- 1Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, Academic Assembly, University of Toyama, Toyama, Japan
- 2Department of Pathology, Faculty of Medicine, Academic Assembly, University of Toyama, Toyama, Japan
- 3Department of Diagnostic Pathology, Ishikawa Prefectural Central Hospital, Kanazawa, Japan
Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma (TL-LGNPPA) is an extremely rare neoplasm of the nasopharynx. Accordingly, its clinical and pathological characteristics are not well-known. We report a case of TL-LGNPPA and review the relevant literature on TL-LGNPPA. A 38-year-old Japanese woman presented with a history of nasal obstruction that had persisted for 1 month after symptoms of a common cold (e.g., low-grade fever, sore throat, and fatigue). A pedunculated tumor of ~20 mm in diameter was found on the posterior edge of the nasal septum. The tumor was endoscopically resected. Based on careful histopathological and immunohistochemical examinations, it was diagnosed as TL-LGNPPA. At 5 years after surgery, the patient remained disease-free. TL-LGNPPA has a very good prognosis, and complete resection with a sufficient safety margin is recommended as the first-line treatment. The morphological characteristics and immunohistochemical findings, especially TTF-1 positivity and thyroglobulin negativity, are important for the diagnosis.
Introduction
Primary nasopharyngeal adenocarcinomas (NPACs) are rare neoplasms, accounting for only 0.38–0.48% of all malignant nasopharyngeal neoplasms (1, 2). NPACs are classified into 2 main groups based on marked differences in their clinical manifestations, histomorphological features, and behavior: the surface origin type, which is usually papillary in configuration and of low-grade malignancy, and the salivary gland type (3). In 1988, Wenig et al. first described low-grade nasopharyngeal papillary adenocarcinomas (LGNPPAs) that had an indolent clinical behavior and low-grade histological features as a distinct entity (4). LGNPPA is histologically characterized by papillary fronds and crowded glandular structures lined by a bland cuboidal to columnar epithelium resembling papillary thyroid carcinoma. In 2005, Carrizo and Luna first reported that two pediatric patients with LGNPPA showed the expression of thyroid transcription factor-1 (TTF-1) in their tumor cell nuclei and named LGNPPA with the expression of TTF-1 “thyroid-like low-grade nasopharyngeal papillary adenocarcinoma” (TL-LGNPPA) (5). TL-LGNPPA is an extremely rare tumor; to date, only 27 cases have been reported (6).
We herein report the case of a 38-year-old Japanese woman with primary TL-LGNPPA and review the relevant literature on TL-LGNPPA.
Case Report
A 38-year-old Japanese woman with a previous history of urticaria caused by an unknown allergen presented to a hospital in 2015 with nasal obstruction that had persisted for 1 month after symptoms of the common cold (e.g., low-grade fever, sore throat, and fatigue). An inspection of the nasal cavity with a soft fiberscope revealed a pedunculated polypoid tumor of ~20 mm in diameter on the posterior edge of nasal septum (Figures 1A,B). Magnetic resonance imaging (MRI) revealed a 20-mm tumor located in the epipharynx that originated from the posterior edge of the nasal septum, and T1- and T2-weighted images showed the same or slightly higher intensities compared to that of the nasal concha (Figures 1C,D). A chest X-ray examination showed no signs of a lung lesion. Enhanced computed tomography (CT) or MRI was not performed because of the patient's history of allergy due to an unknown allergen. A clinical examination revealed no signs of thyroid tumor, cervical lymphadenopathy, or other physical abnormalities. A biopsy of the pedunculated portion of the mass was performed, and it was diagnosed as a benign salivary gland-type tumor. The patient was referred to our hospital and presented for surgical treatment 2 months after first visiting the previous hospital in 2015. On the first inspection of the nasopharynx in our hospital, the main part of the tumor had disappeared and only the pedunculated portion of the tumor remained (Figure 1E). Plain CT revealed no invasive findings or metastatic lesions. The tumor was endoscopically resected 3 weeks after the patient's first visit to our department. In this operation, the tumor was completely excised with a surgical margin of ~5 mm using a needle electrode knife and was removed together with the periosteum from the vomer (Figure 1F).
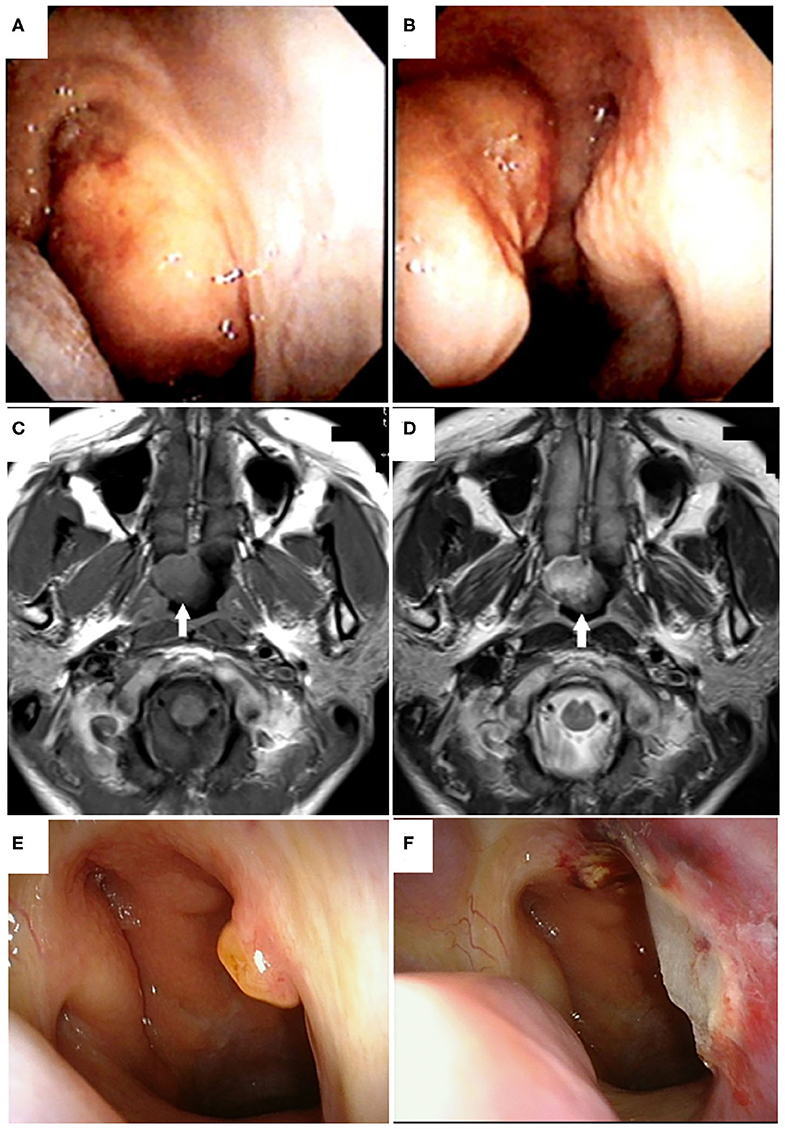
Figure 1. Tumor appearance on nasopharyngeal endoscopy and magnetic resonance imaging (MRI). Endoscopic findings of the nasopharyngeal tumor viewed from the right (A) and left (B) nasal cavities at the first visit to the previous hospital are shown. A pedunculated polypoid tumor originating from the posterior edge of the nasal septum was found in the epipharynx. Horizontal views of plain T1-weighted (C) and T2-weighted magnetic resonance imaging (D) of the head showed a tumor of ~20 mm in diameter located in the epipharynx originating from the posterior edge of the nasal septum without invasive or destructive findings (white arrows). T1- and T2-weighted images showed the same or slightly higher intensities compared to that of the nasal concha. Preoperative (E) and postoperative (F) endoscopic findings of the nasopharyngeal tumor viewed from the right nasal cavity in our hospital. In the preoperative view, the main portion of tumor had disappeared and only the pedunculated portion remained (E). The tumor was endoscopically resected with a 5-mm safety margin (F).
