
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 29 April 2021
Sec. Surgical Oncology
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.604880
 Lei-Lei Wu1,2†
Lei-Lei Wu1,2† Wu-Tao Chen3†
Wu-Tao Chen3† Xuan Liu2
Xuan Liu2 Wen-Mei Jiang2
Wen-Mei Jiang2 Yang-Yu Huang2
Yang-Yu Huang2 Peng Lin2
Peng Lin2 Hao Long2
Hao Long2 Lan-Jun Zhang2
Lan-Jun Zhang2 Guo-Wei Ma2*
Guo-Wei Ma2*Background: In this study, we aim to establish a nomogram to predict the prognosis of non-small cell lung cancer (NSCLC) patients with stage I-IIIB disease after pneumonectomy.
Methods: Patients selected from the Surveillance, Epidemiology, and End Results (SEER, N = 2,373) database were divided into two cohorts, namely a training cohort (SEER-T, N = 1,196) and an internal validation cohort (SEER-V, N = 1,177). Two cohorts were dichotomized into low- and high-risk subgroups by the optimal risk prognostic score (PS). The model was validated by indices of concordance (C-index) and calibration plots. Kaplan-Meier analysis and the log-rank tests were used to compare survival curves between the groups. The primary observational endpoint was cancer-specific survival (CSS).
Results: The nomogram comprised six factors as independent prognostic indictors; it significantly distinguished between low- and high-risk groups (all P < 0.05). The unadjusted 5-year CSS rates of high-risk and low-risk groups were 33 and 60% (SEER-T), 34 and 55% (SEER-V), respectively; the C-index of this nomogram in predicting CSS was higher than that in the 8th TNM staging system (SEER-T, 0.629 vs. 0.584, P < 0.001; SEER-V, 0.609 vs. 0.576, P < 0.001). In addition, the PS might be a significant negative indictor on CSS of patients with white patients [unadjusted hazard ration (HR) 1.008, P < 0.001], black patients (unadjusted HR 1.007, P < 0.001), and Asian or Pacific Islander (unadjusted HR 1.008, P = 0.008). In cases with squamous cell carcinoma (unadjusted HR 1.008, P < 0.001) or adenocarcinoma (unadjusted HR 1.008, P < 0.001), PS also might be a significant risk factor.
Conclusions: For post-pneumonectomy NSCLC patients, the nomogram may predict their survival with acceptable accuracy and further distinguish high-risk patients from low-risk patients.
Lung cancer remains the most common type of cancer, as it contributed to one quarter of all cancer-related deaths in 2019. Overall, 13% of estimated new cancer cases in men and women are lung cancer, which is responsible for ~24% of estimated new deaths among the 10 leading cancer types; lung cancer also has one of the lowest 5-year survival rates at 19% (1). Multiple therapy options are available for patients with non-small cell lung cancer (NSCLC), such as surgery, chemotherapy, radiation, and targeted therapy (2–7). Previous studies revealed that some factors might have an effect on prognoses, such as the lymph nodes ratio (LNR), pleura invasion, and surgical approaches (8–10).
Pneumonectomy, which was first performed by Macewen in 1895, has now become a common procedure in thoracic surgery and has been applied to massive and centrally located carcinoma that cannot be completely resected by lobectomy or lesser resection (11). A research reported that the 30-day mortality rate of patients with pneumonectomy was 16–20% (12). With respect to the side of pneumonectomy, a retrospective study has suggested that right pneumonectomy patients with stage I and II NSCLC had a higher risk in the overall survival, induced partly by the physiological importance of the right lung (13). However, the 5-year overall survival rate of patients receiving pneumonectomy was 32%, which was not satisfying (14).
Nomogram, which is a graphical calculating device, has been widely applied in clinical studies to predict prognosis based on relevant clinical characteristics (15, 16). Consisting of scales for each variable, nomogram provides clinical doctors with a convenient and effective way to estimate risk and make decisions. For patients after pneumonectomy, researchers paid more attention to the perioperative complications and perioperative death rate. Recently, some researchers constructed nomograms to predict long-term survival outcomes of NSCLC patients with pneumonectomy based on hematological indicators (17, 18).
