- 1Department of Neurological Surgery, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, United States
- 2Department of Neurological Surgery, Columbia University Medical Center, New York, NY, United States
- 3Department of Neurobiology and Behavior, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, United States
Objective: Severe traumatic brain injury (sTBI) often results in disorders of consciousness. Patients emerging from coma frequently exhibit aberrant behaviors such as agitation. These non-purposeful combative behaviors can interfere with medical care. Interestingly, agitation is associated with arousal and is often among the first signs of neurological recovery. A better understanding of these behaviors may shed light on the mechanisms driving the return of consciousness in sTBI patients. This study aims to investigate the association between posttraumatic agitation and the recovery of consciousness.
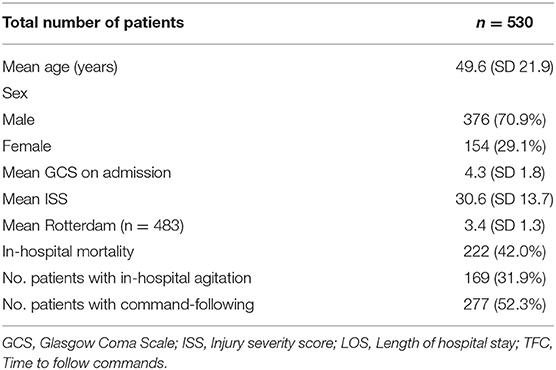
Methods: A retrospective chart review was conducted in 530 adult patients (29.1% female) admitted to Stony Brook University Hospital between January 2011 and December 2019 with a diagnosis of sTBI and Glasgow Coma Scale (GCS) ≤8. Agitation was defined as a Richmond Agitation Sedation Scale (RASS) > +1, or any documentation of equivalently combative and violent behaviors in daily clinical notes. The ability to follow verbal commands was used to define the recovery of consciousness and was assessed daily.
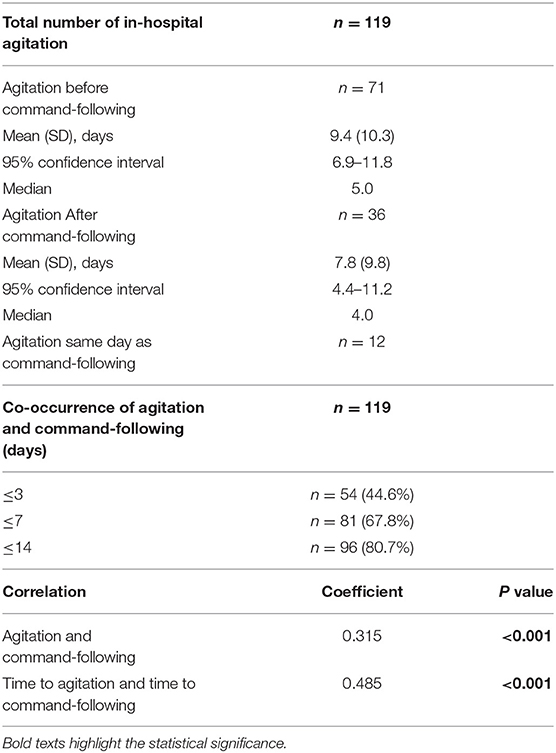
Results: Of 530 total sTBI patients, 308 (58.1%) survived. Agitation was present in 169 of all patients and 162 (52.6%) of surviving patients. A total of 273 patients followed commands, and 159 of them developed agitation. Forty patients developed agitation on hospital arrival whereas 119 developed agitation later during their hospital course. Presence of in-hospital agitation positively correlated with command-following (r = 0.315, p < 0.001). The time to develop agitation and time to follow commands showed positive correlation (r = 0.485, p < 0.001). These two events occurred within 3 days in 54 (44.6%) patients, within 7 days in 81 (67.8%) patients, and within 14 days in 96 (80.2%) patients. In 71 (59.7%) patients, agitation developed before command-following; in 36 (30.2%) patients, agitation developed after command-following; in 12 (10.1%) patients, agitation developed on the same day as command-following.
Conclusion: Posttraumatic agitation in comatose patients following sTBI is temporally associated with the recovery of consciousness. This behavior indicates the potential for recovery of higher neurological functioning. Further studies are required to identify neural correlates of posttraumatic agitation and recovery of consciousness after sTBI.
Introduction
Severe traumatic brain injury (sTBI) often results in disorders of consciousness (1). Patients in comatose states can exhibit aberrant motor behaviors, characterized by hyperactivity, non-purposeful movement, and combativeness that are disruptive to patient care (2, 3). Agitation is frequently encountered in sTBI patients in the hospital setting (4, 5). The prevalence of in-hospital agitation in patients following sTBI is between 10 and 90% (2, 5). This large variability can be explained by differences in diagnostic criteria and under- or over-recognition of these behaviors (6, 7). Interestingly, these behaviors represent fluctuations of consciousness during periods when patients reemerge from posttraumatic coma (8). Thus, a thorough understanding of the mechanisms driving agitation is imperative for patient safety and facilitation of recovery-directed treatment for sTBI.