A histologic examination revealed a papillary structure with hyalinized fibrovascular cores lined by cuboidal to columnar stratified cells with round to oval vesicular nuclei and eosinophilic cytoplasm (Figure 2A). An increase in nuclear chromatin and mild nuclear atypia were found, but no nuclear polymorphism was detected. Some cells had clear chromatin; however, a nuclear groove and nuclear pseudoinclusion were absent. No mitotic figures were found and there was no necrosis (Figure 2B). A streaming pattern lining of the tumor cells with small round to oval nuclei (i.e., spindle cell component) was also found in some areas (Figure 2C). Psammoma bodies were not seen. The tumor showed invasive growth into the underlying fibrous connective tissue (Figure 2A). These morphological findings suggested polymorphous low-grade adenocarcinoma (PLGA) and low-grade nasopharyngeal papillary adenocarcinoma (LGNPPA) as differential diagnoses. Additional immunohistochemical examinations were needed to make a definitive diagnosis.
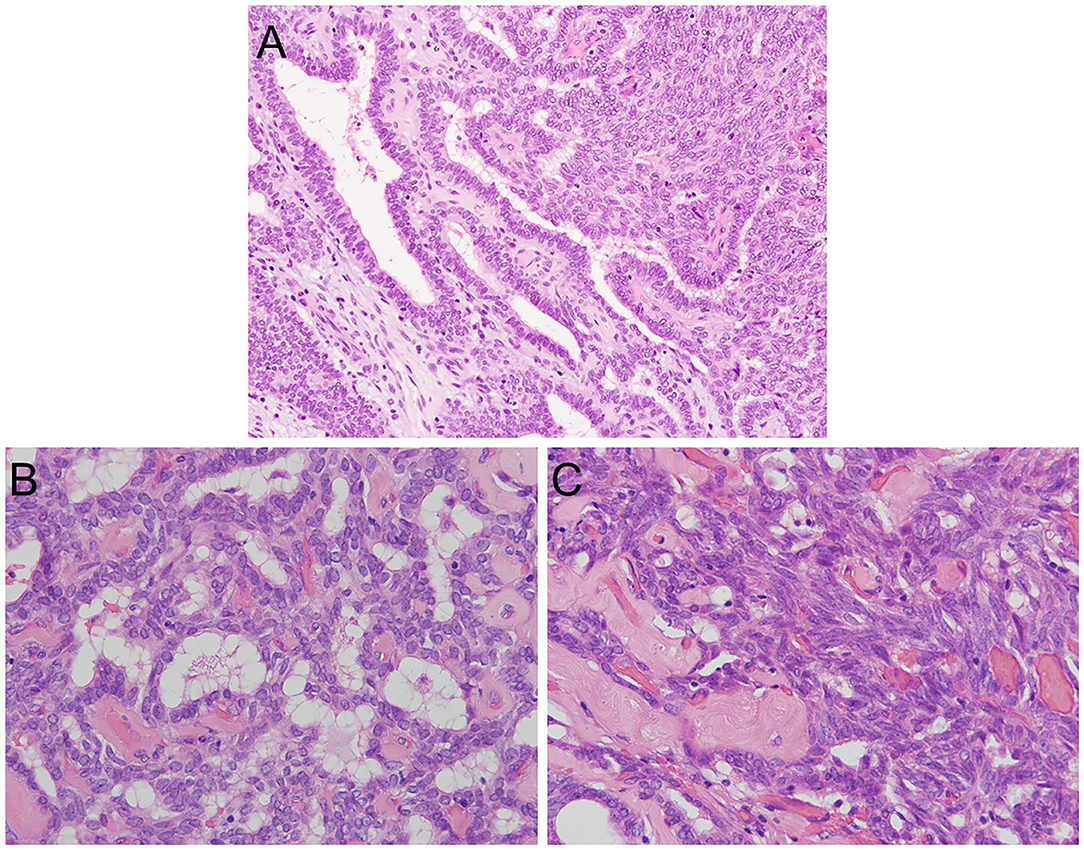
Figure 2. Histopathological features of TL-LGNPPA. (A) Histological examination revealed a papillary structure with fibrovascular cores lined by cuboidal to columnar stratified cells with round to oval vesicular nuclei and eosinophilic cytoplasm. Psammoma bodies were not seen. The tumor showed invasive growth into the underlying fibrous connective tissue. (H&E staining, × 20). (B) An increase in nuclear chromatin and mild nuclear atypia were found, but no nuclear polymorphism was detected. Some cells had clear chromatin; however, the nuclear groove and nuclear pseudoinclusion were absent. No mitotic figures were found, and necrosis was not identified (H&E staining, × 40). (C) A streaming pattern lining of the tumor cells was also found in some areas (H&E staining, × 40).
Immunohistochemistry revealed that the tumor cells were positive for cytokeratin (CK) AE1/AE3 (Figure 3A), CK7, CK19, epithelial membrane antigen (EMA), vimentin (Figure 3B), and thyroid tissue factor-1 (TTF-1) (Figure 3C) but negative for CK5/6, CK20, smooth muscle actin (SMA) (Figure 3D), calponin, p63, glial fibrillary acidic protein (GFAP), S100 (Figure 3E), CDX2, CEA, PAX8, CD10, DOG1, GATA3, SOX10, GCDFP-15, and thyroglobulin (Figure 3F). A pathological diagnosis of LGNPPA with TTF-1 (i.e., TL-LGNPPA) was finally made. The surgical margin was negative. Adjuvant therapy was not performed because of the free histopathological margin and information about the clinical characteristics of TL-LGNPPA reported in the relevant literature. There was no evidence of recurrence or distant metastasis at 5 years after surgery. The patient is currently being followed up and is satisfied with the good clinical course and lack of post-treatment symptoms.
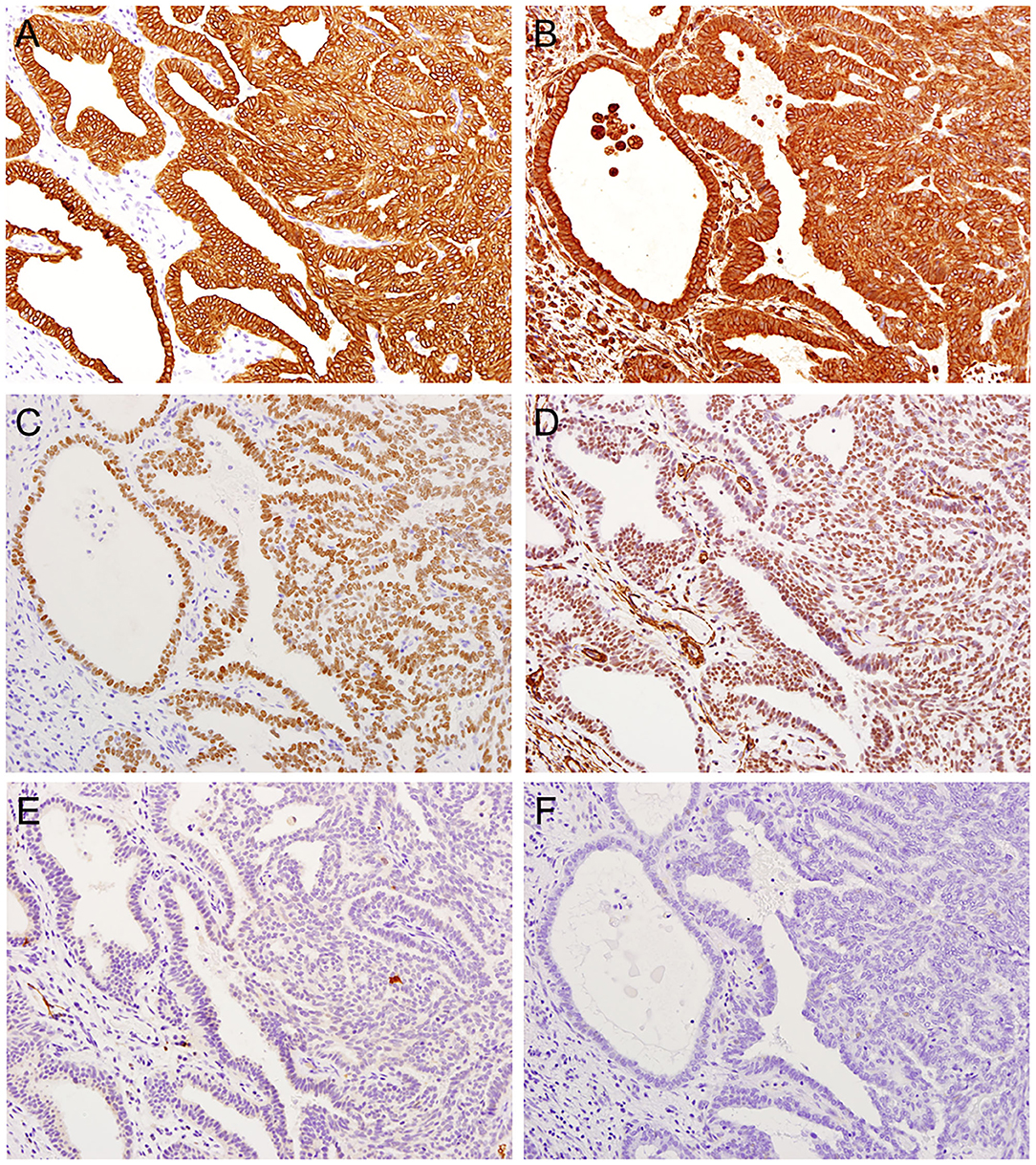
Figure 3. Immunohistochemical features of TL-LGNPPA. (A) Positive staining for cytokeratin (CK) AE1/AE3 (× 20). (B) Positive staining for vimentin (× 20). (C) Positive nuclear staining for thyroid tissue factor-1 (TTF-1) (× 20). (D) Negative staining for smooth muscle actin (SMA) (× 20). (E) Negative staining for S100 (× 20). (F) Negative staining for thyroglobulin (× 20).
