- 1Hospital Universitario del Henares, Madrid, Spain
- 2Universidad Francisco de Vitoria, Madrid, Spain
- 3Hospital Universitario Puerta de Hierro Majadahonda, Madrid, Spain
- 4Ramón y Cajal University Hospital, Madrid, Spain
Objective: The aim of this study is to describe the macroscopic features and histologic details observed after retromuscular abdominal wall reconstruction with the combination of an absorbable mesh and a permanent mesh.
Methods: We have considered all patients that underwent abdominal wall reconstruction (AWR) with the combination of two meshes that required to be reoperated for any reason. Data was extracted from a prospective multicenter study from 2012 to 2019. Macroscopic evaluation of parietal adhesions and histological analysis were carried out in this group of patients.
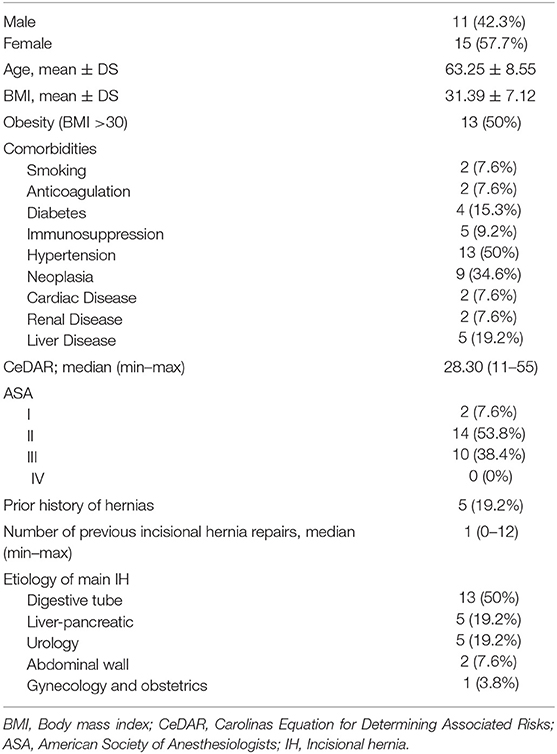
Results: Among 466 patients with AWR, we identified 26 patients that underwent a reoperation after abdominal wall reconstruction using absorbable and permanent mesh. In eight patients, the reoperation was related to abdominal wall issues: four patients were reoperated due to recurrence, three patients required an operation for chronic mesh infection and one patient for symptomatic bulging. A miscellanea of pathologies was the cause for reoperation in 18 patients. During the second surgical procedures made after a minimum of 3 months follow-up, a fibrous tissue between the permanent mesh covering and protecting the peritoneum was identified. This fibrous tissue facilitated blunt dissection between the permanent material and the peritoneum. Samples of this tissue were obtained for histological examination. No case of severe adhesions to the abdominal wall was seen. In four cases, the reoperation could be carried out laparoscopically with minimal adhesions from the previous procedure.
Conclusions: The reoperations performed after the combination of absorbable and permanent meshes have shown that the absorbable mesh acts as a protective barrier and is replaced by a fibrous layer rich in collagen. In the cases requiring new hernia repair, the layer between peritoneum and permanent mesh could be dissected without special difficulty. Few intraperitoneal adhesions to the abdominal wall were observed, mainly filmy, easy to detach, facilitating reoperations.
Introduction
Treatment of complex incisional hernias of the anterolateral abdominal wall is a surgical challenge. These defects are usually repaired with non-absorbable materials to minimize hernia recurrence. There are several surgical planes to insert meshes: intraperitoneal (inlay), preperitoneal, retro-muscular (sublay) or supra-aponeurotic (onlay) (1). Onlay mesh placement presents more postoperative complications but comparable recurrence rate to sublay techniques (2). Synthetic meshes placed intra-peritoneally can cause dense adhesions, bowel injuries, mesh migrations and mesh erosion into abdominal contents, although the risk of enterocutaneous fistula formation remains low. Unlike the aforementioned planes, retrorectal mesh placement might offer advantages especially when dealing with complex hernia repair. Posterior components separation technique (PCS) as described by Novitsky and Rosen has shown beneficial effects (3). In fact, transversus abdominis release (TAR) is nowadays one of the most effective approaches for complex abdominal wall reconstruction (4). Once the plane between the peritoneum/transversalis fascia and the muscular plane has been created, permanent meshes are commonly used. However, performing retromuscular abdominal wall reconstruction (AWR) is a challenging procedure that may lead to serious complications. Bowell injuries, adhesions to intra-abdominal contents, internal hernias through openings on the peritoneum and posterior rectus sheaths, postoperative pain due to transparietal fixations and mesh wrinkling or migration due to permanent mesh structure are the most common. Those reasons guided us to develop a strategy using two types of meshes in the same retro-muscular plane, an absorbable mesh (AM) along with a non-absorbable permanent mesh (PM) (5, 6). There are also concerns about reoperations after retromuscular mesh placement in terms of early reoperations due to complications or late operations for recurrences or another surgical causes. The aim of this study is to report the macroscopic evaluation and histological features observed among 26 patients that underwent a reoperation after retromuscular abdominal wall reconstruction with AM and PM for complex incisional hernia repair.
Methods
From a prospectively maintained database of complex abdominal wall repair in two hospitals, we identified patients who were reoperated for any reason, between April 2012 and December 2019. The two hospitals involved in the study are recognized referral centers for AWR. All patients underwent an AWR using the combination of AM and PM, as previously described (5). The AM used is made of polyglycolic acid and trimethylene carbonate (GORE® BIO-A® Tissue Reinforcement, WL Gore & Associates, Inc. Flagstaff, AZ, USA). We used two types of macroporous PM: a 26 × 36 cm, 60 g/m2 polypropylene mesh (Optilene mesh, B. Braun, Melsungen, Hessen. Germany) or a 50 × 50 48 g/m2 polypropylene mesh (Bulevb®, Dipro Medical Devices SRL, Torino, Italy).
All reoperations except one were performed in the same centers as the index procedures. Demographics and patient characteristics are summarized in Table 1. In those cases, in which the adhesions to the abdominal wall could be re-explored, we used the adhesion tenacity score to assess intraperitoneal attachments to implanted meshes (7, 8).
We obtained samples of tissue in six reoperated patients, that were stained for microscopic analysis with hematoxylin-eosin stain, Masson's trichrome and Picrosirius red stain to asses tissue integration of the meshes (9).
We have adhered to the STROBE Statement recommendations to draft this article (10). All patients provided informed consent to be included in prospective studies prior to surgery. We obtained Institutional Review Board approval.
Statistics
We have used Statistical Package for the Social Sciences (SPSS) program (version 19.0 for Windows) to describe variables and for statistical analysis. Quantitative variables were expressed as mean or median and standard deviation or quartiles, and categorical variables as absolute numbers and percentages.
