
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Stroke , 13 December 2023
Sec. Stroke Recovery and Rehabilitation
Volume 2 - 2023 | https://doi.org/10.3389/fstro.2023.1274555
 Laus M. Broersen1*
Laus M. Broersen1* Sonia Guida1
Sonia Guida1 Aysun Cetinyurek-Yavuz1,2
Aysun Cetinyurek-Yavuz1,2 Nick van Wijk1
Nick van Wijk1 Ardy van Helvoort1,3
Ardy van Helvoort1,3 Adina T. Michael-Titus4
Adina T. Michael-Titus4 Mirian Lansink1
Mirian Lansink1Background: Malnutrition is common after stroke. Stroke patients often have a suboptimal energy intake, body weight and inadequate blood nutrient levels. Nutrient insufficiencies may not be detected, but their recognition is essential to provide adequate nutritional support after a stroke. This comprehensive summary of the literature is a collection of data on blood levels of a broad selection of nutrients involved in restoring cerebral blood flow and functional brain connectivity in stroke patients compared to controls.
Methods: Embase and MEDLINE were searched for studies published in English in the period 1980–2022. Studies including adult stroke subjects and controls whose blood samples were analyzed for docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), uridine, choline, folate, vitamin B6, vitamin B12, vitamin C, vitamin E, selenium, coenzyme Q10 (CoQ10), carnitine, arginine, or taurine were selected. If there were >3 reports (k) per nutrient, results were generated with an unadjusted and age-adjusted random-effects meta-analysis model. Risk of bias was evaluated for relevant domains from the ROBINS-I tool and with Egger's test.
Results: One hundred five reports on blood nutrient levels were extracted from 56 eligible studies. Overall, meta-analyses showed lower blood levels of most nutrients in stroke patients compared to controls. The number of reports and the statistical significance for the unadjusted data were: folate (k = 27; p = 0.005), vitamin B12 (k = 23; p = 0.002), vitamin E (k = 11; p = 0.013), DHA (k = 7, p = 0.015), EPA (k = 7; p = 0.004), vitamin C (k = 6; p = 0.020), and selenium (k = 6; p = 0.018). No significant decreases were observed for vitamin B6 (k = 6; p = 0.52) and arginine (k = 4; p = 0.93). For other selected nutrients, there were insufficient reports to perform a meta-analysis. Available reports pointed toward lower (CoQ10, choline; k = 2), higher (taurine; k = 2), or unchanged (carnitine, uridine; k = 1) blood levels after stroke. In general, risk of bias was low.
Conclusion: Our findings indicate that nutrient insufficiencies occur for many nutrients that are involved in repair processes after stroke. The low blood levels of folate, vitamin B12, EPA, DHA, vitamin C, vitamin E, selenium, and possibly CoQ10 and choline, highlight the presence of a suboptimal nutritional status after stroke. The inclusion of targeted nutritional interventions to further support recovery should receive consideration in the multidisciplinary context of stroke rehabilitation.
Malnutrition after stroke is common (Burgos et al., 2018) and occurs across the continuum of stroke care, from the hyperacute to the chronic phase (Huppertz et al., 2021). Malnourishment in stroke patients may not be limited to protein-energy malnutrition and can also include insufficiencies or deficiencies in specific micronutrients and fatty acids, reflected by their lower blood levels (Cherubini et al., 2000; Han et al., 2002; Ikeya et al., 2013). Reduced levels of circulating nutrients can be related to a reduced dietary intake, be a consequence of previous or current lifestyle, be due to medication influencing the absorption and excretion of nutrients, or be due to condition-specific metabolic changes that alter nutrient requirements (Lieber et al., 2018; Sabbouh and Torbey, 2018; Chen et al., 2019; Wakeman and Archer, 2020).
Impaired nutritional status after stroke has consistently been associated with poor outcome and reduced functional recovery (Gariballa et al., 1998; Martineau et al., 2005; Yoo et al., 2008; Gomes et al., 2016). Apart from protein-energy undernutrition, this may be due to insufficiencies in specific nutrients that are relevant for clinical outcome after this central nervous system injury. The acute response to ischemia is characterized by cell death, the formation of free radicals and reactive oxygen species, and an inflammatory response. In the days and weeks following the ischemic event, adaptive brain plasticity mechanisms come into play, involving the release of different growth factors to support angiogenesis, gliogenesis, neurogenesis, neurite outgrowth, and synaptogenesis, that collectively contribute to the initiation of a (partial) recovery of function (Wieloch and Nikolich, 2006; Dalise et al., 2014). Physiological processes occurring after a stroke, which are part of the injury response and the emergence of repair mechanisms, depend on the presence of compounds derived from the body's nutrient reserves or from dietary intake. For instance, many nutrients are known for their antioxidant (Cheli and Baldi, 2011; Ruskovska et al., 2020), anti-inflammatory (Sanderson and Croft, 2005; Tyrovolas et al., 2018), or immune modulating properties (Suchner et al., 2000; Ruiz-Leon et al., 2019), all of which are relevant after stroke. In addition, nutrients act as cofactors in metabolic processes such as the one-carbon metabolism, where the presence of sufficient B-vitamins regulates homocysteine levels (Taylor et al., 2018). Furthermore, nutrients serve as precursors for signaling molecules and structural components, i.e., the building blocks for glial and neuronal cells, neurite extensions and synaptic connections, and new blood vessels that support their functioning. In order to adequately address the condition-specific nutritional needs of stroke patients and support an optimal rehabilitation, it is important to identify the most relevant nutrients which may be suboptimal.
Nutrient insufficiencies may not be detected, but their recognition is essential to provide adequate nutritional support to patients from the early phase post stroke. Therefore, we performed a systematic review and meta-analysis on reported blood levels of micronutrients, amino acids, and fatty acids shown to be involved in the restoration of cerebral blood flow and functional brain connectivity after stroke (Wurtman et al., 2010; van Wijk et al., 2014; Wiesmann et al., 2017). In particular, we investigated reported blood levels of docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), uridine, choline, folate, vitamin B6, vitamin B12, vitamin C, vitamin E, selenium, coenzyme Q10 (CoQ10), carnitine, arginine, and taurine in stroke cases compared to controls.
The systematic literature search was performed by a librarian specialist in two electronic databases, Embase and MEDLINE, to identify studies published in English in the period 1980–2022 in accordance with the PRISMA guidelines. Additional records were identified from recently published literature in 2023. The search strategy used to retrieve the relevant database records published between January 1980 and September 2022 was composed of a conceptual string with the following combination of terms or their analogs or synonyms: “cerebrovascular” AND “ischemic” AND “malnutrition” AND “DHA OR EPA OR uridine OR choline OR folate OR vitamin B6 OR vitamin B12 OR vitamin C OR vitamin E OR selenium OR taurine OR arginine OR CoQ10 OR carnitine” NOT “animal.” Non peer reviewed sources, such as conference abstracts, book chapters, and video-audio media were excluded. The full search strategy is available in the Supplementary Table S1. The review was not registered; no protocol was prepared for registration.
Records were screened based on title and/or abstract and were excluded in case of reviews, case reports, duplicates and when the search criteria were not met. For example, studies that did not present data on any of the nutrients within the scope of this systematic review were excluded. The studies were assessed based on the full-text and excluded if the study population was <18 years old, or if the population included exclusively subjects with haemorrhagic stroke or transient ischemic attack, or it did not include a control group. The inclusion of a study in the meta-analysis required that the blood levels of the nutrients and the number of subjects assessed were available for the stroke and control group. Studies presenting data for more than one nutrient or for independent subgroups were treated independently and constituted the total number of reports. Study selection and data extraction was performed by three independent reviewers and the final dataset was discussed with the review team.
The blood levels of DHA, EPA, uridine, choline, folate, vitamin B6, vitamin B12, vitamin C, vitamin E, selenium, CoQ10, carnitine, arginine, and taurine in stroke cases and controls were extracted. Data on the type of stroke and timing after stroke were recorded. Timing was based on the definition of the stroke phases provided by the Stroke Recovery and Rehabilitation Roundtable Taskforce (Bernhardt et al., 2017), simplified to: acute (<7 days), subacute (between 7 days and 6 months), and chronic (6 months or more). Data on the type of controls were also extracted. Mean age and the number of subjects assessed were collected for both the stroke and control groups.
Risk of bias (high, unclear, or low) was evaluated by two independent assessors for each study on the basis of 4 domains: selection, detection, attrition, and reporting bias. These were selected from the ROBINS-I tool (Sterne et al., 2016) as being most relevant for non-randomized observational studies. In short, each assessor rated the risk per domain as either high, unclear, or low. Then, all assessments were compared and sporadic disagreements were discussed between assessors to come to an agreement on the final score.
In addition, publication bias was assessed with Egger's test if possible (see Section 2.5).
Blood levels of the nutrients were expressed as mean ± standard deviation (SD). In the studies where the mean ± SD was available in subgroups rather than for the whole group of stroke patients or controls, the weighted mean and the pooled SD were calculated. When a study reported the mean value but the SD was missing the SD was estimated based on the standard error of the mean (SEM) or the figures provided in the study, if available. When a study reported median, first and third quartile, the formulas from Wan et al. (2014) were used to impute mean and SD. In case the median was available with the range of the data, the formulas from Hozo et al. (2005) were used to impute mean and SD.
To allow comparison across studies, the standardized mean difference (SMD) estimated by Hedges' g, was used to standardize the results of the studies to the same scale, which expresses the size of the effect in each study relative to the variability observed in that study.
In the meta-analyses where the number of reports was >3, the overall effect was estimated with a random-effect (RE) meta-analysis model with Hartung Knapp (HK) modification for unadjusted data. The analysis was replicated including age as a covariate if the number of reports was >3; age-adjustment was obtained by correcting for centralized age differences between groups. In case the mean age was not available and the median (range) was reported, or the mean age was available only in the stroke or the control group and these groups were age matched, the mean value of the age was imputed. The between-study variance was estimated using the I2 statistic, where an I2 > 95% indicates considerable amount of heterogeneity.
The publication bias was evaluated with Egger's test (regression test for funnel plot asymmetry) if the number of reports included in the meta-analysis was ≥10. The RE meta-analysis with HK modification and the Egger's test output was generated using Metafor package in R (Viechtbauer, 2010; R Core Team, 2022).
The number of records identified from Embase and Medline was 5,893 for the publication period 1980–2022. A recent study (van Wijk et al., 2023) was identified as an additional record from recently published literature. Screening based on the eligibility criteria resulted in a total of 56 studies and 105 reports published in the period 1980–2023 (Figure 1).