Of note, the impact of adjuvant therapy on patients undergoing pneumonectomy remains unclear and controversial. An international adjuvant lung cancer trial in 2004 demonstrated an improved survival among patients with completely resected NSCLC (P < 0.03) (19); however, pneumonectomy patients did not have a survival benefit from adjuvant chemotherapy. In addition, the benefit of post-operative radiotherapy (PORT) in pN2 NSCLC patients has been established in a large-scale retrospective study (3). Therefore, this study aims to develop a nomogram for predicting prognosis of NSCLC patients who undergo pneumonectomy. We hope that this prognostic model may provide some information for clinicians to decide on the strategy of follow-up management and adjuvant therapy in patients with stage IA-IIIB NSCLC.
We identified eligible patients from the Surveillance, Epidemiology, and End Results (SEER) database. Cases were identified using the NSCLC participant data file from the SEER, which contain deidentified patients level data that do not identify hospitals, health care providers, or patients. Institutional review board approval was waived by the Ethics Committee of Sun Yat-sen University Cancer Center. Tumor, nodes, and metastasis (TNM) stages were determined by the classification reported in the 8th American Joint Committee on Cancer (AJCC) Staging Manual. We enrolled a total of 2,373 patients from the SEER database. Patients were randomly divided into two cohorts: the training cohort (SEER-T, N = 1,196) and an internal validation cohort (SEER-V, N = 1,177), as shown in Figure 1. All patients recruited for this study met the following criteria: (1) patients underwent pneumonectomy; (2) patients had pathological confirmation of NSCLC; and (3) patients with stage IA-IIIB disease, and IIIB only included T3-4N2M0 tumors. Patients were excluded if they met these criteria: (1) patients with another malignant tumor; (2) patients with an incomplete follow-up; (3) patients who died within 1 month after surgery; and (4) patients under 18 years old.
The median follow-up time for patients in the SEER database was 70.0 months. Follow-ups were performed by office visits or telephone interviews. Cancer-specific survival (CSS) was defined as the interval between the time of resection to the time of death from cancer.
A comparison of categorical variables was conducted by Pearson's Chi-square test. A multivariate analysis was performed to evaluate the influences of gender, age, maximum tumor diameter, laterality of pneumonectomy, grade, TNM stage, primary tumor location, race, pleural invasion, LNR, chemotherapy, radiotherapy, and pathological subtypes on CSS. A two-sided P < 0.05 was considered statistically significant. The most valuable prognostic factors identified by the univariate analysis were confirmed by a multivariate Cox regression analysis and excluded other confounding factors that might have affected the survival. In addition, Kaplan–Meier analysis and the log-rank tests were used to compare survival curves between groups. Cases were censored at death or at the end of follow-up. The selection of CSS as a primary clinical end point was considered the most clinically relevant. We adopted a model development and validation approach, and used a randomized method to extract trained and validated data sets to maintain a transparent report of the multivariate predictive model on individual prognostic guidelines.
Patients' demographics and clinical characteristics were reported for the training cohort. We constructed a nomogram based on six factors: gender, age, laterality of pneumonectomy, LNR, tumor size, and TNM stage. The model for CSS was constructed using the linear predictor of the finalized model, which was derived from the training data set. We regarded the prognostic score (PS) derived from the nomogram as an evaluation tool. The PS depended on the contribution of each variable to CSS from the cox regression model we constructed. The variables which contributed to CSS was matched to the horizontal axis at the top of Figure 2A, and the PS each variable presented was added up as total points. Two cohorts (SEER-T, SEER-V) were split into low- and high-risk subgroups by the optimal PS among non-living patients in the training cohort, and a risk score cutoff was defined to classify patients in the validation cohorts. The optimal cutoff point of the PS and LNR was calculated using the “survminer” package in R 3.6.1 software (https://www.r-project.org/). Data were analyzed using R 3.6.1 and SPSS software (version 24; IBM Corp., USA). Indices of concordance (C-index) were generated for the discrimination of the multivariable prognostic model.

