- 1First Department of Surgery, Division of Vascular Surgery, National and Kapodistrian University of Athens, Athens, Greece
- 2Department of Surgery, Duke University Hospital, Durham, NC, United States
The evaluation and management of patients with abdominal vascular trauma or injury requires immediate and effective decision-making in these unfavorable circumstances. The majority of these patients arrive at trauma centers in profound shock, secondary to massive blood loss, which is often unrelenting. Moreover, ischemia, compartment syndrome, thrombosis, and embolization may also be life threatening and require immediate intervention. To minimize the risk of these potentially lethal complications, early understanding of the disease process and emergent therapeutic intervention are necessary. In the literature, the management of acute traumatic vascular injuries is restricted to traditional open surgical techniques. However, in penetrating injuries surgeons often face a potentially contaminated field, which renders the placement of prosthetic grafts inappropriate. Currently, however, there are sparse data on the management of vascular trauma with endovascular techniques. The role of endovascular technique in penetrating abdominal vascular trauma, which is almost always associated with severe active bleeding, is limited. It is worth mentioning that hybrid operating rooms with angiographic radiology capabilities offer more opportunities for the management of this kind of injuries by either temporary control of the devastating bleeding using endovascular balloon tamponade or with embolization and stenting. On the other hand, blunt abdominal injuries are less dangerous and they could be treated at most times by endovascular means. Since surgeons continue to encounter abdominal vascular trauma, open and endovascular techniques will evolve constantly giving us encouraging messages for the near future.
Introduction
The evaluation and management of patients with abdominal vascular trauma or injury requires rapid and effective decision-making in these unfavorable circumstances. Penetrating abdominal trauma is by far the most common and accounts for about 90% of the cases (1, 2). The mortality rate varies widely and may reach 90% (3, 4). More than 70% of deaths can be expected to occur within the first day, whereas late-stage mortality may be attributed to secondary complications, such as sepsis and/or multiple organ failure, due to trauma (4). For this reason, emergent transfer to a trauma center, early assessment of the injury, and surgical intervention are critical for optimizing patient survival (1). Although, the management of acute traumatic vascular injuries was, until recently, restricted to traditional open surgical techniques (1, 2), the use of endovascular techniques provides a reliable alternative (5–8). In this review, we summarize all the available data on abdominal vascular trauma after the introduction of endovascular surgery in the treatment armamentarium, in order to provide surgeons and other physicians with a succinct and focused update.
Surgical Anatomy
The major sites of hemorrhage in patients, victims from blunt or penetrating abdominal trauma, are the viscera, following the mesentery, and the major abdominal vessels (9). For a better estimation and treatment of the injuries, the abdomen was conventionally divided into three zones as follows (10):
• Zone 1: midline retroperitoneum (extending from the aortic hiatus to the sacral promotore). This zone is subdivided into the supramesocolic [suprarenal aorta, celiac axis (CA), superior mesenteric artery (SMA), renal arteries (RAs), the supramesocolic area of inferior vena cava (IVC), superior mesenteric vein (SMV)] area and the inframesocolic area that contains the infrarenal aorta and the IVC.
• Zone 2: upper lateral retroperitoneum (left and right, which contains the kidneys and their vessels)
• Zone 3: pelvic retroperitoneum (including the iliac vessels)
Recently, Feliciano et al. (11) reported a fourth zone in the perihepatic area that includes the hepatic artery, the portal vein (PV), the retrohepatic IVC, and the hepatic veins.
Clinical Presentation
The clinical presentation is variable based on various parameters, such as the event, the involved vessel, the size of the injury, the presence of associated injuries, and the time elapsed since the injury. Abdominal vascular trauma can be presented in one of three ways: as free intraperitoneal hemorrhage, intraperitoneal/retroperitoneal hematoma, or thrombosis of the vessel (12). Under these circumstances, the hemodynamic status of the patients should be rapidly evaluated in order to divide them in two groups, those with ongoing hemorrhage and those without (hematoma/thrombosis). In cases of active hemorrhage, the patients arrive at the emergency department hypotensive and “non-responding” to the infusion of crystalloids and blood due to the presence of active bleeding directly into the peritoneal and/or retroperitoneal cavity. In this critical situation, the patients should undergo rapid assessment and transfer to an operating room for definitive repair of their vascular injuries. In cases of hematoma or thrombosis, their clinical status may be different. They may have only modest hypotension, and they are candidates for further imaging studies, as described below (12).
Diagnostic Evaluation
In most critically ill patients with penetrating abdominal injuries, emergent laparotomy without additional investigations is needed. Rapidly obtained plain X-ray evaluation is of diagnostic value if available, because the location of the missile may be useful in designing the operation (9, 13). Multi-slice computerized tomography (CT) should be strongly considered as diagnostic tool to facilitate initial management decisions in more stable patients. Active bleeding after penetrating injuries is detected as a linear or irregular area of extravascular contrast-enhanced blood (14). Known drawbacks are the radiation exposure and the reactions to contrast material while the time required for transporting and performing CT scanning does not permit its use in unstable patients.
In patients with blunt trauma, radiographic diagnosis of bone injuries may increase the suspicion of vascular injuries. Under these circumstances, CT represents a useful tool for identifying large hematomas, false aneurysms, and/or vessel occlusion. In addition, angiography is an important tool in both diagnostic and therapeutic approaches for patients with blunt trauma. The main sites of application are the infrarenal aorta, RAs, and/or the iliac arteries. However, Maturen et al. (15) suggested that angiography has lower sensitivity and specificity compared to CT in active bleeding situations.
Zone I Injuries
The IVC is the most commonly injured abdominal vessel and accounts for about 25% of abdominal vascular injuries (1). Penetrating injuries are by far the most common and account for 90% of the IVC injuries. Treatment of IVC injuries includes direct repair, patch repair, interposition of vein grafts, atrio caval shunting, and packing. Anterior perforations are repaired best using a continuous suture (venorrhaphy). In some patients with concomitant anterior and posterior perforation of the IVC, the posterior wound can be exposed and repaired from inside the vessel by extending the anterior wound (16). This approach is carried out when the patient is stable. When the patient is hemodynamically unstable with active bleeding or severe infrarenal injuries, or when repair produces stenosis, ligation is a first-option treatment and well tolerated (17). However, caution is required due to the likelihood of appearance of compartment syndrome in the lower limbs. Measurement of the pressures in the anterior compartment of the legs, bilateral fasciotomies if needed, maintaining the circulating volume stable in the postoperative period, and use of elastic compression wraps are some of the main strategies to assist this group of patients (17). Recently, in a review with 100 IVC injuries, ligation was performed in 25 cases with good results and without trace of lower extremity edema (17). However, there have been occasional reports of severe edema in postoperative period that has required later interposition grafting (18). On the other hand, ligation of the suprarenal IVC is not an acceptable option, because it results in renal failure. In these situations, common approaches are the application of a large venous patch taken from either the superior mesenteric or the ovarian vein and the application of a PTFE patch. However, this approach is rarely successful because of the patient’s poor hemodynamic status (18).
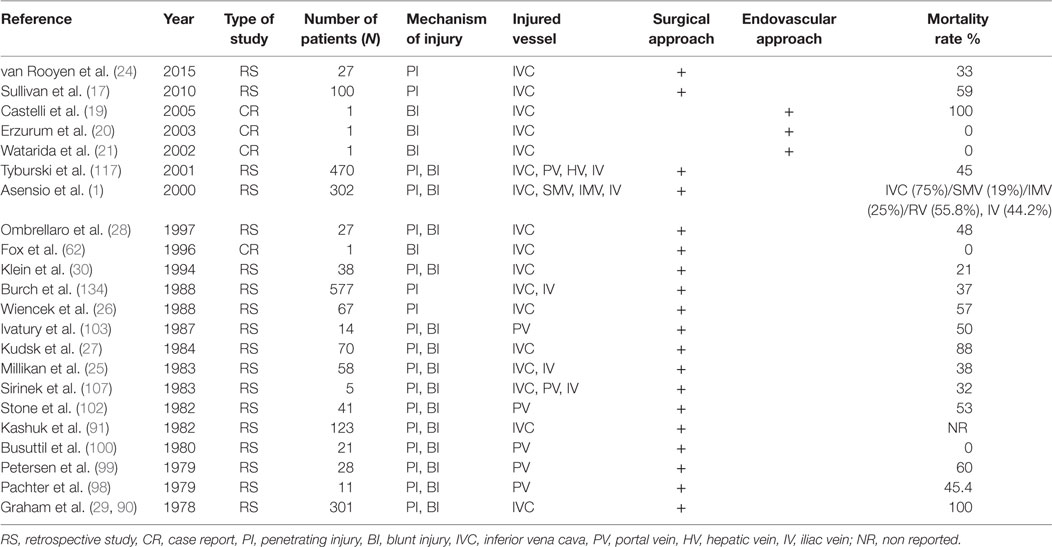
In the last few years, several reports (Table 1) on endovascular techniques based on the management of these complex injuries have been reported. Castelli et al. (19) reported their experience in a patient with blunt trauma. The injury of the IVC revealed by CT angiography was treated using a stent graft. Unfortunately, the patient died due to traumatic brain injury on post trauma day 2. Three other cases with a same similar management approach have recently been published (20–22). The mortality rate of patients, who arrive at the hospital with IVC injuries, ranges between 20 and 57% (23–30). About half of the patients with IVC injuries die before reaching the hospital and before any medical intervention.