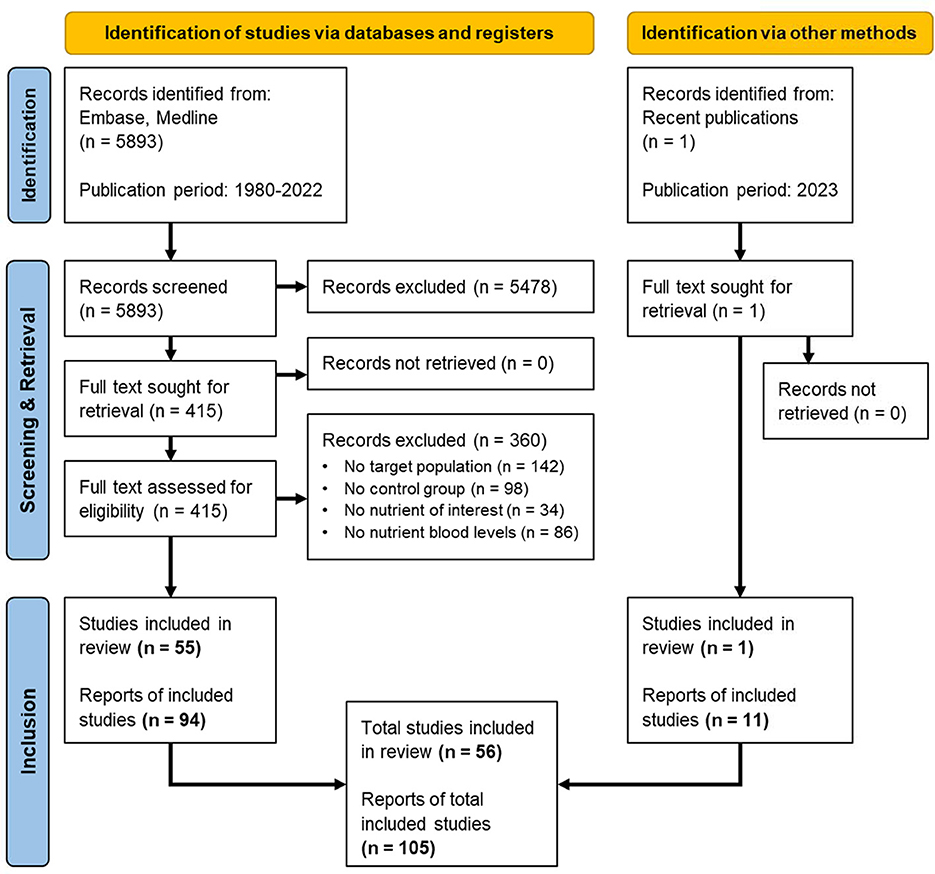
Figure 1. PRISMA flow diagram for the systematic review involving search of databases, registers and other sources, adapted from Page et al. (2021). Breakdown of the screening and retrieval, leading to the inclusion of 56 eligible studies and 105 reports for systematic review and meta-analysis.
The number of stroke subjects included in the 105 reports was 5,725 and the mean age was 64.1 (range: 27.7–77.2) years. Overall, the main diagnosis was ischemic stroke and the assessment 165 of the nutrient blood levels occurred within the acute (k = 59), the subacute (k = 22), or the chronic (k = 11) 166 phase. For the remaining reports (k = 13), the moment of assessment was not indicated. Two studies with assessments overlapping phase borders, were assigned to the best fitting phase, i.e., 1–10 days (Han et al., 2002) to acute, and 21–42 weeks (Brattstrom et al., 1992) to chronic. The number of controls included in the reports was 16,171 and it primarily included relatively healthy subjects whose mean age was 60.6 (range: 27.0–77.8) years. The results of the 105 reports consisted of the following meta-analysis groups: folate (k = 27), vitamin B12 (k = 23), vitamin E (k = 11), DHA (k = 7), EPA (k = 7), vitamin B6 (k = 6), vitamin C (k = 6), selenium (k = 6), arginine (k = 4). Nutrients with three or fewer available reports, i.e., taurine (k = 2), CoQ10 (k = 2), choline (k = 2), carnitine (k = 1), and uridine (k = 1), were not included in the meta-analysis and were treated separately. A summary of the results is reported in Supplementary Table S2.
Risk of selection bias was present in 16%, and unclear in 38% of the studies. Selection bias was obvious where specific patients with a higher risk of malnutrition due to dysphagia or the use of specific medication were excluded. Similarly, the selection of relatively unhealthy control subjects, such as elderly patients without cerebrovascular diagnosis, was considered a source of bias. Risk of detection bias was present in 9%, and unclear in 32% of the studies. In most cases detection bias was due to a lack of fasting in one or both groups (stroke patients and controls) before blood sampling. In addition, unblinded handling or analysis of samples could have introduced bias. Risk of attrition bias and risk of reporting bias were very low to absent in the selected studies; there were hardly any missing data, and all outcomes were clearly reported. Overall results of the risk of bias assessment are summarized in Figure 2.
Twenty-seven reports with a total of 3,279 stroke cases and 2,679 controls were included in the meta-analysis (Brattstrom et al., 1992; Hultberg et al., 1997; Williams et al., 2001; Han et al., 2002; Karttunen et al., 2002; Kelly et al., 2003; Sachdev et al., 2003; Glew et al., 2004; Kocer et al., 2004; Fang et al., 2005; Liu et al., 2005; Urbanska et al., 2006; Angelova et al., 2008; Mojiminiyi et al., 2008; Kara et al., 2009; Kim et al., 2010; Moghaddasi et al., 2010; Mohapatra and Sarangi, 2010; Osunkalu et al., 2010; Mejia Mohamed et al., 2011; Omrani et al., 2011; Ashjazadeh et al., 2013; Jiang et al., 2014; Cho et al., 2015a,b; Rudreshkumar et al., 2018; van Wijk et al., 2023). The mean number of subjects was 90.2 (range: 20–579) in the stroke groups and 99.2 (range: 20–427) in the control groups. The mean age was 60.8 (range: 27.7–72.7) years in the stroke cases and 58.6 (range: 27.0–71.7) years in the controls. Blood levels of folate were lower in stroke patients compared to controls for unadjusted [t(26) = −3.03, p = 0.006] and age-adjusted [t(25) = −2.97, p = 0.006] model (Figure 3). The Egger's test [t(25) = −0.59, p = 0.56] indicated no evidence of publication bias (Supplementary Figure S1).
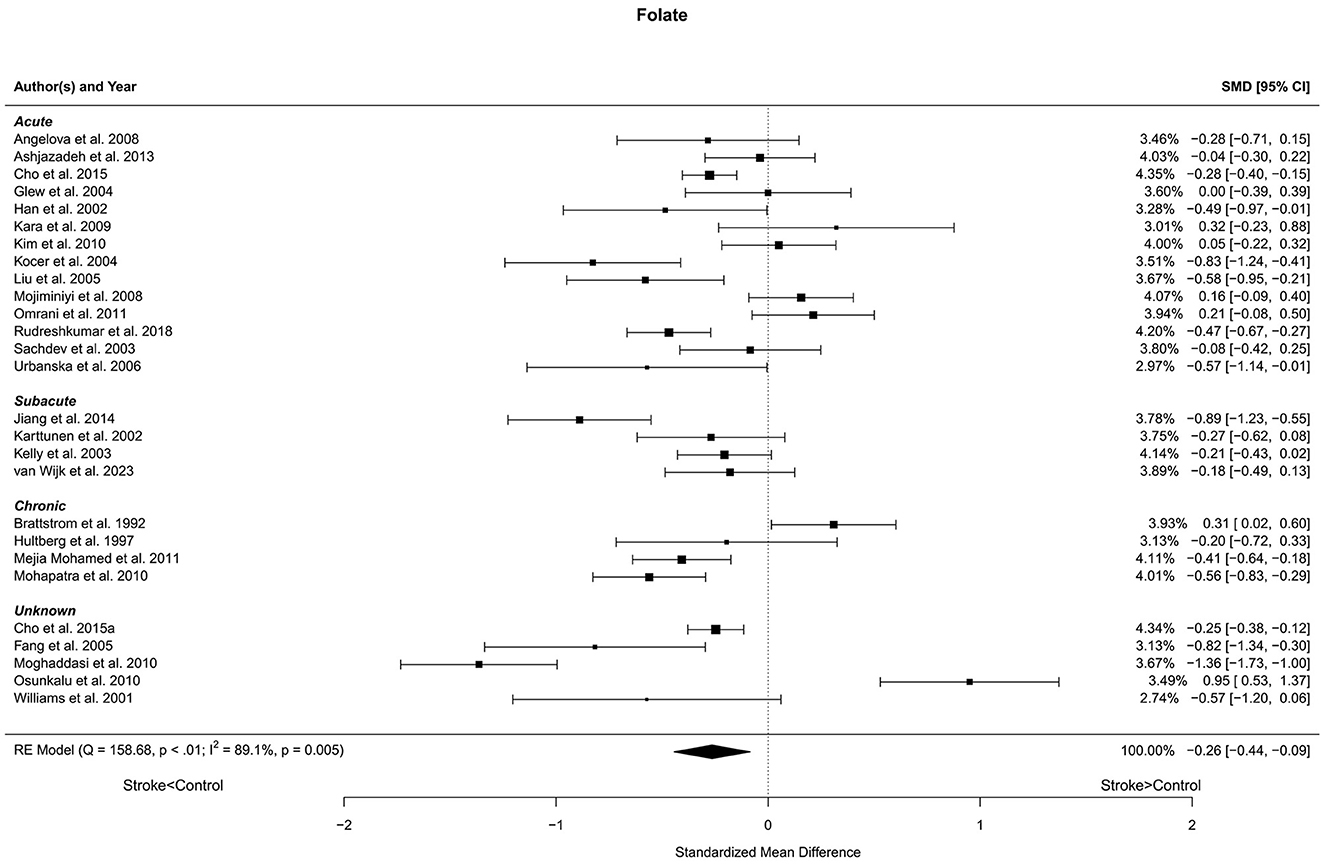
Figure 3. Comparison of blood folate levels in stroke patients and controls. Included reports are organized in the forest plot by phase (acute—subacute—chronic—unknown) to visualize possible effects of time after stroke. Relative weights (%) and standardized mean differences (plus confidence interval) for each report are indicated at the right. Characteristics and result of the RE meta-analysis are in the lower left corner.