Figure 2. (A) Post-operative prognostic nomogram for patients after pneumonectomy with stage I-IIIB non-small-cell lung cancer. (B) Calibration curves for predicting cancer-specific survival of patients at each time point in the training cohort. (C) Calibration curves for predicting cancer-specific survival of patients at each time point in the internal validation cohort.
Overall, 2,373 patients were identified from the SEER database, including 1,196 patients in the training cohort (SEER-T) and 1,177 patients in the internal validation cohort (SEER-V).
In the SEER-T and SEER-V cohorts, a large proportion of patients was under the age of 65 years (60.9% in SEER-T and 64.5% in SEER-V). Male patients were predominant in both cohorts (67.6% in the SEER-T and 66.0% in the SEER-V). A tumor located in the left lung was quite common and had a distribution of 57.9% in the SEER-T and 59.0% in the SEER-V. The median survival time was 56.0 months for patients in the SEER-T and 48.0 months in the SEER-V. The clinical information of patients from the SEER database is shown in Table 1.
The results of univariate and multivariate analyses are presented in Table 2. In the univariate analysis, female sex (vs. male; P = 0.003), left-side pneumonectomy (vs. right-side; P = 0.008), and younger age (≤65 vs. >65 years; P < 0.001) appeared as protective factors. Among other factors, grade (P < 0.001), TNM stage (P < 0.001), tumor size (P = 0.006), and LNR (P < 0.001) had an impact on survival, while information related to race, tumor location, histological types, chemotherapy record, radiotherapy record, and pleural invasion were considered insignificant. In the multivariate analysis, after adjustment for other factors, sex, age, laterality, tumor size, LNR, and TNM stage were established as independent factors and thus used to construct the nomogram.
Considering the statistical significance of the risk factors in the multivariate analysis, we developed a nomogram to predict the prognosis of patients undergoing pneumonectomy using eligible variables (sex, age, laterality, tumor size, LNR, and TNM stage) (Figure 2A). The calibration plot showed good concordance with respect to prediction accuracy in both SEER-T and SEER-V with a C-index of 0.629 and 0.609, respectively (Figures 2B,C). The constructed prognostic score is shown in the Table 3. The “survminer” package in R was applied to determine the optimal cutoff value of the PS. Thus, the training data were divided into a high-risk group (N = 479) and a low-risk group (N = 717) by setting the cutoff point at 165. The C-index of 0.629 indicated an acceptable discrimination ability of this model. The Kaplan-Meier curve showed that patients in the high-risk group had significantly poor survival (Figure 3A, unadjusted HR = 2.219; 95% CI 1.887–2.609, P < 0.001). In view of the survival probability, the 1-year CSS was 68% in the high-risk group vs. 86% in the low-risk group, the 3-year CSS was 40 vs. 69%, and the 5-year CSS was 33 vs. 60%. The median survival time in the high- and low-risk group was 24.0 months and 108.0 months, respectively.
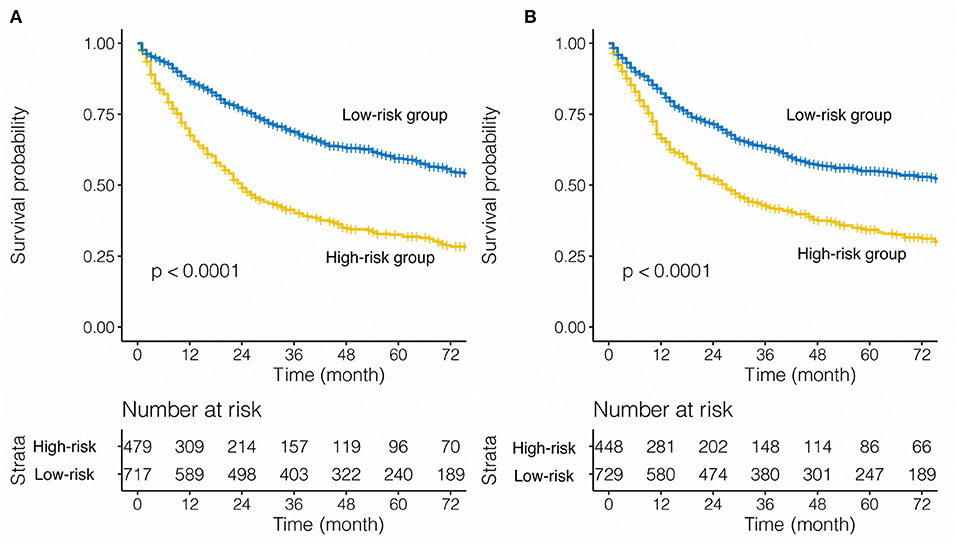
Figure 3. Stratified effect of the nomogram on the training cohort (A) and internal validation cohort (B).
