- 1Department of Neurosurgery, University of Florida, Gainesville, FL, United States
- 2Norman Fixel Institute for Neurological Diseases, University of Florida Health, Gainesville, FL, United States
- 3Department of Psychiatry, University of Florida, Gainesville, FL, United States
Background: In February 2009, the US Food and Drug Administration (FDA) granted Humanitarian Device Exemption (HDE) for deep brain stimulation (DBS) in the anterior limb of the internal capsule (ALIC) for the treatment of severely debilitating, treatment refractory obsessive–compulsive disorder (OCD). Despite its promise as a life altering treatment for patients with otherwise refractory, severely debilitating OCD, the use of DBS for the treatment of OCD has diminished since the FDA HDE endorsement and is now rarely performed even at busy referral centers. We sought to identify factors hindering OCD patients from receiving DBS therapy.
Materials and Methods: University of Florida (UF) clinical research databases were queried to identify patients evaluated as potential candidates for OCD DBS from January 1, 2002 to July 30, 2020. A retrospective review of these patients' medical records was performed to obtain demographic information, data related to their OCD, and details relevant to payment such as third-party payer, study participation, evaluation prior to or after HDE approval, and any stated factors prohibiting surgical intervention.
Results: Out of 25 patients with severe OCD identified as candidates for DBS surgery during the past 18 years, 15 underwent surgery. Prior to FDA HDE approval, 6 out of 7 identified candidates were treated. After the HDE, only 9 out of 18 identified candidates were treated. Seven of the 9 were funded by Medicare, 1 paid out of pocket, and 1 had “pre-authorization” from her private insurer who ultimately refused to pay after the procedure. Among the 10 identified OCD DBS candidates who were ultimately not treated, 7 patients—all with private health insurance—were approved for surgery by the interdisciplinary team but were unable to proceed with surgery due to lack of insurance coverage, 1 decided against surgical intervention, 1 was excluded due to medical comorbidities and excessive perceived surgical risk, and no clear reason was identified for 1 patient evaluated in 2004 during our initial NIH OCD DBS trial.
Conclusion: Based on compelling evidence that DBS provides substantial improvement of OCD symptoms and markedly improved functional capacity in 2 out of 3 patients with severely debilitating, treatment refractory OCD, the FDA approved this procedure under a Humanitarian Device Exemption in 2009, offering new hope to this unfortunate patient population. A careful review of our experience with OCD DBS at the University of Florida shows that since the HDE approval, only 50% of the severe OCD patients (9 of 18) identified as candidates for this potentially life altering treatment have been able to access the therapy. We found the most common limiting factor to be failure of private insurance policies to cover DBS for OCD, despite readily covering DBS for Parkinson's disease, essential tremor, and even dystonia—another HDE approved indication for DBS. We have identified an inherent discrimination in the US healthcare system against patients with medication-refractory OCD who are economically challenged and do not qualify for Medicare. We urge policy makers, insurance companies, and hospital administrations to recognize this health care disparity and seek to rectify it.
Introduction
In February 2009, the US Food Drug Administration (FDA) approved the use of Medtronic “Reclaim” bilateral anterior limb of internal capsule (ALIC) deep brain stimulation (DBS) (Medtronic, Minneapolis, MN) for the treatment of chronic, severe, treatment-resistant obsessive compulsive disorder (OCD) in adult patients who have failed at least three selective serotonin reuptake inhibitors (SSRIs) under an humanitarian device exemption (HDE) (1). This approval came after reviewing the preliminary data of multiple prospective trials dating as far back as 1999 showing responder rates from 33 to 78% (“responder” ≥35% reduction in Yale-Brown Obsessive–Compulsive Scale (YBOCS) scores) (2–6). These results compare favorably to ablative therapies that have been practiced for many years (7). The initial OCD DBS studies intended to recapitulate the targets ablated during classic capsulotomy procedures, targeting the entirety of the anterior limb of the internal capsule (ALIC). With ongoing clinical experience, it was determined that the majority of the clinical benefit was achieved through more ventral and posterior stimulation within the ALIC, and the target was refined to focus on the ventral ALIC and the nucleus accumbens (NA)—now commonly referred to as the ventral capsule/ventral striatum (VC/VS) (8). Over the years, other targets such as the nucleus accumbens (NA) (9–13), subthalamic nucleus (STN) (12, 14, 15), bed nucleus of stria terminalis (BNST) (9, 10, 16), inferior thalamic peduncle (ITP) (17, 18), thalamus (19), medial forebrain bundle (MFB) (20), caudate (12), and anteromedial globus pallidus internus (amGPi) (21) have been proposed as potential targets for OCD DBS. Long term data shows continued benefit for many of the initial responders (22–32). As one of the early centers to build on the pioneering work of Bart Nuttin and his team in Belgium (2, 3) and Ben Greenberg et al. in the US (5), our team at UF was the first to perform an NIH-sponsored trial of DBS for OCD (launched in 2002) (6) and one of the centers providing early evidence in support of the ultimate HDE approval. We experienced first-hand the frequently dramatic, and intensely gratifying improvement in symptoms and quality of life that this therapy commonly provides to desperate patients with severely debilitating OCD and to their families. Despite our experienced team, a very high-volume clinical DBS program, and unrelenting efforts to provide DBS therapy to appropriately selected patients with severe OCD, we have managed to perform only a few OCD DBS procedures at our center since the HDE approval in 2009. We sought to identify factors that are limiting patient access to this potentially life altering therapy.