Twenty-three reports with a total of 2,075 stroke cases and 1,707 controls were included in the meta-analysis (Brattstrom et al., 1992; Hultberg et al., 1997; Williams et al., 2001; Han et al., 2002; Karttunen et al., 2002; Kelly et al., 2003; Sachdev et al., 2003; Glew et al., 2004; Kocer et al., 2004; Liu et al., 2005; Urbanska et al., 2006; Angelova et al., 2008; Kara et al., 2009; Salemi et al., 2009; Moghaddasi et al., 2010; Mohapatra and Sarangi, 2010; Mejia Mohamed et al., 2011; Omrani et al., 2011; Ashjazadeh et al., 2013; Jiang et al., 2014; Rudreshkumar et al., 2018; Kweon et al., 2021; van Wijk et al., 2023). The mean number of subjects was 90.2 (range: 20–199) in the stroke groups and 74.2 (range: 20–249) in the control groups. The mean age was 61.5 (range: 27.7–72.7) years for stroke cases and 59.7 (27.0–71.7) years for controls. Blood levels of vitamin B12 were lower in stroke patients compared to controls for unadjusted [t(22) = −3.58, p = 0.002] and age-adjusted [t(21) = −3.55, p = 0.002] model (Figure 4). The Egger's test [t(21) = −0.27, p = 0.79] indicated no evidence of publication bias (Supplementary Figure S2).
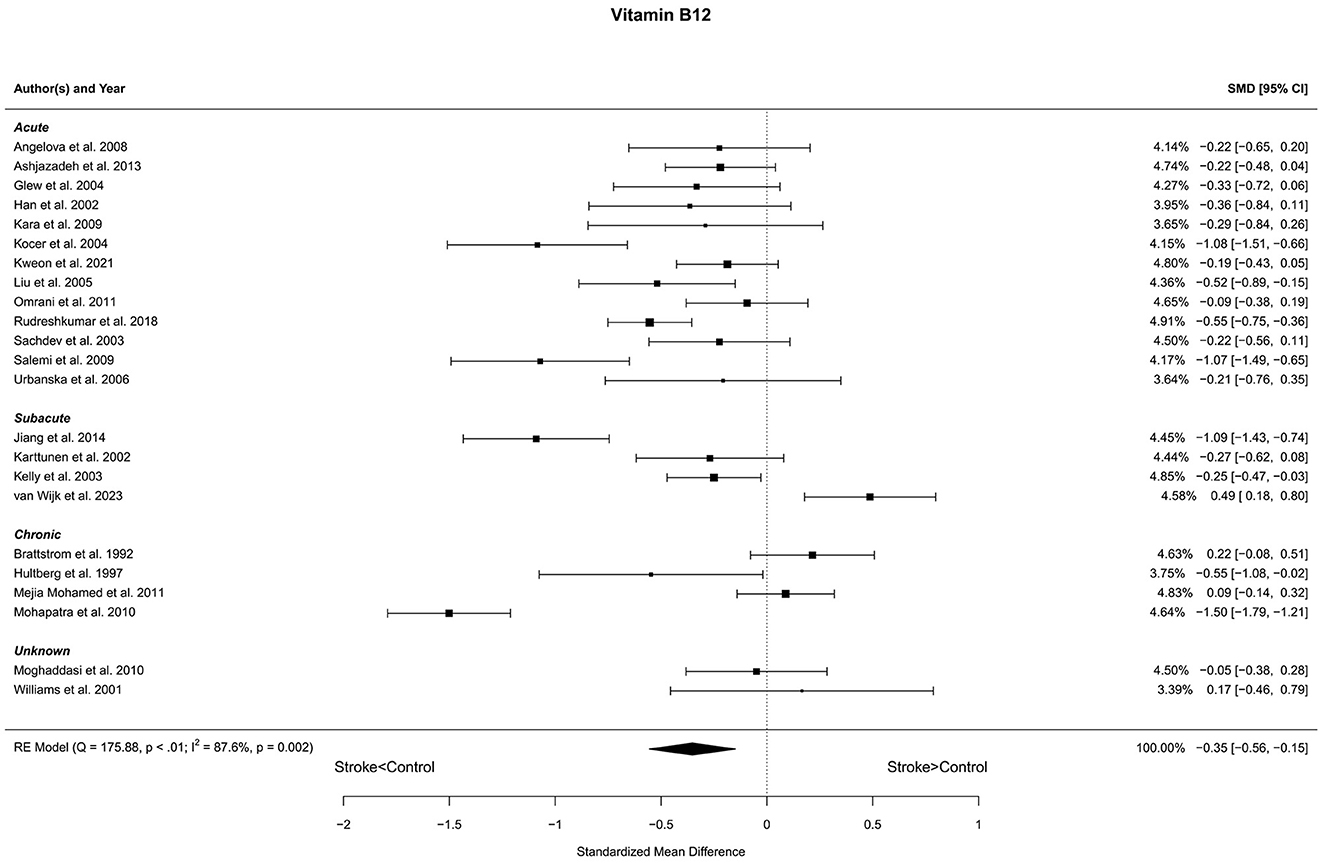
Figure 4. Comparison of blood vitamin B12 levels in stroke patients and controls. Included reports are organized in the forest plot by phase (acute—subacute—chronic—unknown) to visualize possible effects of time after stroke. Relative weights (%) and standardized mean differences (plus confidence interval) for each report are indicated at the right. Characteristics and result of the RE meta-analysis are in the lower left corner.
Eleven reports with a total of 457 stroke cases and 484 controls were included in the meta-analysis (Singh et al., 1995; Daga and Madhuchhanda, 1997; Chang et al., 1998, 2005; Cherubini et al., 2000; Gariballa et al., 2002; Sanchez-Moreno et al., 2004; Mao et al., 2008; van Wijk et al., 2023). Mao et al. (2008) reported three subgroups and one control group. Therefore, the control group was divided into three equal subgroups and using the same mean and SD for each control group. The mean number of subjects was 41.5 (range: 12–110) in the stroke groups and 53.8 (range: 20–202) in the control groups. The mean age was 65.9 (range: 52.5–77.2) years for stroke cases and 62.9 (range: 46.2–77.8) years for controls. The blood levels of vitamin E were lower in stroke patients compared to controls for the unadjusted [t(10) = −3.00, p = 0.013] and for the age-adjusted [t(8) = −2.56, p = 0.034] model (Figure 5). The Egger's test [t(9) = 0.93, p = 0.38] indicated no evidence of publication bias (Supplementary Figure S3).
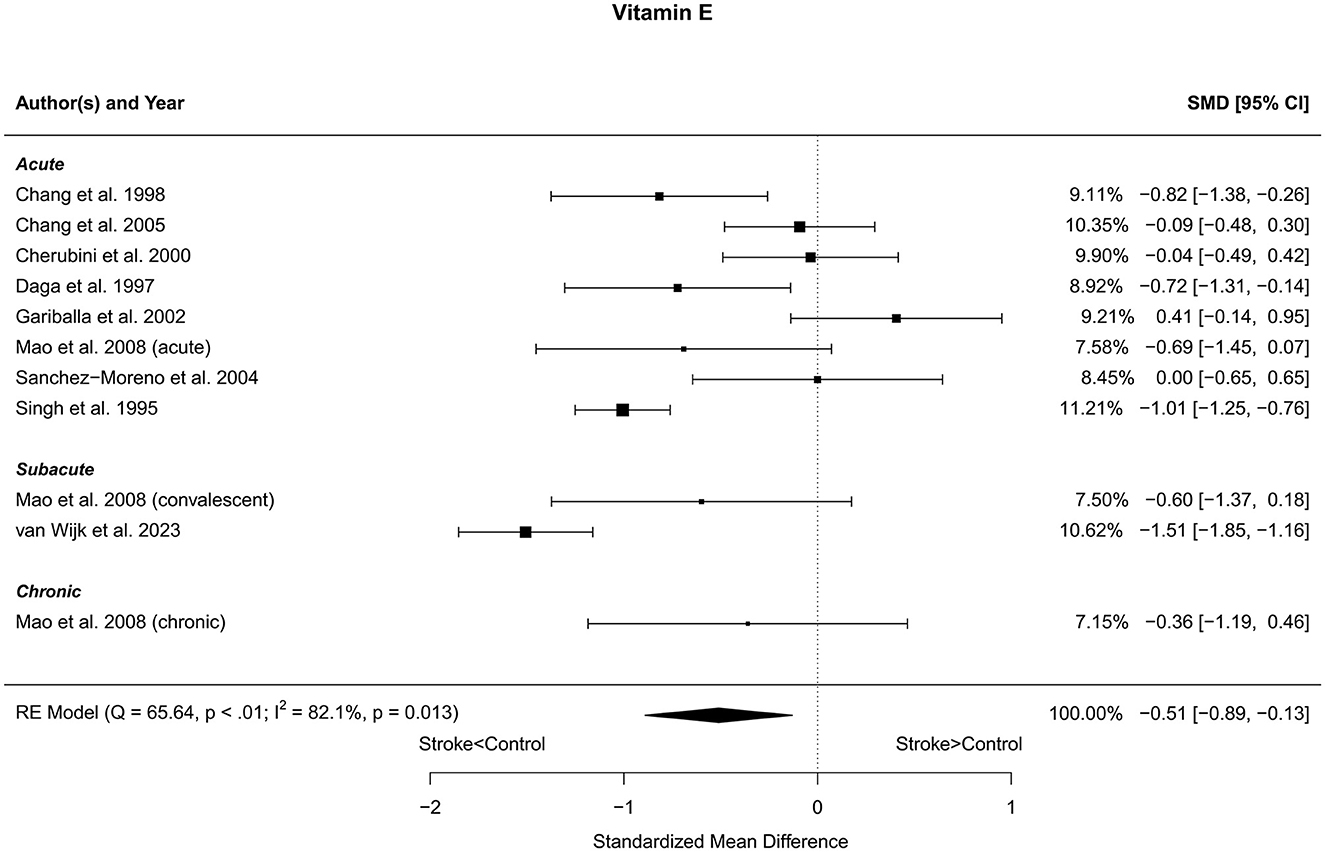
Figure 5. Comparison of blood vitamin E levels in stroke patients and controls. Included reports are organized in the forest plot by phase (acute—subacute—chronic) to visualize possible effects of time after stroke. Relative weights (%) and standardized mean differences (plus confidence interval) for each report are indicated at the right. Characteristics and result of the RE meta-analysis are in the lower left corner.