For the internal validation cohort, the Kaplan-Meier curve demonstrated poorer survival in the high-risk group (Figure 3B, SEER-V: unadjusted HR = 1.780; 95% CI 1.515–2.092, P < 0.001) than low-risk group. The C-index of the validation cohort was acceptable and reached 0.609. For SEER-V, the 1-year CSS was 66 vs. 82%, the 3-year CSS was 43 vs. 63%, and the 5-year CSS was 34 vs. 55% (high-risk vs. low-risk). The median survival time was 27.0 months in the high-risk group and 87.0 months in the low-risk group.
We found that our prognostic model of nomogram had a better predictive ability than that in the 8th AJCC TNM staging system (SEER-T, C-index = 0.629 vs. 0.584, respectively, P < 0.001; SEER-V, C-index = 0.609 vs. 0.576, respectively, P < 0.001).
Based on the 8th TNM stage, patients with IA-IIIB cancer stage were included in our study. Categorized by pathological TNM stage, the model was applied to all patients from the SEER database. In stage IB, IIB, IIIA, and IIIB, PS performed as a risk prognostic factor (all HR >1, all P < 0.05, Figure 4). However, based on the Kaplan–Meier analysis and log-rank tests, we found that patients with stage IA, IB, and IIA disease belonged to the low-risk group (Figure 5). The nomogram had obvious stratified effect on CSS of patients with stage IIB, IIIA, and IIIB (all P < 0.01, Figure 5). As for different race/ethnicity, we suggested that the PS might have a negative effect on CSS of patients with white patients, black patients, and Asian or Pacific Islander (Figure 4). In addition, PS had poor prognosis in patients with squamous cell carcinoma (SCC) or adenocarcinoma.
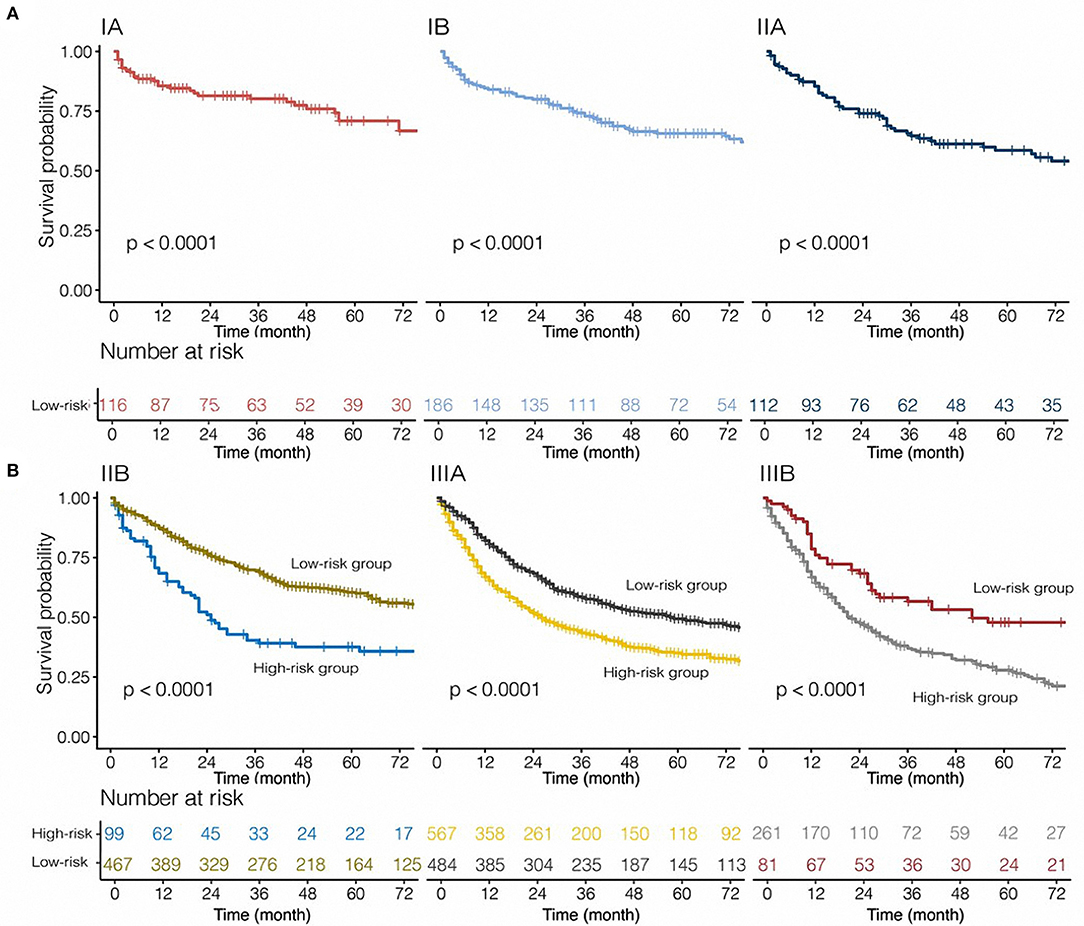
Figure 5. Risk group stratification within each stage for tumor, nodes, and metastasis [(A) patients with stage IA-IIA non-small-cell lung cancer; (B) patients with stage IIB-IIIB non-small-cell lung cancer] in this cohort.
This study aimed to develop a nomogram to predict the CSS of patients with NSCLC who underwent pneumonectomy. Altogether, six variables were considered. After performing univariate and multivariate analyses on the SEER-T cohort, independent prognostic factors such as sex, age, laterality, tumor size, LNR, and TNM stage were identified, while factors such as grade, tumor location, pathology, chemotherapy, radiotherapy, pleural