Review of the Relevant Literature on TL-LGNPPA
We identified only 27 case reports of TL-LGNPPAs or LGNPPAs with the expression of TTF-1 that included detailed patient information in 23 English-language articles published up to 2019 (5–26). We reviewed the relevant literature on TL-LGNPPAs or LGNPPAs with the expression of TTF-1 in terms of the clinicopathological and immunohistochemical characteristics, including our case (Tables 1, 2).
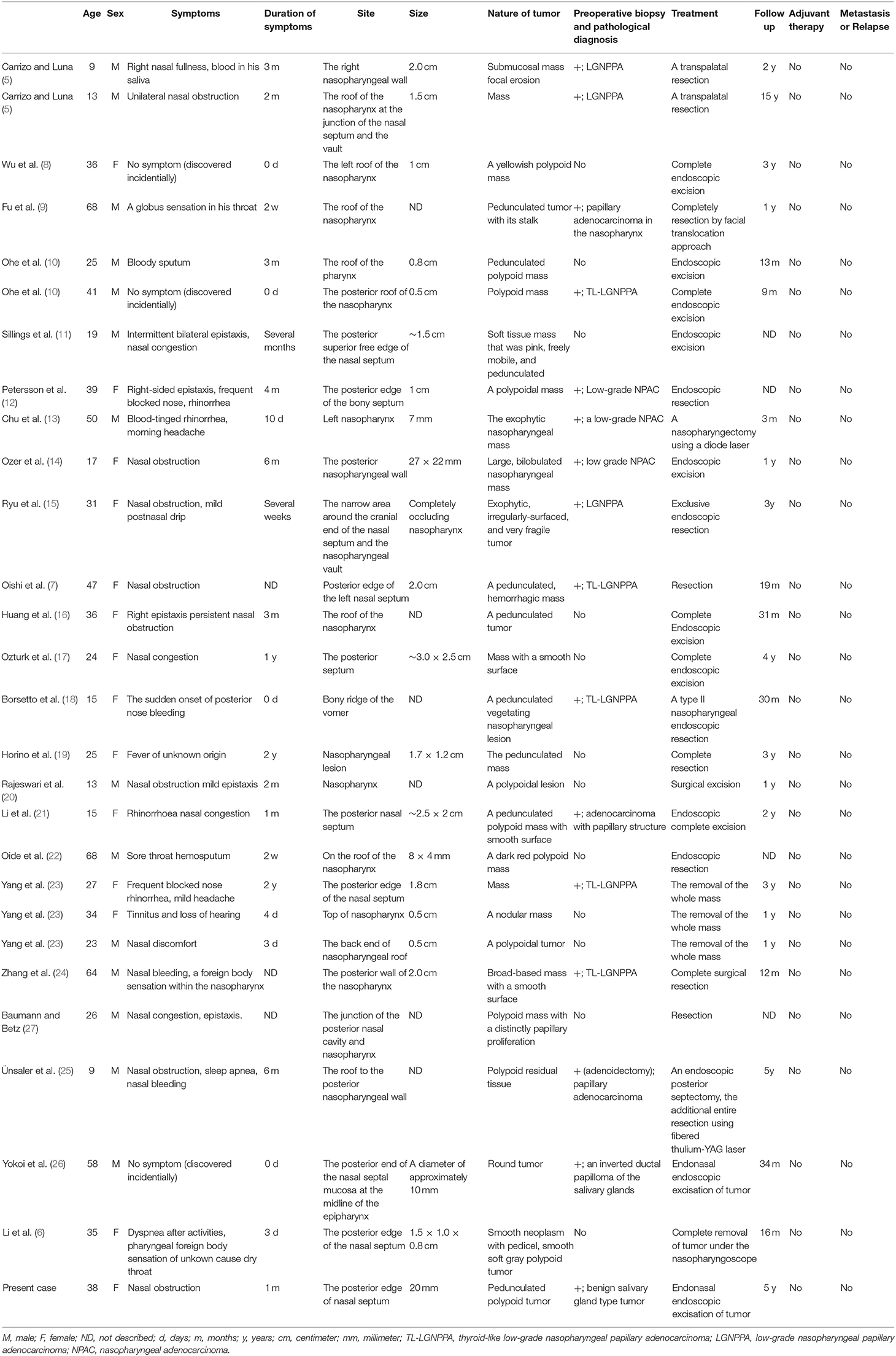
Table 1. Summary of clinical characteristics of previously reported thyroid-like low-grade nasopharyngeal papillary adenocarcinoma.
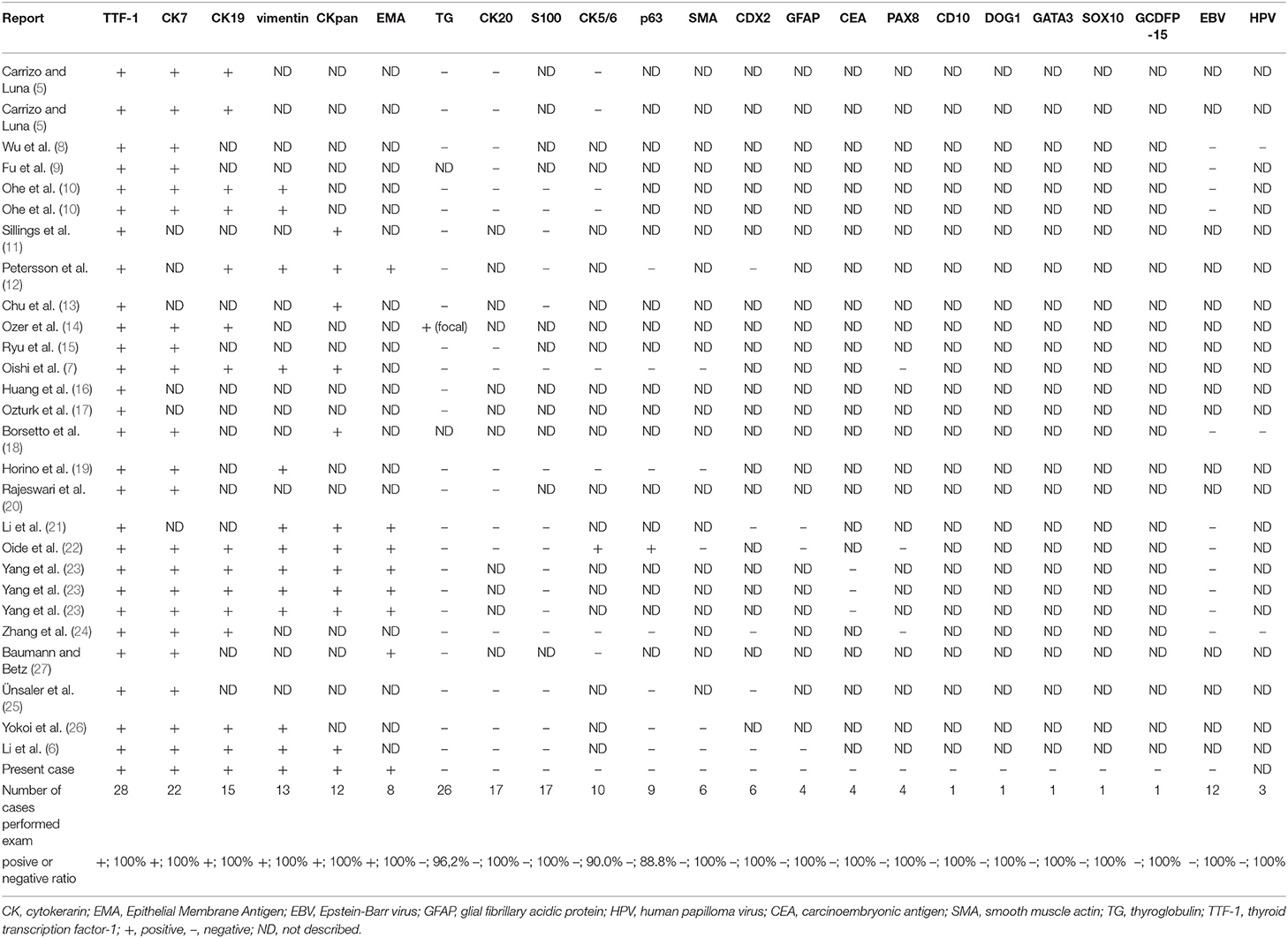
Table 2. Summary of characteristics of immunohistochemical and in situ hybridization investigations of previously reported thyroid-like low-grade nasopharyngeal papillary adenocarcinoma.