The abdominal aorta represents the second most common site of injuries (21%) reported after the IVC (25%) (1). For better estimation and approach, aorta injuries were classified based on CT findings and the presence of free rupture during laparotomy. Azizzadeh et al. (31) initially and Starnes et al. later (32) proposed a classification based on the alteration of the symmetric aortic shape observed in CT as follows:
• Intimal tear/minimal aortic injury: absence of aortic external contour abnormality and intimal defect and/or thrombus of <10 mm in length or width.
• Large intimal flap: absence of aortic external contour abnormality and intimal defect and/or thrombus of ≥10 mm in length or width
• Pseudoaneurysm: external contour abnormality and contained rupture
• Rupture: external contour abnormality with free contrast extravasation or hemoperitoneum found upon laparotomy
In a review of abdominal gunshot injuries, 2.7% were localized in the abdominal aorta (33). The infrarenal part was found injured in 50% of the patients, the supraceliac in 25%, and the remained space between celiac trunk and RAs was injured in 25% of patients (33, 34). Blunt abdominal aortic injury is rare and is related to biomechanical direct and indirect forces that take effect on the abdominal aorta, situated between the spinal column and the peritoneum and abdominal viscera. Intimal dissection is the result of these forces that can also lead to aortic transection. Moreover, thrombosis in the abdominal aorta due to the same mechanism or as a complication of aortic dissection has also been reported (34, 35).
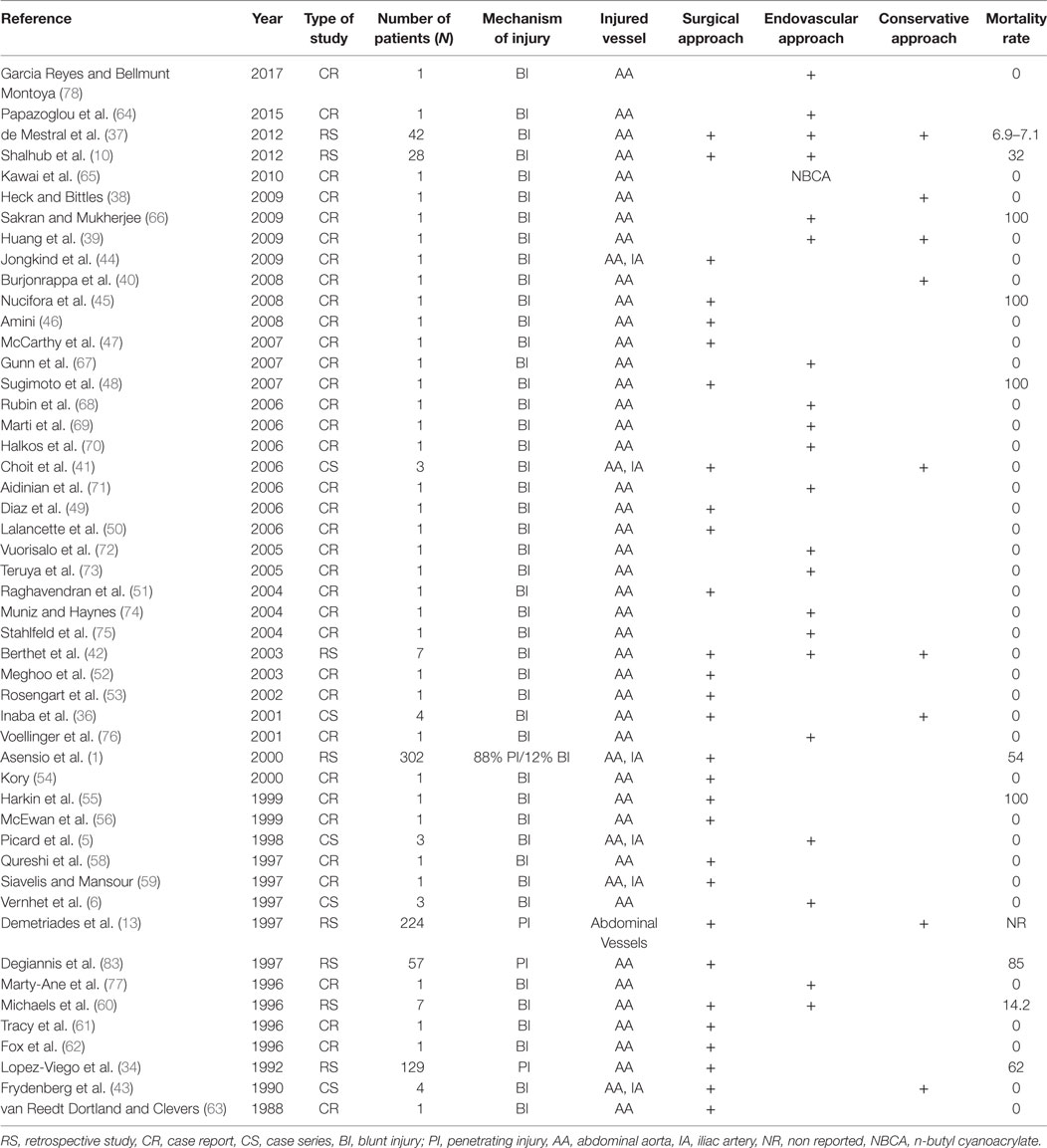
The management of aortic injuries is complex and depends on various factors, such as the type, size, and location of the injury. Penetrating aortic injuries obviously require open repair due to the rapid extravasation of blood in the peritoneal and/or retroperitoneal cavity, which leads to life-threatening conditions. In case of blunt trauma, management may be less urgent. Blunt aortic injuries with small intimal tears could be managed conservatively, with closely follow-up (36–43). In cases of large intimal flaps or free rupture, open (1, 2, 10, 34, 36, 41, 43–63) or endovascular repair are required (5, 6, 10, 36, 38, 41, 60, 64–78) (Table 2).
Traditionally, aortic injuries are approached with the division of the midline of the retroperitoneum by the transverse mesocolon into the supramesocolic and inframesocolic regions. In the supramesocolic area, when hematoma is present, the surgeon has the time to mobilize all left-sided intra-abdominal viscera, including the colon, kidney, spleen, tail of the pancreas, and fundus of the stomach to the midline (left-sided medial visceral rotation) (79). This technique permits extensive exposure and visualization of the entire abdominal aorta from the aortic hiatus of the diaphragm to the aortic bifurcation. Drawbacks of this technique include the risk to damage the spleen and/or left kidney, accessories vessels originate from kidney, and the time to complete the maneuver (80). The bilateral subcostal abdominal approach with left medial rotation (“roof-top” approach) has been described as an alternative technique for treating various complex abdominal aortic pathologies. The main advantage is the avoidance of entering into the left thoracic cavity and that it may potentially be an attractive technique in cases of injuries in supramesocolic region (81). In the presence of active bleeding, immediate priority for the surgeon is the control of the bleeding by direct compression. Once this critical step is achieved, the next thought is to identify the bleeding vessel and to obtain proximal and distal control. However, due to the dense concentration of the major vessels (abdominal aorta, CA, SMA) in this area and the dense nature of the celiac plexus that surrounds the supraceliac aorta, in some cases, left thoracotomy traditionally seems the only safe way to obtain proximal aortic control (82). Injuries restricted within the inframesocolic area could be approached by retracting the transverse colon cephalad and mobilizing the small bowel to the right (79).
Supramesocolic injuries have a significantly worse outcome than inframesocolic injuries due to the viscera’s location, which makes aortic exposure challenging (34). The prognosis is better in case of blunt abdominal aortic trauma compared to penetrating trauma (37). Overall mortality after blunt and penetrating aortic injuries is estimated at 30 and 85%, respectively (37, 83, 84).