Materials and Methods
After Institutional Review Board (IRB) approval, queries of the University of Florida INFORM clinical research database and our Integrated Data Repository (IDR) were performed to identify patients who met criteria for OCD DBS therapy as follows: age over 18 years, ICD code for OCD (300.3, F42.9, F42.8, F42.2, F42.1, F42.0, or F42), Yale-Brown Obsessive–Compulsive Scale (YBOCS) score greater than 28, and evaluation for DBS by our primary psychiatrist (HW) and either primary neurosurgeon (KF) or our primary neuropsychologist (Dawn Bowers) between January 1, 2002 and July 30, 2020. During chart review, to narrow the evaluation to only patients deemed appropriate candidates for OCD DBS therapy by the psychiatrist “gatekeeper,” patients seen only by the primary psychiatrist (HW) with no referral to remaining members of the interdisciplinary board for evaluation as a candidate for DBS therapy, were excluded, as were those presented to the interdisciplinary board as candidates for DBS to treat disorders other than OCD. A retrospective review of the medical record of each of the patients identified by this query was performed to gather the patient's demographic information (age, sex, comorbidities, and disease duration), pre- and post-operative YBOCS score, payer source, participation in either our initial single-center or subsequent multi-center NIH OCD DBS trial, timing of surgery relative to the 2009 HDE (before or after), and any factors in the record reported to prevent a patient from proceeding with surgical intervention.
Results
Query of our databases identified 28 patients who were appropriate OCD DBS candidates based on the stated inclusion criteria. Retrospective chart review resulted in elimination of 3 patients with (comorbid) OCD who were evaluated by the primary psychiatrist and the primary neurosurgeon as candidates for DBS to treat disorders other than OCD (essential tremor, Parkinson's Disease, and Tourette's Syndrome).
Out of 25 patients with severe OCD identified as appropriate candidates for DBS surgery during the past 18 years, 15 underwent surgery. Prior to FDA HDE approval, 6 out of 7 identified candidates (86%) were treated (all funded by our NIH grant). After the HDE, only 9 out of 18 identified candidates (50%) were treated. Six of the 9 post HDE patients treated surgically were funded by Medicare, 1 patient who underwent surgery had Medicaid, but his coverage was denied post-operatively and the procedure was written off by the hospital, 1 wealthy patient with a private insurance policy that denied coverage paid out of pocket, and 1 patient with private insurance obtained “pre-authorization” for the surgery after several appeals to her insurer, underwent DBS surgery in 2011, only to have payment ultimately denied by the insurer (“bait and switch”) (33).
Among the 10 identified OCD DBS candidates who were ultimately not treated, 7 patients—all with private health insurance—were approved for surgery by the interdisciplinary board, but were unable to proceed with surgery due to lack of insurance coverage for this specific procedure by their insurer, 1 patient decided the risk of DBS surgery was unacceptable and opted to try transcranial magnetic stimulation (TMS), 1 was excluded by the interdisciplinary DBS board because of excessive perceived surgical risk due to significant medical comorbidities, and no clear reason was identified for 1 patient from 2004 during our initial NIH OCD DBS trial.