Clinicopathological Characteristics of TL-LGNPPA
In the 28 case reports included in our literature review (Table 1), the mean age of patients with TL-LGNPPA was 32.3 years (range: 9–68 years). Fourteen of the patients were men and 14 were women; thus, the incidence of TL-LGNPPA did not differ according to sex. The clinical symptoms of the patients included nasal obstruction or nasal congestion (n = 14, 50.0%), bleeding (including epistaxis and bloody sputum; n = 12, 42.9%), nasal or pharyngeal discomfort (including foreign body sensation; n = 5, 17.9%), rhinorrhea (n = 3, 10.7%), and headache (n = 2, 7.1%). Three patients had no clinical symptoms (n = 3, 10.7%). Sleep apnea, dry throat, postnasal drip, dyspnea, sore throat, prolonged fever for 2 years, and loss of hearing were also found in one case each (3.6%). Regarding the duration of symptoms, 1 year was defined as 12 months and 1 month was defined as 30 days when the duration was indicated in years and months, respectively. Several weeks and several months were defined as 3 weeks and 3 months, respectively. The mean and median duration of symptoms was 116.6 and 30 days (range, 0–730 days), respectively. The mean maximum tumor diameter was 14.8 mm (range, 5–30 mm).
Regarding appearance of the tumor, the TL-LGNPPAs tended to be pedunculated (n = 10, 35.7%) and polypoid (n = 12, 42.9%) tumors. The tumor locations included the posterior edge of the nasal septum (n = 10, 35.7%), the roof or vault of the nasopharynx (n = 9, 32.1%), the narrow area around the cranial end of the nasal septum and the nasopharyngeal vault (n = 3, 10.7%), and the posterior wall of the nasopharynx (n = 2, 7.1%). The tumor was located in the right or left wall of the nasopharynx in one case each (3.6%). Incisional biopsy was performed before treatment in 16 cases. TI-LGNPPA or LGNPPA was histopathologically diagnosed in 14 patients (87.5%); however, 2 patients including our case were diagnosed as having benign tumors.
All cases were treated with complete resection and without adjuvant treatment. Endoscopic surgery was performed in 16 cases (57.1%). Transpalatal and facial translocation approaches were used in 2 and 1 case, respectively. No detailed information about the surgical approach in the remaining 9 cases was found in the literature. In four cases, secondary resection was performed due to an insufficient surgical margin after the first operation. No patients received adjuvant therapy after complete resection. The mean follow-up period was 31.0 months [range, 3–148 months (15 years)]. No distant metastasis or recurrence was found in any of the cases.
Characteristics of Immunohistochemical and in situ Hybridization Investigations of TL-LGNPPA
We reviewed the results of the immunohistochemical examinations in the 28 cases of TL-LGNPPA, including our case (Table 2). These examinations indicated the positive expression of CK7 (100%, 22/22 cases), CK19 (100%, 15/15 cases), vimentin (100%, 13/13 cases), CKpan (100%, 12/12 cases), and EMA (100%, 8/8 cases) and the negative expression of thyroglobulin (96.2%, 25/26 cases), CK20 (100%, 17/17 cases), S100 (100%, 17/17 cases), CK5/6 (90.0%, 9/10 cases), p63 (88.8%, 8/9 cases), SMA (100%, 6/6 cases), CDX2 (100%, 6/6 cases), GFAP (100%, 4/4 cases), PAX8 (100%, 4/4 cases), and CEA (100%, 4/4 cases). The expression of CD10, DOG1, GATA3, SOX10, and GCDFP-15 was examined only in our case, and all were negative.
In situ hybridization to detect Epstein-Barr Virus (EBV) and human papilloma virus (HPV) was negative in all cases (12/12 cases [100%] and 3/3 cases [100%], respectively).
Discussion
Primary nasopharyngeal adenocarcinomas (NPACs) can be classified by their morphological features and clinical behavior into 2 main categories: the conventional or mucosal surface origin type and the salivary gland type (3). The former is usually a low-grade tumor with papillary configuration and likely originates from the nasopharyngeal surface mucosa (i.e., LGNPPA), whereas the latter consists of tumors such as mucoepidermoid adenocarcinoma, adenoid cystic carcinoma, and polymorphous low-grade adenocarcinoma (3). TL-LGNPPA is an extremely rare neoplasm that exhibits morphologic features that are analogous to papillary thyroid carcinoma and the abnormal expression of TTF-1.
From our review of the 28 cases reported in the literature (including the present case), most TL-LGNPPAs had a pedunculated and polypoid shape and were located at the vault of epipharynx and the posterior edge of the nasal septum. Patients with TL-LGNPPA were most commonly in their thirties, and there was no sex difference in its incidence. The main symptoms of TL-LGNPPA were nasal obstruction or nasal congestion, bleeding, and nasal or pharyngeal discomfort. In our case, the main symptom was nasal obstruction, which persisted for 1 month after the onset of symptoms of a common cold. Horino et al. (19) reported the case of a 25-year-old woman who had long-lasting fever of unknown origin. They hypothesized that the overexpression of TTF-1 in the tumor might have induced the local expression of interleukin-6, resulting in the fever. Furthermore, it was suggested that TL-LGNPPA has an excellent prognosis and that local invasion or distant metastasis of TL-LGNPPA was extremely rare. Some studies have reported the local extension of LGNPPA into the sphenoid sinus (28) or parapharyngeal space (29); however, an immunohistochemical study was negative for TTF-1 in these two cases of LGNPPA. TL-LGNPPA might tend to grow more slowly than LGNPPA without the expression of TTF-1, and the frequency of local invasion might differ from that in LGNPPA without the expression of TTF-1.
Endoscopic complete resection would be recommended as the first choice of treatment for TL-LGNPPA. In 4 of 28 cases of TL-LGNPPA, additional resection of the tumor was performed after insufficient primary surgery; however, none of these patients developed local recurrence or distant metastasis. In our case, endoscopic resection with a 5-mm safety margin was performed, and 5-year disease-free survival was achieved. Some studies reported cases of LGNPPA treated with adjuvant therapy after complete resection. Wang et al. (28) used photodynamic therapy as a postoperative adjuvant therapy for an incompletely resected primary nasopharyngeal papillary adenocarcinoma and achieved 5-year disease-free survival. In other reports, adjuvant radiotherapy was performed, and no patients developed recurrent disease or distant metastasis (4, 30). These LGNPPAs were negative for TTF-1, or TTF-1 expression was not investigated. In TL-LGNPPA or LGNPPA with TTF-1, serial adjuvant therapy might be excessive after complete resection. We cannot draw any complete conclusions regarding the need for adjuvant therapy due to the small number of case reports of TL-LGNPPA. Further accumulation of cases and long-term follow-up will be necessary.