The underlying mechanisms of posttraumatic agitation are poorly understood. Several theories have proposed that this behavior is likely secondary to alterations in brain metabolism, dysregulation of neural transmission, and remodeling of neural network (9). Dysregulation of dopaminergic neurotransmission, for example, often correlates with behavioral changes after neurologic insults (10). Pharmacotherapies, such as amantadine, have been used to facilitate arousal by stimulating the dopaminergic pathway (11). Randomized controlled clinical trials have shown that amantadine promotes cognitive recovery in sTBI patients who are in prolonged coma (12). Amantadine has also been reported to increase the incidence of in-hospital agitation when used in the early stage after TBI (13). These findings suggest that agitation and arousal likely share the same neurological pathways and are associated with the recovery of consciousness.
The first-line treatment for agitation is by providing patient-centered care through environmental changes, such as sufficient pain relief, light-dark cycle simulation, and nursing staff consistency (14). Antipsychotics are commonly used to manage agitation in critically ill patients who are unresponsive to conservative measures (15). Both typical and atypical antipsychotics suppress dopaminergic neurotransmission and have been used as prophylaxis and/or treatment for confusion and agitation, but recent studies suggest that the efficacy is limited (16). Importantly, the safety of using antipsychotics in sTBI patients is not known with certainty (17–19). Retrospective clinical studies have shown that using antipsychotics to treat posttraumatic delirium can impair cognitive recovery (18). Evidence from TBI animal models suggests that both typical and atypical antipsychotics hinder recovery from TBI-related deficits (20–22). These results suggest that dopaminergic suppression interferes with arousal and consequently recovery of consciousness. Currently, two randomized controlled clinical trials are being conducted to evaluate the safety and efficacy of antipsychotics in sTBI patients in rehabilitation settings (23, 24). Results from these studies can shed light on the underlying mechanism of agitation during the early stage of recovery from sTBI.
Given the clinical significance of posttraumatic agitation in patients emerging from disorders of consciousness, early recognition and appropriate management will benefit neurological outcomes. The balance between promoting arousal in early stage of recovery from sTBI and the risk of developing agitation is often patient-specific and is difficult to achieve. Understanding the clinical features of posttraumatic agitation and its relationship to arousal can establish patient-specific treatment plans, as these goal-directed behaviors are often among the first signs of returning of consciousness. In the present study, we evaluate the relationship between posttraumatic agitation and the recovery of consciousness in comatose patients following sTBI in the acute hospital setting. By identifying the association between the two, we emphasize the clinical importance of agitated behaviors, and advise prudent clinical management.
Methods
Subjects
Adult patients (age ≥18 at time of injury) admitted to Stony Brook University Hospital, a level 1 trauma center in Long Island, New York, from January 2011 to December 2019 with a clinical diagnosis of sTBI and Glasgow Coma Scale (GCS) ≤ 8 were included in the analysis. All study procedures were approved by the Stony Brook University Committee on Research in Human Subjects, in accordance with federal guidelines.
Data Collection
Data was collected retrospectively from individual patient charts. Admission profiles such as age, gender, GCS, injury severity score (ISS), and mechanisms of injury were used to describe baseline patient characteristics. When available, non-contrast CT scans were reviewed and evaluated using the Rotterdam head CT scoring system (25). Levels of agitation and sedation were assessed hourly by nursing stuff using the Richmond Agitation Sedation Scale (RASS) (6). The RASS is a validated tool used to guide sedation therapy in hospitalized patients. The scale uses a rating severity from−5 (unarousable) to +4 (combative). RASS > +1, or documentation of agitated behaviors that were equivalently combative was used to define agitation in our cohort. These behaviors were characterized by non-purposeful movement, patient-ventilator dyssynchrony, or self-harming behaviors, and required immediate medical intervention, such as higher dose of sedation or physical restraints. The ability to follow verbal commands (GCS motor score = 6) was used to define recovery of consciousness and was evaluated daily by clinicians. The date of first reported agitation and the date of following commands were used to analyze correlation between the two. Length of hospital stay (LOS), time to follow commands (TFC), discharge GCS, and discharge location were used as outcome measures.
Statistical Analysis
Descriptive statistics are reported as percentage, mean, or standard deviation wherever appropriate. Extreme values (≥3 standard deviations from the mean of the original dataset) were removed for more accurate data representation. For between-group comparisons, two-tailed Student's t-test and Chi-square were used to test statistical significance of continuous and categorical variables, respectively. Statistical significance was defined as p < 0.05. Statistical analysis was performed using SPSS, version 26 (IBM Corp).
Results
Patient Characteristics
A total of 530 patients (29.1% female) were included in this study based on the eligibility criteria (Table 1). The most common cause of injury was road traffic accidents including motor vehicle, motorcycle, bicycle accidents, and pedestrian struck, accounting for a total of 282 (53.7%) cases. Fall from standing and elevated heights account for 196 (37.3%) cases. Other mechanisms of injury included violence and gunshot wounds (Figure 1). Of the 530 patients, 222 (41.9%) patients died as a result of their injuries. Of the 308 surviving patients, the average age was 44.8 (SD 19.6) years, ranging from 18 to 94 years old. The mean GCS on admission was 4.6 (SD 1.9), the mean injury severity score (ISS) was 27.9 (SD 13.0), and the mean Rotterdam score was 2.9 (SD 1.0). The mean length of hospital stay (LOS) was 34.4 (SD 36.3) days, ranging from 2 to 245 days. Among all surviving patients, 273 (88.6%) individuals recovered consciousness by following verbal commands during their hospitalization. The mean time to follow commands (TFC) was 10.1 (SD 12.3) days, ranging from 1 to 72 days. A majority of patients were discharged to rehabilitation facilities (63.0%) or home (26.6%), and the rest to nursing home/hospice (8.1%) or another hospital (2.3%). Most patients had a favorable course of recovery and were discharged with a significantly higher GCS compared to that on admission. Discharge GCS 13–15 was seen in 249 (80.8%) patients, GCS 9–12 seen in 33 (10.7%) patients, and GCS 3–8 seen in 26 (8.5%) patients (Table 2).