Endovascular management is used in selected cases, mainly in blunt aortic trauma. Any significant injury may be managed, if amenable, with stenting (64–78) or embolization (65). Unfortunately, existing data are limited to case reports (CRs) (Table 2) that describe injuries like limited infrarenal dissection and large intimal flaps (64–68, 70–78). Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) offers a new tool for control of non-compressible abdominal vascular injuries. Recent advances in device technology have permitted for rapid deployment through smaller delivery (7 Fr) systems without dependence on fluoroscopy (85). Current guidelines through the American College of Surgeons find REBOA to represent a less invasive means of providing thoracic aortic occlusion compared to left thoracotomy with cross clamp to decrease blood loss from abdominal hemorrhage. Pelvic hemorrhage may also be selectively controlled through inflation of the device in the infrarenal aorta (86). While these two deployment strategies are promising, no data are currently available to demonstrate a mortality benefit compared to traditional techniques of initial vascular control. With this new technology comes consideration of new potential complications including femoral arterial injuries as well as extremity ischemia. Despite these points, REBOA offers a novel, non-invasive means of obtaining rapid vascular control in the exsanguinating abdominal trauma patient (85).
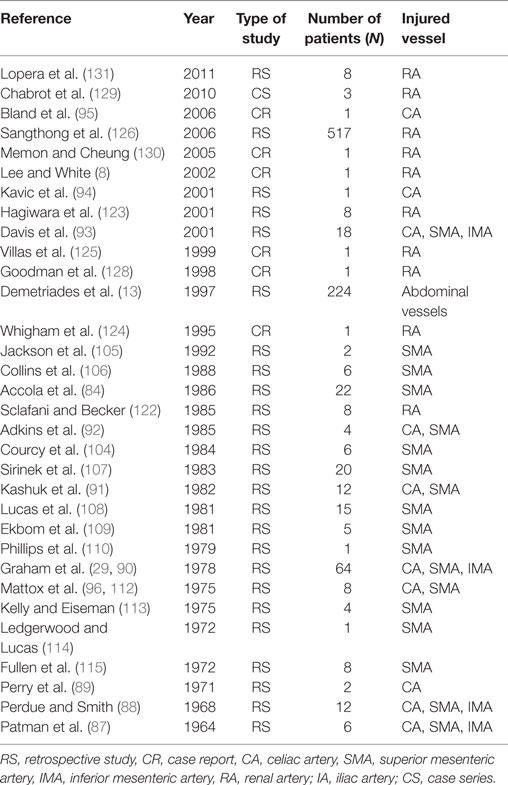
Injury of the CA is rare and may occur at the main trunk or any of its branches (1). Since Patman et al. (87) reported the first case, few studies (88–95) are available in the literature (Table 2). Asensio et al. (1) presented the largest series in the literature, in which 12 patients suffered from CA injuries after penetrating trauma. Eleven were treated with ligation and one with primary repair. The authors concluded that patients are not amenable to simple arteriorrhaphy should undergo ligation, which should not cause any short morbidity other than the risk of gallbladder necrosis (1). However, few data exist in the literature describing the consequences of this procedure, and surgeons should not worry about ligating the hepatic artery proper proximal to the origin of gastroduodenal artery, since the collateral flow through this vessel will maintain the viability of the liver. The reported mortality rate ranges from 38 to 75% (87, 88, 90–92). Therefore, the hemodynamic status of the patient, the complexity of the indicated operation (direct repair or conduit/graft needed for repair) and the associated injuries taking priority on operation plan should contribute to the final decision-making of repairing the celiac artery.
Injury to the PV trunk is relatively rare. Penetrating injuries are responsible for about 90% of the cases, as they are confirmed after laparotomy (96). The major percentage of patients, victims of PV penetrating injuries, present signs of hemorrhagic shock and require emergency laparotomy (97). On the other hand, blunt trauma often provokes thrombosis of the vessel and occasionally avulsion and bleeding (97).
Due to the location (retro/suprapancreatic) of the PV, the friability of its wall and the greater volume of blood through it, the management of this vessel injury is challenging. Exposure of retro-pancreatic PV and its major branches can be achieved by mobilization and medial rotation of the right colon and hepatic flexure of the colon, in association with extensive Kocher mobilization of the duodenum (26, 91). More often, stapled division of the neck of the pancreas is necessary for successful exposure (98–100). The same technique for dissection of the suprapancreatic PV has also been described (98, 99). There are several PV repair techniques, but lateral venorrhaphy is preferred (98, 99). Complex reconstruction using resection with end-to-end anastomosis, interposition grafting, transposition of splenic vein down to the SMV to replace the proximal PV, an end-to-side portocaval shunt, and a veno-venous shunt from the SMV to the distal PV or IVC have also been described (26, 91, 98–100). In addition, ligation of the vein is another surgical option compatible with survival, but in such cases bowel edema and wall necrosis are often complications. The experience after portal ligation is restrictive in the literature; however, there is no evidence of development portal hypertension in such cases (91, 98, 99). The mortality rate in PV injuries is high and ranges between 50 and 72% (101–103).
Several studies on SMA injuries have been conducted at experienced trauma centers (1, 84, 87, 91–93, 104–115) (Table 2). Since Fullen et al. (115) proposed an anatomic classification of injuries to the SMA, their management depends on the level of injury. Injuries in zone 1 (trunk proximal to the inferior pancreaticoduodenal artery) and zone 2 (between the inferior pancreaticoduodenal artery and the middle colic artery) can be achieved by left-sided medial visceral rotation and dissection anteriorly to the left kidney. Then the anterior aspect of the aorta including the SMA will be visible (79). However, these zones are characterized by high level of surgical difficulties, and under active bleeding in an unstable patient, the use of intraluminal shunt into the debrided ends of SMA should be considered. If the patient is stable and the proximal part of the SMA should be replaced, saphenous vein or prosthetic grafts have been described (84). On the other hand, injuries in zones III (distal to middle colic artery) and IV (the segmental intestinal branches) should be approached directly without any additional surgical manipulation.
Injuries of the inferior mesenteric artery (IMA) are rare. All the published studies (1, 87, 88, 93) reported results of IMA associated with other visceral vessels (Table 3). The main described mechanism was penetrating injury and accounted for 1% of all abdominal injuries (1). In critical situations, experts proposed ligation of the IMA with remarkable results for the bowel due to the rich collateral blood supply (1, 93).
Zone 2 Injuries
The management of renovascular injuries depends on the mechanism of injury, the time of diagnosis, the ischemia time, the general condition of the patient, and the presence of a contralateral normal kidney. Injuries to the renal vasculature (zone 2) are difficult to manage due to the small size of the vessel and its location deep in the retroperitoneum. Occasionally, small injuries after penetrating trauma can be repaired by lateral arteriorrhaphy or resection and end-to-end anastomosis (11, 93). Interposition grafting with saphenous vein, PTFE graft, and rarely harvesting of the splenic and hepatic arteries in order to replace the left/right RAs, respectively, has also been presented (116). However, the latter approach is not advisable under hemodynamic instability but when the patient is stable. In cases with penetrating wounds associated with hemodynamic instability and with significant renovascular injuries or long period of ischemia, nephrectomy may be the better choice, as long as a normal contralateral kidney was confirmed (11). The survival rate after penetrating injuries ranges between 65 and 87% (117) with renal salvage in only 30–40% of the cases (118, 119).
The management of blunt injuries to the RAs is complicated by the often-delayed diagnosis and prolonged ischemia of the kidney. The patients often complain about abdominal and flank pain, whereas associated signs like gross or microscopic hematuria are also reported. The management of isolated main RA occlusion remains controversial. Treatment options include immediate nephrectomy, non-operative management, or revascularization by surgical or endovascular techniques (11). In stable patients with short warm ischemia time (<5 h), revascularization should be performed (11, 16, 93). In cases of delayed diagnosis (>5 h), most surgeons avoid revascularization, unless the injury involves both kidneys or solitary kidney, due to the disappointing results after revascularization (120, 121). Other patients, assuming they have a normally functioning contralateral kidney, should be either monitored or considered for endovascular procedures. At least, patients with bilateral RA injuries or those with solitary kidney should be strongly considered for revascularization (11).