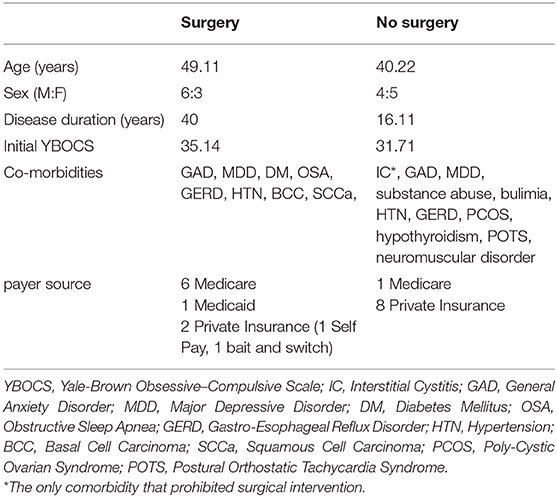
OCD DBS responders are defined as patients who have at least a 35% reduction in their YBOCS score at 1 year. Of the 15 OCD patients implanted at the University of Florida, pre-operative and 1-year post-operative YBOCS scores were available via retrospective chart review for 11 patients. Seven (63.64%) of these patients were responders at 1 year. The average age of those patients who underwent surgery was 49 vs. 40 in those candidates who did not proceed to surgery. There were 6 males in the surgical group and 3 in the non-surgical group. The average disease duration was 40 years in the surgical group and 16.11 years in the non-surgical group. Average pre-operative YBOCS score for the surgical group was 35 and 32 in the non-surgical group. Co-morbidities were similar between groups and are listed in Table 1. In the non-surgical group, one patient was deemed an inappropriate candidate for surgical intervention due to severe interstitial cystitis that interfered with her ability to interact outside the home even more than her OCD.
Discussion
When a novel therapeutic intervention is developed for the treatment of patients with a rare disorder, it is difficult to gather enough clinical evidence to meet the FDA standard of reasonable assurance of safety and effectiveness. Not only is it impractical to perform a large prospective randomized trial of the intervention due to insufficient numbers of cases, but the small prospective market for any therapeutic device involved represents a strong disincentive for those who might invest in the development of such therapies. In order to address these challenges that disadvantage patients with rare disorders, Congress included a provision in the Safe Medical Devices Act of 1990 to create a new regulatory pathway for products intended for diseases or conditions that affect small (rare) populations—the Humanitarian Device Exemption (HDE) Program (34).
Humanitarian Device Exemptions were created to improve access to therapies for patients with rare disorders. While OCD is not an uncommon disorder, the number of people with severely debilitating, medication refractory OCD who would be appropriate candidates for DBS therapy is certainly well below 8,000 per year in the US (the legally defined threshold for HDE qualification). The quality of life of patients afflicted with such treatment refractory, severe cases of OCD is abysmally poor, but two out of three of these severely afflicted patients respond to DBS. The therapeutic benefit we have observed in most patients is typically dramatic, restoring function and quality of life to a degree that has been extremely gratifying. Those of us involved in formal studies were encouraged when the HDE was granted, expecting that third party payers would now cover the cost of the operations and we would no longer need to include that cost in our NIH budgets. Unfortunately, this expectation was not met. Insurers refused to pay for the procedure despite the HDE, and our large multicenter OCD DBS trial essentially died due to inability to recruit subjects under the third-party payer model. The genesis of the current project stems from our frustration with the observation that we are performing far fewer OCD DBS cases per year since the HDE than we did prior to its approval. The main goal of the HDE is improved access, but we have demonstrated with this analysis that access to OCD DBS has actually diminished in our cohort of patients with medication refractory, severely debilitating obsessive–compulsive disorder since the HDE was granted.
The HDE mechanism requires that each institution performing the procedure covered by the HDE have local IRB approval and follow a minimum protocol for quality assurance and the gathering of some standardized outcomes data regarding the effects of the HDE approved intervention. Realistically, centers with higher volume and experience with clinical research tend to be best equipped to manage these protocols and maintain compliance with an FDA HDE. By design, the overhead required to maintain approval and compliance with an HDE tends to discourage smaller, less experienced centers, and has the desirable effect of steering patients with rare disorders to larger regional referral centers. Although it is markedly inferior evidence to that generated by a prospective trial, the HDE, if protocols are followed, can encourage the generation of some useful, shareable data on outcomes of the intervention in question. Many centers are working together to review combined data and determine predictive factors for patient response to therapy (35–40) and possible mechanisms of action (38, 41–49). The more data available for review, the more we can understand these mechanisms and tailor the therapy to the needs of individual patients to improve outcomes.
