio-Briones J, Solsona Narbon E. AdVance/AdVance XP transobturator male slings: preoperative degree of incontinence as predictor of surgical outcome. Urology. (2013) 81:1034–9. doi: 10.1016/j.urology.2013.01.007
52. Friedl A, Muhlstadt S, Zachoval R, Giammo A, Kivaranovic D, Rom M, et al. Long-term outcome of the adjustable transobturator male system (ATOMS): results of a European multicentre study. BJU Int. (2017) 119:785–92. doi: 10.1111/bju.13684
53. Muhlstadt S, Friedl A, Mohammed N, Schumann A, Weigand K, Kawan F, et al. Five-year experience with the adjustable transobturator male system for the treatment of male stress urinary incontinence: a single-center evaluation. World J Urol. (2017) 35:145–51. doi: 10.1007/s00345-016-1839-x
54. Cornel EB. Argus-T adjustable male sling: the influence of surgical technique on complications and short-term efficacy. Urol Int. (2016) 96:164–70. doi: 10.1159/000443673
55. Lima JP, Pompeo AC, Bezerra CA. Argus T(R) versus Advance(R) Sling for postprostatectomy urinary incontinence: a randomized clinical trial. Int Braz J Urol. (2016) 42:531–9. doi: 10.1590/S1677-5538.IBJU.2015.0075
56. Leizour B, Chevrot A, Wagner L, Droupy S, Costa P. Adjustable retropubic suburethral sling Remeex(®) in the treatment of male stress urinary incontinence: one-year results. Prog Urol. (2017) 27:238–43. doi: 10.1016/j.purol.2016.11.006
57. Trigo Rocha F, Gomes CM, Mitre AI, Arap S, Srougi M. A prospective study evaluating the efficacy of the artificial sphincter AMS 800 for the treatment of postradical prostatectomy urinary incontinence and the correlation between preoperative urodynamic and surgical outcomes. Urology. (2008) 71:85–9. doi: 10.1016/j.urology.2007.09.009
58. Kaiho Y, Masuda H, Takei M, Hirayama T, Mitsui T, Yokoyama M, et al. Surgical and patient reported outcomes of artificial urinary sphincter implantation: a multicenter, prospective, observational study. J Urol. (2018) 199:245–50. doi: 10.1016/j.juro.2017.08.077
59. Yiou R, Butow Z, Baron T, Salomon L, Audureau E. Adjustable continence therapy (ProACT) after male sling failure for patients with post-radical prostatectomy urinary incontinence: a prospective study with one-year follow-up. World J Urol. (2015) 33:1331–6. doi: 10.1007/s00345-014-1447-6
60. Crivellaro S, Singla A, Aggarwal N, Frea B, Kocjancic E. Adjustable continence therapy (ProACT) and bone anchored male sling: comparison of two new treatments of post prostatectomy incontinence. Int J Urol. (2008) 15:910–4. doi: 10.1111/j.1442-2042.2008.02161.x
61. Toia B, Gresty H, Pakzad M, Hamid R, Ockrim J, Greenwell T. Bulking for stress urinary incontinence in men: a systematic review. Neurourol Urodyn. (2019) 38:1804–11. doi: 10.1002/nau.24102
62. Chughtai B, Sedrakyan A, Isaacs AJ, Mao J, Lee R, Te A, et al. National study of utilization of male incontinence procedures. Neurourol Urodyn. (2016) 35:74–80. doi: 10.1002/nau.22683
63. Rehder P, Gozzi C. Transobturator sling suspension for male urinary incontinence including post-radical prostatectomy. Eur Urol. (2007) 52:860–6. doi: 10.1016/j.eururo.2007.01.110
64. Bauer RM, Grabbert MT, Klehr B, Gebhartl P, Gozzi C, Homberg R, et al. 36-month data for the AdVance XP(R) male sling: results of a prospective multicentre study. BJU Int. (2017) 119:626–30. doi: 10.1111/bju.13704
65. Meisterhofer K, Herzog S, Strini KA, Sebastianelli L, Bauer R, Dalpiaz O. Male slings for postprostatectomy incontinence: a systematic review and meta-analysis. Eur Urol Focus. (2020) 6:575–92. doi: 10.1016/j.euf.2019.01.008