Results
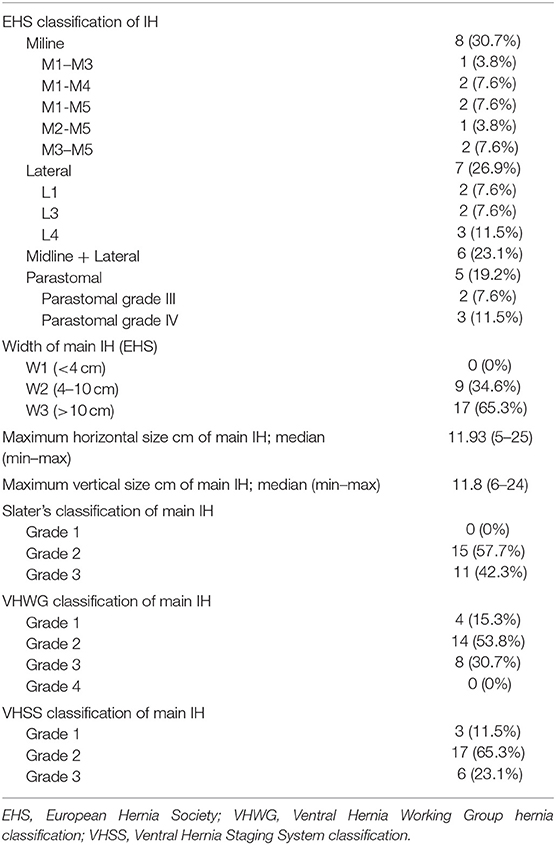
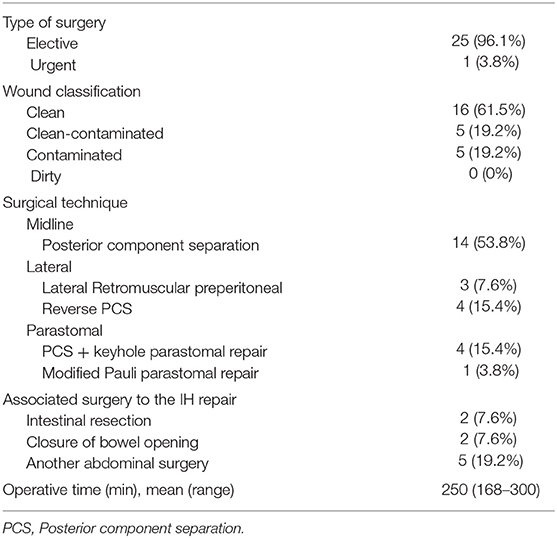
We identified 26 patients that had previously undergone AWR with PCS and reconstruction with the combination of AM and PM repair and required ensuing reoperation. Patient demographics and characteristics are shown in Tables 1, 2. Operative details of previous AWR are summarized in Table 3. It is worth noting that among the 26 selected patients, most of the patients underwent a posterior component separation technique as previously described (6, 11, 12). In three cases, lateral retromuscular preperitoneal approach was performed and, in a patient with parastomal hernia, modified Pauli technique was achieved (13).
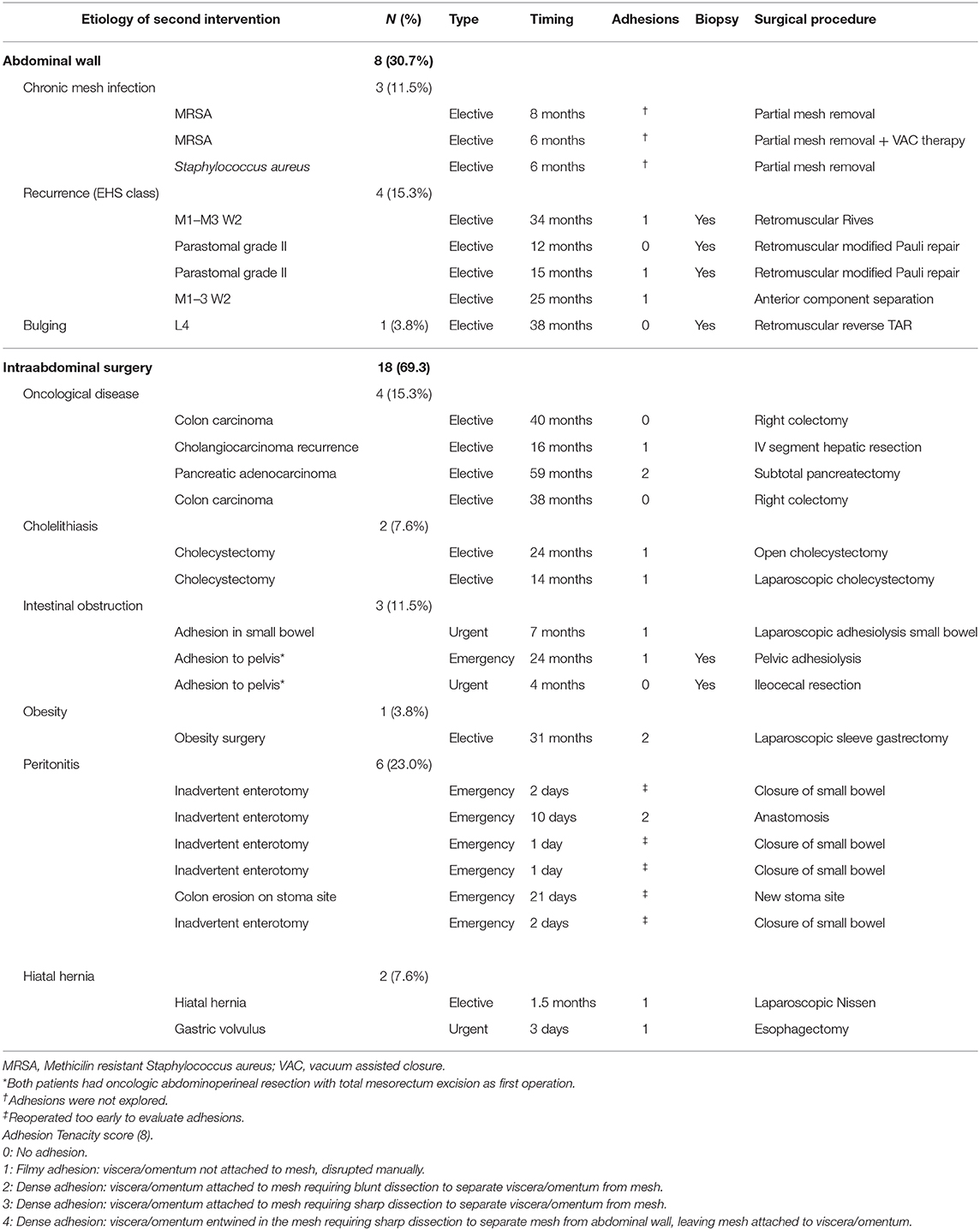
In eight cases, the reoperation was centered on the abdominal wall as four patients presented hernia recurrence, three patients chronic mesh infection, and one patient abdominal postoperative symptomatic bulging. The remaining 18 patients were reoperated for a variety of diseases that included intestinal leak, intestinal obstruction, bariatric surgery, oncological disease, symptomatic cholelithiasis and hiatal hernia. Operative data regarding reoperations are summarized in Table 4. Among those five that required new abdominal wall surgery, we performed a retromuscular redo repair in four cases: two patients underwent retromuscular modified Pauli repair (13), one patient retromuscular reverse TAR (12) and one patient a redo Rives-Stoppa procedure. We accomplished anterior components separation technique in one patient.