With an HDE in place designed to improve access, the question becomes, “Why are so few patients with severely debilitating, medication refractory OCD, actually getting the treatment that could provide them such important benefit?” This review sought to identify common limiting factors. Only half of the patients deemed candidates for OCD DBS at our center since the HDE were treated. Because of policies widely adopted by private insurers, access to the procedure appears to be essentially limited to patients with Medicare, or those with uncommon wealth sufficient to pay out of pocket. Medicare coverage is limited to US citizens >65 years of age (not the typical age group of these patients), or those collecting Social Security Disability Insurance (SSDI) insurance for a minimum of 24 months. Qualifying for SSDI for psychiatric disorders is challenging, and requires extensive medical documentation of symptoms, treatment, and inability to function outside the home or inability to work for a minimum of 12 months. OCD patients who are appropriate candidates for DBS should generally qualify for SSDI, but to do so they must not work for 12 months, then be on disability for 24 months prior to qualifying for Medicare coverage for DBS. This represents a typical delay in treatment of over 3 years for patients with extraordinarily poor quality of life.
If a patient does not have, or cannot qualify for, Medicare coverage, they have two remaining options: pay out of pocket or travel to the nearest center conducting a fully funded clinical trial. Currently there are only six open trials of DBS for OCD according to ClinicalTrials.gov, and most presumably do not cover the cost of the procedure. Even if the cost of the procedure were covered, extensive travel is both logistically and financially prohibitive for most patients with severely debilitating OCD. Our one self-pay patient was required to pay $104,000 out of pocket prior to scheduling her surgery. Such out-of-pocket expenses are unobtainable for the vast majority of Americans, let alone those encumbered with burden of debilitating OCD. Based on our analysis, it appears that access to this potentially life altering therapy is extremely limited for the non-wealthy—an example of the far too common healthcare disparity in our current healthcare system.
How then do we correct this discrimination against the non-wealthy and avoid the prolonged suffering and treatment delays? Short of radical health care reform, the solution appears to be to persuade private insurers to cover DBS for OCD. We have tried traditional methods of appealing insurer's denial with evidence by published quantitative trials, cost-effectiveness trials, and peer-to-peer reviews with little avail. We are hopeful that highlighting the problem with publications like this one will have some persuasive effect. Helping policy makers to understand the terrible plight of these patients and the dramatically beneficial effect the therapy provides to most patients would presumably have a positive effect. Though citation of quantitative data, such as the fact that two thirds of otherwise treatment refractory patients respond to the treatment with >35% reduction in YBOCS scores, is helpful, sharing qualitative data describing patients' experiences may more powerfully illustrate the true benefit of the therapy. A few studies provide such qualitative data (25, 50–52), but are not published in high impact journals and probably have not been viewed by many insurance company executives or their medical consultants. Media attention to the issue might help advance the cause as might the publication of more cost-effectiveness studies such as those by Moon and Ooms (53, 54). These studies were carried out in Korea, United Kingdom (UK), and the Netherlands, but none have been carried out in the United States. Moon et al. used a Markov model to estimate cost-effectiveness of DBS vs. traditional therapy over a 10 year horizon and report a ratio based on quality-adjusted life-year (QALY). They found a cost-effectiveness ratio of $37,865/QALY in Korea and $34,462/QALY in the UK. Ooms et al. used a 2-year prospective model and found €65,394 gain per QALY when using a rechargeable generator as is recommended in this subgroup given their high frequency use. These are substantial cost savings that could also be relayed to the insurers if they chose to evaluate long term benefits.
Though this review of our experience clearly shows that non-Medicare insurance is the most important factor limiting access to OCD DBS among patients deemed appropriate candidates for this therapy at our center, there are limitations that should be considered when interpreting our results. The cohort studied here is small with only 25 patients reviewed. Despite best efforts, the retrospective nature of the study leaves some incomplete data including the inability to determine the limiting factor in 1 untreated patient. Our inclusion criteria required the patient be evaluated by both the primary psychiatrist (HW) and another key member of the interdisciplinary board as a surrogate marker of being deemed an appropriate candidate for OCD DBS therapy because there was not a direct order that was trackable in the database. Through discussions with the primary psychiatrist, we learned that after several patients were unable to access the surgery due to poor insurance coverage, he began to routinely discuss this potential problem with patients prior to referring them for further evaluation by the interdisciplinary DBS board. After this discussion, some indeterminate number of patients opted not to proceed with interdisciplinary evaluation for DBS knowing they could not afford the therapy. We can therefore assume that our methodology in this study underestimates the problem, and the percentage of appropriate OCD DBS candidates who are ultimately denied access to the therapy is even greater than documented here.