infiltration, and race were excluded due to statistical insignificance. The nomogram for CSS was constructed using the previously stated eligible variables and achieved an acceptable C-index of 0.629 (95% CI 0.627–0.630). A prognostic score was calculated for each patient using the model and was dichotomized by setting the cutoff point to 165. The high- and low-risk groups were derived from the SEER-T based on the dichotomized PS, which showed good distinction with ~20% increase in the 1, 3, 5-year survival rates in the low-risk group compared with the high-risk group. In order to verify this result, the model was applied to the SEER-V, which showed good distinction and achieved acceptable discrimination with a C-index of 0.609 (95% CI 0.607–0.610). The results demonstrated that this model could be used well in another internal validation cohort. In addition, the model fitted well in patients with stage IIB, IIIA, and IIIB disease and showed evident differences in the 1, 3, 5-year CSS rates in the low-risk group compared with the high-risk group. We suggested that this model, which was based on six indicators, could distinguish high-risk post-operative patients from low-risk patients. Nomogram was used to calculate individual risk scores for cancer prognoses. Therefore, it is possible to predict the prognosis of an eligible patient, thus providing more direction for individualized follow-up management and therapy. We hope to build an easy model by using commonly obtained patient information. Eventually, six meaningful indicators were selected using univariate and multivariate analyses of the training cohort. We constructed a nomogram based on the above six factors and successfully identified high-risk and low-risk populations in the training and validation cohorts. Our model had a significant impact on patient differentiation (Figure 3), because the C index for predicting CSS rates reached 0.629 (SEER-T), and 0.609 (SEER-V) in the training and validation cohorts, respectively. In terms of the clinical application, these indicators can be easily assessed. Clinicians could use the above information and our model to calculate scores of NSCLC patients with pneumonectomy, and give patients advice on whether adjuvant therapy and the strategy of follow-up management.