Seven reports with a total of 773 stroke cases and 608 controls were included in the meta-analysis (Park et al., 2009; Kim et al., 2012; Ikeya et al., 2013; Shang et al., 2018; Szczuko et al., 2020; Hutanu et al., 2021; van Wijk et al., 2023). The mean number of subjects was 110.4 (range: 40–248) in the stroke groups and 86.9 (range: 35–215) in the control groups. The mean age was 66.8 (range: 59.0–72.5) years for stroke cases and 65.3 (range: 54.4–75.4) years for controls. Blood levels of DHA were lower in stroke patients compared to controls for both the unadjusted [t(6) = −3.37, p = 0.015] and age-adjusted [t(5) = −3.66, p = 0.015] model (Figure 6).
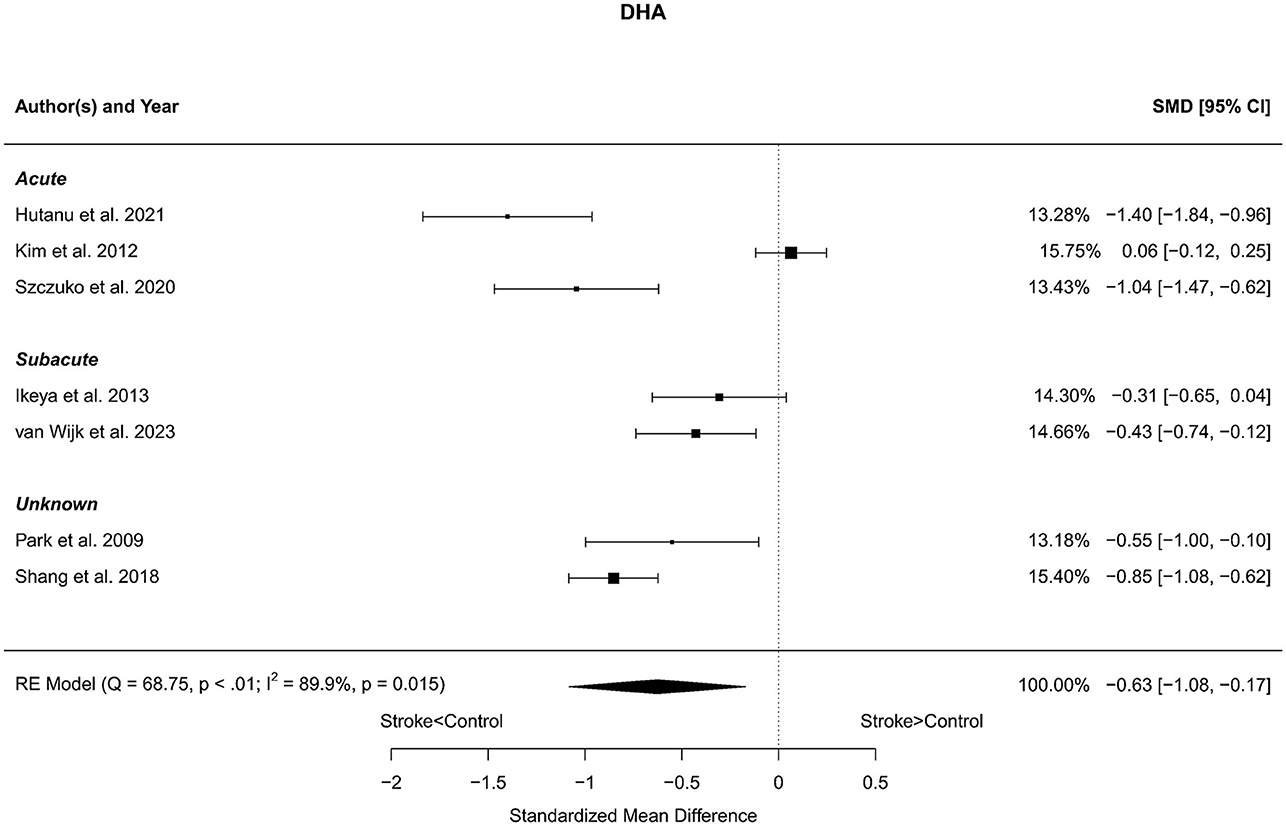
Figure 6. Comparison of blood DHA levels in stroke patients and controls. Included reports are organized in the forest plot by phase (acute—subacute—unknown) to visualize possible effects of time after stroke. Relative weights (%) and standardized mean differences (plus confidence interval) for each report are indicated at the right. Characteristics and result of the RE meta-analysis are in the lower left corner.
Seven reports with a total of 773 stroke cases and 608 controls were included in the meta-analysis (Park et al., 2009; Kim et al., 2012; Ikeya et al., 2013; Shang et al., 2018; Szczuko et al., 2020; Hutanu et al., 2021; van Wijk et al., 2023). The mean number of subjects was 110.4 (range: 40–248) in the stroke groups and 86.9 (range: 35–215) in the control groups. The mean age was 66.8 (range: 59.0–72.5) years for stroke cases and 65.3 (range: 54.4–75.4) years for controls. Blood levels of EPA were lower in stroke patients compared to controls for both the unadjusted [t(6) = −4.44, p = 0.004] and age-adjusted [t(5) = −4.20, p = 0.009] model (Figure 7).
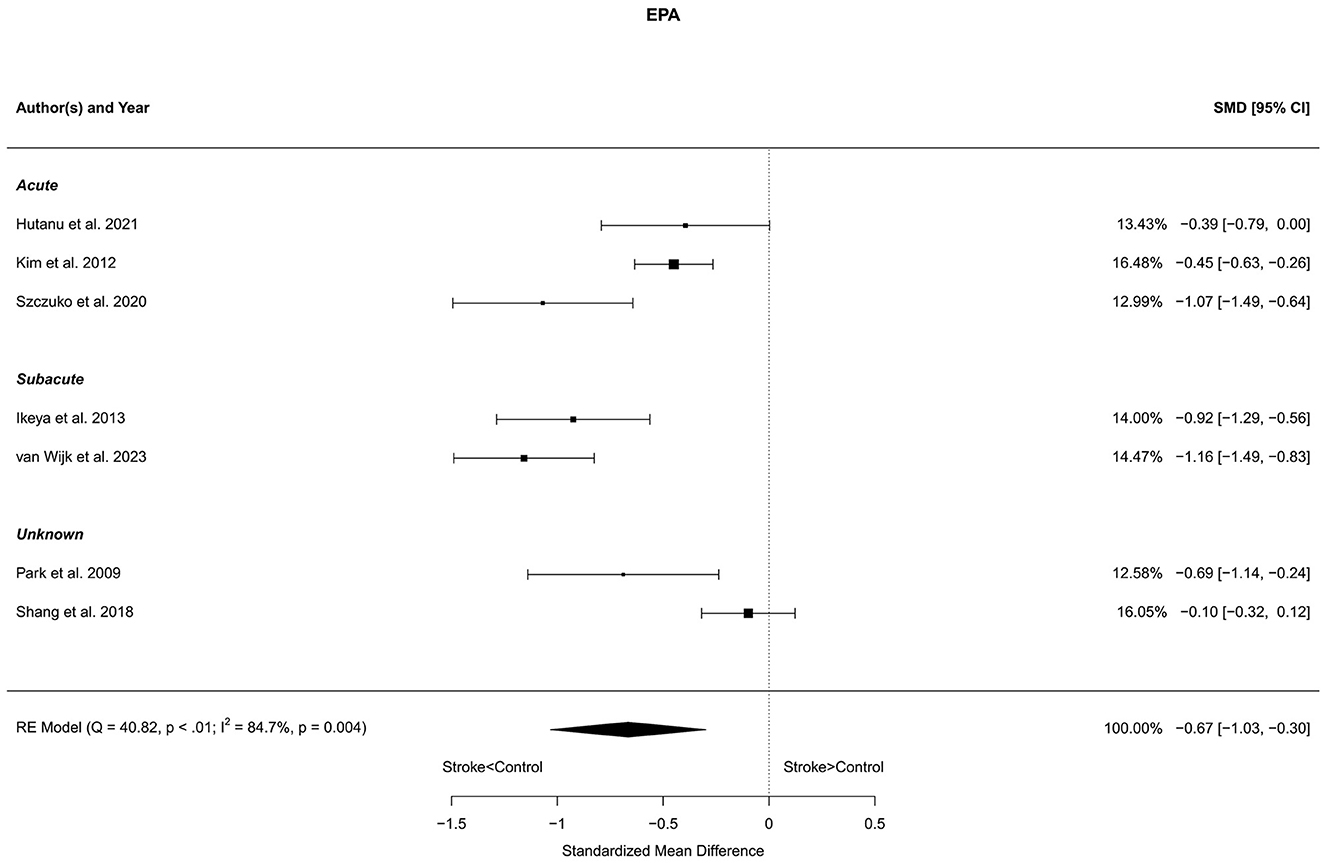
Figure 7. Comparison of blood EPA levels in stroke patients and controls. Included reports are organized in the forest plot by phase (acute—subacute—unknown) to visualize possible effects of time after stroke. Relative weights (%) and standardized mean differences (plus confidence interval) for each report are indicated at the right. Characteristics and result of the RE meta-analysis are in the lower left corner.
Six reports with a total of 631 stroke cases and 604 controls were included in the meta-analysis (Brattstrom et al., 1992; Williams et al., 2001; Kelly et al., 2003; Angelova et al., 2008; Rudreshkumar et al., 2018; van Wijk et al., 2023). The mean number of subjects was 105.2 (range: 20–180) in the stroke groups and 100.7 (range: 20–249) in the control groups. The mean age was 56.1 (range: 27.7–70.7) years for stroke cases and 53.6 (range: 27.0–68.4) years for controls. Blood levels of vitamin B6 were not different in stroke patients compared to controls; neither for the unadjusted [t(5) = −0.69, p = 0.52], nor for age-adjusted [t(4) = −0.62, p = 0.57] model (Figure 8).