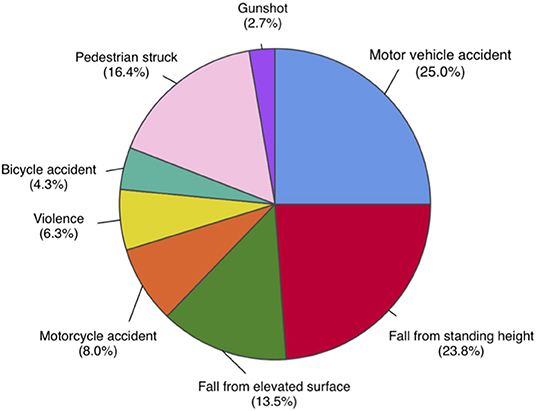
Figure 1. Distribution of mechanisms of injury in total study population, n = 530. Road traffic accidents, including motor vehicle, motorcycle, bicycle accidents, and pedestrian struck, are most common mechanisms of injury, accounting for a total of 282 (53.7%) cases. Fall from elevated and standing heights is second most common, accounting for 196 (37.3%) cases. Other mechanisms include violence and gunshot wounds.
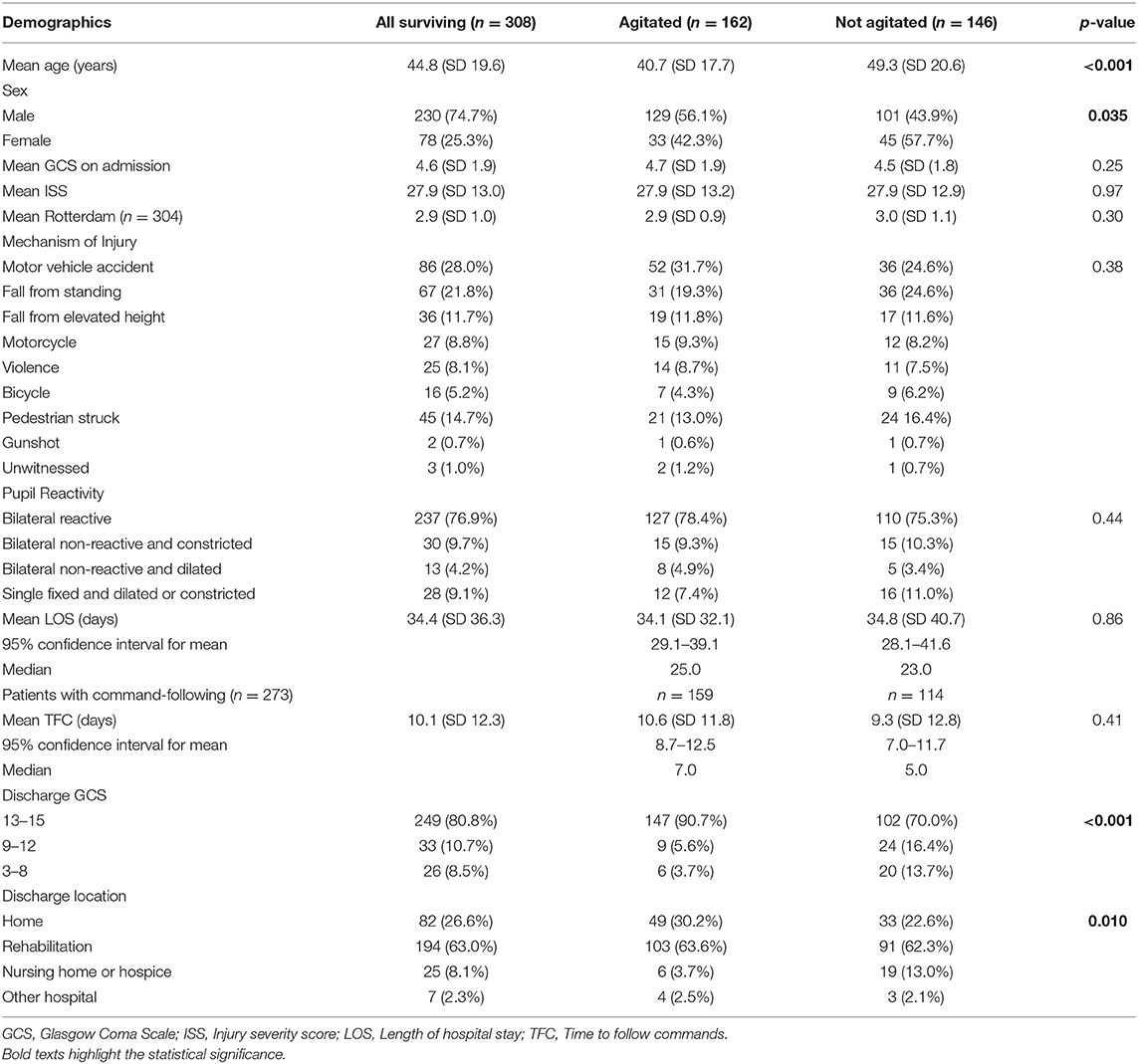
Table 2. Patient characteristics and clinical outcomes of surviving agitated and non-agitated patients, n = 308.