The advancement of endovascular techniques has opened new horizons in the management of renovascular injuries. However, experience with this technique is still limited (8, 122–127). First, Sclafani and Becker (122) reported eight patients with penetrating (n = 6) and blunt trauma (n = 2), who were treated with angiographic embolization. Seven of these eight patients underwent successful procedures with preservation of the renal function, and only one nephrectomy due to hematuria was performed. Later, another study (123) reported the same results after angiographic embolization, obviating the need for open surgery. All patients presented normal functioning status at discharge. In more recent years, few CRs (Table 3) document the successful use of various stents to obliterate intimal flaps after RA injuries without short-term complications (8, 124–131). Thus, endovascular treatment may play an important role in selected cases of blunt renovascular trauma, when patients are stable with injuries like intimal tears, acute occlusions, false aneurysms, and arteriovenous fistulae.
Injury of the renal vein is the result of penetrating and blunt trauma mechanisms. Although, blunt avulsion injuries result in exsanguination, under penetrating trauma, the patient may be stabilized with retroperitoneal tamponade (1). Lateral venorrhaphy remains the first treatment choice; however, it is not possible in case of extensive injuries. In these cases, ligation of the renal vein has been proposed unless the collateral vein circulation is inappropriate, to maintain stable and vital the kidneys. Ligation of the left renal vein is tolerated well due to satisfactory venous drainage through the left gonadal vein, left adrenal vein, and lumbar veins. This choice is not feasible in cases of the right renal vein, when the collateral vein flow is absent, and nephrectomy is the only choice (93). The survival rate ranged from 44 to 70% with a mean of 60% (1, 93).
Zone 3 Injuries
Injuries of the iliac arteries due to penetrating or blunt trauma are described in the literature (Table 2). Penetrating trauma is by far the most common mechanism of injury, whereas blunt trauma remains uncommon; the only available data on blunt trauma come from CRs. Velmahos et al. (132) published their experience in 30 patients suffering from blunt injury to the iliac arteries. Seventeen patients (56.6%) underwent embolization of the bleeding internal iliac arteries as primary treatment while the rest of the patients had undergone to laparotomies before the embolization. The success rate was 97% (29–30) in controlling pelvic hemorrhage. Later, Cestero et al. (133) reported that iliac artery injury occurred after penetrating trauma in 10% of the cases, whereas 26% of the patients had combined arterial and venous injuries. The common involved site is the common iliac artery (CIA) and the branches of the internal iliac artery for penetrating and blunt trauma, respectively. Often the patient shows severe hypotension and abdominal distension in case of penetrating trauma and with signs of absent or diminished femoral pulses (1, 41, 44). Thrombosis is observed in later stages of blunt injuries through diagnostic imaging such as Duplex ultrasound and/or CT (41).
Surgical exploration should be carried out in cases of active bleeding after penetrating trauma and if blunt trauma persists with associated intraperitoneal leak, hematoma that creates absent or diminished femoral pulses or it has continuing expansion (11, 44). Arterial injuries could be repaired with vascular sutures (arteriorrhaphy) or interposition of venous/PTFE grafts. Special consideration should be taken to avoid graft infection, for which some authors proposed an extra-anatomic bypass as the method of treatment (134). On the other side, supporters of the endovascular technique proposed this approach in order to avoid complications due to the contamination of the graft.
Thus, endovascular treatment is a trustworthy alternative in cases of further graft infection and in cases with false aneurysm, arteriovenous fistulae, major intimal tears, and vessel’s thrombosis (135). Moreover, it was also proposed by some experts as the first-line therapeutic option due to the low complication rate in cases of chronic traumatic injuries of the CIA or external iliac artery (7, 136).
Iliac venous injuries are technically challenging due to the difficult exposure caused by anatomic placement behind the arteries. Injuries on the common and/or external iliac veins could be managed with lateral repair with polypropylene sutures (4-0, 5-0) or with ligation (1). In case of significant narrowing after lateral repair, treatment with anticoagulants is appropriate to reduce the risk of thrombosis and/or pulmonary embolism. Ligation is usually well tolerated although many patients develop transient leg edema. Complex reconstruction with spiral grafts or prosthetic materials is not recommended.
The traditional pre-peritoneal pelvic packing represents a trustworthy alternative to address venous hemorrhage in complex pelvic fractures (137). The “trigger” to perform this technique is the persistent hemodynamic instability of the patient despite two units of red blood cells transfusion during the initial resuscitation (137). Other proposed indications were unstable patients with pelvic hematoma diagnosed on focused assessment with sonography in trauma exam and in cases of unavailable angiographic embolization in some centers (138). The survival rate of patients with iliac venous injuries ranges between 65 and 95% (1, 25, 93, 107, 117, 134).
Treatment Algorithm
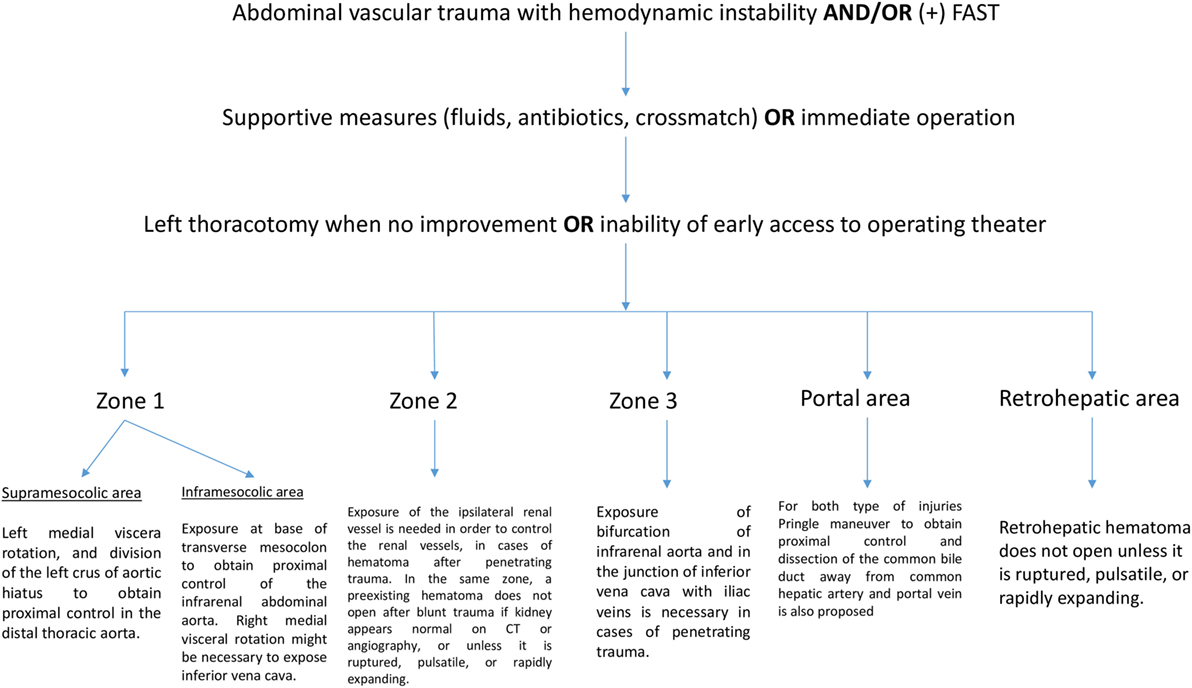
Abdominal vascular injuries represent a devastating situation, requiring immediate and effective decision-making. Penetrating injuries are most common and present either active bleeding or a contained retroperitoneal, mesenteric, or portal hematoma. Recently, experts proposed a treatment algorithm based on the management of abdominal vascular trauma for better understanding and surgical approach (11). In brief, for penetrating injuries in supramesocolic area (zone 1), left medial viscera rotation and division of the left crus of aortic hiatus to obtain proximal control of the distal thoracic aorta have been proposed. Another means of proximal aortic control may be obtained adjacent to the esophagus and stomach through the lesser omentum. Hematoma located in inframesocolic area requires exposure at base of the transverse mesocolon to obtain proximal control of the infrarenal abdominal aorta by means of evisceration of the small bowel to the right and opening the retroperitoneum at base of transverse mesocolon. Unless aortic injury has been detected, right medial visceral rotation is necessary to expose IVC. For penetrating zone 2 injuries, exposure of the ipsilateral renal vessel is needed in order to control the renal vessels. In the same zone, a preexisting hematoma does not require exploration after blunt trauma if the kidney appears normal on CT or angiography. Active bleeding confined to zone 2 may be approached with division of lateral peritoneum and Gerota’s fascia whereas some situations dictate a medial to lateral approach for early control of the hilum. Figure 1 illustrates the algorithm described above. Exposure of the bifurcation of infrarenal aorta and in the junction of IVC with iliac veins is necessary in cases of penetrating trauma confined in zone 3.