According to the adhesion tenacity classification used: 10 patients presented filmy adhesions that were manually disrupted (grade 1), three dense adhesions that required blunt dissection (grade 2) and five presented no adhesions (grade 0). It is particularly important to emphasize that none of the patients presented grade 3 or 4 adhesions that required sharp dissection or leaving fragments of mesh attached to the viscera. From this analysis, the three patients with chronic infection were not included as the abdominal cavity were not re-explored. Those patients reoperated in the immediate postoperative period (<7 days) were also excluded.
In the patients reoperated, we macroscopically observed a layer of fibrous tissue between the peritoneum and PM (Figure 1). This layer could be smoothly separated from the PM with blunt dissection (Figure 2). In fact, in four out of five patients with recurrence, the retromuscular preperitoneal plane could be dissected again without difficulty and used one more time for reconstruction (Figure 3).
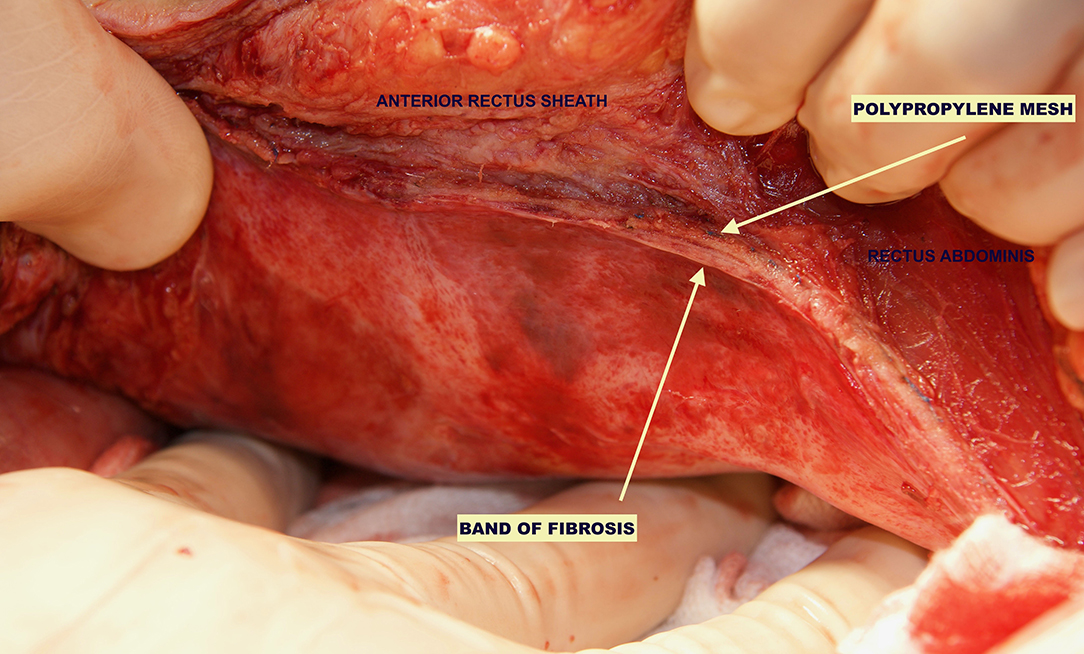
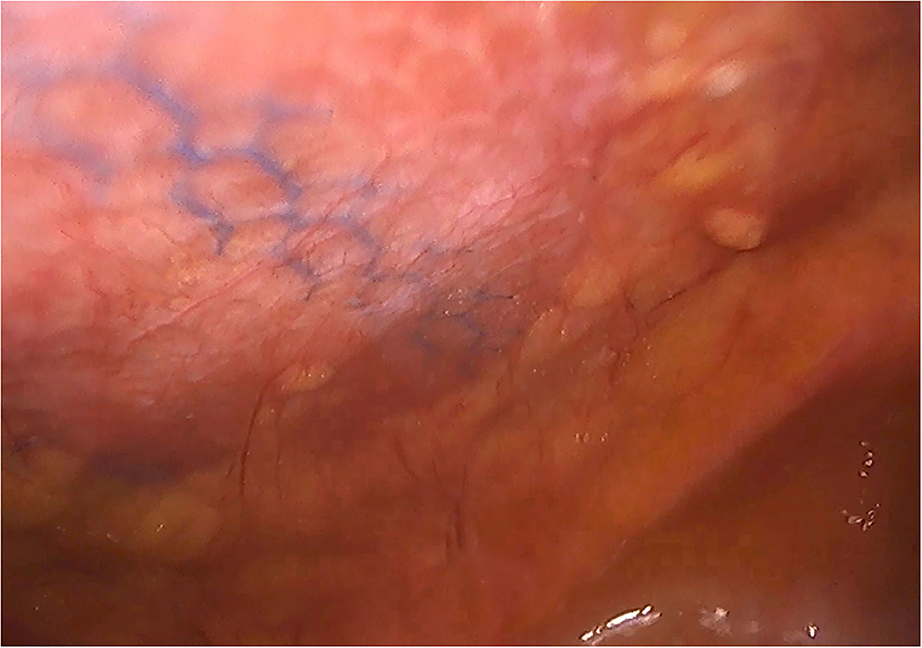
Figure 1. Case of reoperation at 4 months for intestinal obstruction to pelvic floor. No intra-abdominal adhesions to the abdominal wall were seen. The permanent mesh could not be discerned through the peritoneum. A layer of fibrosis between the peritoneum and the permanent mesh is observed.