66. Esquinas C, Angulo JC. Effectiveness of Adjustable Transobturator Male System (ATOMS) to Treat Male Stress Incontinence: a systematic review and meta-analysis. Adv Ther. (2019) 36:426–41. doi: 10.1007/s12325-018-0852-4
67. Hüsch T, Kretschmer A, Obaje A, Kirschner-Hermanns R, Anding R, Pottek T, et al. Fixed or adjustable sling in the treatment of male stress urinary incontinence: results from a large cohort study. Transl Androl Urol. (2020) 9:1099–107. doi: 10.21037/tau-19-852
68. Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. J Urol. (1974) 112:75–80. doi: 10.1016/S0022-5347(17)59647-0
69. Leicht W, Thuroff J. Therapy of male urinary incontinence: artificial sphincter versus male slings. Der Urologe Ausg A. (2012) 51:341–7. doi: 10.1007/s00120-012-2820-y
70. Van der Aa F, Drake MJ, Kasyan GR, Petrolekas A, Cornu JN. The artificial urinary sphincter after a quarter of a century: a critical systematic review of its use in male non-neurogenic incontinence. Eur Urol. (2013) 63:681–9. doi: 10.1016/j.eururo.2012.11.034
71. Hüsch T, Kretschmer A, Thomsen F, Kronlachner D, Kurosch M, Obaje A, et al. Risk factors for failure of male slings and artificial urinary sphincters: results from a large middle european cohort study. Urol Int. (2017) 99:14–21. doi: 10.1159/000449232
72. Crivellaro S, Morlacco A, Bodo G, Agro EF, Gozzi C, Pistolesi D, et al. Systematic review of surgical treatment of post radical prostatectomy stress urinary incontinence. Neurourol Urodyn. (2016) 35:875–81. doi: 10.1002/nau.22873
73. Parsons BA, Evans S, Wright MP. Prostate cancer and urinary incontinence. Maturitas. (2009) 63:323–8. doi: 10.1016/j.maturitas.2009.06.005
74. Tantawy SA, Elgohary HMI, Abdelbasset WK, Kamel DM. Effect of 4 weeks of whole-body vibration training in treating stress urinary incontinence after prostate cancer surgery: a randomised controlled trial. Physiotherapy. (2019) 105:338–45. doi: 10.1016/j.physio.2018.07.013
75. Mitterberger M, Marksteiner R, Margreiter E, Pinggera GM, Frauscher F, Ulmer H, et al. Myoblast and fibroblast therapy for post-prostatectomy urinary incontinence: 1-year followup of 63 patients. J Urol. (2008) 179:226–31. doi: 10.1016/j.juro.2007.08.154
76. Gotoh M, Yamamoto T, Kato M, Majima T, Toriyama K, Kamei Y, et al. Regenerative treatment of male stress urinary incontinence by periurethral injection of autologous adipose-derived regenerative cells: 1-year outcomes in 11 patients. Int J Urol. (2014) 21:294–300. doi: 10.1111/iju.12266
77. Apostolidis A, Dasgupta P, Denys P, Elneil S, Fowler CJ, Giannantoni A, et al. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. (2009) 55:100–19. doi: 10.1016/j.eururo.2008.09.009
Keywords: prostate cancer, incontinence (male), detrusor activity, stress incontinence, prostatectomy
Citation: Rahnama'i MS, Marcelissen T, Geavlete B, Tutolo M and Hüsch T (2021) Current Management of Post-radical Prostatectomy Urinary Incontinence. Front. Surg. 8:647656. doi: 10.3389/fsurg.2021.647656
Received: 30 December 2020; Accepted: 03 March 2021;
Published: 09 April 2021.
Edited by:
Luis Alex Kluth, University Hospital Frankfurt, GermanyReviewed by:
Juan Gomez Rivas, Hospital Clínico San Carlos, SpainCopyright © 2021 Rahnama'i, Marcelissen, Geavlete, Tutolo and Hüsch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad S. Rahnama'i, c2FqamFkX3JAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.