Histopathologically, TL-LGNPPAs are composed of a complex, arborizing papillary configuration with hyalinized fibrovascular cores and glands lined by cuboidal to columnar cells with a moderate amount of eosinophilic cytoplasm and round to oval nuclei with vesicular to clear chromatin (31). Psammomatoid calcifications are seen in approximately one-third of cases. With the exception of psammoma bodies, most findings were present in our case. The spindle cell component was found in our case. In the previous literature up to 2019, only five cases of TL-LGNPPA with a prominent spindle cell component, named “biphasic low-grade nasopharyngeal papillary adenocarcinoma,” had been reported (7, 10, 12, 26). Whether the etiology of biphasic LGNPPA differs from TL-LGNPPA without the spindle cell component and the reason why it develops in TL-LGNPPA remain unknown. Petersson et al. discussed tumors with a biphasic appearance, such as a biphasic synovial sarcoma, a medullary thyroid carcinoma, or a group of enigmatic neoplasms that show “thymic-like or brachial pouch differentiation” as differential diagnoses of biphasic TL-LGNPPA (12). In their report, biphasic LGNPPA was differentiated from these tumors based on diffuse positivity of CK and no evidence of gene alternations such as SYT-SSX1/2 fusion transcripts t(X; 18) (p11.2; q11.2) against a biphasic synovial sarcoma, the absence of immunopositivity for CEA, calcitonin, and chromogranin A against medullary thyroid carcinoma, and the difference in originating regions or the expression of TTF-1 against a group of enigmatic neoplasms, respectively (12). Only one report mentioned TL-LGNPPA with squamous differentiation (22); however, Yokoi et al. posited that the development of spindle cells occurred independently of squamous differentiation as the tumors were negative for CK5/6 and p40 (26). Further research and accumulation of cases are needed to elucidate the pathogenesis of TL-LGNPPA with the spindle cell component.
Immunohistochemically, the most characteristic finding of TL-LGNPPA is TTF-1 positivity (5). TTF-1 is a member of the homeodomain transcription factor family that regulates genes expressed within the thyroid, lung, and brain, including thyroglobulin, thyroid peroxidase, Clara cell secretory protein, and surfactant proteins (32). TTF-1 is not only found in TL-LGNPPA but also in primary thyroid papillary adenocarcinoma or lung cancer, suggesting that metastatic thyroid papillary adenocarcinoma or lung cancer in the nasopharynx is important as a differential diagnosis of TL-LGNPPA. Immunostaining of thyroglobulin is highly recommended to differentiate TL-LGNPPA from metastatic thyroid papillary adenocarcinoma (6). Thyroglobulin is usually detected in metastatic thyroid papillary adenocarcinoma, whereas TL-LGNPPA is negative for thyroglobulin. These findings were also observed in our case.
As an additional differential diagnosis of TL-LGNPPA, clinicians should also consider low-grade sinonasal adenocarcinoma, which is divided into salivary type, intestinal type, and non-intestinal type adenocarcinoma (33).
The papillary variant of PLGA is one of the most important differential diagnoses of TL-LGNPPA. PLGA is a minor salivary gland neoplasm that is characterized by morphologic variability, cytologic uniformity, and an infiltrating growth pattern (34). In the WHO classification of salivary gland tumors that was updated in 2017, PLGA was renamed and shortened to polymorphous adenocarcinoma (PAC) (35, 36). PLGA may also have a papillary architecture with an invasive growth pattern and slight nuclear atypia; as a result, it is difficult to distinguish PLGA from TL-LGNPPA based on the morphology alone (3, 24). Immunohistochemical examinations are important to distinguish PLGA from TL-LGNPPA. PLGA (PAC, classical variant) is positive for CK7, CK8, CK18, vimentin, p63 (a myoepithelial marker), and S100 and negative for CK19, p40, and TTF-1 (3, 36). In our case, PLGA and LGNPPA were suggested as differential diagnoses based on the morphology; however, immunohistochemistry was negative for p63 and S100 and positive for TTF-1; thus, the final diagnosis was TL-LGNPPA.
The latest WHO classification of salivary gland tumors includes under the PAC heading not only classical PLGA but also the so-called “cribriform adenocarcinoma of minor salivary glands” (CAMSG) (35, 36). Clinically, CASMG often shows more aggressive regional and distant spread compared to PLGA (PAC, classical variant) (35, 36). Microscopically, the tumor cells of CAMSG show pale and vesicular nuclei with a ground-glass appearance that resembles the Orphan Annie Eye-nuclei of papillary thyroid carcinoma (PTC) (36). CAMSG shows architectural uniformity with a predominant cribriform and solid growth pattern, sometimes with peripheral palisading, peripheral clefting, and glomeruloid appearance (37). Immunohistochemically, CAMSG is strongly positive for CK7, CK8, CK18, S100, and vimentin. Furthermore, AE1/AE3 and SOX10 are strongly expressed in CAMSG (36). Basal and myoepithelial markers such as p63, SMA, and CK5/6 are variably positive in all CASMGs. As with PAC, CAMSG is also consistently negative for p40. CK19 stains in most CAMSG although in a mild-to-moderate manner (36). Most importantly, CAMSG is consistently negative for both thyroglobulin and TTF-1 (38). Despite the histologic and immunophenotypic similarity between PLGA and CAMSG, partially different genetic alterations in PRKD genes were found in PLGA and CAMSG (36). More than 70% of PACs exhibit activating mutations in the PRKD1 gene, which is a single-nucleotide variant (E710D) that affects a highly conserved amino acid in the catalytic loop of the kinase domain (36). In contrast to PAC, 80% of CAMSGs show rearrangements rather than mutations in PRKD genes (PRKD1-3) (36). From our review of the literature and our case, we could not find any results of the examination of PRKD gene alteration including in our case. However, we could also differentiate CAMSG in the final diagnosis because of the difference in immunohistochemical findings in our case (i.e., positive for TTF-1 and negative for S100, CK5/6, p63, SMA, and SOX10) despite that lack of PKRD studies.
Papillary variants of intestinal-type adenocarcinoma (ITAC) should also be excluded in the diagnosis of TL-LGNPPA (6). ITACs comprise a significant proportion of primary adenocarcinomas of the sinonasal tract, which show histological features that are reminiscent of colonic adenoma and adenocarcinoma (39, 40). ITACs are characteristically associated with occupational exposure to wood and leather dust, and have more aggressive behavior, characterized by repeated local recurrence and an ominous outcome in comparison to low-grade papillary adenocarcinoma (39, 40). Immunohistochemically, ITACs are positive for the expression of CK7, CDX-2, villin, MUC2, SATB-2, and CK20, whereas TL-LGNPPAs are negative for CDX-2 and CK20 (39).
Sinonasal renal cell-like adenocarcinoma (SNRLCA) is a variant of low-grade, non-intestinal sinonasal adenocarcinoma that mimics renal cell carcinoma (41). This entity histologically resembles clear cell renal cell carcinoma, with nests and follicles of polyhedral cells with abundant optically clear cytoplasm (41). Nuclear pleomorphism and mitotic activity are minimal (41). Immunochemically, it has been reported to be positive for CK7 and carbonic anhydrase IX (CAIX) and negative for PAX8, RCC, and vimentin (41, 42). The expression of S100 is variable and inconsistent in SNRLCA (42). Some case reports of SNRCLA indicated positive expression for DOG1 and SOX 10 (43, 44). Positivity for S100, DOG1, and SOX10, which are markers of seromucinous differentiation, might depend on the degree of seromucinous differentiation of SNRLCA (33, 44). Other reports indicated that TTF-1 was completely negative in SNRCLA (43–46). We could not find any examinations of CAIX from our review of the literature including our case, but SNRCLA could be differentiated in the final diagnosis because our case was positive for TTF-1 and vimentin.