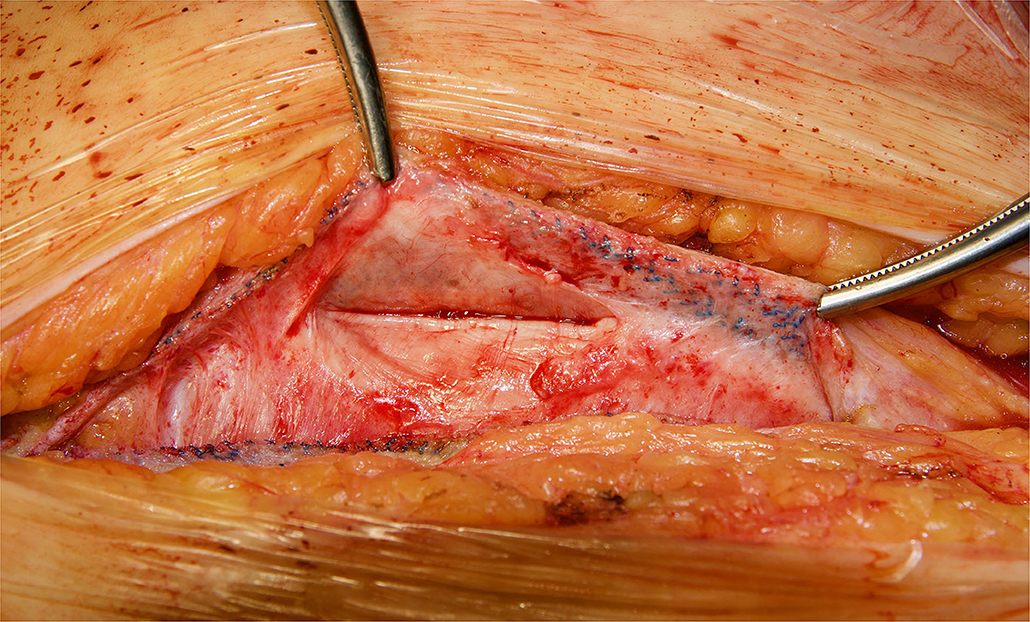
Figure 2. Reoperation through previous midline incision at 12 months. A dense fibrosis covering the peritoneum is observed.
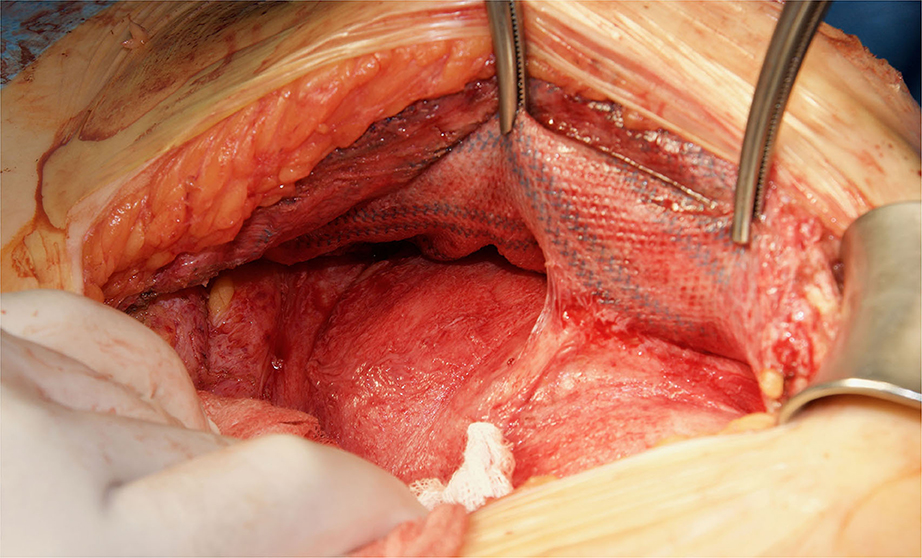
Figure 3. Redo retromuscular dissection in the case of Figure 2. Easy blunt dissection between peritoneum and permanent mesh could be achieved.
Histologically, a thick fibrous capsule over the peritoneum was found in all specimens analyzed with hematoxylin-eosin stain (Figure 4). The identification of the fibrous tissue was confirmed in Masson's trichrome (Figure 5). Picrosirius red staining revealed that the composition consisted of mainly collagen scaffold (Figure 6). In the interesting case reoperated at 4 months due to intestinal obstruction for adhesion to the pelvic floor, an amount of amorphous material was still present with residual foreign body reaction among the fibrous layer, showing that the AM had only been partially integrated (Figure 7).
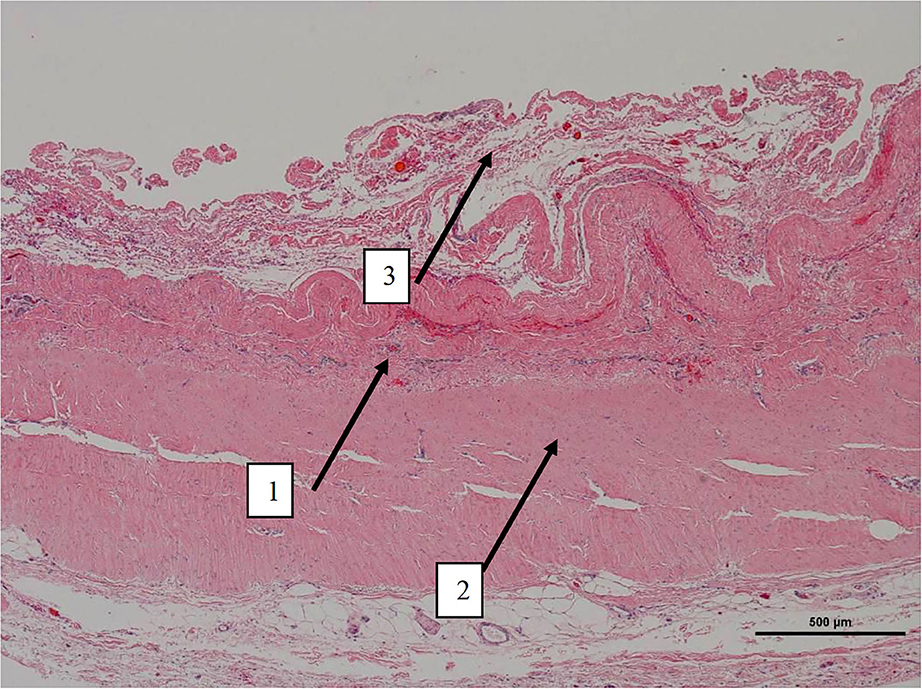
Figure 4. Low magnification picture of representative section on slide with hematoxylin & eosin staining. Arrow 1 denotes the layer where macrophages with intracytoplasmic particles are present as well as monofilament biomaterial. Arrow 2 represents an area with highly oriented and densely packed collagen. Arrow 3 on the periphery is composed of loosely arranged collagen fibers.