Characteristics of Agitated Patients
Agitation was observed in 162 (52.6%) of 308 surviving patients, and 159 of these individuals followed commands (Figure 2). The agitated cohort was on average 8.6 years younger than the non-agitated one (40.7 vs. 49.3 years, p < 0.001, Table 2). Males were more likely to develop agitation than females (56.1 vs. 42.3%, p = 0.035). There were no significant differences between agitated and non-agitated patients in admission GCS (4.7 vs. 4.5, p = 0.25), ISS (27.9 vs. 27.9, p = 0.97), and Rotterdam scores (2.9 vs. 3.0, p = 0.30), and pupil reactivity (p = 0.44). The mechanisms of injury have similar distributions between agitated and non-agitated patients, with road traffic accidents being the most common, followed by falls. A larger proportion of agitated patients were discharged with GCS 13–15 compared to the non-agitated ones (90.7 vs. 70.0%, p < 0.001, Table 2). Agitated patients were more frequently discharged home or to rehabilitation facilities, whereas a larger proportion of non-agitated patients were discharged to nursing home or hospice (p = 0.010). There were no significant differences in LOS (34.1 vs. 34.8 days, p = 0.86) or TFC (10.6 vs. 9.3 days, p = 0.41) between agitated and non-agitated patients (Table 2).
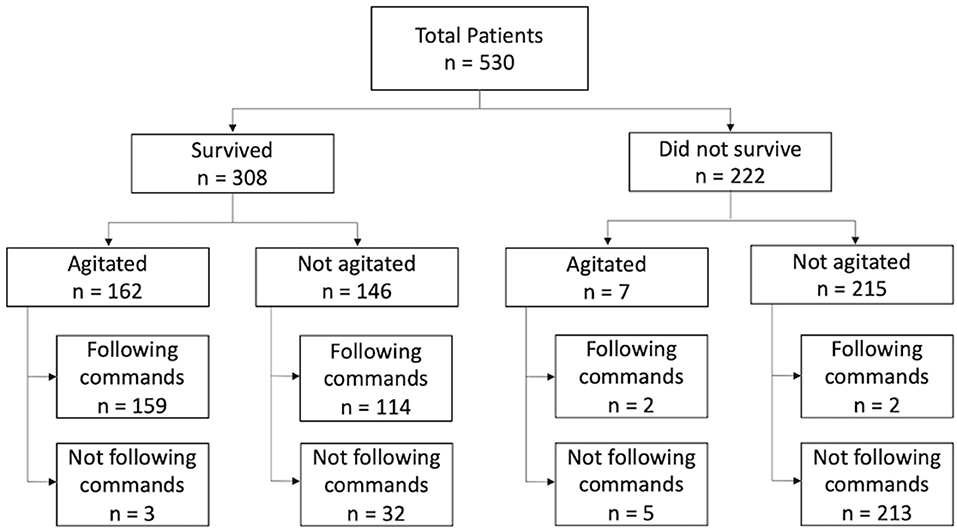
Figure 2. Flowchart of total study population, n = 530. Survival status was determined by retrospective chart review. Seven patients developed agitation but subsequently expired due to failure to wean off mechanical ventilation.
Agitation Correlates With Recovery of Consciousness
The ability to follow commands (GCS = 6) was achieved in 273 of all surviving patients, and agitation was observed in 159 (58.2%) of them (Figure 3). A positive correlation was observed between the presence of agitation and that of command-following (r = 0.315, p < 0.001). Time to develop agitation and time to follow commands was also positively correlated (r = 0.485, p < 0.001). Of these patients, 40 (24.7%) showed signs of agitation upon arrival to the hospital, whereas 119 (75.3%) patients arrived in a comatose state and developed agitation later during their hospital course. In these 119 patients, a temporal association was observed between the time of agitation onset and time to follow commands (Figure 3). In 54 (44.6%) of these patients, agitation and command-following were observed within 3 days of each other; in 81 (67.8%) patients, these two events occurred within 7 days; and in 96 (80.7%) patients, co-occurrence was observed within 14 days. Among these 119 patients, 71 (59.7%) developed signs of agitation before command-following by an average of 9.4 (SD 10.3) days, ranging from 1 to 47 days (95% CI: 6.9–11.8 days); 36 (30.2%) patients developed agitation after command-following by an average of 7.8 (SD 9.8) days, ranging from 1 to 40 days (95% CI: 4.4–11.2 days); and 12 (10.1%) patients developed agitation on the same day as command-following (Table 3).