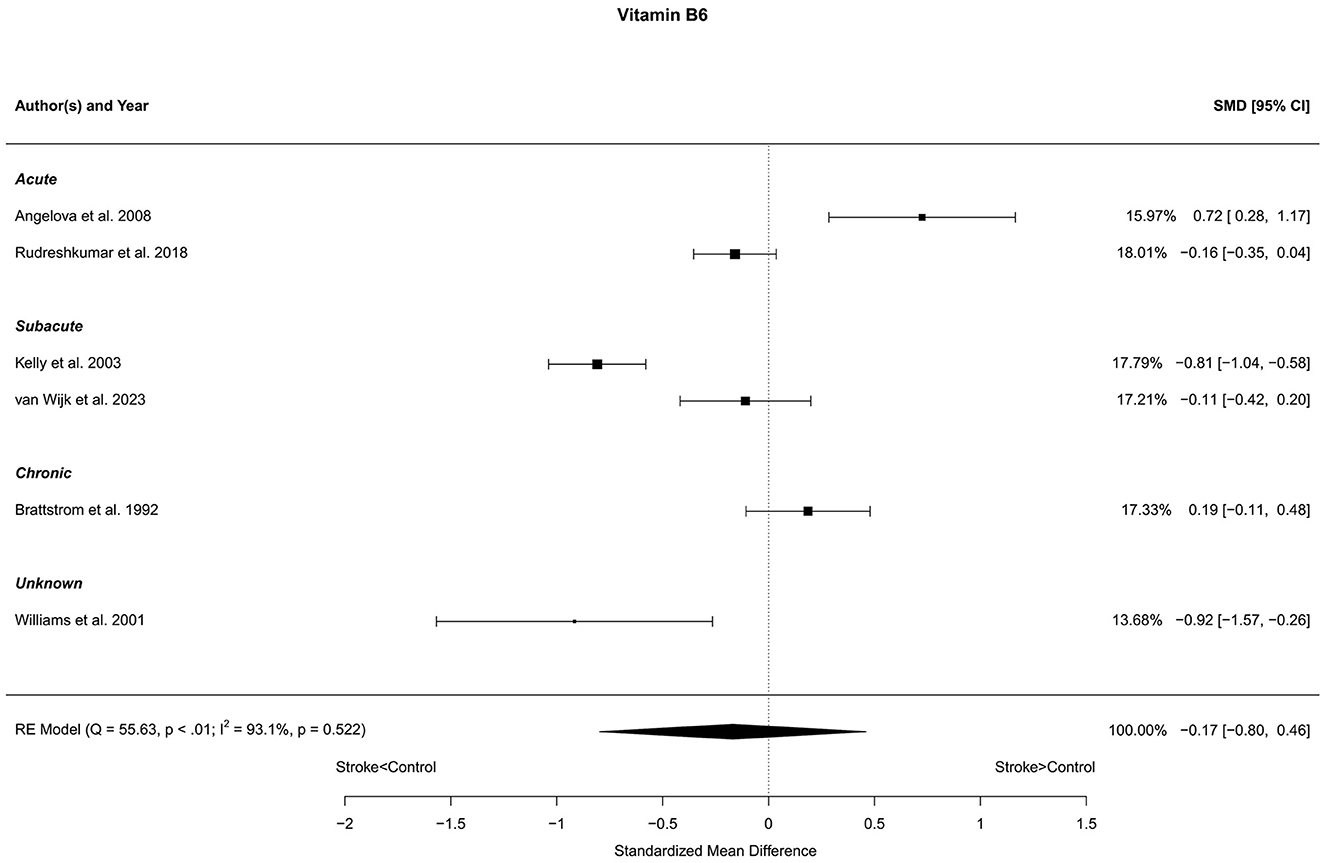
Figure 8. Comparison of blood vitamin B6 levels in stroke patients and controls. Included reports are organized in the forest plot by phase (acute—subacute—chronic—unknown) to visualize possible effects of time after stroke. Relative weights (%) and standardized mean differences (plus confidence interval) for each report are indicated at the right. Characteristics and result of the RE meta-analysis are in the lower left corner.
Six reports with a total of 248 stroke cases and 342 controls were included in the meta-analysis (Hume et al., 1982; Sharpe et al., 1994; Singh et al., 1995; Cherubini et al., 2000; El Kossi and Zakhary, 2000; Sanchez-Moreno et al., 2004). The mean number of subjects was 41.3 (range: 10–110) in the stroke groups and 57.0 (range: 12–202) in the control groups. The mean age was 65.0 (range: 52.5–77.2) years for stroke cases and 62.0 (range: 46.2–77.8) years for controls. Blood levels of vitamin C were significantly lower in stroke patients compared to controls, with statistical significance for the unadjusted [t(5) = −3.36, p = 0.020] and a trend in the age-adjusted [t(3) = −2.49, p = 0.088] model (Figure 9).

Figure 9. Comparison of blood vitamin C levels in stroke patients and controls. Included reports are all from the acute phase after stroke. Relative weights (%) and standardized mean differences (plus confidence interval) for each report are indicated at the right. Characteristics and result of the RE meta-analysis are in the lower left corner.
Six reports with a total of 581 stroke cases and 12,030 controls were included in the meta-analysis (Chang et al., 1998; Angelova et al., 2008; Hu et al., 2019; Mironczuk et al., 2021; van Wijk et al., 2023). The mean number of subjects was 96.8 (range: 36–202) in the stroke groups and 2,005 (range: 21–6,983) in the control groups. The mean age was 63.0 (range: 46.9–70.8) years for stroke cases and 52.9 (range: 46.0–63.1) years for controls. Blood levels of selenium were lower in stroke patients compared to controls, for both unadjusted [t(5) = −3.47, p = 0.018] and age-adjusted [t(4) = −3.19, p = 0.033] model (Figure 10).
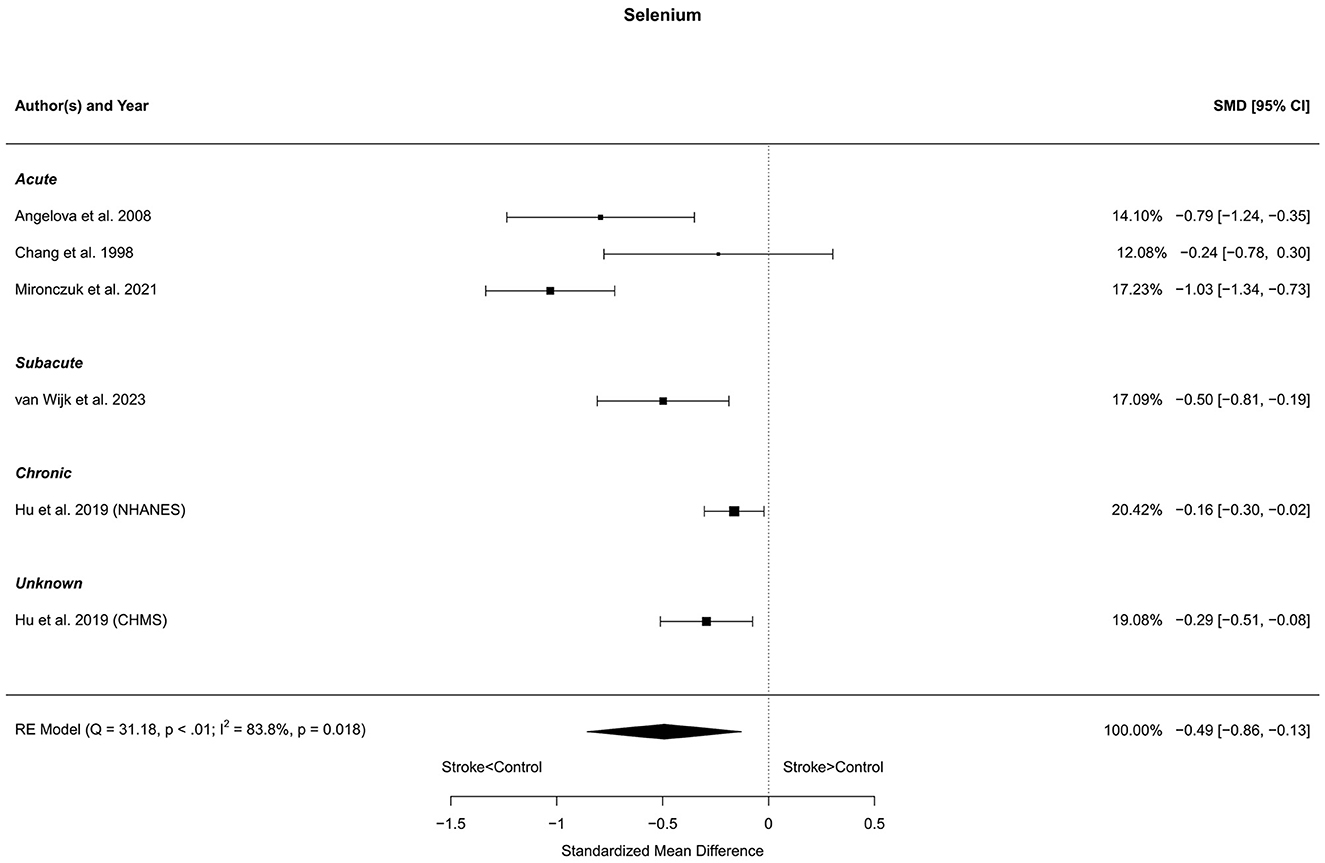
Figure 10. Comparison of blood selenium levels in stroke patients and controls. Included reports are organized in the forest plot by phase (acute—subacute—chronic—unknown) to visualize possible effects of time after stroke. Relative weights (%) and standardized mean differences (plus confidence interval) for each report are indicated at the right. Characteristics and result of the RE meta-analysis are in the lower left corner.
Four reports with a total of 358 stroke cases and 158 controls were included in the meta-analysis (Rashid et al., 2003; Hosinian et al., 2016; Szpetnar et al., 2016; Ercan et al., 2019). The mean number of subjects was 89.5 (range: 18–228) in the stroke groups and 39.5 (range: 12–60) in the control groups. The mean age was 71.0 (range: 64.0–75.3) years for stroke cases and 65.7 (range: 60.1–71.3) years for controls. Blood levels of arginine were not significantly different in stroke patients and controls; neither for unadjusted [t(3) = −0.10, p = 0.93], nor for age-adjusted [t(2) = −0.12, p = 0.92] model (Figure 11).

Figure 11. Comparison of blood arginine levels in stroke patients and controls. Included reports are all from the acute phase after stroke. Relative weights (%) and standardized mean differences (plus confidence interval) for each report are indicated at the right. Characteristics and result of the RE meta-analysis are in the lower left corner.
For five of the preselected nutrients, i.e., taurine, CoQ10, choline, carnitine, and uridine, an insufficient number of reports was found to perform the meta-analysis.