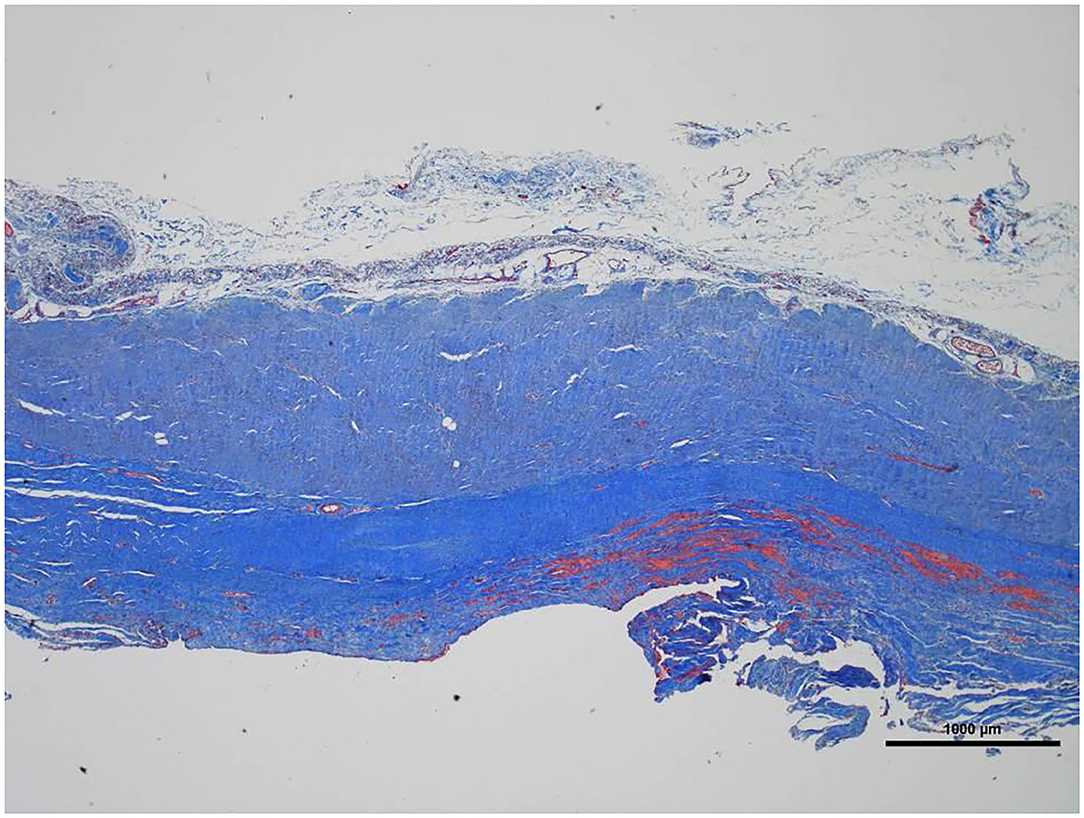
Figure 5. Low magnification picture of representative section on slide with Masson's trichrome stain. The connective tissue is composed almost entirely of collagen (blue staining tissue).
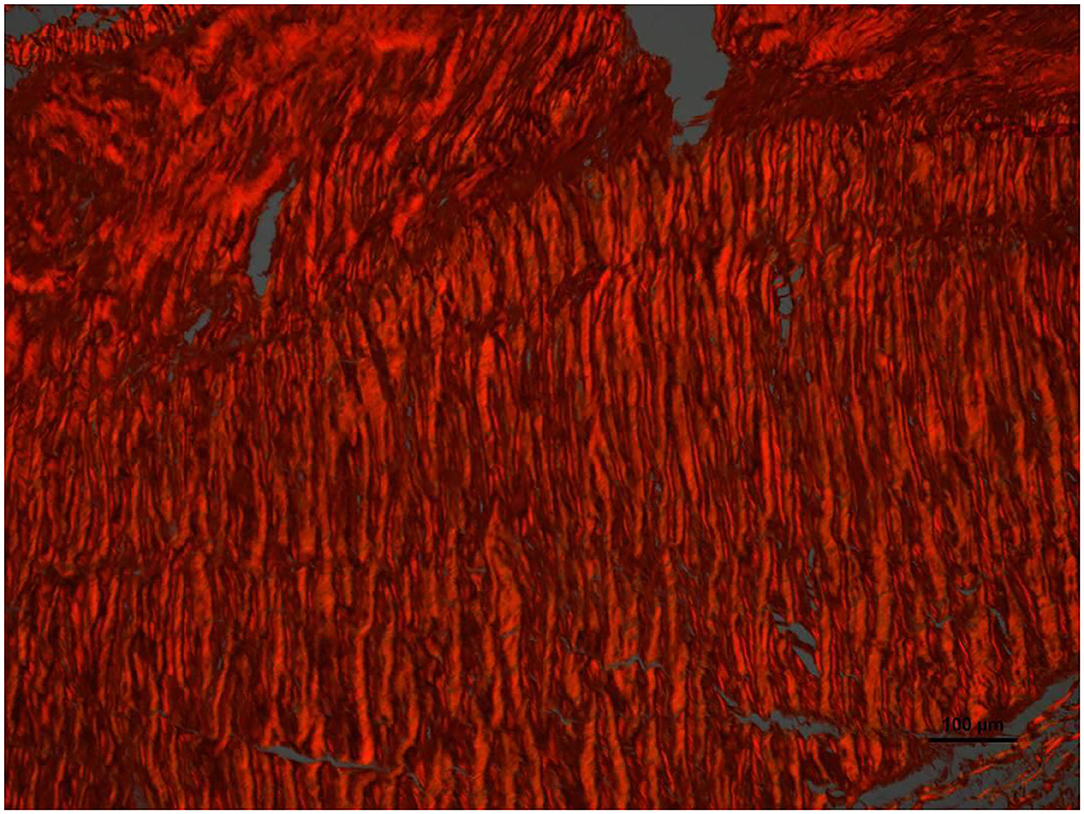
Figure 6. High magnification image of the area pointed with arrow number 2 in Figure 4. With Picrosirius red stain and polarized light microscopy the collagen is birefringent orange-red colored, highly oriented and densely packed.