ETV6 rearranged sinonasal low-grade non-intestinal type adenocarcinoma is one of the differential diagnoses of TL-LGNPPA. Three cases of this novel entity restricted to the sinonasal tract were first reported by Andreasen et al. in 2017 (47). Morphologically, this tumor is tubular, composed of cuboidal to cylindrical tumor cells with bright eosinophilic cytoplasm, which is positive for periodic acid-Schiff with diastase in the atypical portion. Nuclei are basally located, compact, or vesicular with granular chromatin and a single centrally located nucleoli. Tumor stroma is sparse, but fine fibrovascular strands are seen between tubular formations. Mitoses are rare, and no vascular or perineural growth is observed. Immunohistochemically, the tumor cells are positive for CK7, SOX10, DOG1, vimentin, S100, and GCDFP-15 and negative for CK20, GATA3, and mammaglobin (47, 48). This entity is characterized by the gene rearrangement of ETC variant 6 (ETV6), which is a fusion between ETV6 and the Neurotrophic receptor tyrosine kinase type 3 (NTRK3) or RET gene identified with fluorescence in situ hybridization (FISH) (47, 48). FISH for ETV6 rearrangement in previous reports was not performed in our case or in the previous literature for TL-LGNPPA. However, we could differentiate TL-LGNPPA from ETV6 rearranged sinonasal low-grade non-intestinal type adenocarcinoma on the basis of the immunohistochemical findings in our case (i.e., positive for TTF-1 and negative for S100, CK5/6, DOG1, SOX10, and GCDFP-15).
Nasopharyngeal carcinoma has been shown to be strongly associated with Epstein-Barr virus (EBV) (49). In situ hybridization reveals that EBV-encoded RNA (EBER) is strongly expressed by the tumor cells in these cases, indicating the presence of EBV RNA (50). From our review of the literature, all 12 cases of TL-LGNPPA, including our case and the cases reported in 8 studies in the literature, were completely negative for EBER (8, 9, 11, 18, 21–24). Furthermore, 3 cases reported in 3 studies in the literature were negative for human papilloma virus (HPV) (5, 18, 24). These findings suggest that the origin of TL-LGNPPA might not be associated with EBV or HPV infection; however, further investigations are needed due to the relatively small number of cases.
Gene alterations of TL-LGNPPA were only analyzed in 3 cases in 3 studies (7, 12, 22). BRAF V600E mutations, the most common gene alteration in PTC, were not detected in any of the 3 reported cases of TL-LGNPPA in which genetic analyses were performed. Oide et al. also performed sequencing for mutations in N-RAS (codon 61) genes, which are known to be mutated in PTC, in a case of NPAC (22). However, the results were negative, and they concluded that although morphologically similar, NPAC and PTC do not share the same molecular pathogenesis (22). Furthermore, they analyzed the EGFR and ALK genes, which are involved in some populations of TTF-1-positive lung adenocarcinoma, and found no mutations (22). Petersson et al. investigated BRAF and KIT mutations and SYT-SSX1/2 rearrangement, but they did not identify any particular genetic alterations (12). As shown in these reports, no specific gene alterations have been found in TL-LGNPPA, and the tumorigenesis of TL-LGNPPA remains unclear. We summarize the differentiation of immunohistochemical findings and gene alternations between TL-LGNPPA and other sinonasal adenocarcinomas in Table 3 (33–48, 51–56). The accumulation of more cases and further investigations will be needed to elucidate the pathogenesis of this rare neoplasm.
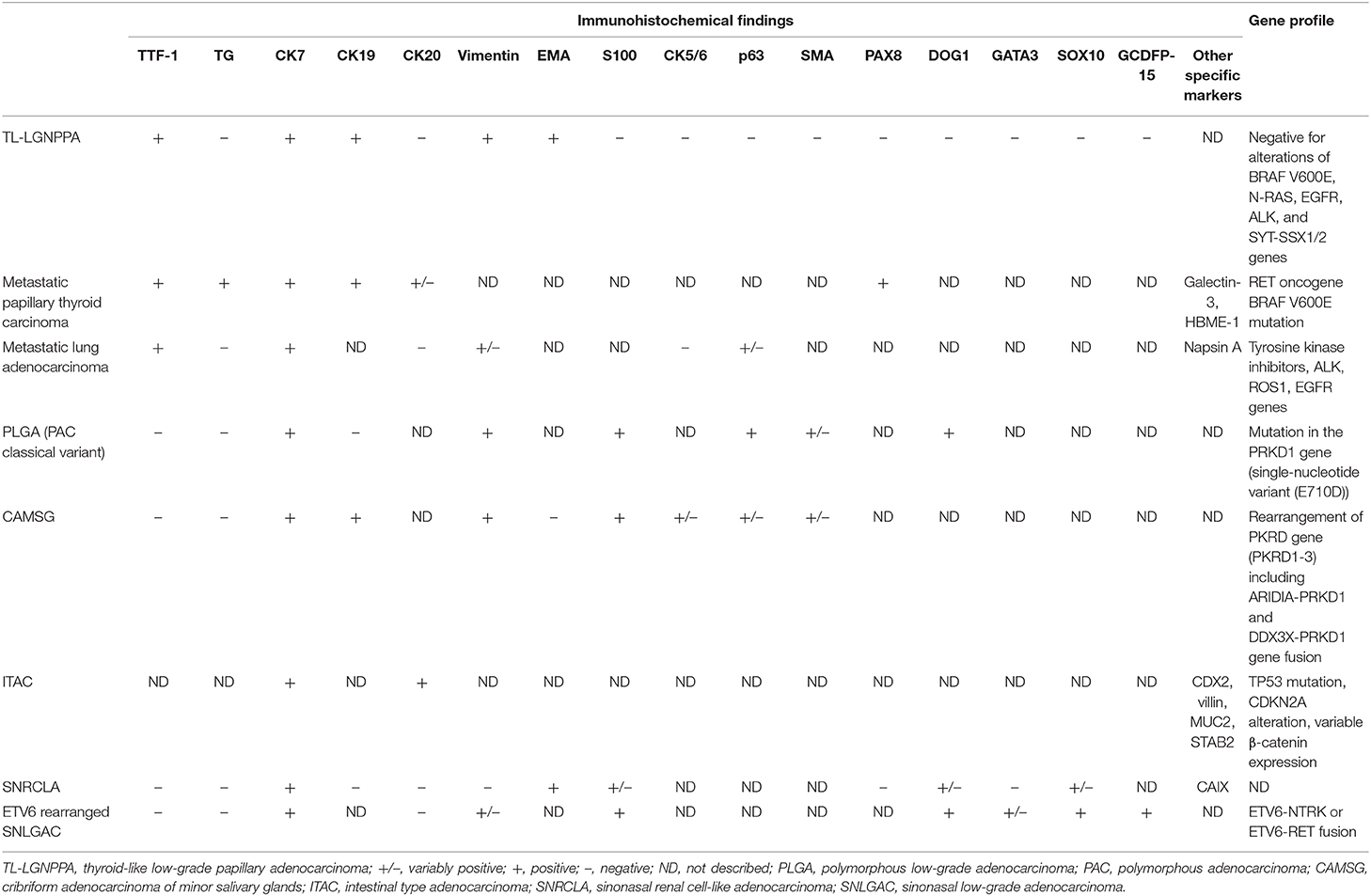
Table 3. Differentiation between TL-LGNPPA and other sinonasal adenocarcinomas [Refer to (33–48, 51–56)].