The impact of adjuvant chemotherapy on patients undergoing pneumonectomy remains unclear and controversial. An international adjuvant lung cancer trial in 2004 demonstrated an improved survival among patients with completely resected NSCLC (P < 0.03) (19); however, pneumonectomy patients did not have a survival benefit from adjuvant chemotherapy. Similarly, in our study, chemotherapy did not act as a significant prognostic factor on CSS. In addition, PORT was not identified as an independent prognostic factor in this study. The benefit of PORT in pN2 NSCLC patients has been established in a large-scale retrospective study (3). In our study, however, PORT was more of a risk factor in the univariate analysis of the pneumonectomy cohort (HR = 1.250; 95% CI 1.000–1.550, P = 0.047). The LNR was regarded as a valuable prognostic factor in NSCLC patients, although it is not well-understood in patients who undergo pneumonectomy. Taylor et al. claimed that a higher LNR was correlated with a worse prognosis and a higher rate of local recurrence and vice versa (20). However, there were only 88 patients with pneumonectomy included in their study. Besides, Han et al. explained that the LNR could predict the survival probability of pIIIA-N2 patients after surgery and post-operative chemotherapy (21). There were 2804 patients (including 27 patients with pneumonectomy) for main analyses. Chiappetta et al. also suggested that a high LNR was a risk prognostic factor, and was related to a poor survival outcome. Their findings were based on a multicenter analysis (22). Nevertheless, in the above-mentioned studies, pneumonectomy was not discussed separately. In our study, the prognostic impact of LNR was similar to their results that a high LNR correlated with poor prognosis for patients after pneumonectomy. In addition, the effect of LNR on pneumonectomy was shown in Figure 3 and Table 2.
The impact of right or left pneumonectomy on prognosis remains controversial. Riquet et al. discovered that the overall survival after pneumonectomy among the 10-year survivors (N = 250) could not be meaningfully distinguished by the laterality of tumor/surgery (14). Similarly, in 2019, Yang et al. reported that right-sided or left-sided pneumonectomy after induction chemotherapy was not beneficial for long-term survival (23). Wang et al. also analyzed the data of 100 cases with pneumonectomy and found that the right- or left-side resection didn't give a long-term survival benefit for those patients (24). However, according to the study conducted by Dhanasopon et al., right pneumonectomy patients had worse prognosis compared to left pneumonectomy patients (25). Their study included 79,953 patients, 4,245 of whom underwent pneumonectomy. Simón et al. reported that stage I and II NSCLC patients had better survival with left pneumonectomy based on data of 1,475 patients (including 421 patients after pneumonectomy) (13). The results of our study were similar to those of Dhanasopon et al. and Simón et al. and showed that right-sided pneumonectomy was an independent risk factor for CSS (Table 2).
Nevertheless, several drawbacks exist in this study. Above all, this study was restricted by its retrospective design. Above six independent prognostic factors were derived from a retrospective study. Thus, a prospective study is required to fully validate the model in the clinical application. Confounding errors and biases were limited to an extent by the univariate and multivariate analyses, yet they were inevitable due to the disproportionately distributed variables, such as the TNM stage, races, and tumor location. Hopefully, with a larger sample size, the problem of disproportionate distribution could be solved. Moreover, due to the nature of the SEER database, we could not acquire complete information on the chemotherapy, radiotherapy, and perioperative complications. Therefore, it remained unclear whether chemotherapy, radiotherapy, or perioperative complications had an impact on long-term survival in this pneumonectomy cohort study. Further studies are needed with elaborate medical records to assess the impact of above these factors.
We developed a nomogram for patients with NSCLC undergoing pneumonectomy, which achieved acceptable discrimination in the internal validation cohort. For eligible patients who would be classified in the high-risk group, supplemental individualized follow-up management and therapy should be provided in order to achieve better survival. The study also reported that right lung pneumonectomy was a high-risk prognostic factor compared to the left lung pneumonectomy. In addition, multicenter prospective studies and large number of cases are needed to confirm our findings.