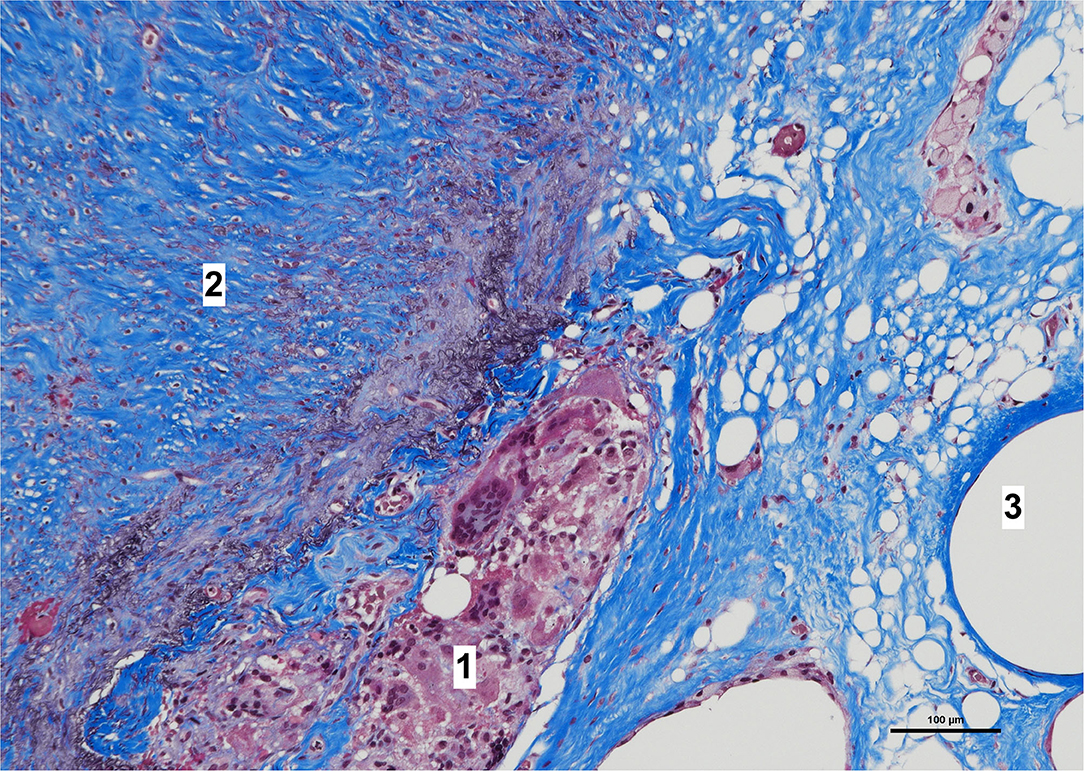
Figure 7. High magnification image of biopsy taken of case in Figure 1, with Masson's trichrome stain. Remnants of amorphous material (absorbable mesh) (1), between the fibrosis over the peritoneum, (2) and fibers of the permanent mesh (3).
Discussion
We started to use an AM along with a PM mainly to provide a barrier between intra-abdominal contents and PM in urologic cases. In these patients with previous cystectomies, where the peritoneum between both epigastric vessels is frequently removed, it was impossible for us to close the posterior layer despite the posterior component separation. We had the initial impression that the use of AM not only helped us to extend a very wide piece of PM without the need of transparietal fixations, but also that it could reinforce the posterior layer covering the inadvertent tears in the peritoneum. So, this became our model of AWR after posterior component separation. The satisfactory clinical results after using the combination of these meshes have already been published (5, 6, 12). Apart from the previous cystectomy cases, there are other circumstances that may expose the PM to the visceral content after a PCS (14). Peritoneal tears can be very common during a TAR procedure, meaning expert surgeons describe them as inevitable (15). The peritoneal layer is extremely thin and vulnerable to disruption between the midline preperitoneal fat and the anterior axillary line (5). The problem is not the tears that are seen and closed, but those unnoticed. Previous mesh implantations with or without infection, multi-recurrent hernias, AWR after open abdomen or resection of parietal tumors are other reasons that may prevent an adequate closure of the visceral sac or posterior layer. Acute internal hernias, that may occur in the immediate postoperative period, represent another complication after retromuscular hernia repair (16). All these problems can be solved by the placement of an AM between PM and peritoneum, creating an additional barrier facing intra-abdominal contents. The AM does not work like a conventional mesh and should be considered only as a tissue scaffold to reinforce the posterior layer and to cover inadvertent tears in the peritoneum. Based in our experience (5, 6, 12), we advocate to use the combination of meshes in complex abdominal wall reconstruction, particularly when there are concerns about appropriate closure of posterior layer. In our opinion, an incisional hernia that can be solved with a simple Rives-Stoppa procedure does not need this double mesh reconstruction.
We have then used this tissue scaffold, made of glycolic acid and trimethylene carbonate, that primarily degrades by hydrolysis and facilitates tissue generation and healing, as it has been observed in experimental settings (17, 18). These experimental findings have been confirmed in our clinical scenarios, as we have observed that AM is replaced by a fibrous tissue (Figures 6, 7). Apart from this clinical confirmation, we have also noticed that, when a new surgery in the abdominal wall was performed, a plane between PM and peritoneum could be dissected with blunt dissection (Figure 3). This maneuver is particularly important, as it may facilitate new retromuscular procedure to treat hernia recurrence or bulging. Masson's trichrome and Picrosirius red staining showed that a thick fibrous tissue had replaced the AM (Figures 6, 7). Rests of amorphous material still to be phagocyted, observed at 4 months, supports that the AM in combination with PM is, at least, partially responsible for this fibrosis (Figure 7). Here, it is important to remember that the histological analysis was made using the combination of AM and PM, and we do not know if the AM alone would generate the same response. Nonetheless, we do know, from our experience operating recurrences after retromuscular approach with PM, that dissecting a layer between peritoneum and previous retromuscular PM is almost impossible to achieve.
This is the first clinical report of macroscopic and histological analysis after the implantation of the combination of an AM and a PM. However, we are not the only ones that have used an AM as a barrier when performing retromuscular PM repairs. Liu et al. suggested, in an experimental model, that a physical barrier made of an AM may prevent adhesions to the PM (19). In this porcine model, self-made composite meshes made of non-absorbable and absorbable synthetic polyglactin materials were used in an intra-abdominal setting (intraperitoneal onlay). Although the use of an AM did not reduce adhesions to the PM, a thick fibrous capsule replaced the AM and therefore might represent an important layer to prevent intestinal erosion. The histological pictures shown in this experimental study are quite similar to those observed in our current study. Consequently, we propose that this important experimental analysis has a clinical correlation in surgical practice. Later, the same group published a multicenter experience using the AM as an interface between visceral contents and PM in cases where the peritoneum could not be completely restored (14). Probably looking for similar results, there are now several meshes that incorporate the combination of 3D absorbable or biological scaffold along with a PM that have been approved for clinical use in abdominal wall reinforcement by FDA (United States Food and Drug) administration: GORE® SYNECORD® Preperitoneal Biomaterial (WL Gore & Associates, Inc. Flagstaff, AZ, USA) and Ovitex® Tissue Matrix (Tela Bio Inc. Malvern, PA, USA). The possibility of using the combination of 3D absorbable and permanent biomaterials might allow redoing the preperitoneal plane in case of reoperation for recurrence or bulging, but we have to wait for clinical results with these other biomaterials as the composition of them differs from the combination that we are currently using.