Conclusion
We treated an extremely rare case of TL-LGNPPA originating from the posterior edge of the nasal septum. From our experience and a review of the literature, TL-LGNPPA has a very good prognosis, and endoscopic complete resection would be recommended as the first-line treatment. In addition to the morphological characteristics, immunohistochemistry is important for the diagnosis, including the differential diagnosis, as TL-LGNPPA is immunohistochemically positive for CK7 and TTF-1 and negative for thyroglobulin, CK20, and S100. Clinicians should pay attention to the possibility of this rare entity if they detect a pedunculated or polypoid mass in the nasopharynx.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HTak, TH, HTac, AN, HM, MS, and HS: conception and design. HTak, TH, HM, MS, and HS: literature search and obtaining of images. HTac, TH, MS, and HS: writing the article. All authors: critical revision and final approval of the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CAIX, carbonic anhydrase IX; CAMSG, cribriform adenocarcinoma of minor salivary glands; CK, cytokeratin; CT, computed tomography; EBER, EBV encoded RNA; EBV, Epstein-Barr virus; EMA, epithelial membrane antigen; GFAP, glial fibrillary acidic protein; HPV, human papilloma virus; ITAC, intestinal-type adenocarcinoma; LGNPPA, low-grade nasopharyngeal papillary adenocarcinoma; MRI, magnetic resonance imaging; NPAC, nasopharyngeal adenocarcinoma; PAC, polymorphous adenocarcinoma; PLGA, polymorphous low-grade adenocarcinoma; PTC, papillary thyroid carcinoma; SMA, smooth muscle actin; SNRLCA, sinonasal renal cell-like adenocarcinoma; TL-LGNPPA, thyroid-like low-grade nasopharyngeal papillary adenocarcinoma; TTF-1, thyroid transcription factor-1.
References
1. He JH, Zong YS, Luo RZ, Liang XM, Wu QL, Liang YJ. [Clinicopathological characteristics of primary nasopharyngeal adenocarcinoma]. Ai Zheng. (2003) 22:753–7. [Article in Chinese]
2. McGuire LJ, Lee JK. The histopathologic diagnosis of nasopharyngeal carcinoma. Ear Nose Throat J. (1990) 69:229–36.
3. Pineda-Daboin K, Neto A, Ochoa-Perez V, Luna MA. Nasopharyngeal adenocarcinomas: a clinicopathologic study of 44 cases including immunohistochemical features of 18 papillary phenotypes. Ann Diagn Pathol. (2006) 10:215–21. doi: 10.1016/j.anndiagpath.2005.11.002
4. Wenig BM, Hyams VJ, Heffner DK. Nasopharyngeal papillary adenocarcinoma. a clinicopathologic study of a low-grade carcinoma. Am J Surg Pathol. (1988) 12:946–53. doi: 10.1097/00000478-198812000-00005
5. Carrizo F, Luna MA. Thyroid transcription factor-1 expression in thyroid-like nasopharyngeal papillary adenocarcinoma: report of 2 cases. Ann Diagn Pathol. (2005) 9:189–92. doi: 10.1016/j.anndiagpath.2005.04.019
6. Li L, Zhou F, Lin F, Han C. Clinicopathologic characteristics of thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: a case report. Appl Immunohistochem Mol Morphol. (2019) 28:e81–4. doi: 10.1097/PAI.0000000000000545
7. Oishi N, Kondo T, Nakazawa T, Mochizuki K, Kasai K, Inoue T, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: case report and literature review. Pathol Res Pract. (2014) 210:1142–5. doi: 10.1016/j.prp.2014.04.020
8. Wu PY, Huang CC, Chen HK, Chien CY. Adult thyroid-like low-grade nasopharyngeal papillary adenocarcinoma with thyroid transcription factor-1 expression. Otolaryngol Head Neck Surg. (2007) 137:837–8. doi: 10.1016/j.otohns.2007.06.725
9. Fu CH, Chang KP, Ueng SH, Wu CC, Hao SP. Primary thyroid-like papillary adenocarcinoma of the nasopharynx. Auris Nasus Larynx. (2008) 35:579–82. doi: 10.1016/j.anl.2007.10.009
10. Ohe C, Sakaida N, Tadokoro C, Fukui H, Asako M, Tomoda K, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: report of two cases. Pathol Int. (2010) 60:107–11. doi: 10.1111/j.1440-1827.2009.02480.x
11. Sillings CN, Weathers DR, Delgaudio JM. Thyroid-like papillary adenocarcinoma of the nasopharynx: a case report in a 19-year-old male. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2010) 110:e25–8. doi: 10.1016/j.tripleo.2010.04.029
12. Petersson F, Pang B, Loke D, Hao L, Yan B. Biphasic low-grade nasopharyngeal papillary adenocarcinoma with a prominent spindle cell component: report of a case localized to the posterior nasal septum. Head Neck Pathol. (2011) 5:306–13. doi: 10.1007/s12105-011-0252-4
13. Chu YT, Yue CT. Nasopharyngeal papillary adenocarcinoma: a case report and clinicopathologic review. Tzu Chi Med J. (2012) 24:19–21. doi: 10.1016/j.tcmj.2011.11.003
14. Ozer S, Kayahan B, Cabbarzade C, Bugdayci M, Kosemehmetoglu K, Yucel OT. Thyroid-like papillary adenocarcinoma of the nasopharynx with focal thyroglobulin expression. Pathology. (2013) 45:622–4. doi: 10.1097/PAT.0b013e32836536e9
15. Ryu J, Park WS, Jung YS. Exclusive endoscopic resection of nasopharyngeal papillary adenocarcinoma via combined transnasal and transoral approach. Clin Exp Otorhinolaryngol. (2013) 6:48–51. doi: 10.3342/ceo.2013.6.1.48
16. Huang CH, Chang YL, Wang CP, Wu HP. Positive immunostaining of thyroid transcription factor-1 in primary nasopharyngeal papillary adenocarcinoma. J Formos Med Assoc. (2015) 114:473–4. doi: 10.1016/j.jfma.2013.04.012
17. Ozturk K, Midilli R, Veral A, Ertan Y, Karci B. Primary thyroid-like papillary adenocarcinoma of the nasal septum: a case report. Ear Nose Throat J. (2015) 94:E19–21. doi: 10.1177/014556131509400213
18. Borsetto D, Cazzador D, Prosenikliev V, Zamon A, Volo T, Marino F, et al. Nasopharyngeal thyroid-like low-grade papillary adenocarcinoma. B-ENT. (2016) 12:235–40.
19. Horino T, Ichii O, Hamada-Ode K, Matsumoto T, Shimamura Y, Inoue K, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: a case report. Mol Clin Oncol. (2016) 5:693–6. doi: 10.3892/mco.2016.1056
20. Rajeswari B, Sukumaran Nair RK, Parukuttyamma K, Mathews A. Low-grade papillary adenocarcinoma of nasopharynx with expression of thyroid transcription factor-1: case report and review of literature. Indian J Pathol Microbiol. (2016) 59:518–20. doi: 10.4103/0377-4929.191809
21. Li M, Wei J, Yao X, Wang C. Clinicopathological features of low-grade thyroid-like nasopharyngeal papillary adenocarcinoma. Cancer Res Treat. (2017) 49:213–8. doi: 10.4143/crt.2016.195
22. Oide T, Kadosono O, Matsushima J, Wu D, Nagashima H, Saigusa H, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma with squamous differentiation: a novel histological finding. Hum Pathol. (2017) 70:43–8. doi: 10.1016/j.humpath.2017.05.020
23. Yang S, Huang Y, Lu X, Chen Y, Wang J, Chen W. Thyroid-like low-grade nasopharyngeal papillary carcinoma with a “biphasic” morphology: report of 3 cases and literature review. Int J Clin Exp Pathol. (2017) 10:6038–46.
24. Zhang WL, Ma S, Havrilla L, Cai L, Yu CQ, Shen S, et al. Primary thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: a case report and literature review. Medicine. (2017) 96:e8851. doi: 10.1097/MD.0000000000008851
25. Ünsaler S, Başaran B, Aslan I, Yilmazbayhan D. Endonasal endoscopic nasopharyngectomy for the treatment of nasopharyngeal papillary adenocarcinoma: report of a rare case. Int J Pediatr Otorhinolaryngol. (2018) 104:51–3. doi: 10.1016/j.ijporl.2017.10.041
26. Yokoi H, Terado Y, Fujiwara M, Matsumoto Y, Ikeda T, Saito K. Biphasic low-grade nasopharyngeal papillary adenocarcinoma: a case report and literature review. BMC Clin Pathol. (2018) 18:10. doi: 10.1186/s12907-018-0076-1
27. Baumann KB, Betz SJ. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma. Head Neck Pathol. (2019) 13:661–3. doi: 10.1007/s12105-018-0947-x
28. Wang CP, Chang YL, Chen CT, Yang TH, Lou PJ. Photodynamic therapy with topical 5-aminolevulinic acid as a post-operative adjuvant therapy for an incompletely resected primary nasopharyngeal papillary adenocarcinoma: a case report. Lasers Surg Med. (2006) 38:435–8. doi: 10.1002/lsm.20291
29. Kakkar A, Sakthivel P, Mahajan S, Thakar A. Nasopharyngeal papillary adenocarcinoma as a second head and neck malignancy. Head Neck Pathol. (2019) 13:699–704. doi: 10.1007/s12105-018-0944-0
30. Wang X, Yan H, Luo Y, Fan T. Low-grade nasopharyngeal papillary adenocarcinoma: a case report and review of the literature. Onco Targets Ther. (2016) 9:2955–9. doi: 10.2147/OTT.S100447
31. Stelow EB, Bell D, Wenig BM. Nasopharyngeal papillary adenocarcinoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO Classification of Head and Neck Tumours. 4th edn. Lyon: International Agency for Research on Cancer (2017). p. 70.