For taurine, two reports with a total of 188 stroke cases and 97 controls were selected (Castillo et al., 1996; Ghandforoush-Sattari et al., 2011). The mean number of subjects was 94.0 (range: 60–128) in the stroke groups and 48.5 (range: 43–54) in the control groups. The mean reported age was 68.0 years for stroke cases and 56.0 years for controls. In both studies blood levels of taurine in stroke patients were elevated and reached statistical significance in one out of two (p < 0.0001 vs. p = 0.170) studies.
For CoQ10, two reports with a total of 157 stroke cases and 117 controls were selected (Simani et al., 2018; van Wijk et al., 2023). The mean number of subjects was 78.5 (range: 76–81) in the stroke groups and 58.5 (range: 34–83) in the control groups. The mean age was 63.6 (range: 63.0–64.2) years for stroke cases and 62.0 (range: 60.8–63.1) years for controls. Both studies reported lower blood levels of CoQ10 in stroke patients compared to controls (p < 0.05 and p < 0.001).
For choline, two reports with a total of 145 stroke cases and 148 controls were selected (Lee et al., 2019; van Wijk et al., 2023). The mean number of subjects was 72.5 (range: 65–80) in the stroke groups and 74.0 (range: 65–83) in the control groups. The mean age was 63.7 (range: 63.2–64.2) years for stroke cases and 62.2 (range: 61.3–63.1) years for controls. Both studies reported lower blood levels of choline in stroke compared to controls (p < 0.002 and p < 0.008).
Only one report (van Wijk et al., 2023) showed that blood levels of uridine was 4.01 ± 1.28 μmol/L in 78 stroke cases and 4.17 ± 1.24 μmol/L in 82 controls (p > 0.1). The blood levels of total carnitine reported in the same study for 81 stroke cases and 82 controls were 49.2 ±13.6 and 51.3 ± 9.4 μmol/L (p > 0.1), respectively.
The main findings of our systematic review are that stroke patients have lower blood levels of many nutrients that are involved in repair processes after stroke, including folate, vitamin B12, EPA, DHA, vitamin C, vitamin E, selenium, and possibly CoQ10 and choline. These low blood levels highlight the presence of a suboptimal nutritional status after stroke. Our findings indicate that the inclusion of targeted nutritional interventions to further support recovery should receive consideration in the multidisciplinary context of stroke rehabilitation.
This systematic review shows that nutritional insufficiencies are quite common in stroke patients. From a selected group of nutrients relevant for their involvement in the response to injury and repair processes in the central nervous system, nine nutrients yielded a sufficient number of reports to be included in the meta-analysis. For seven out of these nine nutrients, the blood levels were found to be significantly lower in stroke patients compared to controls. These nutrients comprised B-vitamins (vitamin B12, folate), antioxidants (vitamin C, vitamin E, selenium), and long chain n-3 polyunsaturated fatty acids (n-3 PUFAs: DHA, EPA). In addition, there were five nutrients with an insufficient number of reports to perform the meta-analysis. For two of these nutrients, choline and CoQ10, studies predominantly reported lower blood levels in stroke patients compared to controls. For the nutrient taurine, studies reported higher blood levels in stroke patients compared to controls. Overall, the risk of bias in the selected studies was low, due to the non-randomized observational nature of the studies and the use of objective analytical assessments. If the present bias had affected the result of the meta-analysis, it is expected to have resulted in a slight moderation of the observed insufficiencies.
The majority of reports included in the present meta-analysis studied blood nutrient levels in the acute phase, i.e., within the first week after the stroke. However, we observed no clear differences between findings from the acute, subacute or chronic phases; in every phase post-stroke, lower levels are observed. Furthermore, individual findings that seemed to deviate from the observed trends could not be linked to a specific period of measurement. It is important to realize that individual nutrients may differ considerably in kinetic properties related to their uptake, storage, and secretion. Nevertheless, even blood levels of nutrients with larger storage capacities, like vitamin B12 and the n3-PUFAs EPA and DHA, were found to be low in stroke patients. Thus, the present observations suggest that specific nutrient insufficiencies are already present at the time of stroke and/or quickly develop after stroke.
The observed number of nutritional insufficiencies after stroke raises the question whether the present findings merely reflect the high prevalence of malnutrition, a reduced dietary intake, and the presence of dysphagia that is commonly observed after stroke. Indeed, a recent systematic review and meta-analysis showed that an impaired nutritional condition may already be present at the time of a stroke, but is also known to develop or to deteriorate in the months after stroke (Huppertz et al., 2021). Unfortunately, reports included in the present analysis have, in general, not provided specific information on the prevalence of dysphagia and/or malnutrition in their patient populations. It is clear that there is a need for longitudinal studies to provide more detail on the development of specific nutritional insufficiencies over time and their link to protein-energy malnutrition. Still, nutritional insufficiencies after stroke are also observed in non-dysphagic patients and even in well-nourished patients without overt signs of malnutrition (van Wijk et al., 2023), indicating that additional factors contribute to the weakened nutritional status of stroke patients. Stroke is an acute condition that may have long-term consequences; it triggers a cascade of physiological responses and, depending on the site and the size of the infarcted area, may result in long-term metabolic changes related to inactivity-related muscle wasting, wound healing and chronic inflammation (Wieloch and Nikolich, 2006; Aquilani et al., 2011; Dalise et al., 2014; Hajsl et al., 2020).
Because nutritional insufficiencies can already be observed in acute stroke patients, they could have been present before the stroke and could be the result of an interplay of pre-stroke lifestyle factors, associated comorbidities, and the use of related medication. It is well-known that lifestyle factors including diet and aerobic exercise contribute to important vascular and metabolic risk factors for stroke (Suter, 1999; Karttunen et al., 2002; He et al., 2004; Myint et al., 2008; Brouwer et al., 2021). Similarly, such lifestyle factors contribute to the presence and severity of several common comorbidities in stroke patients, including hyperhomocysteinemia, type 2 diabetes mellitus, high blood pressure, and high plasma lipids. As a consequence, polypharmacy is not uncommon in stroke patients, and the regular use of certain medication is known to influence the intake, metabolism, and excretion of nutrients (Gervasio, 2010; Wakeman and Archer, 2020). Deficiencies in the affected nutrients, e.g., vitamin B12, are likely to contribute to an exacerbation of problems, such as diabetic neuropathy (Wakeman and Archer, 2020). It therefore appears that stroke patients display nutritional insufficiencies that not only increase the risk of stroke and stroke recurrence, but also decrease the likelihood of functional recovery after stroke. There is strong preclinical evidence obtained in the transient middle cerebral artery occlusion mouse model of stroke, showing that a therapeutic dietary supplementation containing eight of the identified nutrients, i.e., DHA, EPA, vitamin C, vitamin E, selenium, vitamin B12, folic acid, and choline, was able to restore brain structure and function after ischemic stroke (Wiesmann et al., 2017). In this study, dietary supplementation decreased neuroinflammation, improved functional and structural brain connectivity, improved cerebral blood flow in the affected area, and also improved motor function in the mice.
Given the importance of lifestyle factors in the causation of stroke, changes in lifestyle are likely to support the recovery process. Adherence to healthier diets, such as the Mediterranean diet, DASH diet, or MIND diet, that would be expected to increase the dietary intake of vegetables, fruits, and fatty fish, i.e., sources of B-vitamins, antioxidants, and n-3 PUFAs, could help to maintain the improved nutritional status after recovery. Since the implementation of and the adherence to dietary changes may take considerable time and effort, targeted nutritional products or dedicated food supplements could be considered to help addressing the specific nutritional needs.
To our knowledge, this systematic review has been performed in the most appropriate way to provide a transparent and comprehensive overview of the existing evidence. Nevertheless, this study has some limitations. The preselected list of nutrients that was used for the systematic review is certainly not exhaustive. It is therefore likely that additional nutritional insufficiencies will be discovered with further searches. Historically, some nutrients have been studied more often than others, due to their involvement in stroke-relevant processes or risk factors, and were therefore more likely to be included in this review. For future studies we recommend the inclusion of other nutrients that may be involved in recovery. In addition, we note that the included reports may have studied heterogenous populations and used somewhat different control groups, which may have affected the conclusions. Given the worldwide differences in lifestyle and dietary habits, it would be interesting to investigate the influence of geographical differences in the prevalence of specific nutritional insufficiencies. The relative low number of observations per country and nutrient did not allow further analysis in this direction. Similarly, the severity of the stroke and the associated complications may have an important impact on the nutritional status of stroke patients. Unfortunately, the included reports did not provide sufficient information to assess the potential influence of this factor. Despite these limitations, all eligible literature on blood levels of the selected nutrients in stroke was considered valuable and included in the analysis of the review. More research should be carried out to further clarify changes in nutrient levels over time after stroke. Nevertheless, our results shed light on a problem that is often underestimated or even unnoticed, but is likely to be most relevant for functional recovery after stroke.
The current findings may have important consequences for how we appreciate the value of adequate nutrition and the supplementation of those nutrients that can address the condition-specific nutritional needs of patients in recovery after stroke. Current international stroke guidelines do not recommend routine administration of generic oral nutritional supplements in well-nourished stroke patients (Burgos et al., 2018; Powers et al., 2018; NICE, 2019). Based on our findings, it can be argued that the assessment of nutritional status in stroke should not be limited to the detection of protein-energy malnutrition and that tailored nutritional supplementation should be considered as a possibility in order to support optimal functional recovery after stroke.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
LB: Investigation, Visualization, Writing—original draft, Writing—review & editing, Validation. SG: Writing— original draft, Writing—review & editing, Investigation. AC-Y: Formal analysis, Methodology, Validation, Visualization, Writing—review & editing. NW: Investigation, Visualization, Writing—review & editing. AH: Conceptualization, Writing—review & editing. AM-T: Conceptualization, Writing— review & editing. ML: Conceptualization, Supervision, Writing—review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to thank Daan Snoeks for all literature searches.
LB, SG, AC-Y, NW, AH, and ML are employees of Danone Nutricia Research.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fstro.2023.1274555/full#supplementary-material
CoQ10, coenzyme Q10; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HK, Hartung Knapp; PUFAs, polyunsaturated fatty acids; RE, random-effects meta-analysis model.
Angelova, E. A., Atanassova, P. A., Chalakova, N. T., and Dimitrov, B. D. (2008). Associations between serum selenium and total plasma homocysteine during the acute phase of ischaemic stroke. Eur. Neurol. 60, 298–303. doi: 10.1159/000157884
Aquilani, R., Sessarego, P., Iadarola, P., Barbieri, A., and Boschi, F. (2011). Nutrition for brain recovery after ischemic stroke: an added value to rehabilitation. Nutr. Clin. Pract. 26, 339–345. doi: 10.1177/0884533611405793
Ashjazadeh, N., Fathi, M., and Shariat, A. (2013). Evaluation of homocysteine level as a risk factor among patients with ischemic stroke and its subtypes. Iran. J. Med. Sci. 38, 233–239.