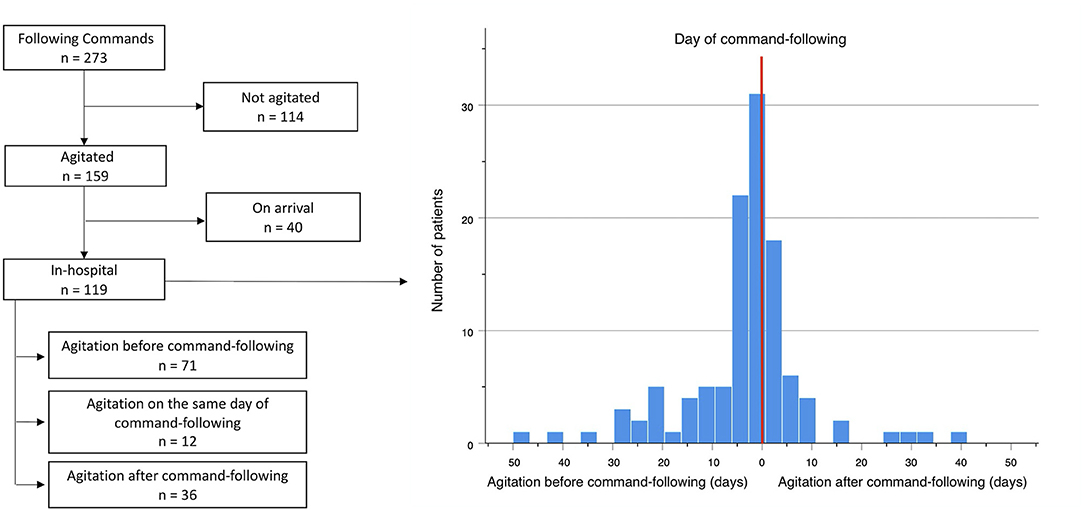
Figure 3. Temporal association between onset of agitation and command-following, n = 119. Red line represents the day the patient begins following commands. X-axis shows numbers of days from agitation onset to command-following. Left to the red line represents days from agitation to command-following. Right to the red line represents days from command following to agitation. Y-axis corresponds to the number of patients.
Antipsychotic Use in Agitated Patients Does Not Affect Clinical Outcomes
Seventy-five (47.2%) patients with agitation received antipsychotic medication as a treatment for agitated symptoms, and 32 of them received more than one type of antipsychotics. Haloperidol was the most commonly administered antipsychotic medication (n = 43, 57.3%), followed by quetiapine (n = 39, 52.0%). Other antipsychotics included olanzapine (n = 26, 34.7%), risperidone (n = 6, 8.0%), ziprasidone (n = 1, 1.3%) and aripiprazole (n = 1, 1.3%). Baseline patient characteristics, such as age, sex, admission GCS, ISS, or Rotterdam score, were not different between patients who received antipsychotics and those who did not (Table 4). There were no significant differences in LOS (34.5 vs. 28.7 days, p = 0.16), TFC (9.6 vs. 10.7 days, p = 0.55), discharge GCS (p = 0.13), or discharge location (p = 0.19) between patients who received antipsychotics vs. those who did not (Table 4). Administration of multiple types of antipsychotics compared to a single type or the use of haloperidol did not correlate with a change in LOS, TFC, discharge GCS, or discharge location.
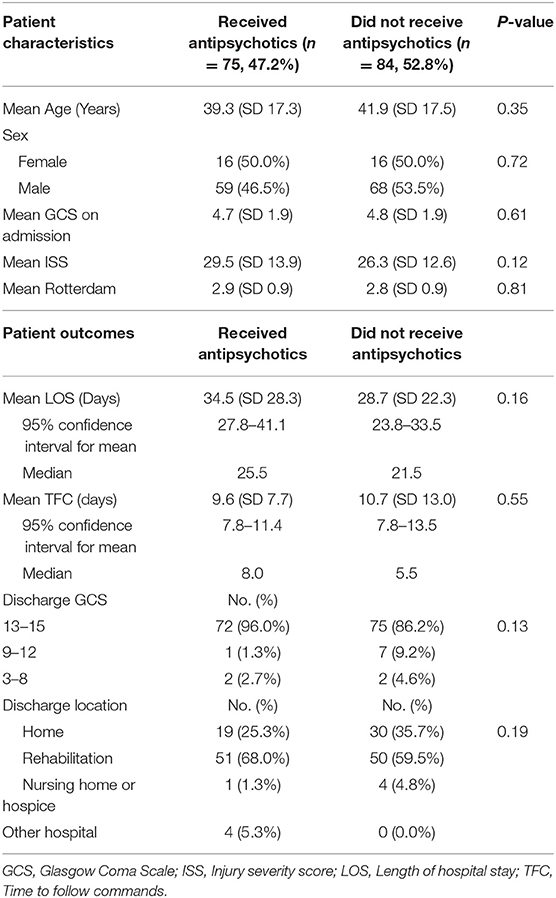
Table 4. Comparing patient characteristics and clinical outcomes between patients who received antipsychotics for agitation vs. those who did not receive antipsychotics, n = 159.
Discussion
The present study is by far the largest series reporting the association between posttraumatic agitation and recovery of consciousness during early stage of sTBI in adult patients. Our data shows that agitation often accompanies the ability to respond to verbal commands and can be viewed an early sign of neurological recovery. Among all 169 patients who developed agitation, 162 (95.5%) survived, and 159 (94.1%) followed commands. This result suggests that posttraumatic agitation strongly correlates with command-following. Thus, in minimally conscious patients, agitation can be viewed as a positive sign that these patients are more likely to “wake up” and exhibit more meaningful signs of neurological recovery. Following severe trauma, the brain undergoes rapid biochemical, hormonal, and structural network remodeling (9), and these processes may manifest as agitated behaviors. Our results show that agitated patients often have better clinical outcomes at hospital discharge compared to non-agitated individuals. A higher percentage of agitated patients were discharged with GCS 13–15; agitated patients were more frequently discharged home or to rehabilitation facilities instead of nursing home or hospice. This difference may be partly explained by the younger age of the agitated group seen in our analysis. The length of hospital stay (LOS) and time to follow commands (TFC) did not differ between the agitated and the non-agitated groups. Although agitated behaviors pose challenges to medical care, their presence appears to reassure favorable prognoses.