Conclusion
DBS is an effective, often life-altering therapy for most appropriately-selected patients with severely debilitating, treatment refractory OCD, and appears to remain effective in limited long-term studies. Initial effectiveness studies provided sufficient evidence to persuade FDA reviewers to grant HDE approval in 2009 for use of Medtronic “Reclaim” bilateral anterior limb of internal capsule (ALIC) deep brain stimulation for the treatment of chronic, severe, treatment-resistant OCD in adult patients who have failed at least three SSRIs. Despite HDE approval, only 9 of 18 patients deemed appropriate candidates for OCD DBS therapy at the University of Florida since 2009 have undergone surgical intervention. This review identified inability to pay due to non-Medicare insurance coverage (private insurers refuse to pay) as the most common factor limiting access to the procedure. Our findings here provide a poignant example of the unfortunate disparities in access to quality care in the US healthcare system, which tends to discriminate against those with psychiatric disorders, and against the non-wealthy. Patients afflicted with severely debilitating OCD generally fall into both of these categories, and typically do not qualify for Medicare coverage. We urge policy makers, insurance companies, and hospital administrations to recognize this health care disparity and seek to rectify it.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Florida Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
HP-D and KF conceived the project idea. HP-D collected the data and wrote the draft. HW and KF were the treating physicians and edited the paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge University of Florida staff members Charles Jacobson, Franztler Pierre, Angela Fogarty, and Dana Mason who helped with data acquisition and provided insights to the individual patient situations.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.642503/full#supplementary-material
References
1. Humanitarian Device Exemption [Internet]. (2009). [cited 11/21/2020]. Available from: Humanitarian Device Exemption (HDE) (fda.gov).
2. Nuttin BCP, Demeulemeester H, Gybels J, Meyerson B. Electrical Stimulation in anterior limbs of internal capsules in patients with obsessive–compulsive disorder. Lancet. (1999) 354:1526. doi: 10.1016/S0140-6736(99)02376-4
3. Nuttin BJ, Gabriëls LA, Cosyns PR, Meyerson BA, Andréewitch S, Sunaert SG, et al. Long-term electrical capsular stimulation in patients with obsessive–compulsive disorder. Neurosurgery. (2003) 52:1263–74. doi: 10.1227/01.NEU.0000064565.49299.9A
4. Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, et al. Deep brain stimulation for refractory obsessive–compulsive disorder. Biol Psychiatry. (2005) 57:510–6. doi: 10.1016/j.biopsych.2004.11.042
5. Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive–compulsive disorder. Neuropsychopharmacology. (2006) 31:2384–93. doi: 10.1038/sj.npp.1301165
6. Goodman WK, Foote KD, Greenberg BD, Ricciuti N, Bauer R, Ward H, et al. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. (2010) 67:535–42. doi: 10.1016/j.biopsych.2009.11.028
7. Kumar KK, Appelboom G, Lamsam L, Caplan AL, Williams NR, Bhati MT, et al. Comparative effectiveness of neuroablation and deep brain stimulation for treatment-resistant obsessive–compulsive disorder: a meta-analytic study. J Neurol Neurosurg Psychiatry. (2019) 90:469–73. doi: 10.1136/jnnp-2018-319318
8. Greenberg BD, Gabriels LA, Malone DA, Rezai AR, Friehs GM, Okun MS, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive–compulsive disorder: worldwide experience. Mol Psychiatry. (2010) 15:64–79. doi: 10.1038/mp.2008.55
9. Islam L, Franzini A, Messina G, Scarone S, Gambini O. Deep brain stimulation of the nucleus accumbens and bed nucleus of stria terminalis for obsessive–compulsive disorder: a case series. World Neurosurg. (2015) 83:657–63. doi: 10.1016/j.wneu.2014.12.024
10. Farrand S, Evans AH, Mangelsdorf S, Loi SM, Mocellin R, Borham A, et al. Deep brain stimulation for severe treatment-resistant obsessive–compulsive disorder: an open-label case series. Aust N Z J Psychiatry. (2018) 52:699–708. doi: 10.1177/0004867417731819
11. Huys D, Kohl S, Baldermann JC, Timmermann L, Sturm V, Visser-Vandewalle V, et al. Open-label trial of anterior limb of internal capsule-nucleus accumbens deep brain stimulation for obsessive–compulsive disorder: insights gained. J Neurol Neurosurg Psychiatry. (2019) 90:805–12. doi: 10.1136/jnnp-2018-318996
12. Welter ML, Alves Dos Santos JF, Clair AH, Lau B, Diallo HM, Fernandez-Vidal S, et al. Deep brain stimulation of the subthalamic, accumbens, or caudate nuclei for patients with severe obsessive–compulsive disorder: a randomized crossover controlled study. Biol Psychiatry. (2020). doi: 10.1016/j.biopsych.2020.07.013. [Epub ahead of print].