Conclusion
Abdominal vascular injuries are commonly seen in daily clinical practice. Active bleeding after penetrating injuries is the most dreadful scenario that surgeons have to face. Hematoma in most times spares time to the surgeon for better decision-making and surgical approach. Vascular repair are generally carried out with arteriorrhaphy and/or venorrhaphy or even with insertion of substitute vascular conduits. Endovascular technique gains more and more field in the management of blunt trauma and in most cases with delayed vascular complications, such as aneurysms, arteriovenous fistulae, and arterial occlusion. The role of endovascular technique in penetrating abdominal vascular trauma, which is almost always associated with severe active bleeding, is limited. It is worth mentioning that hybrid operating rooms with angiographic radiology capabilities offer more opportunities for the management of this kind of injuries by either temporary control of the devastating bleeding using endovascular balloon tamponade or with embolization and stenting. Abdominal vascular trauma continues to represent a difficult problem and, open and endovascular technique continue to evolve to address this complex disease process.
Author Contributions
CB: organized the study and reviewed the manuscript. GK: gathered the data, organized the study, and drafted the manuscript. CM and DM: gathered the data. DT and SG: reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AML declared a shared affiliation, with no collaboration, with the authors to the handling Editor.
References
1. Asensio JA, Chahwan S, Hanpeter D, Demetriades D, Forno W, Gambaro E, et al. Operative management and outcome of 302 abdominal vascular injuries. Am J Surg (2000) 180:528–33; discussion 33–4. doi:10.1016/S0002-9610(00)00519-5
2. Cox EF. Blunt abdominal trauma. A 5-year analysis of 870 patients requiring celiotomy. Ann Surg (1984) 199:467–74. doi:10.1097/00000658-198404000-00015
3. Patterson BO, Holt PJ, Cleanthis M, Tai N, Carrell T, Loosemore TM, et al. Imaging vascular trauma. Br J Surg (2012) 99:494–505. doi:10.1002/bjs.7763
4. Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg (2012) 255:379–85. doi:10.1097/SLA.0b013e318235d9e6
5. Picard E, Marty-Ane CH, Vernhet H, Sessa C, Lesnik A, Senac JP, et al. Endovascular management of traumatic infrarenal abdominal aortic dissection. Ann Vasc Surg (1998) 12:515–21. doi:10.1007/s100169900194
6. Vernhet H, Marty-Ane CH, Lesnik A, Chircop R, Serres-Cousine O, Picard E, et al. Dissection of the abdominal aorta in blunt trauma: management by percutaneous stent placement. Cardiovasc Intervent Radiol (1997) 20:473–6. doi:10.1007/s002709900197
7. Lyden SP, Srivastava SD, Waldman DL, Green RM. Common iliac artery dissection after blunt trauma: case report of endovascular repair and literature review. J Trauma (2001) 50:339–42. doi:10.1097/00005373-200102000-00024
8. Lee JT, White RA. Endovascular management of blunt traumatic renal artery dissection. J Endovasc Ther (2002) 9:354–8. doi:10.1177/152660280200900315
9. Salazar GM, Walker TG. Evaluation and management of acute vascular trauma. Tech Vasc Interv Radiol (2009) 12:102–16. doi:10.1053/j.tvir.2009.08.004
10. Shalhub S, Starnes BW, Tran NT, Hatsukami TS, Lundgren RS, Davis CW, et al. Blunt abdominal aortic injury. J Vasc Surg (2012) 55:1277–85. doi:10.1016/j.jvs.2011.10.132
11. Feliciano DV, Moore EE, Biffl WL. Western Trauma Association Critical Decisions in Trauma: management of abdominal vascular trauma. J Trauma Acute Care Surg (2015) 79:1079–88. doi:10.1097/TA.0000000000000869
12. Eachempati SR, Robb T, Ivatury RR, Hydo LJ, Barie PS. Factors associated with mortality in patients with penetrating abdominal vascular trauma. J Surg Res (2002) 108:222–6. doi:10.1006/jsre.2002.6543
13. Demetriades D, Velmahos G, Cornwell E III, Berne TV, Cober S, Bhasin PS, et al. Selective nonoperative management of gunshot wounds of the anterior abdomen. Arch Surg (1997) 132:178–83. doi:10.1001/archsurg.1997.01430260076017
14. Castrillon GA, Soto JA. Multidetector computed tomography of penetrating abdominal trauma. Semin Roentgenol (2012) 47:371–6. doi:10.1053/j.ro.2012.05.006
15. Maturen KE, Adusumilli S, Blane CE, Arbabi S, Williams DM, Fitzgerald JT, et al. Contrast-enhanced CT accurately detects hemorrhage in torso trauma: direct comparison with angiography. J Trauma (2007) 62:740–5. doi:10.1097/01.ta.0000235508.11442.a8
16. Feliciano DV. Abdominal vascular injuries. Surg Clin North Am (1988) 68:741–55. doi:10.1016/S0039-6109(16)44583-4
17. Sullivan PS, Dente CJ, Patel S, Carmichael M, Srinivasan JK, Wyrzykowski AD, et al. Outcome of ligation of the inferior vena cava in the modern era. Am J Surg (2010) 199:500–6. doi:10.1016/j.amjsurg.2009.05.013
18. Degiannis E, Velmahos GC, Levy RD, Souter I, Benn CA, Saadia R. Penetrating injuries of the abdominal inferior vena cava. Ann R Coll Surg Engl (1996) 78:485–9.
19. Castelli P, Caronno R, Piffaretti G, Tozzi M. Emergency endovascular repair for traumatic injury of the inferior vena cava. Eur J Cardiothorac Surg (2005) 28:906–8. doi:10.1016/j.ejcts.2005.09.001
20. Erzurum VZ, Shoup M, Borge M, Kalman PG, Rodriguez H, Silver GM. Inferior vena cava endograft to control surgically inaccessible hemorrhage. J Vasc Surg (2003) 38:1437–9.
21. Watarida S, Nishi T, Furukawa A, Shiraishi S, Kitano H, Matsubayashi K, et al. Fenestrated stent-graft for traumatic juxtahepatic inferior vena cava injury. J Endovasc Ther (2002) 9:134–7. doi:10.1177/152660280200900122
22. Cheaito A, Tillou A, Lewis C, Cryer H. Corrigendum to “management of traumatic blunt IVC injury” [Int. J. Surg. Case Rep. 28 (2016) 26-30]. Int J Surg Case Rep (2017) 42:212–3. doi:10.1016/j.ijscr.2017.12.021
23. Kuehne J, Frankhouse J, Modrall G, Golshani S, Aziz I, Demetriades D, et al. Determinants of survival after inferior vena cava trauma. Am Surg (1999) 65:976–81.
24. van Rooyen PL, Karusseit VO, Mokoena T. Inferior vena cava injuries: a case series and review of the South African experience. Injury (2015) 46:71–5. doi:10.1016/j.injury.2014.06.016
25. Millikan JS, Moore EE, Cogbill TH, Kashuk JL. Inferior vena cava injuries – a continuing challenge. J Trauma (1983) 23:207–12. doi:10.1097/00005373-198303000-00005
26. Wiencek RG Jr, Wilson RF. Inferior vena cava injuries – the challenge continues. Am Surg (1988) 54:423–8.
27. Kudsk KA, Bongard F, Lim RC Jr. Determinants of survival after vena caval injury. Analysis of a 14-year experience. Arch Surg (1984) 119:1009–12. doi:10.1001/archsurg.1984.01390210013004
28. Ombrellaro MP, Freeman MB, Stevens SL, Diamond DL, Goldman MH. Predictors of survival after inferior vena cava injuries. Am Surg (1997) 63:178–83.