32. Bingle CD. Thyroid transcription factor-1. Int J Biochem Cell Biol. (1997) 29:1471–3. doi: 10.1016/S1357-2725(97)00007-1
33. Leivo I. Sinonasal adenocarcinoma: update on classification, immunophenotype and molecular features. Head Neck Pathol. (2016) 10:68–74. doi: 10.1007/s12105-016-0694-9
34. Wenig BM, Harpaz N, Delbridge C. Polymorphous low-grade adenocarcinoma of seromucous glands of the nasopharynx: a report of a case and a discussion of the morphologic and immunohistochemical features. Am J Clin Pathol. (1989) 92:104–9. doi: 10.1093/ajcp/92.1.104
35. Seethala RR, Stenman G. Update from the 4th Edition of the World Health Organization classification of head and neck tumours: tumors of the Salivary Gland. Head Neck Pathol. (2017) 11:55–67. doi: 10.1007/s12105-017-0795-0
36. Vander Poorten V, Triantafyllou A, Skálová A, Stenman G, Bishop JA, Hauben E, et al. Polymorphous adenocarcinoma of the salivary glands: reappraisal and update. Eur Arch Otorhinolaryngol. (2018) 275:1681–95. doi: 10.1007/s00405-018-4985-5
37. Mimica X, Katabi N, McGill MR, Hay A, Zanoni DK, Shah JP, et al. Polymorphous adenocarcinoma of salivary glands. Oral Oncol. (2019) 95:52–8. doi: 10.1016/j.oraloncology.2019.06.002
38. Michal M, Kacerovska D, Kazakov DV. Cribriform adenocarcinoma of the tongue and minor salivary glands: a review. Head Neck Pathol. (2013) 7:S3–11. doi: 10.1007/s12105-013-0457-9
39. Franchi A, Massi D, Palomba A, Biancalani M, Santucci M. CDX-2, Cytokeratin 7 and Cytokeratin 20 immunohistochemical expression in the differential diagnosis of primary adenocarcinomas of the sinonasal tract. Virchows Arch. (2004) 445:63–7. doi: 10.1007/s00428-004-1030-4
40. Leivo I. Intestinal-type adenocarcinoma: classification, immunophenotype, molecular features and differential diagnosis. Head Neck Pathol. (2017) 11:295–300. doi: 10.1007/s12105-017-0800-7
41. Stelow EB, Bishop JA. Update from the 4th Edition of the World Health Organization classification of head and neck tumours: tumors of the nasal cavity, paranasal sinuses and skull base. Head Neck Pathol. (2017) 11:3–15. doi: 10.1007/s12105-017-0791-4
42. Shen T, Shi Q, Velosa C, Bai S, Thompson L, Simpson R, et al. Sinonasal renal cell-like adenocarcinomas: robust carbonic anhydrase expression. Hum Pathol. (2015) 46:1598–606. doi: 10.1016/j.humpath.2015.06.017
43. Chen Z, Wang Z, Shi H, Liu Q. Renal cell-like carcinoma of the nasal cavity: a case report and review of the literature. Diagn Pathol. (2017) 12:75. doi: 10.1186/s13000-017-0660-1
44. Kubik M, Barasch N, Choby G, Seethala R, Snyderman C. Sinonasal renal cell-like carcinoma: case report and review of the literature. Head Neck Pathol. (2017) 11:333–7. doi: 10.1007/s12105-016-0774-x
45. Wu J, Fang Q, He YJ, Chen WX, Qi YK, Ding J. Local recurrence of sinonasal renal cell-like adenocarcinoma: a CARE compliant case report. Medicine. (2019) 98:e14533. doi: 10.1097/MD.0000000000014533
46. Storck K, Hadi UM, Simpson R, Ramer M, Brandwein-Gensler M. Sinonasal renal cell-like adenocarcinoma: a report on four patients. Head Neck Pathol. (2008) 2:75–80. doi: 10.1007/s12105-008-0047-4
47. Andreasen S, Skálová A, Agaimy A, Bishop JA, Laco J, Leivo I, et al. ETV6 gene rearrangements characterize a morphologically distinct subset of sinonasal low-grade non-intestinal-type adenocarcinoma: a novel translocation-associated carcinoma restricted to the sinonasal tract. Am J Surg Pathol. (2017) 41:1552–60. doi: 10.1097/PAS.0000000000000912
48. Andreasen S, Kiss K, Melchior LC, Laco J. The ETV6-RET gene fusion is found in ETV6-rearranged low-grade sinonasal adenocarcinoma without NTRK3 involvement. Am J Surg Pathol. (2018) 42:985–8. doi: 10.1097/PAS.0000000000001069
49. Tsao SW, Tsang CM, Lo KW. Epstein–Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. (2017) 372:20160270. doi: 10.1098/rstb.2016.0270
50. Takada K. Role of EBER and BARF1 in Nasopharyngeal Carcinoma (NPC) tumorigenesis. Semin Cancer Biol. (2012) 22:162–5. doi: 10.1016/j.semcancer.2011.12.007
51. Liu H, Lin F. Application of immunohistochemistry in thyroid pathology. Arch Pathol Lab Med. (2015) 139:67–82. doi: 10.5858/arpa.2014-0056-RA
52. Fischer S, Asa AL. Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med. (2008) 132:359–72. doi: 10.1043/1543-2165(2008)132[359:AOITTN]2.0.CO;2
53. Inamura K. Update on immunohistochemistry for the diagnosis of lung cancer. Cancers. (2018) 10:72. doi: 10.3390/cancers10030072
54. Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol. (2011) 24:1348–59. doi: 10.1038/modpathol.2011.92
55. Yatabe Y, Dacic S, Borczuk AC, Warth A, Russell PA, Lantuejoul S, et al. Best practices recommendations for diagnostic immunohistochemistry in lung cancer. J Thorac Oncol. (2019) 14:377–407. doi: 10.1016/j.jtho.2018.12.005
Keywords: thyroid transcription factor-1, thyroglobulin, endoscopic resection, clinicopathological features, thyroid-like low-grade nasopharyngeal papillary adenocarcinoma
Citation: Takakura H, Hamashima T, Tachino H, Nakazato A, Minato H, Sasahara M and Shojaku H (2020) Clinicopathological Features of Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma: A Case Report and Review of the Literature. Front. Surg. 7:596796. doi: 10.3389/fsurg.2020.596796
Received: 20 August 2020; Accepted: 26 October 2020;
Published: 19 November 2020.
Edited by:
Vincent Vander Poorten, KU Leuven, BelgiumReviewed by:
Piero Nicolai, University of Padua, ItalyDiana Bell, University of Texas MD Anderson Cancer Center, United States
Piet Slootweg, Radboud University Nijmegen, Netherlands
Copyright © 2020 Takakura, Hamashima, Tachino, Nakazato, Minato, Sasahara and Shojaku. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hideo Shojaku, aHNob2pha3VAbWVkLnUtdG95YW1hLmFjLmpw
 Hiromasa Takakura
Hiromasa Takakura Takeru Hamashima
Takeru Hamashima Hirohiko Tachino
Hirohiko Tachino Akira Nakazato
Akira Nakazato Hiroshi Minato
Hiroshi Minato Masakiyo Sasahara
Masakiyo Sasahara Hideo Shojaku
Hideo Shojaku