Bernhardt, J., Hayward, K. S., Kwakkel, G., Ward, N. S., Wolf, S. L., Borschmann, K., et al. (2017). Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Recovery and Rehabilitation Roundtable taskforce. Int. J. Stroke 12, 444–450. doi: 10.1177/1747493017711816
Brattstrom, L., Lindgren, A., Israelsson, B., Malinow, M. R., Norrving, B., Upson, B., et al. (1992). Hyperhomocysteinaemia in stroke: prevalence, cause, and relationships to type of stroke and stroke risk factors. Eur. J. Clin. Invest. 22, 214–221. doi: 10.1111/j.1365-2362.1992.tb01829.x
Brouwer, R., Wondergem, R., Otten, C., and Pisters, M. F. (2021). Effect of aerobic training on vascular and metabolic risk factors for recurrent stroke: a meta-analysis. Disabil. Rehabil. 43, 2084–2091. doi: 10.1080/09638288.2019.1692251
Burgos, R., Breton, I., Cereda, E., Desport, J. C., Dziewas, R., Genton, L., et al. (2018). ESPEN guideline clinical nutrition in neurology. Clin. Nutr. 37, 354–396. doi: 10.1016/j.clnu.2017.09.003
Castillo, J., Davalos, A., Naveiro, J., and Noya, M. (1996). Neuroexcitatory amino acids and their relation to infarct size and neurological deficit in ischemic stroke. Stroke 27, 1060–1065. doi: 10.1161/01.STR.27.6.1060
Chang, C.-Y., Chen, J.-Y., Ke, D., and Hu, M.-L. (2005). Plasma levels of lipophilic antioxidant vitamins in acute ischemic stroke patients: correlation to inflammation markers and neurological deficits. Nutrition 21, 987–993. doi: 10.1016/j.nut.2005.02.010
Chang, C. Y., Lai, Y. C., Cheng, T. J., Lau, M. T., and Hu, M. L. (1998). Plasma levels of antioxidant vitamins, selenium, total sulfhydryl groups and oxidative products in ischemic-stroke patients as compared to matched controls in Taiwan. Free Radic. Res. 28, 15–24. doi: 10.3109/10715769809097872
Cheli, F., and Baldi, A. (2011). Nutrition-based health: cell-based bioassays for food antioxidant activity evaluation. J. Food Sci. 76, R197–R205. doi: 10.1111/j.1750-3841.2011.02411.x
Chen, N., Li, Y., Fang, J., Lu, Q., and He, L. (2019). Risk factors for malnutrition in stroke patients: a meta-analysis. Clin. Nutr. 38, 127–135. doi: 10.1016/j.clnu.2017.12.014
Cherubini, A., Polidori, M. C., Bregnocchi, M., Pezzuto, S., Cecchetti, R., Ingegni, T., et al. (2000). Antioxidant profile and early outcome in stroke patients. Stroke 31, 2295–2300. doi: 10.1161/01.STR.31.10.2295
Cho, Y., Kim, J. O., Jeon, Y. J., Choi, G. H., Shin, J. S., Cho, S. H., et al. (2015a). Predisposing roles of paraoxonase-1 genetic variants in Korean ischemic stroke patients. Genes Genomics 37, 579–586. doi: 10.1007/s13258-015-0287-0
Cho, Y., Kim, J. O., Lee, J. H., Park, H. M., Jeon, Y. J., Oh, S. H., et al. (2015b). Association of reduced folate carrier-1 (RFC-1) polymorphisms with ischemic stroke and silent brain infarction. PLoS ONE 10, e0115295. doi: 10.1371/journal.pone.0115295
Daga, M. K., Madhuchhanda, Mishra, TK, and Mohan A. (1997). Lipid peroxide, beta-carotene and alpha-tocopherol in ischaemic stroke and effect of exogenous vitamin E supplementation on outcome. J. Assoc. Physicians India 45, 843–846.
Dalise, S., Ambrosio, F., and Modo, M. (2014). Brain plasticity and recovery in preclinical models of stroke. Arch. Ital. Biol. 152, 190–215. doi: 10.12871/00039829201442
El Kossi, M. M., and Zakhary, M. M. (2000). Oxidative stress in the context of acute cerebrovascular stroke. Stroke 31, 1889–1892. doi: 10.1161/01.STR.31.8.1889
Ercan, M., Mungan, S., Guzel, I., Celik, H. T., Bal, C., Abusoglu, S., et al. (2019). Serum asymmetric dimethylarginine and nitric oxide levels in Turkish patients with acute ischemic stroke. Adv. Clin. Exp. Med. 28, 693–698. doi: 10.17219/acem/78360
Fang, X., Namba, H., Akamine, S., and Sugiyama, K. (2005). Methylenetetrahydrofolate reductase gene polymorphisms in patients with cerebral hemorrhage. Neurol. Res. 27, 73–76. doi: 10.1179/016164105X18313
Gariballa, S. E., Hutchin, T. P., and Sinclair, A. J. (2002). Antioxidant capacity after acute ischaemic stroke. QJM 95, 685–690. doi: 10.1093/qjmed/95.10.685
Gariballa, S. E., Parker, S. G., Taub, N., and Castleden, M. (1998). Nutritional status of hospitalized acute stroke patients. Br. J. Nutr. 79, 481–487. doi: 10.1079/BJN19980085
Gervasio, J. M. (2010). “Drug-induced changes to nutritional status,” in Handbook of Drug-Nutrient Interactions. Nutrition and Health, eds J. I. Boullata, and V. T. Armenti (New York, NY: Humana Press), 427–445. doi: 10.1007/978-1-60327-362-6_15
Ghandforoush-Sattari, M., Mashayekhi, S. O., Nemati, M., and Ayromlou, H. (2011). Changes in plasma concentration of taurine in stroke. Neurosci. Lett. 496, 172–175. doi: 10.1016/j.neulet.2011.04.010
Glew, R. H., Okolie, H., Crossey, M., Suberu, O., Trujillo, M., Pereyra, M., et al. (2004). Serum lipid profiles and homocysteine levels in adults with stroke or myocardial infarction in the town of Gombe in northern Nigeria. J. Health Popul. Nutr. 22, 341–347.
Gomes, F., Emery, P. W., and Weekes, C. E. (2016). Risk of malnutrition is an independent predictor of mortality, length of hospital stay, and hospitalization costs in stroke patients. J. Stroke Cerebrovasc. Dis. 25, 799–806. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.017
Hajsl, M., Hlavackova, A., Broulikova, K., Sramek, M., Maly, M., Dyr, J. E., et al. (2020). Tryptophan metabolism, inflammation, and oxidative stress in patients with neurovascular disease. Metabolites 10. doi: 10.3390/metabo10050208
Han, S., Guo, Y., Sun, G., and Gu, Y. (2002). Relation of plasma homocystein with folic acid and vitamine B12 in patients with cerebral infarction. Chin. J. Clin. Rehabil. 6, 2970–2971.
He, K., Merchant, A., Rimm, E. B., Rosner, B. A., Stampfer, M. J., Willett, W. C., et al. (2004). Folate, vitamin B6, and B12 intakes in relation to risk of stroke among men. Stroke 35, 169–174. doi: 10.1161/01.STR.0000106762.55994.86
Hosinian, M., Qujeq, D., and Ahmadi Ahangar, A. (2016). The relation between GABA and L-arginine levels with some stroke risk factors in acute ischemic stroke patients. Int.J. Mol. Cell. Med. 5, 100–105.
Hozo, S. P., Djulbegovic, B., and Hozo, I. (2005). Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 5, 13. doi: 10.1186/1471-2288-5-13
Hu, X. F., Stranges, S., and Chan, L. H. M. (2019). Circulating selenium concentration is inversely associated with the prevalence of stroke: results from the canadian health measures survey and the national health and nutrition examination survey. J. Am. Heart Assoc. 8, e012290. doi: 10.1161/JAHA.119.012290
Hultberg, B., Andersson, A., and Lindgren, A. (1997). Marginal folate deficiency as a possible cause of hyperhomocystinaemia in stroke patients. Eur. J. Clin. Chem. Clin. Biochem. 35, 25–28. doi: 10.1515/cclm.1997.35.1.25
Hume, R., Vallance, B. D., and Muir, M. M. (1982). Ascorbate status and fibrinogen concentrations after cerebrovascular accident. J. Clin. Pathol. 35, 195–199. doi: 10.1136/jcp.35.2.195
Huppertz, V., Guida, S., Holdoway, A., Strilciuc, S., Baijens, L., Schols, J., et al. (2021). Impaired nutritional condition after stroke from the hyperacute to the chronic phase: a systematic review and meta-analysis. Front. Neurol. 12, 780080. doi: 10.3389/fneur.2021.780080
Hutanu, A., Iancu, M., Dobreanu, M., Oprea, O. R., Barbu, S., Maier, S., et al. (2021). Extended lipid profile in Romanian ischemic stroke patients in relation to stroke severity and outcome: a path analysis model. Arch. Med. Sci. 17, 864–873. doi: 10.5114/aoms.2019.89302
Ikeya, Y., Fukuyama, N., Kitajima, W., Ogushi, Y., and Mori, H. (2013). Comparison of eicosapentaenoic acid concentrations in plasma between patients with ischemic stroke and control subjects. Nutrition 29, 127–131. doi: 10.1016/j.nut.2012.05.003
Jiang, B., Chen, Y., Yao, G., Yao, C., Zhao, H., Jia, X., et al. (2014). Effects of differences in serum total homocysteine, folate, and vitamin B12 on cognitive impairment in stroke patients. BMC Neurol. 14, 217. doi: 10.1186/s12883-014-0217-9
Kara, N., Senes, M., Coskun, O., Inan, L., Saydam, G., Yucel, D., et al. (2009). Urinary methylmalonic acid levels in patients with acute ischemic stroke. Clin. Biochem. 42, 578–583. doi: 10.1016/j.clinbiochem.2009.02.018
Karttunen, V., Alfthan, G., Hiltunen, L., Rasi, V., Kervinen, K., Kesaniemi, Y. A., et al. (2002). Risk factors for cryptogenic ischaemic stroke. Eur. J. Neurol. 9, 625–632. doi: 10.1046/j.1468-1331.2002.00464.x
Kelly, P. J., Shih, V. E., Kistler, J. P., Barron, M., Lee, H., Mandell, R., et al. (2003). Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke 34, e51–e54. doi: 10.1161/01.STR.0000071109.23410.AB
Kim, O. J., Lee, J. H., Choi, J. K., Oh, S. H., Hong, S. H., Oh, D., et al. (2010). Association between tumor necrosis factor-alpha (-308G–>A and−238G–>A) polymorphisms and homocysteine levels in patients with ischemic strokes and silent brain infarctions. Cerebrovasc. Dis. 30, 483–490. doi: 10.1159/000319023
Kim, Y. J., Kim, O. Y., Cho, Y., Chung, J. H., Jung, Y. S., Hwang, G. S., et al. (2012). Plasma phospholipid fatty acid composition in ischemic stroke: importance of docosahexaenoic acid in the risk for intracranial atherosclerotic stenosis. Atherosclerosis 225, 418–424. doi: 10.1016/j.atherosclerosis.2012.09.007
Kocer, A., Ince, N., Canbulat, C. E., and Sargin, M. (2004). Serum vitamin B12 and folic Acid levels in acute cerebral atherothrombotic infarction. Tohoku J. Exp. Med. 204, 155–161. doi: 10.1620/tjem.204.155
Kweon, O. J., Lim, Y. K., Lee, M. K., and Kim, H. R. (2021). Clinical utility of serum holotranscobalamin measurements in patients with first-ever ischemic stroke. Dis. Markers. 2021, 9914298. doi: 10.1155/2021/9914298
Lee, T. H., Cheng, M. L., Shiao, M. S., and Lin, C. N. (2019). Metabolomics study in severe extracranial carotid artery stenosis. BMC Neurol. 19, 138. doi: 10.1186/s12883-019-1371-x
Lieber, A. C., Hong, E., Putrino, D., Nistal, D. A., Pan, J. S., Kellner, C. P., et al. (2018). Nutrition, energy expenditure, dysphagia, and self-efficacy in stroke rehabilitation: a review of the literature. Brain Sci. 8. doi: 10.3390/brainsci8120218
Liu, J. G., Zhang, Z. C., Gao, H. F., Liu, H. X., and Tan, X. M. (2005). Relationship between hyperhomocysteinemia and cerebral stroke in young and middle-aged people. Chin. J. Clin. Rehabil. 9, 221–223.