The configuration of the AM that we use is very different to the conventional macroporous woven knitted absorbable meshes (Vycril® mesh) or to the more recent macroporous meshes with longer absorption times: Tigr® Matrix or Phasix™. BIO-A® Tissue Reinforcement is a 3D microscopic scaffold, 1.7 mm thick, that provides physical support to the extension of large PM, avoiding foldings, transparietal fixations and facilitating Stoppa and Taco configurations of PM at inguinal areas and posterior abdominal wall (12, 20). All these absorbable meshes are degraded by hydrolysis. Vycril® is made of polyglycolic acid and polylactic acid; BIO-A® Tissue Reinforcement, polyglycolic acid and trimethylene carbonate; Tigr® Matrix, polyglycolic acid, polylactic acid and trimethylene carbonate; and Phasix™, poly-4-hydroxybutyrate. While complete resorption of Vycril® mesh is at 2–3 months, BIO-A® Tissue Reinforcement is around 6 months, Phasix™ 12–18 months and Tigr® Matrix 3 years. Interestingly, we have observed some microscopic traces of the absorbable mesh at 1-year reoperations.
Although there are currently several adhesions classifications (21), we have chosen the classification that detail the tenacity of adhesion to previous mesh to better assess the reoperation findings (7, 8). As expected, we did not observe strong adhesions or viscera attached in any of the reoperated cases (Table 4). No adhesions (Figure 8) or filmy adhesions that only required blunt dissections (Figure 9) allowed four cases to be reoperated by laparoscopic approach despite the previous posterior component separation technique (Figure 10).
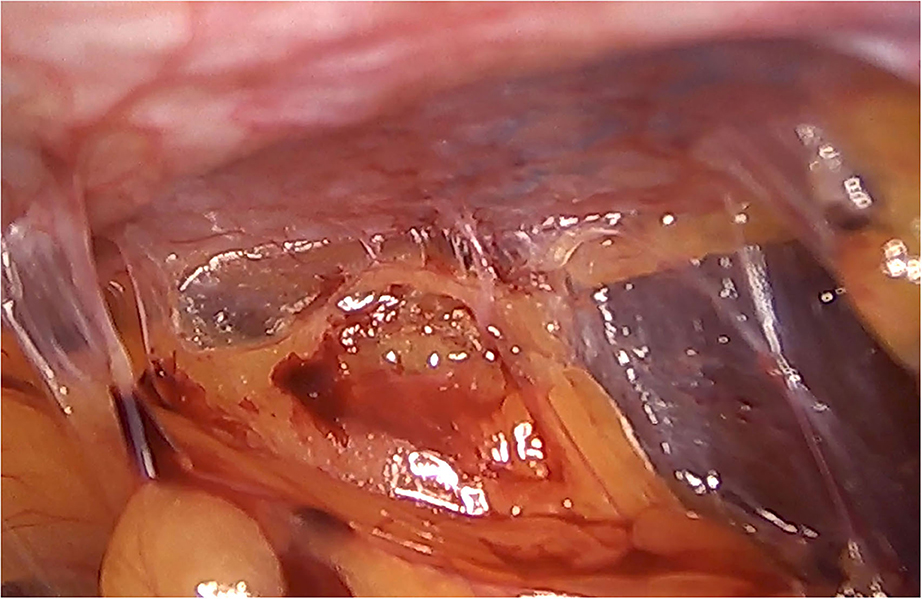
Figure 9. Intra-abdominal adhesions in another case of laparoscopic surgery after PCS technique with the combination of meshes. Tenacity score 2.
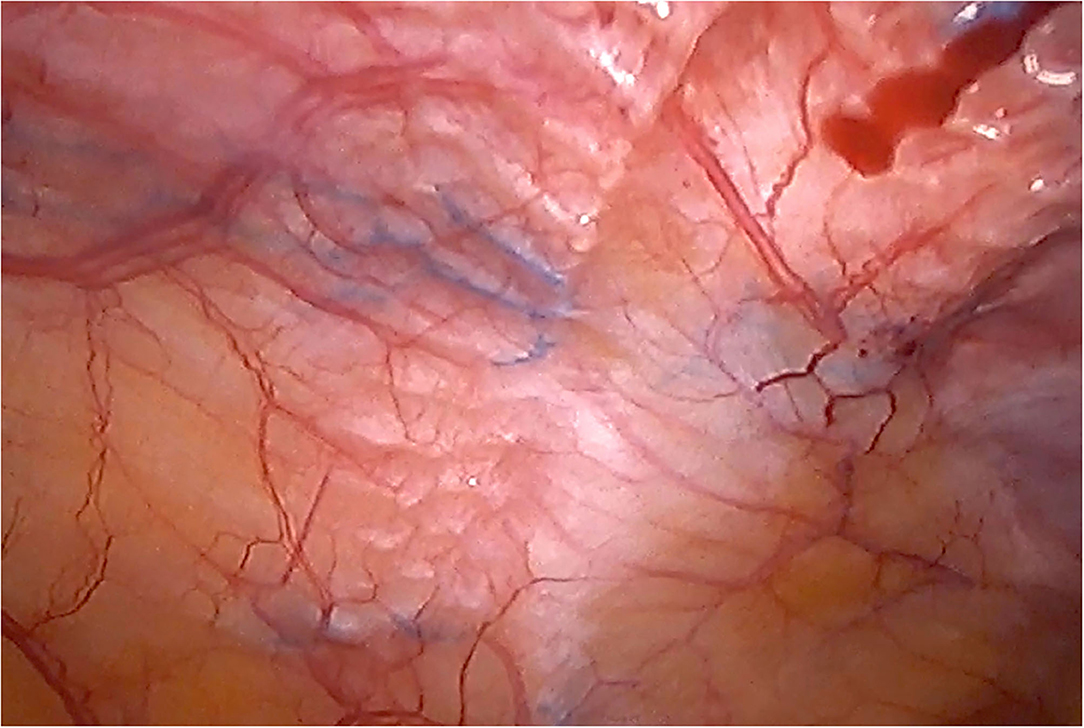
Figure 10. Laparoscopic reoperation after PCS technique with the combination of meshes. View of the edge of the permanent mesh without adhesions.
Our study has several significant limitations. Firstly, there is a lack of homogeneity in the causes that motivated a new surgical procedure. However, this study is focused on intraoperative macroscopic and microscopic features not related to its etiology. Secondly, no comparison that could provide useful information can be made among cases operated only using AM or PM. So that, no control group with only permanent mesh was analyzed. Nonetheless, these reoperations come from an international multicenter study that takes into account a large volume of patients with complex abdominal wall reconstruction using this technique.