The etiology of agitated behaviors in critically ill patients following sTBI is multifactorial (26), Pain and infection, for example, cause systemic catecholamine dysregulation and psychomotor disturbance. In comatose patients emerging from disorders of consciousness, being able to regain awareness to immediate surrounding is a sign of arousal (27). As patients undergo further neurological recovery, they develop the ability to interfere with medical treatment to minimize discomfort. The reemergence of these goal-directed behaviors, clinically present as severe agitation, strongly suggests the recovery of consciousness (27). In the sTBI population, continuous sedation is administered due to the need for mechanical ventilation, and clinical improvement prompts discontinuation of these interventions. Interestingly, agitation is often observed during these periods, as patients demonstrate non-purposeful movements and self-harming behaviors when recovering from coma. Attempts to disrupt patient-ventilator synchrony to minimize discomfort is an example of early goal-directed behavior that indicates one's awareness of immediate surroundings. These behaviors are highly suggestive of the potential for more complex and meaningful recovery of higher-level neurological functions.
Clinical management of agitation in sTBI patients consists of environmental changes and adequate sedation to minimize patient discomfort (14). When these conservative measures fail, antipsychotics are the mainstay of treatment for agitation. In our study, nearly half of agitated patients received antipsychotics. A previous study suggests that antipsychotics have neuroprotective effects in critically-ill TBI patients, and thus improve in-hospital outcomes (28). In the present study, the use of antipsychotics did not correlates with LOS, TFC, discharge GCS, or discharge location. These outcomes were also not different between groups that received multiple types vs. single type, or those who received typical vs. atypical antipsychotics. Despite that antipsychotics may reduce symptoms of agitation in acute settings (29), its long-term effects on overall cognitive functions have not been thoroughly investigated (30, 31). Moreover, suppression of dopaminergic neurotransmission can be unfavorable for posttraumatic arousal as clinical trials in amantadine have shown efficacy (12). The ongoing randomized controlled clinical trials using antipsychotics to treat posttraumatic agitation in subacute rehabilitation settings may shed light on the management of emotional dysregulation as a result of sTBI (23, 24). The treatment of posttraumatic agitation in acute hospital setting requires further large-scale prospective randomized studies with long-term follow up. When managing agitated symptoms, clinicians should bear in mind that they are often a positive clinical sign that indicates a vibrant state of recovering consciousness.
Finally, posttraumatic agitation was found in 31.9% of our total study population, and 95.9% of these patients survived. The agitated cohort was on average 8.6 years younger than the non-agitated cohort. This result is consistent with an observational study conducted in a trauma intensive care unit that investigated all types of traumatic injuries (32). Additionally, we found that a larger proportion of male patients developed posttraumatic agitation compared to females. Sex hormones are recognized as key modulators of the pathogenic process after TBI by affecting brain metabolism and neural repair (33). During the early phase after TBI, neuroendocrine dysregulation alters the ratios of estrogen, progesterone, and testosterone. This process plays an important role in TBI recovery and rehabilitation (34). Further research is required to understand these molecular and biochemical mechanisms in order to develop effective treatments that facilitate both short-term recovery of consciousness and long-term cognitive functioning.
Future studies using advanced neuroimaging have the potential to reveal patient-specific factors and aberrant neural activity that predispose sTBI patients to posttraumatic agitation. In our study, 41.5% of patients recovered consciousness but did not develop agitation. This result may be explained by patient-specific characteristics and/or lesions to specific locations or neural network. Previous studies conducted in patients with penetrating TBI suggest that lesions in the prefrontal cortex are highly predictive of long-term agitation and aggression (35). Identification of neural correlates of agitation in sTBI patients can facilitate the development of therapeutic strategies for treating these disruptive behaviors.
Limitations
There are several limitations to our study. First, this study is based on a retrospective chart review of adults (≥18 years) with a diagnosis of sTBI (GCS ≤ 8). The results only infer associations but not causal relationships within this population. Second, in our study, the Richmond Agitation Sedation Scale (RASS) and daily clinical assessment were used to diagnose agitation. Other measures, such as the Agitated Behavior Scale, use different criteria to characterize agitation and delirium when compared with the RASS. This makes comparisons with other studies difficult. Third, injury site was not included in the analysis. Many patients suffered injuries at various locations and subcategorizing patients to isolate the effects of injury site was difficult. Finally, the return of consciousness was defined as the ability to follow motor commands. Some patients may recover from comatose state, yet, be unable to consistently follow commands due to neuromuscular insufficiency (36). Outcome data were limited to the hospital stay only, and the authors cannot comment on the impact of the above factors on any longer-term outcome measures. Future prospective studies with long-term follow up are needed to reveal the underlying neurological mechanisms of in-hospital agitation in sTBI patients to better understand its clinical indication and to provide appropriate management.