Publicly available datasets were analysed in this study. This data can be found here: https://seer.cancer.gov/.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
G-WM and L-LW: conceptualization. L-LW, W-MJ, XL, Y-YH, and W-TC: data curation. W-TC, L-LW, and G-WM: formal analysis. G-WM, L-LW, and W-TC: methodology. G-WM, L-JZ, HL, and PL: project administration and supervision. L-LW, G-WM, and XL: validation. W-TC and L-LW: roles/writing—original draft. G-WM, L-LW, XL, and W-MJ: writing—review and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors greatly appreciate all patients who contributed to this study. The authors thank all staff from Surveillance, Epidemiology, and End Results for their contribution in recording medical records. The authors appreciate Editage (https://app.editage.com/) for the English language review. L-LW sincerely thanks Prof. Tie-Hua Rong for teaching surgical oncology.
NSCLC, non-small cell cancer; SEER, Surveillance, Epidemiology, and End Results; PS, prognostic score; LNR, lymph node ratio; HR, hazard ratio; CIs, confidence intervals; C-index, indices of concordance; SD, standard deviation; CSS, cancer-specific survival; AJCC, American Joint Committee on Cancer; TNM, tumor, nodes, and metastasis; PORT, post-operative radiotherapy; SCC, squamous cell carcinoma; Adeno, adenocarcinoma; LCC, large cell carcinoma; SC, sarcomatoid carcinoma.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. (2019) 69:363–85. doi: 10.3322/caac.21565
3. Lally BE, Zelterman D, Colasanto JM, Haffty BG, Detterbeck FC, Wilson LD. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. (2006) 24:2998–3006. doi: 10.1200/JCO.2005.04.6110
4. Salazar MC, Rosen JE, Wang Z, Arnold BN, Thomas DC, Herbst RS, et al. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol. (2017) 3:610–9. doi: 10.1001/jamaoncol.2016.5829
5. Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Shen Y, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. (2018) 19:139–48. doi: 10.1016/S1470-2045(17)30729-5
6. Kim H, Lussier YA, Noh OK, Li H, Oh YT, Heo J. Prognostic implication of pulmonary function at the beginning of postoperative radiotherapy in non-small cell lung cancer. Radiother Oncol. (2014) 113:374–8. doi: 10.1016/j.radonc.2014.11.007
7. Kunitoh H, Kato H, Tsuboi M, Shibata T, Asamura H, Ichinose Y, et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol. (2008) 26:644–9. doi: 10.1200/JCO.2007.14.1911
8. Yao J, Wang S, Zhu X, Huang J. Imaging biomarker discovery for lung cancer survival prediction. In: International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer (2016). p. 649–57.