Conclusions
The observation of reoperated cases of abdominal wall reconstruction with the combination of an absorbable scaffold as an adjunct to permanent synthetic mesh represents an appropriate approach for complex incisional hernias as it may facilitate dissection between AM and PM granting new retro-muscular pre-peritoneal hernia redo in emergency or planned situations. We have witnessed that a fibrous protective layer rich in collagen fibers replaced the absorbable material. Few intra-peritoneal postoperative adhesions were observed, which were mainly filmy and easy to detach with blunt dissection, facilitating reoperations.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Comité Ético de la Universidad Francisco de Vitoria, proyecto 39/2019. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MG-U, JL-M, AR, AM, and LB have contributed operating patients and writing draft of manuscript. JM-R and MP-F has elaborated tables and statistics. CS has elaborated figures, analyzed microscopic slides preparation, and review final version of manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
MG-U, LB, JL-M, AR, and CS received honorarium for educational workshops organized by Gore. MG-U received speaker fees from Dipromed and B Braun.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Parker SG, Halligan S, Liang MK, Muysoms FE, Adrales GL, Boutall A, et al. International classification of abdominal wall planes (ICAP) to describe mesh insertion for ventral hernia repair. Br J Surg. (2020) 107:209–17. doi: 10.1002/bjs.11400
2. Köckerling F. Onlay technique in incisional hernia repair-A systematic review. Front Surg. (2018) 5:71. doi: 10.3389/fsurg.2018.00071
3. Novitsky YW, Elliott HL, Orenstein SB, Rosen MJ. Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. Am J Surg. (2012) 204:709–16. doi: 10.1016/j.amjsurg.2012.02.008
4. Poulose BK. Transversus abdominis muscle release. Innovations in technique can still happen. Ann Surg. (2016) 264:233–4. doi: 10.1097/SLA.0000000000001734
5. Robin-Lersundi A, Blazquez Hernando L, López-Monclús J, Cruz Cidoncha A, San Miguel Méndez C, Jimenez Cubedo E, et al. How we do it: down to up posterior components separation. Langenbecks Arch Surg. (2018) 403:539–46. doi: 10.1007/s00423-018-1655-4
6. García-Ureña MA, López-Monclús J, Cuccurullo D, Blazquez Hernando LA, García Pastor P, Reggio S, et al. Abdominal wall reconstruction utilizing the combination of absorbable and permanent mesh in a retromuscular position: a multicentre prospective study. World J Surg. (2019) 43:149–58. doi: 10.1007/s00268-018-4765-9
7. Matthews BD. Enterotomy during hernia repair: prevention and management. In: Novitsky YW, editor. Hernia Surgery. Current Principles. Cham: Springer International Publishing Switzerland (2016). p. 371–8.
8. Jenkins ED, Yom V, Melman L, Brunt LM, Eagon JC, Frisella MM, et al. Prospective evaluation of adhesion characteristics to intraperitoneal mesh and adhesiolysis-related complications during laparoscopic re-exploration after prior ventral hernia repair. Surg Endosc. (2010) 24:3002–7. doi: 10.1007/s00464-010-1076-0
9. Puchtler H, Waldrop FS, Valentine LS. Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beitr Pathol. (1973) 150:174–87. doi: 10.1016/S0005-8165(73)80016-2
10. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. (2007) 16:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010
11. Reinpold W. Transversus abdominis muscle release: technique, indication, and results. Int J Abdom Wall and Hernia Surg. (2018) 1:79–86. doi: 10.4103/ijawhs.ijawhs_27_18
12. Muñoz Rodriguez JM, López-Monclús J, San Miguel Méndez C, Pérez-Flecha M, Robin Valle de Lersundi A, Blazquez Hernando LA, et al. Outcomes of abdominal wall reconstruction in patients with the combination of complex midline and lateral incisional hernias. Surgery. (2020) 168:532–42. doi: 10.1016/j.surg.2020.04.045
13. San Miguel C, Muñoz J, Robin Á, Minaya AM, Cruz A, Pérez-Flecha M, et al. Modified Pauli abdominal wall reconstruction for recurrent parastomal hernia after a Sugarbaker repair - a video vignette. Colorectal Dis. (2019) 21:1224–5. doi: 10.1111/codi.14783
14. Winder JS, Majumder A, Fayezizadeh M, Novitsky Y, Pauli EM. Outcomes of utilizing absorbable mesh as an adjunct to posterior sheath closure during complex posterior component separation. Hernia. (2018) 22:303–9. doi: 10.1007/s10029-018-1732-1
15. Novitsky YW. Posterior component separation via transversus abdominis muscle release: the TAR procedure. In: Novitsky YN, editor. Hernia Surgery. Current Principles. Cham: Springer International Publishing Switzerland (2016). p. 116–35.
16. Davis JR, Villarreal JE, Cobb WS, Carbonell AM, Warren JA. Interparietal hernia complicating retromuscular ventral hernia repair. Am Surg. (2016) 82:658–9. doi: 10.1177/000313481608200736
17. Peeters E, van Barneveld KWY, Schreinemacher MH, Hertogh GD, Ozog Y, Bouvy N, et al. One-year outcome of biological and synthetic bioabsorbable meshes for augmentation of large abdominal wall defects in a rabbit model. J Surg Res. (2013) 180:274–83. doi: 10.1016/j.jss.2013.01.025
18. López-Cano M, Armengol M, Teresa Quiles M, Biel A, Velasco J, Huguet P, et al. Preventive midline laparotomy closure with a new bioabsorbable mesh: an experimental study. J Surg Res. (2013) 181:160–9. doi: 10.1016/j.jss.2012.05.041
19. Liu L, Petro C, Majumder A, Fayezizadeh M, Anderson J, Novitsky YW. The use of Vicryl mesh in a porcine model to assess its safety as an adjunct to posterior fascial closure during retromuscular mesh placement. Hernia. (2016) 20:289–95. doi: 10.1007/s10029-016-1469-7
20. La Pinska MP. Open flank hernia repair. In: Rosen M, editor. Atlas of Abdominal Wall Reconstruction. 2nd ed. Amsterdam: Elsevier (2016). p. 183–94.
Keywords: second look, absorbable mesh, polypropylene mesh, mesh integration, posterior component separation, transversus abdominis release
Citation: Robin Valle de Lersundi A, Munoz-Rodriguez J, Lopez-Monclus J, Blazquez Hernando LA, San Miguel C, Minaya A, Perez-Flecha M and Garcia-Urena MA (2021) Second Look After Retromuscular Repair With the Combination of Absorbable and Permanent Meshes. Front. Surg. 7:611308. doi: 10.3389/fsurg.2020.611308
Received: 28 September 2020; Accepted: 09 December 2020;
Published: 08 January 2021.
Edited by:
Alexander H. Petter-Puchner, Institute for Experimental and Clinical Traumatology (LBG), AustriaCopyright © 2021 Robin Valle de Lersundi, Munoz-Rodriguez, Lopez-Monclus, Blazquez Hernando, San Miguel, Minaya, Perez-Flecha and Garcia-Urena. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miguel Angel Garcia-Urena, bWFndXJlbmFAZ21haWwuY29t
 Alvaro Robin Valle de Lersundi
Alvaro Robin Valle de Lersundi Joaquín Munoz-Rodriguez
Joaquín Munoz-Rodriguez Javier Lopez-Monclus3
Javier Lopez-Monclus3 Miguel Angel Garcia-Urena
Miguel Angel Garcia-Urena