13. Huff W, Lenartz D, Schormann M, Lee SH, Kuhn J, Koulousakis A, et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive–compulsive disorder: Outcomes after one year. Clin Neurol Neurosurg. (2010) 112:137–43. doi: 10.1016/j.clineuro.2009.11.006
14. Chabardès S, Polosan M, Krack P, Bastin J, Krainik A, David O, et al. Deep brain stimulation for obsessive–compulsive disorder: subthalamic nucleus target. World Neurosurg. (2013) 80:S31.e1–8. doi: 10.1016/j.wneu.2012.03.010
15. Tyagi H, Apergis-Schoute AM, Akram H, Foltynie T, Limousin P, Drummond LM, et al. A Randomized trial directly comparing ventral capsule and anteromedial subthalamic nucleus stimulation in obsessive–compulsive disorder: clinical and imaging evidence for dissociable effects. Biol Psychiatry. (2019) 85:726–34. doi: 10.1016/j.biopsych.2019.01.017
16. Winter L, Heitland I, Saryyeva A, Lütjens G, Schwabe K, Heissler HE, et al. Acute effects of electrical stimulation of the bed nucleus of the stria terminalis/internal capsule in obsessive–compulsive disorder. World Neurosurg. (2018) 111:e471-e7. doi: 10.1016/j.wneu.2017.12.084
17. Lee DJ, Dallapiazza RF, De Vloo P, Elias GJB, Fomenko A, Boutet A, et al. Inferior thalamic peduncle deep brain stimulation for treatment-refractory obsessive–compulsive disorder: a phase 1 pilot trial. Brain Stimul. (2019) 12:344–52. doi: 10.1016/j.brs.2018.11.012
18. Jiménez-Ponce F, Velasco-Campos F, Castro-Farfán G, Nicolini H, Velasco AL, Salín-Pascual R, et al. Preliminary study in patients with obsessive–compulsive disorder treated with electrical stimulation in the inferior thalamic peduncle. Neurosurgery. (2009) 65(6 Suppl.):203–9. discussion: 9. doi: 10.1227/01.NEU.0000345938.39199.90
19. Maarouf M, Neudorfer C, El Majdoub F, Lenartz D, Kuhn J, Sturm V. Deep Brain stimulation of medial dorsal and ventral anterior nucleus of the thalamus in OCD: a retrospective case series. PLoS ONE. (2016) 11:e0160750. doi: 10.1371/journal.pone.0160750
20. Coenen VA, Schlaepfer TE, Goll P, Reinacher PC, Voderholzer U, Tebartz van Elst L, et al. The medial forebrain bundle as a target for deep brain stimulation for obsessive–compulsive disorder. CNS Spectr. (2017) 22:282–9. doi: 10.1017/S1092852916000286
21. Nair G, Evans A, Bear RE, Velakoulis D, Bittar RG. The anteromedial GPi as a new target for deep brain stimulation in obsessive compulsive disorder. J Clin Neurosci. (2014) 21:815–21. doi: 10.1016/j.jocn.2013.10.003
22. Winter L, Saryyeva A, Schwabe K, Heissler HE, Runge J, Alam M, et al. Long-term deep brain stimulation in treatment-resistant obsessive–compulsive disorder: outcome and quality of life at four to eight years follow-up. Neuromodulation. (2021) 22:324–30. doi: 10.1111/ner.13232
23. Chabardes S, Krack P, Piallat B, Bougerol T, Seigneuret E, Yelnik J, et al. Deep brain stimulation of the subthalamic nucleus in obsessive–compulsive s disorders: long-term follow-up of an open, prospective, observational cohort. J Neurol Neurosurg Psychiatry. (2020) 91:1349–56. doi: 10.1136/jnnp-2020-323421
24. Graat I, Mocking R, Figee M, Vulink N, de Koning P, Ooms P, et al. Long-term outcome of deep brain stimulation of the ventral part of the anterior limb of the internal capsule in a cohort of 50 patients with treatment-refractory obsessive–compulsive disorder. Biol Psychiatry. (2020). doi: 10.1016/j.biopsych.2020.08.018. [Epub ahead of print].