Conclusion
Severe traumatic brain injury (sTBI) commonly results in disorders of consciousness. This study suggests that the presence of agitation during the early stage after sTBI is a clinical sign associated with command-following and is associated with recovery of consciousness. Additionally, patients with agitation often demonstrate more favorable clinical outcomes. Management of agitation should be based on the consideration of patient characteristics, risk and benefit of pharmacological intervention, and effects on long-term cognitive functioning. Using antipsychotics to reduce agitated symptoms does not affect in-hospital outcomes, but its effects on neurocognitive recovery remain inconclusive. Clinicians should bear in mind that agitation is a sign of recovery of consciousness when treating this condition and counseling families.
Data Availability Statement
The data supporting the conclusions of this article will be made available by the authors at reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Stony Brook University Committee on Research in Human Subjects. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
ZW, NW, ZZ, and MC collected data. ZW completed statistical calculations and wrote the manuscript. NW, CM and SM assisted in study design and critically revised the manuscript. TG, JS, and RM revised the manuscript. SF managed the database. CM and SM provided overall supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Growing Convergence Research program (NSF Award 2021002). A SEED grant funding from the Office of the Vice President for Research at Stony Brook University. A FUSION-TRO award (63845) from the Renaissance School of Medicine at Stony Brook University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Bradley Ashcroft, Justine Liang, and Matthew Morris for thoughtful discussion and data collection.
References
1. Bruns J Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. (2003) 44:2–10. doi: 10.1046/j.1528-1157.44.s10.3.x
2. Stefan A, Mathe JF, Group S. What are the disruptive symptoms of behavioral disorders after traumatic brain injury? A systematic review leading to recommendations for good practices. Ann Phys Rehabil Med. (2016) 59:5–17. doi: 10.1016/j.rehab.2015.11.002
3. Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. (2018) 33:1428–57. doi: 10.1002/gps.4823
4. Mcnett M, Sarver W, Wilczewski P. The prevalence, treatment and outcomes of agitation among patients with brain injury admitted to acute care units. Brain Inj. (2012) 26:1155–62. doi: 10.3109/02699052.2012.667587
5. Pouwels CGJG, Wolters Grégorio G, Spauwen PJJ, Bus BAA, Winkens I, Ponds RWHM. Prevalentie en uitingsvormen van agressie bij volwassen patiënten met niet-aangeboren hersenletsel: een literatuuroverzicht [Prevalence and manifestations of aggression in adult patients with acquired brain injury: a review]. Tijdschr Psychiatr. (2019) 61:862–78.
6. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. (2002) 166:1338–44. doi: 10.1164/rccm.2107138
7. Bogner JA, Corrigan JD, Stange M, Rabold D. Reliability of the agitated behavior scale. J Head Trauma Rehabil. (1999) 14:91–6. doi: 10.1097/00001199-199902000-00012
8. Lombard LA, Zafonte RD. Agitation after traumatic brain injury: considerations and treatment options. Am J Phys Med Rehabil. (2005) 84:797–812. doi: 10.1097/01.phm.0000179438.22235.08
9. Ganau M, Lavinio A, Prisco L. Delirium and agitation in traumatic brain injury patients: an update on pathological hypotheses and treatment options. Minerva Anestesiol. (2018) 84:632–40. doi: 10.23736/S0375-9393.18.12294-2
10. Jenkins PO, Mehta MA, Sharp DJ. Catecholamines and cognition after traumatic brain injury. Brain. (2016) 139:2345–71. doi: 10.1093/brain/aww128
11. Sawyer E, Mauro LS, Ohlinger MJ. Amantadine enhancement of arousal and cognition after traumatic brain injury. Ann Pharmacother. (2008) 42:247–52. doi: 10.1345/aph.1K284
12. Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. (2012) 366:819–26. doi: 10.1056/NEJMoa1102609
13. Gramish JA, Kopp BJ, Patanwala AE. Effect of amantadine on agitation in critically ill patients with traumatic brain injury. Clin Neuropharmacol. (2017) 40:212–6. doi: 10.1097/WNF.0000000000000242
14. Oddo M, Crippa IA, Mehta S, Menon D, Payen JF, Taccone FS, et al. Optimizing sedation in patients with acute brain injury. Crit Care. (2016) 20:128. doi: 10.1186/s13054-016-1294-5
15. Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the american association for emergency psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. (2012) 13:26–34. doi: 10.5811/westjem.2011.9.6866
16. Herling SF, Greve IE, Vasilevskis EE, Egerod I, Bekker Mortensen C, Moller AM, et al. Interventions for preventing intensive care unit delirium in adults. Cochrane Database Syst Rev. (2018) 11:CD009783. doi: 10.1002/14651858.CD009783.pub2
17. Williamson D, Frenette AJ, Burry LD, Perreault M, Charbonney E, Lamontagne F, et al. Pharmacological interventions for agitated behaviours in patients with traumatic brain injury: a systematic review. BMJ Open. (2019) 9:e029604. doi: 10.1136/bmjopen-2019-029604