29. Graham JM, Mattox KL, Beall AC Jr, DeBakey ME. Traumatic injuries of the inferior vena cava. Arch Surg (1978) 113:413–8. doi:10.1001/archsurg.1978.01370160071011
30. Klein SR, Baumgartner FJ, Bongard FS. Contemporary management strategy for major inferior vena caval injuries. J Trauma (1994) 37:35–41; discussion 41–2. doi:10.1097/00005373-199407000-00008
31. Azizzadeh A, Keyhani K, Miller CC III, Coogan SM, Safi HJ, Estrera AL. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg (2009) 49:1403–8. doi:10.1016/j.jvs.2009.02.234
32. Starnes BW, Lundgren RS, Gunn M, Quade S, Hatsukami TS, Tran NT, et al. A new classification scheme for treating blunt aortic injury. J Vasc Surg (2012) 55:47–54. doi:10.1016/j.jvs.2011.07.073
33. Feliciano DV, Burch JM, Spjut-Patrinely V, Mattox KL, Jordan GL Jr. Abdominal gunshot wounds. An urban trauma center’s experience with 300 consecutive patients. Ann Surg (1988) 208:362–70. doi:10.1097/00000658-198809000-00014
34. Lopez-Viego MA, Snyder WH III, Valentine RJ, Clagett GP. Penetrating abdominal aortic trauma: a report of 129 cases. J Vasc Surg (1992) 16:332–5; discussion 35–6. doi:10.1016/0741-5214(92)90365-F
35. Lock JS, Huffman AD, Johnson RC. Blunt trauma to the abdominal aorta. J Trauma (1987) 27:674–7. doi:10.1097/00005373-198706000-00015
36. Inaba K, Kirkpatrick AW, Finkelstein J, Murphy J, Brenneman FD, Boulanger BR, et al. Blunt abdominal aortic trauma in association with thoracolumbar spine fractures. Injury (2001) 32:201–7. doi:10.1016/S0020-1383(00)00203-5
37. de Mestral C, Dueck AD, Gomez D, Haas B, Nathens AB. Associated injuries, management, and outcomes of blunt abdominal aortic injury. J Vasc Surg (2012) 56:656–60. doi:10.1016/j.jvs.2012.02.027
38. Heck JM, Bittles MA. Traumatic abdominal aortic dissection in a 16-month-old child. Pediatr Radiol (2009) 39:750–3. doi:10.1007/s00247-009-1224-7
39. Huang JT, Heckman JT, Gunduz Y, Ohki T. Endovascular management of stenosis of the infrarenal aorta secondary to blunt abdominal aortic trauma in a multiply injured patient. J Trauma (2009) 66:E81–5. doi:10.1097/01.ta.0000238651.56585.e4
40. Burjonrappa S, Vinocur C, Smergel E, Chhabra A, Galiote J. Pediatric blunt abdominal aortic trauma. J Trauma (2008) 65:E10–2. doi:10.1097/01.ta.0000208140.50947.4e
41. Choit RL, Tredwell SJ, Leblanc JG, Reilly CW, Mulpuri K. Abdominal aortic injuries associated with chance fractures in pediatric patients. J Pediatr Surg (2006) 41:1184–90. doi:10.1016/j.jpedsurg.2006.01.069
42. Berthet JP, Marty-Ane CH, Veerapen R, Picard E, Mary H, Alric P. Dissection of the abdominal aorta in blunt trauma: endovascular or conventional surgical management? J Vasc Surg (2003) 38:997–1003; discussion 04.
43. Frydenberg M, Royle JP, Hoare M. Blunt abdominal aortic trauma. Aust N Z J Surg (1990) 60:347–50. doi:10.1111/j.1445-2197.1990.tb07382.x
44. Jongkind V, Linsen MA, Diks J, Vos AW, Klinkert P, Rauwerda JA, et al. Aortoiliac reconstruction for abdominal aortic rupture after blunt trauma. J Trauma (2009) 66:1248–50. doi:10.1097/01.ta.0000236010.41841.7a
45. Nucifora G, Hysko F, Vasciaveo A. Blunt traumatic abdominal aortic rupture: CT imaging. Emerg Radiol (2008) 15:211–3. doi:10.1007/s10140-007-0649-2
46. Amini M. Pseudoaneurysm of the abdominal aorta following blunt trauma. J Coll Physicians Surg Pak (2008) 18:115–7. doi:02.2008/JCPSP.115117
47. McCarthy MC, Price SW, Rundell WK, Lehner JT, Barney LM, Ekeh AP, et al. Pediatric blunt abdominal aortic injuries: case report and review of the literature. J Trauma (2007) 63:1383–7. doi:10.1097/01.ta.0000224912.06226.2c
48. Sugimoto T, Omura A, Kitade T, Takahashi H, Koyama T, Kurisu S. An abdominal aortic rupture due to seatbelt blunt injury: report of a case. Surg Today (2007) 37:86–8. doi:10.1007/s00595-006-3314-6
49. Diaz JA, Campbell BT, Moursi MM, Boneti C, Kokoska ER, Jackson RJ, et al. Delayed manifestation of abdominal aortic stenosis in a child presenting 10 years after blunt abdominal trauma. J Vasc Surg (2006) 44:1104–6. doi:10.1016/j.jvs.2006.06.040
50. Lalancette M, Scalabrini B, Martinet O. Seat-belt aorta: a rare injury associated with blunt abdominal trauma. Ann Vasc Surg (2006) 20:681–3. doi:10.1007/s10016-006-9058-3
51. Raghavendran K, Singh G, Arnoldo B, Flynn WJ. Delayed development of infrarenal abdominal aortic pseudoaneurysm after blunt trauma: a case report and review of the literature. J Trauma (2004) 57:1111–4. doi:10.1097/01.TA.0000053399.86908.11
52. Meghoo CA, Gonzalez EA, Tyroch AH, Wohltmann CD. Complete occlusion after blunt injury to the abdominal aorta. J Trauma (2003) 55:795–9. doi:10.1097/01.TA.0000039053.32562.C0
53. Rosengart MR, Zierler RE. Fractured aorta – a case report. Vasc Endovascular Surg (2002) 36:465–7. doi:10.1177/153857440203600608
54. Kory LA. Thrombosis of the abdominal aorta in a child after blunt trauma. AJR Am J Roentgenol (2000) 175:553–4. doi:10.2214/ajr.175.2.1750553
55. Harkin DW, Kirk G, Clements WD. Abdominal aortic rupture in a child after blunt trauma on a soccer field. Injury (1999) 30:303–4. doi:10.1016/S0020-1383(99)00081-9
56. McEwan L, Woodruff P, Archibald C. Lap belt abdominal aortic trauma. Australas Radiol (1999) 43:369–71. doi:10.1046/j.1440-1673.1999.433689.x
57. Borioni R, Garofalo M, Seddio F, Colagrande L, Marino B, Albano P. Posttraumatic infrarenal abdominal aortic pseudoaneurysm. Tex Heart Inst J (1999) 26:312–4.
58. Qureshi A, Roberts N, Nicholson A, Johnson B. Three-dimensional reconstruction by spiral computed tomography to locate aortic tear following blunt abdominal trauma. Eur J Vasc Endovasc Surg (1997) 14:316–7. doi:10.1016/S1078-5884(97)80246-8
59. Siavelis HA, Mansour MA. Aortoiliac dissection after blunt abdominal trauma: case report. J Trauma (1997) 43:862–4. doi:10.1097/00005373-199711000-00022
60. Michaels AJ, Gerndt SJ, Taheri PA, Wang SC, Wahl WL, Simeone DM, et al. Blunt force injury of the abdominal aorta. J Trauma (1996) 41:105–9. doi:10.1097/00005373-199607000-00016
61. Tracy TF Jr, Silen ML, Graham MA. Delayed rupture of the abdominal aorta in a child after a suspected handlebar injury. J Trauma (1996) 40:119–20. doi:10.1097/00005373-199601000-00022
62. Fox JT, Huang YC, Barcia PJ, Beresky RE, Olsen D. Blunt abdominal aortic transection in a child: case report. J Trauma (1996) 41:1051–3. doi:10.1097/00005373-199612000-00020
63. van Reedt Dortland RW, Clevers GJ. Crush accident resulting in isolated abdominal aortic injury. Neth J Surg (1988) 40:108–9.