25. Holland MT, Trapp NT, McCormick LM, Jareczek FJ, Zanaty M, Close LN, et al. Deep brain stimulation for obsessive–compulsive disorder: a long term naturalistic follow up study in a single institution. Front Psychiatry. (2020) 11:55. doi: 10.3389/fpsyt.2020.00055
26. Mallet L, Du Montcel ST, Clair AH, Arbus C, Bardinet E, Baup N, et al. Long-term effects of subthalamic stimulation in obsessive–compulsive disorder: follow-up of a randomized controlled trial. Brain Stimul. (2019) 12:1080–2. doi: 10.1016/j.brs.2019.04.004
27. Polosan M, Chabardes S, Bougerol T, Ardouin C, Pollak P, Benabid AL, et al. Long-term improvement in obsessions and compulsions with subthalamic stimulation. Neurology. (2016) 87:1843–4. doi: 10.1212/WNL.0000000000003248
28. Fayad SM, Guzick AG, Reid AM, Mason DM, Bertone A, Foote KD, et al. Six-nine year follow-up of deep brain stimulation for obsessive–compulsive disorder. PLoS ONE. (2016) 11:e0167875. doi: 10.1371/journal.pone.0167875
29. Grant JE, Odlaug BL, Chamberlain SR. Long-term deep-brain stimulation treatment for obsessive–compulsive disorder. J Clin Psychiatry. (2016) 77:132–3. doi: 10.4088/JCP.15cr09931
30. Ooms P, Mantione M, Figee M, Schuurman PR, van den Munckhof P, Denys D. Deep brain stimulation for obsessive–compulsive disorders: long-term analysis of quality of life. J Neurol Neurosurg Psychiatry. (2014) 85:153–8. doi: 10.1136/jnnp-2012-302550
31. Roh D, Chang WS, Chang JW, Kim CH. Long-term follow-up of deep brain stimulation for refractory obsessive–compulsive disorder. Psychiatry Res. (2012) 200:1067–70. doi: 10.1016/j.psychres.2012.06.018
32. Denys D, Graat I, Mocking R, de Koning P, Vulink N, Figee M, et al. Efficacy of deep brain stimulation of the ventral anterior limb of the internal capsule for refractory obsessive–compulsive disorder: a clinical cohort of 70 patients. Am J Psychiatry. (2020) 177:265–71. doi: 10.1176/appi.ajp.2019.19060656
33. Rossi JP GJ, Okun MS. The problem of funding off-label deep brain stimulation: bait-and-switch tactics and the need for policy reform. JAMA Neurol. (2017) 74:9–10. doi: 10.1001/jamaneurol.2016.2530
34. Administration UFaD. Humanitarian Device Exemption 2019 [Available from: www.fda.gov/medical-device/premarket-submissions/humanitarian-device-exemption.
35. Guzick A, Hunt PJ, Bijanki KR, Schneider SC, Sheth SA, Goodman WK, et al. Improving long term patient outcomes from deep brain stimulation for treatment-refractory obsessive–compulsive disorder. Expert Rev Neurother. (2020) 20:95–107. doi: 10.1080/14737175.2020.1694409
36. Baldermann JC, Melzer C, Zapf A, Kohl S, Timmermann L, Tittgemeyer M, et al. Connectivity profile predictive of effective deep brain stimulation in obsessive–compulsive disorder. Biol Psychiatry. (2019) 85:735–43. doi: 10.1016/j.biopsych.2018.12.019
37. Barcia JA, Avecillas-Chasín JM, Nombela C, Arza R, García-Albea J, Pineda-Pardo JA, et al. Personalized striatal targets for deep brain stimulation in obsessive–compulsive disorder. Brain Stimul. (2019) 12:724–34. doi: 10.1016/j.brs.2018.12.226
38. Liebrand LC, Caan MWA, Schuurman PR, van den Munckhof P, Figee M, Denys D, et al. Individual white matter bundle trajectories are associated with deep brain stimulation response in obsessive–compulsive disorder. Brain Stimul. (2019) 12:353–60. doi: 10.1016/j.brs.2018.11.014
39. Voon V. Toward precision medicine: prediction of deep brain stimulation targets of the ventral internal capsule for obsessive–compulsive disorder. Biol Psychiatry. (2019) 85:708–10. doi: 10.1016/j.biopsych.2019.03.969
40. Gentil AF, Lopes AC, Dougherty DD, Rück C, Mataix-Cols D, Lukacs TL, et al. Hoarding symptoms and prediction of poor response to limbic system surgery for treatment-refractory obsessive–compulsive disorder. J Neurosurg. (2014) 121:123–30. doi: 10.3171/2014.2.JNS131423
41. Karas PJ, Lee S, Jimenez-Shahed J, Goodman WK, Viswanathan A, Sheth SA. Deep brain stimulation for obsessive–compulsive disorder: evolution of surgical stimulation target parallels changing model of dysfunctional brain circuits. Front Neurosci. (2018) 12:998. doi: 10.3389/fnins.2018.00998