9. Wu LL, Liu X, Jiang WM, Huang W, Lin P, Long H, et al. Stratification of patients with stage IB NSCLC based on the 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual. Front Oncol. (2020) 10:571. doi: 10.3389/fonc.2020.00571
10. Qiu C, Dong W, Su B, Liu Q, Du J. The prognostic value of ratio-based lymph node staging in resected non-small-cell lung cancer. J Thorac Oncol. (2013) 8:429–35. doi: 10.1097/JTO.0b013e3182829c16
11. Fell SC. A brief history of pneumonectomy. 1999. Chest Surg Clin N Am. (2002) 12:541–63. doi: 10.1016/S1052-3359(02)00023-6
12. Melloul E, Egger B, Krueger T, Cheng C, Mithieux F, Ruffieux C, et al. Mortality, complications and loss of pulmonary function after pneumonectomy vs. sleeve lobectomy in patients younger and older than 70 years. Interact Cardiovasc Thorac Surg. (2008) 7:986–9. doi: 10.1510/icvts.2008.182279
13. Simón C, Moreno N, Peñalver R, González G, Alvarez-Fernández E, González-Aragoneses F. The side of pneumonectomy influences long-term survival in stage I and II non-small cell lung cancer. Ann Thorac Surg. (2007) 84:952–8. doi: 10.1016/j.athoracsur.2007.04.075
14. Riquet M, Mordant P, Pricopi C, Legras A, Foucault C, Dujon A, et al. A review of 250 ten-year survivors after pneumonectomy for non-small-cell lung cancer. Eur J Cardiothorac Surg. (2014) 45:876–81. doi: 10.1093/ejcts/ezt494
15. Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. (2015) 33:861–9. doi: 10.1200/JCO.2014.56.6661
16. Zheng XQ, Huang JF, Lin JL, Chen L, Zhou TT, Chen D, et al. Incidence, prognostic factors, and a nomogram of lung cancer with bone metastasis at initial diagnosis: a population-based study. Transl Lung Cancer Res. (2019) 8:367–79. doi: 10.21037/tlcr.2019.08.16
17. Cheng B, Wang C, Zou B, Huang D, Yu J, Cheng Y, et al. A nomogram to predict outcomes of lung cancer patients after pneumonectomy based on 47 indicators. Cancer Med. (2020) 9:1430–40. doi: 10.1002/cam4.2805
18. Liu Y, Li X, Yin Z, Lu P, Ma Y, Kai J, et al. Prognostic prediction models based on clinicopathological indices in patients with resectable lung cancer. Front Oncol. (2020) 10:571169. doi: 10.3389/fonc.2020.571169
19. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. (2004) 350:351–60. doi: 10.1056/NEJMoa031644
20. Taylor MD, LaPar DJ, Thomas CJ, Persinger M, Stelow EB, Kozower BD, et al. Lymph node ratio predicts recurrence and survival after R0 resection for non-small cell lung cancer. Ann Thorac Surg. (2013) 96:1163–70. doi: 10.1016/j.athoracsur.2013.04.031
21. Han H, Zhao Y, Gao Z, Zheng D, Fu F, Zhao Z, et al. A prognostic score system with lymph node ratio in stage IIIA-N2 NSCLC patients after surgery and adjuvant chemotherapy. J Cancer Res Clin Oncol. (2019) 145:2115–22. doi: 10.1007/s00432-019-02952-w
22. Chiappetta M, Leuzzi G, Sperduti I, Bria E, Mucilli F, Lococo F, et al. Lymph-node ratio predicts survival among the different stages of non-small-cell lung cancer: a multicentre analysis. Eur J Cardiothorac Surg. (2019) 55:405–12. doi: 10.1093/ejcts/ezy311
23. Yang CJ, Shah SA, Lin BK, Van Dusen KW, Chan DY, Tan WD, et al. Right-sided versus left-sided pneumonectomy after induction therapy for non-small cell lung cancer. Ann Thorac Surg. (2019) 107:1074–81. doi: 10.1016/j.athoracsur.2018.10.009
24. Wang G, Liu L, Zhang J, Li S. The analysis of prognosis factor in patients with non-small cell lung cancer receiving pneumonectomy. J Thorac Dis. (2020) 12:1366–73. doi: 10.21037/jtd.2020.02.33
Keywords: pneumonectomy, non-small cell lung cancer, nomogram, cancer-specific survival, stage I-IIIB
Citation: Wu L-L, Chen W-T, Liu X, Jiang W-M, Huang Y-Y, Lin P, Long H, Zhang L-J and Ma G-W (2021) A Nomogram to Predict Long-Term Survival Outcomes of Patients Who Undergo Pneumonectomy for Non-small Cell Lung Cancer With Stage I-IIIB. Front. Surg. 8:604880. doi: 10.3389/fsurg.2021.604880
Received: 10 September 2020; Accepted: 09 April 2021;
Published: 29 April 2021.
Edited by:
Marco Scarpa, University Hospital of Padua, ItalyReviewed by:
Giuseppe Mangiameli, University of Milan, ItalyCopyright © 2021 Wu, Chen, Liu, Jiang, Huang, Lin, Long, Zhang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-Wei Ma, bWFnd0BzeXN1Y2Mub3JnLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.