64. Papazoglou KO, Karkos CD, Kalogirou TE, Giagtzidis IT. Endovascular management of lap belt-related abdominal aortic injury in a 9-year-old child. Ann Vasc Surg (2015) 29(365):e11–5. doi:10.1016/j.avsg.2014.09.026
65. Kawai N, Sato M, Tanihata H, Sahara S, Takasaka I, Minamiguchi H, et al. Repair of traumatic abdominal aortic pseudoaneurysm using N-butyl-2-cyano-acrylate embolization. Cardiovasc Intervent Radiol (2010) 33:406–9. doi:10.1007/s00270-009-9593-8
66. Sakran JV, Mukherjee D. Four-year follow-up of endograft repair of traumatic aortic transection in a 10-year-old. Vasc Endovascular Surg (2009) 43:597–8. doi:10.1177/1538574409334832
67. Gunn M, Campbell M, Hoffer EK. Traumatic abdominal aortic injury treated by endovascular stent placement. Emerg Radiol (2007) 13:329–31. doi:10.1007/s10140-006-0556-y
68. Rubin S, Pages ON, Poncet A, Baehrel B. Endovascular treatment of an acute subdiaphragmatic aortic rupture. Ann Thorac Surg (2006) 82:2276–8. doi:10.1016/j.athoracsur.2006.03.088
69. Marti M, Pinilla I, Baudraxler F, Simon MJ, Garzon G. A case of acute abdominal aortic dissection caused by blunt trauma. Emerg Radiol (2006) 12:182–5. doi:10.1007/s10140-006-0473-0
70. Halkos ME, Nicholas J, Kong LS, Burke JR, Milner R. Endovascular management of blunt abdominal aortic injury. Vascular (2006) 14:223–6. doi:10.2310/6670.2006.00038
71. Aidinian G, Karnaze M, Russo EP, Mukherjee D. Endograft repair of traumatic aortic transection in a 10-year-old – a case report. Vasc Endovascular Surg (2006) 40:239–42. doi:10.1177/153857440604000310
72. Vuorisalo S, Railo M, Lappalainen K, Aho P, Lepantalo M. Low-energy blunt abdominal aortic trauma in an underweighted man. Eur J Vasc Endovasc Surg (2005) 29:595–6. doi:10.1016/j.ejvs.2005.02.013
73. Teruya TH, Bianchi C, Abou-Zamzam AM, Ballard JL. Endovascular treatment of a blunt traumatic abdominal aortic injury with a commercially available stent graft. Ann Vasc Surg (2005) 19:474–8. doi:10.1007/s10016-005-4653-2
74. Muniz AE, Haynes JH. Delayed abdominal aortic rupture in a child with a seat-belt sign and review of the literature. J Trauma (2004) 56:194–7. doi:10.1097/01.TA.0000033141.40817.E9
75. Stahlfeld KR, Mitchell J, Sherman H. Endovascular repair of blunt abdominal aortic injury: case report. J Trauma (2004) 57:638–41. doi:10.1097/01.TA.0000042018.39379.10
76. Voellinger DC, Saddakni S, Melton SM, Wirthlin DJ, Jordan WD, Whitley D. Endovascular repair of a traumatic infrarenal aortic transection: a case report and review. Vasc Surg (2001) 35:385–9. doi:10.1177/153857440103500509
77. Marty-Ane CH, Alric P, Prudhomme M, Chircop R, Serres-Cousine O, Mary H. Intravascular stenting of traumatic abdominal aortic dissection. J Vasc Surg (1996) 23:156–61. doi:10.1016/S0741-5214(05)80047-3
78. Garcia Reyes ME, Bellmunt Montoya S. Aortic seat belt injury in a 13 year old girl. Eur J Vasc Endovasc Surg (2017) 53:558. doi:10.1016/j.ejvs.2017.01.021
79. Mattox KL, McCollum WB, Jordan GL Jr, Beall AC Jr, DeBakey ME. Management of upper abdominal vascular trauma. Am J Surg (1974) 128:823–8. doi:10.1016/0002-9610(74)90079-8
80. Fry WR, Fry RE, Fry WJ. Operative exposure of the abdominal arteries for trauma. Arch Surg (1991) 126:289–91. doi:10.1001/archsurg.1991.01410270029004
81. Lazaris AM, Mastoraki S, Seretis K, Karouki M, Matsagkas M, Vasdekis SN. How to do a ’roof-top’ approach to the supraceliac aorta. ANZ J Surg (2015) 85:386–7. doi:10.1111/ans.13031
82. Debakey ME, Creech O Jr, Morris GC Jr. Aneurysm of thoracoabdominal aorta involving the celiac, superior mesenteric, and renal arteries; report of four cases treated by resection and homograft replacement. Ann Surg (1956) 144:549–73. doi:10.1097/00000658-195610000-00004
83. Degiannis E, Levy RD, Florizoone MG, Badicel TV, Badicel M, Saadia R. Gunshot injuries of the abdominal aorta: a continuing challenge. Injury (1997) 28:195–7. doi:10.1016/S0020-1383(96)00189-1
84. Accola KD, Feliciano DV, Mattox KL, Bitondo CG, Burch JM, Beall AC Jr, et al. Management of injuries to the suprarenal aorta. Am J Surg (1987) 154:613–8. doi:10.1016/0002-9610(87)90227-3
85. Moore LJ, Brenner M, Kozar RA, Pasley J, Wade CE, Baraniuk MS, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg (2015) 79:523–30; discussion 30–2. doi:10.1097/TA.0000000000000809
86. Brenner M, Bulger EM, Perina DG, Henry S, Kang CS, Rotondo MF, et al. Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Trauma Surg Acute Care Open (2018) 3:e000154. doi:10.1136/tsaco-2017-000154
87. Patman RD, Poulos E, Shires GT. The management of civilian arterial injuries. Surg Gynecol Obstet (1964) 118:725–38.
89. Perry MO, Thal ER, Shires GT. Management of arterial injuries. Ann Surg (1971) 173:403–8. doi:10.1097/00000658-197103000-00011
90. Graham JM, Mattox KL, Beall AC Jr, DeBakey ME. Injuries to the visceral arteries. Surgery (1978) 84:835–9.
91. Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma – a unified approach. J Trauma (1982) 22:672–9. doi:10.1097/00005373-198208000-00004
92. Adkins RB Jr, Bitseff EL Jr, Meacham PW. Abdominal vascular injuries. South Med J (1985) 78:1152–60. doi:10.1097/00007611-198510000-00003
93. Davis TP, Feliciano DV, Rozycki GS, Bush JB, Ingram WL, Salomone JP, et al. Results with abdominal vascular trauma in the modern era. Am Surg (2001) 67:565–70; discussion 70–1.
94. Kavic SM, Atweh N, Ivy ME, Possenti PP, Dudrick SJ. Celiac axis ligation after gunshot wound to the abdomen: case report and literature review. J Trauma (2001) 50:738–9. doi:10.1097/00005373-200104000-00025
95. Bland ZM, Dobos N, Gosselin MV. Case of the season: imaging of blunt traumatic injury to the abdominal aorta. Semin Roentgenol (2006) 41:157–8. doi:10.1053/j.ro.2006.04.002
96. Mattox KL, Espada R, Beall AR Jr. Traumatic injury to the portal vein. Ann Surg (1975) 181:519–22. doi:10.1097/00000658-197505000-00003
97. Buckman RF, Pathak AS, Badellino MM, Bradley KM. Portal vein injuries. Surg Clin North Am (2001) 81:1449–62. doi:10.1016/S0039-6109(01)80017-7
98. Pachter HL, Drager S, Godfrey N, LeFleur R. Traumatic injuries of the portal vein. The role of acute ligation. Ann Surg (1979) 189:383–5.
99. Petersen SR, Sheldon GF, Lim RC Jr. Management of portal vein injuries. J Trauma (1979) 19:616–20. doi:10.1097/00005373-197908000-00009
100. Busuttil RW, Kitahama A, Cerise E, McFadden M, Lo R, Longmire WP Jr. Management of blunt and penetrating injuries to the porta hepatis. Ann Surg (1980) 191:641–8. doi:10.1097/00000658-198005000-00017
101. Jurkovich GJ, Hoyt DB, Moore FA, Ney AL, Morris JA Jr, Scalea TM, et al. Portal triad injuries. J Trauma (1995) 39:426–34. doi:10.1097/00005373-199509000-00005
102. Stone HH, Fabian TC, Turkleson ML. Wounds of the portal venous system. World J Surg (1982) 6:335–41. doi:10.1007/BF01653551
103. Ivatury RR, Nallathambi M, Lankin DH, Wapnir I, Rohman M, Stahl WM. Portal vein injuries. Noninvasive follow-up of venorrhaphy. Ann Surg (1987) 206:733–7. doi:10.1097/00000658-198712000-00008
104. Courcy PA, Brotman S, Oster-Granite ML, Soderstrom CA, Siegel JH, Cowley RA. Superior mesenteric artery and vein injuries from blunt abdominal trauma. J Trauma (1984) 24:843–5. doi:10.1097/00005373-198409000-00012
105. Jackson MR, Olson DW, Beckett WC Jr, Olsen SB, Robertson FM. Abdominal vascular trauma: a review of 106 injuries. Am Surg (1992) 58:622–6.