42. Rappel P, Marmor O, Bick AS, Arkadir D, Linetsky E, Castrioto A, et al. Subthalamic theta activity: a novel human subcortical biomarker for obsessive compulsive disorder. Transl Psychiatry. (2018) 8:118. doi: 10.1038/s41398-018-0165-z
43. Baldermann JC, Bohn KP, Hammes J, Schüller CB, Visser-Vandewalle V, Drzezga A, et al. Local and global changes in brain metabolism during deep brain stimulation for obsessive–compulsive disorder. Brain Sci. (2019) 9:220. doi: 10.3390/brainsci9090220
44. Calzà J, Gürsel DA, Schmitz-Koep B, Bremer B, Reinholz L, Berberich G, et al. Altered cortico-striatal functional connectivity during resting state in obsessive–compulsive disorder. Front Psychiatry. (2019) 10:319. doi: 10.3389/fpsyt.2019.00319
45. Park HR, Kim IH, Kang H, McCairn KW, Lee DS, Kim BN, et al. Electrophysiological and imaging evidence of sustained inhibition in limbic and frontal networks following deep brain stimulation for treatment refractory obsessive compulsive disorder. PLoS ONE. (2019) 14:e0219578. doi: 10.1371/journal.pone.0219578
46. Fridgeirsson EA, Figee M, Luigjes J, van den Munckhof P, Schuurman PR, van Wingen G, et al. Deep brain stimulation modulates directional limbic connectivity in obsessive–compulsive disorder. Brain. (2020) 143:1603–12. doi: 10.1093/brain/awaa100
47. Li N, Baldermann JC, Kibleur A, Treu S, Akram H, Elias GJB, et al. A unified connectomic target for deep brain stimulation in obsessive–compulsive disorder. Nat Commun. (2020) 11:3364. doi: 10.1038/s41467-020-16734-3
48. Smith EE, Schüller T, Huys D, Baldermann JC, Andrade P, Allen JJ, et al. A brief demonstration of frontostriatal connectivity in OCD patients with intracranial electrodes. Neuroimage. (2020) 220:117138. doi: 10.1016/j.neuroimage.2020.117138
49. Sullivan CRP, Olsen S, Widge AS. Deep brain stimulation for psychiatric disorders: From focal brain targets to cognitive networks. Neuroimage. (2020) 225:117515. doi: 10.1016/j.neuroimage.2020.117515
50. Bosanac P, Hamilton BE, Lucak J, Castle D. Identity challenges and 'burden of normality' after DBS for severe OCD: a narrative case study. BMC Psychiatry. (2018) 18:186. doi: 10.1186/s12888-018-1771-2
51. de Haan S, Rietveld E, Stokhof M, Denys D. Effects of deep brain stimulation on the lived experience of obsessive–compulsive disorder patients: in-depth interviews with 18 Patients. PLoS ONE. (2015) 10:e0135524. doi: 10.1371/journal.pone.0135524
52. de Haan S, Rietveld E, Stokhof M, Denys D. Becoming more oneself? Changes in personality following DBS treatment for psychiatric disorders: Experiences of OCD patients and general considerations. PLoS ONE. (2017) 12:e0175748. doi: 10.1371/journal.pone.0175748
53. Moon W, Kim SN, Park S, Paek SH, Kwon JS. The cost-effectiveness of deep brain stimulation for patients with treatment-resistant obsessive–compulsive disorder. Medicine (Baltimore). (2017) 96:e7397. doi: 10.1097/MD.0000000000007397
Keywords: deep brain stimulation, obsessive—compulsive disorder, humanitarian device exemption (HDE), healthcare disparities, YBOCS = Yale-Brown Obsessive Compulsive Scale
Citation: Pinckard-Dover H, Ward H and Foote KD (2021) The Decline of Deep Brain Stimulation for Obsessive–Compulsive Disorder Following FDA Humanitarian Device Exemption Approval. Front. Surg. 8:642503. doi: 10.3389/fsurg.2021.642503
Received: 16 December 2020; Accepted: 10 February 2021;
Published: 12 March 2021.
Edited by:
Nicholas Thomas Trapp, Stanford University, United StatesReviewed by:
Darin D. Dougherty, Massachusetts General Hospital and Harvard Medical School, United StatesMartijn Figee, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2021 Pinckard-Dover, Ward and Foote. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heather Pinckard-Dover, aHBpbmNrYXJkQGdtYWlsLmNvbQ==
 Heather Pinckard-Dover
Heather Pinckard-Dover Herbert Ward2,3
Herbert Ward2,3 Kelly D. Foote
Kelly D. Foote