18. Nikooie R, Neufeld KJ, Oh ES, Wilson LM, Zhang A, Robinson KA, et al. Antipsychotics for treating delirium in hospitalized adults: a systematic review. Ann Intern Med. (2019) 171:485–95. doi: 10.7326/M19-1860
19. Elovic EP, Jasey NN Jr, Eisenberg ME. The use of atypical antipsychotics after traumatic brain injury. J Head Trauma Rehabil. (2008) 23:132–5. doi: 10.1097/01.HTR.0000314532.07530.e5
20. Hoffman AN, Cheng JP, Zafonte RD, Kline AE. Administration of haloperidol and risperidone after neurobehavioral testing hinders the recovery of traumatic brain injury-induced deficits. Life Sci. (2008) 83:602–7. doi: 10.1016/j.lfs.2008.08.007
21. Wilson MS, Gibson CJ, Hamm RJ. Haloperidol, but not olanzapine, impairs cognitive performance after traumatic brain injury in rats. Am J Phys Med Rehabil. (2003) 82:871–9. doi: 10.1097/01.PHM.0000091982.33232.CB
22. Folweiler KA, Bondi CO, Ogunsanya EA, Laporte MJ, Leary JB, Radabaugh HL, et al. Combining the antipsychotic drug haloperidol and environmental enrichment after traumatic brain injury is a double-edged sword. J Neurotrauma. (2017) 34:451–8. doi: 10.1089/neu.2016.4417
23. Deb S, Leeson V, Aimola L, Bodani M, Li L, Weaver T, et al. Aggression following traumatic brain injury: effectiveness of risperidone (AFTER): study protocol for a feasibility randomised controlled trial. Trials. (2018) 19:325. doi: 10.1186/s13063-018-2601-z
24. Phyland RK, Mckay A, Olver J, Walterfang M, Hopwood M, Hicks AJ, et al. Use of olanzapine to treat agitation in traumatic brain injury: study protocol for a randomised controlled trial. Trials. (2020) 21:662. doi: 10.1186/s13063-020-04553-2
25. Maas AI, Steyerberg EW, Butcher I, Dammers R, Lu J, Marmarou A, et al. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. (2007) 24:303–14. doi: 10.1089/neu.2006.0033
26. Arciniegas DB, Mcallister TW. Neurobehavioral management of traumatic brain injury in the critical care setting. Crit Care Clin. (2008) 24:737–65, viii. doi: 10.1016/j.ccc.2008.06.001
27. Martens G, Bodien Y, Sheau K, Christoforou A, Giacino JT. Which behaviours are first to emerge during recovery of consciousness after severe brain injury? Ann Phys Rehabil Med. (2020) 63:263–9. doi: 10.1016/j.rehab.2019.10.004
28. Asmar S, Lokhandwala A, Richards J, Bible L, Avila M, Castanon L, et al. The neuroprotective effect of quetiapine in critically-ill traumatic brain injury patients. J Trauma Acute Care Surg. (2020) 89:775–82. doi: 10.1097/TA.0000000000002866
29. Rosati DL. Early polyneuropharmacologic intervention in brain injury agitation. Am J Phys Med Rehabil. (2002) 81:90–3. doi: 10.1097/00002060-200202000-00003
30. Kim E, Bijlani M. A pilot study of quetiapine treatment of aggression due to traumatic brain injury. J Neuropsychiatry Clin Neurosci. (2006) 18:547–9. doi: 10.1176/jnp.2006.18.4.547
31. Stanislav SW. Cognitive effects of antipsychotic agents in persons with traumatic brain injury. Brain Inj. (1997) 11:335–41. doi: 10.1080/026990597123494
32. Mahmood S, Mahmood O, El-Menyar A, Asim M, Al-Thani H. Predisposing factors, clinical assessment, management and outcomes of agitation in the trauma intensive care unit. World J Emerg Med. (2018) 9:105–12. doi: 10.5847/wjem.j.1920-8642.2018.02.004
33. Spani CB, Braun DJ, Van Eldik LJ. Sex-related responses after traumatic brain injury: considerations for preclinical modeling. Front Neuroendocrinol. (2018) 50:52–66. doi: 10.1016/j.yfrne.2018.03.006
34. Agha A, Rogers B, Mylotte D, Taleb F, Tormey W, Phillips J, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol. (2004) 60:584–91. doi: 10.1111/j.1365-2265.2004.02023.x
35. Pardini M, Krueger F, Hodgkinson C, Raymont V, Ferrier C, Goldman D, et al. Prefrontal cortex lesions and MAO-A modulate aggression in penetrating traumatic brain injury. Neurology. (2011) 76:1038–45. doi: 10.1212/WNL.0b013e318211c33e
Keywords: severe traumatic brain injury, coma, posttraumatic agitation, recovery of consciousness, antipsychotics
Citation: Wang Z, Winans NJ, Zhao Z, Cosgrove ME, Gammel T, Saadon JR, Mani R, Ravi B, Fiore SM, Mikell CB and Mofakham S (2021) Agitation Following Severe Traumatic Brain Injury Is a Clinical Sign of Recovery of Consciousness. Front. Surg. 8:627008. doi: 10.3389/fsurg.2021.627008
Received: 07 November 2020; Accepted: 22 March 2021;
Published: 21 April 2021.
Edited by:
Ziya Levent Gokaslan, Brown University, United StatesReviewed by:
Sabino Luzzi, University of Pavia, ItalyElias Shaaya, Rhode Island Hospital, United States
Copyright © 2021 Wang, Winans, Zhao, Cosgrove, Gammel, Saadon, Mani, Ravi, Fiore, Mikell and Mofakham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sima Mofakham, sima.mofakham@stonybrookmedicine.edu
 Zhe Wang
Zhe Wang Nathan J. Winans1,2
Nathan J. Winans1,2 Theresa Gammel
Theresa Gammel Bharadwaj Ravi
Bharadwaj Ravi Charles B. Mikell
Charles B. Mikell Sima Mofakham
Sima Mofakham