106. Collins PS, Golocovsky M, Salander JM, Champion H, Rich NM. Intra-abdominal vascular injury secondary to penetrating trauma. J Trauma (1988) 28:S165–70. doi:10.1097/00005373-198801001-00034
107. Sirinek KR, Gaskill HV III, Root HD, Levine BA. Truncal vascular injury – factors influencing survival. J Trauma (1983) 23:372–7. doi:10.1097/00005373-198305000-00003
108. Lucas AE, Richardson JD, Flint LM, Polk HC Jr. Traumatic injury of the proximal superior mesenteric artery. Ann Surg (1981) 193:30–4. doi:10.1097/00000658-198101000-00005
109. Ekbom GA, Towne JB, Majewski JT, Woods JH. Intra-abdominal vascular trauma-a need for prompt operation. J Trauma (1981) 21:1040–4. doi:10.1097/00005373-198112000-00007
110. Phillips CV, Jacobsen DC, Brayton DF, Bloch JH. Central vessel trauma. Am Surg (1979) 45:517–30.
111. Elkins R, DeMeester TR, Brawley RK. Surgical exposure of the upper abdominal aorta and its branches. Surgery (1971) 70:622–7.
112. Mattox KL, McCollum WB, Beall AC Jr, Jordan GL Jr, Debakey ME. Management of penetrating injuries of the suprarenal aorta. J Trauma (1975) 15:808–15. doi:10.1097/00005373-197509000-00009
113. Kelly GL, Eiseman B. Civilian vascular injuries. J Trauma (1975) 15:507–14. doi:10.1097/00005373-197506000-00010
114. Ledgerwood A, Lucas CE. Survival following proximal superior mesenteric artery occlusion from trauma. J Trauma (1974) 14:622–6. doi:10.1097/00005373-197407000-00011
115. Fullen WD, Hunt J, Altemeier WA. The clinical spectrum of penetrating injury to the superior mesenteric arterial circulation. J Trauma (1972) 12:656–64. doi:10.1097/00005373-197208000-00003
116. Barone GW, Kahn MB, Cook JM, Thompson BW, Barnes RW, Eidt JF. Traumatic left renal artery stenosis managed with splenorenal bypass: case report. J Trauma (1990) 30:1594–6. doi:10.1097/00005373-199012000-00030
117. Tyburski JG, Wilson RF, Dente C, Steffes C, Carlin AM. Factors affecting mortality rates in patients with abdominal vascular injuries. J Trauma (2001) 50:1020–6. doi:10.1097/00005373-200106000-00008
118. Carroll PR, McAninch JW, Klosterman P, Greenblatt M. Renovascular trauma: risk assessment, surgical management, and outcome. J Trauma (1990) 30:547–52; discussion 53–4. doi:10.1097/00005373-199005000-00004
119. Brown MF, Graham JM, Mattox KL, Feliciano DV, DeBakey ME. Renovascular trauma. Am J Surg (1980) 140:802–5. doi:10.1016/0002-9610(80)90121-X
120. Tillou A, Romero J, Asensio JA, Best CD, Petrone P, Roldan G, et al. Renal vascular injuries. Surg Clin North Am (2001) 81:1417–30. doi:10.1016/S0039-6109(01)80015-3
121. Haas CA, Dinchman KH, Nasrallah PF, Spirnak JP. Traumatic renal artery occlusion: a 15-year review. J Trauma (1998) 45:557–61. doi:10.1097/00005373-199809000-00024
122. Sclafani SJ, Becker JA. Interventional radiology in the treatment of retroperitoneal trauma. Urol Radiol (1985) 7:219–30. doi:10.1007/BF02926889
123. Hagiwara A, Sakaki S, Goto H, Takenega K, Fukushima H, Matuda H, et al. The role of interventional radiology in the management of blunt renal injury: a practical protocol. J Trauma (2001) 51:526–31. doi:10.1097/00005373-200109000-00017
124. Whigham CJ Jr., Bodenhamer JR, Miller JK. Use of the Palmaz stent in primary treatment of renal artery intimal injury secondary to blunt trauma. J Vasc Interv Radiol (1995) 6:175–8. doi:10.1016/S1051-0443(95)71088-0
125. Villas PA, Cohen G, Putnam SG III, Goldberg A, Ball D. Wallstent placement in a renal artery after blunt abdominal trauma. J Trauma (1999) 46:1137–9. doi:10.1097/00005373-199906000-00035
126. Sangthong B, Demetriades D, Martin M, Salim A, Brown C, Inaba K, et al. Management and hospital outcomes of blunt renal artery injuries: analysis of 517 patients from the National Trauma Data Bank. J Am Coll Surg (2006) 203:612–7. doi:10.1016/j.jamcollsurg.2006.07.004
127. Sprouse LR II, Hamilton IN Jr. The endovascular treatment of a renal arteriovenous fistula: placement of a covered stent. J Vasc Surg (2002) 36:1066–8. doi:10.1067/mva.2002.127969
128. Goodman DN, Saibil EA, Kodama RT. Traumatic intimal tear of the renal artery treated by insertion of a Palmaz stent. Cardiovasc Intervent Radiol (1998) 21:69–72. doi:10.1007/s002709900215
129. Chabrot P, Cassagnes L, Alfidja A, Mballa JC, Nasser S, Guy L, et al. Revascularization of traumatic renal artery dissection by endoluminal stenting: three cases. Acta Radiol (2010) 51:21–6. doi:10.3109/02841850903473314
130. Memon S, Cheung BY. Long-term results of blunt traumatic renal artery dissection treated by endovascular stenting. Cardiovasc Intervent Radiol (2005) 28:668–9. doi:10.1007/s00270-004-0296-x
131. Lopera JE, Suri R, Kroma G, Gadani S, Dolmatch B. Traumatic occlusion and dissection of the main renal artery: endovascular treatment. J Vasc Interv Radiol (2011) 22:1570–4. doi:10.1016/j.jvir.2011.08.002
132. Velmahos GC, Chahwan S, Hanks SE, Murray JA, Berne TV, Asensio J, et al. Angiographic embolization of bilateral internal iliac arteries to control life-threatening hemorrhage after blunt trauma to the pelvis. Am Surg (2000) 66:858–62.
133. Cestero RF, Plurad D, Green D, Inaba K, Putty B, Benfield R, et al. Iliac artery injuries and pelvic fractures: a national trauma database analysis of associated injuries and outcomes. J Trauma (2009) 67:715–8. doi:10.1097/TA.0b013e3181af6e88
134. Burch JM, Richardson RJ, Martin RR, Mattox KL. Penetrating iliac vascular injuries: recent experience with 233 consecutive patients. J Trauma (1990) 30:1450–9. doi:10.1097/00005373-199012000-00003
135. Haidar GM, Hicks TD, Strosberg DS, El-Sayed HF, Davies MG. “In situ” endografting in the treatment of arterial and graft infections. J Vasc Surg (2017) 65:1824–9. doi:10.1016/j.jvs.2016.12.134
136. Balogh Z, Voros E, Suveges G, Simonka JA. Stent graft treatment of an external iliac artery injury associated with pelvic fracture. A case report. J Bone Joint Surg Am (2003) 85-A:919–22. doi:10.2106/00004623-200305000-00025
137. Burlew CC. Preperitoneal pelvic packing for exsanguinating pelvic fractures. Int Orthop (2017) 41:1825–9. doi:10.1007/s00264-017-3485-3
Keywords: abdominal vascular trauma, penetrating injuries, blunt injuries, abdominal aorta injury, venous injuries, arterial injuries, open approach, endovascular approach
Citation: Karaolanis G, Moris D, McCoy CC, Tsilimigras DI, Georgopoulos S and Bakoyiannis C (2018) Contemporary Strategies in the Management of Civilian Abdominal Vascular Trauma. Front. Surg. 5:7. doi: 10.3389/fsurg.2018.00007
Received: 30 March 2017; Accepted: 29 January 2018;
Published: 19 February 2018
Edited by:
Efthymios Avgerinos, University of Pittsburgh Medical Center, United StatesReviewed by:
Andreas M. Lazaris, National and Kapodistrian University of Athens, GreeceMarina Kafeza, Imperial College London, United Kingdom
Copyright: © 2018 Karaolanis, Moris, McCoy, Tsilimigras, Georgopoulos and Bakoyiannis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgios Karaolanis, ZHJnaWthcmFvbGFuaXNAZ21haWwuY29t
 Georgios Karaolanis
Georgios Karaolanis Dimitrios Moris
Dimitrios Moris C. Cameron McCoy2
C. Cameron McCoy2 Sotirios Georgopoulos
Sotirios Georgopoulos Chris Bakoyiannis
Chris Bakoyiannis