Mao, H., Zhuang, P., Chen, X., Yu, Y., and Chen, B. (2008). Dynamic changes of lipid acylhydroperoxide and vitamin E plasma levels in ischemic stroke patients. Neural Regen. Res. 3, 687–689.
Martineau, J., Bauer, J. D., Isenring, E., and Cohen, S. (2005). Malnutrition determined by the patient-generated subjective global assessment is associated with poor outcomes in acute stroke patients. Clin. Nutr. 24, 1073–1077. doi: 10.1016/j.clnu.2005.08.010
Mejia Mohamed, E. H., Tan, K. S., Ali, J. M., and Mohamed, Z. T. T. (2011). genotype of the methylenetetrahydrofolate reductase C677T polymorphism is an important determinant for homocysteine levels in multi-ethnic Malaysian ischaemic stroke patients. Ann. Acad. Med. Singap. 40, 186–191. doi: 10.47102/annals-acadmedsg.V40N4p186
Mironczuk, A., Kapica-Topczewska, K., Socha, K., Soroczynska, J., Jamiolkowski, J., Kulakowska, A., et al. (2021). Selenium, copper, zinc concentrations and Cu/Zn, Cu/Se molar ratios in the serum of patients with acute ischemic stroke in Northeastern Poland-A new insight into stroke pathophysiology. Nutrients 13, 2139. doi: 10.3390/nu13072139
Moghaddasi, M., Mamarabadi, M., Mirzadeh, S., Freydoonnejad, A. A., and Razjouyan, H. (2010). Homocysteine, vitamin B12 and folate levels in Iranian patients with ischemic stroke. Neurol. Res. 32, 953–956. doi: 10.1179/016164110X12644252260475
Mohapatra, E., and Sarangi, L. (2010). Effect of supplementation of folic acid and mecobalamine in ischemic stroke patients with hyperhomocysteinemia. Pharmacology 2, 774–779.
Mojiminiyi, O. A., Marouf, R., Al Shayeb, A. R., Qurtom, M., Abdella, N. A., Al Wazzan, H., et al. (2008). Determinants and associations of homocysteine and prothrombotic risk factors in Kuwaiti patients with cerebrovascular accident. Med. Princ. Pract. 17, 136–142. doi: 10.1159/000112968
Myint, P. K., Luben, R. N., Welch, A. A., Bingham, S. A., Wareham, N. J., Khaw, K. T., et al. (2008). Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer Norfolk prospective population study. Am. J. Clin. Nutr. 87, 64–69. doi: 10.1093/ajcn/87.1.64
NICE (2019). Stroke and Transient Ischaemic Attack in Over 16s: Diagnosis and Initial Management (NG128). Available online at: wwwniceorguk/guidance/ng128 (accessed July 12, 2023).
Omrani, H. Q., Shandiz, E. E., Qabai, M., Chaman, R., Fard, H. A., Qaffarpoor, M., et al. (2011). Hyperhomocysteinemia, folateo and B12 vitamin in Iranian patients with acute ischemic stroke. ARYA Atheroscler. 7, 97–101.
Osunkalu, V., Onajole, A., Odeyemi, K., Ogunnowo, B., Sekoni, A., Ayoola, G., et al. (2010). Homocysteine and folate levels as indicators of cerebrovascular accident. J. Blood Med. 1, 131–134. doi: 10.2147/JBM.S9529
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372, n71. doi: 10.1136/bmj.n71
Park, Y., Park, S., Yi, H., Kim, H. Y., Kang, S. J., Kim, J., et al. (2009). Low level of n-3 polyunsaturated fatty acids in erythrocytes is a risk factor for both acute ischemic and hemorrhagic stroke in Koreans. Nutr. Res. 29, 825–830. doi: 10.1016/j.nutres.2009.10.018
Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., Bambakidis, N. C., Becker, K., et al. (2018). 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49, e46–e110. doi: 10.1161/STR.0000000000000158
R Core Team (2022). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://wwwR-projectorg (accessed July 12, 2023).
Rashid, P. A., Whitehurst, A., Lawson, N., and Bath, P. M. (2003). Plasma nitric oxide (nitrate/nitrite) levels in acute stroke and their relationship with severity and outcome. J. Stroke Cerebrovasc. Dis. 12, 82–87. doi: 10.1053/jscd.2003.9
Rudreshkumar, K. J., Majumdar, V., Nagaraja, D., and Christopher, R. (2018). Relevance of plasma levels of free homocysteine and methionine as risk predictors for ischemic stroke in the young. Clin. Nutr. 37, 1715–1721. doi: 10.1016/j.clnu.2017.07.005
Ruiz-Leon, A. M., Lapuente, M., Estruch, R., and Casas, R. (2019). Clinical advances in immunonutrition and atherosclerosis: a review. Front. Immunol. 10, 837. doi: 10.3389/fimmu.2019.00837
Ruskovska, T., Maksimova, V., and Milenkovic, D. (2020). Polyphenols in human nutrition: from the in vitro antioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability - an overview and perspective. Br. J. Nutr. 123, 241–254. doi: 10.1017/S0007114519002733
Sabbouh, T., and Torbey, M. T. (2018). Malnutrition in stroke patients: risk factors, assessment, and management. Neurocrit. Care 29, 374–384. doi: 10.1007/s12028-017-0436-1
Sachdev, P. S., Valenzuela, M. J., Brodaty, H., Wang, X. L., Looi, J., Lorentz, L., et al. (2003). Homocysteine as a risk factor for cognitive impairment in stroke patients. Dement. Geriatr. Cogn. Disord. 15, 155–162. doi: 10.1159/000068481
Salemi, G., Gueli, M. C., D'Amelio, M., Saia, V., Mangiapane, P., Aridon, P., et al. (2009). Blood levels of homocysteine, cysteine, glutathione, folic acid, and vitamin B12 in the acute phase of atherothrombotic stroke. Neurol. Sci. 30, 361–364. doi: 10.1007/s10072-009-0090-2
Sanchez-Moreno, C., Dashe, J. F., Scott, T., Thaler, D., Folstein, M. F., Martin, A., et al. (2004). Decreased levels of plasma vitamin C and increased concentrations of inflammatory and oxidative stress markers after stroke. Stroke 35, 163–168. doi: 10.1161/01.STR.0000105391.62306.2E
Sanderson, I. R., and Croft, N. M. (2005). The anti-inflammatory effects of enteral nutrition. JPEN J. Parenter. Enteral Nutr. 29, S134–S138. doi: 10.1177/01486071050290S4S134
Shang, J., Yamashita, T., Fukui, Y., Song, D., Li, X., Zhai, Y., et al. (2018). Different associations of plasma biomarkers in Alzheimer's disease, mild cognitive impairment, vascular dementia, and ischemic stroke. J. Clin. Neurol. 14, 29–34. doi: 10.3988/jcn.2018.14.1.29
Sharpe, P. C., Mulholland, C., and Trinick, T. (1994). Ascorbate and malondialdehyde in stroke patients. Ir. J. Med. Sci. 163, 488–491. doi: 10.1007/BF02967089
Simani, L., Ryan, F., Hashemifard, S., Hooshmandi, E., Madahi, M., Sahraei, Z., et al. (2018). Serum coenzyme Q10 is associated with clinical neurological outcomes in acute stroke patients. J. Mol. Neurosci. 66, 53–58. doi: 10.1007/s12031-018-1115-1
Singh, R. B., Niaz, M. A., Ghosh, S., Beegum, R., Bishnoi, I., Agarwal, P., et al. (1995). Dietary intake and plasma levels of antioxidant vitamins in health and disease: a hospital-based case-control study. J. Nutr. Environ. Med. 5, 235–242. doi: 10.3109/13590849509000224
Sterne, J. A., Hernan, M. A., Reeves, B. C., Savovic, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919. doi: 10.1136/bmj.i4919
Suchner, U., Kuhn, K. S., and Furst, P. (2000). The scientific basis of immunonutrition. Proc. Nutr. Soc. 59, 553–563. doi: 10.1017/S0029665100000793
Suter, P. M. (1999). The effects of potassium, magnesium, calcium, and fiber on risk of stroke. Nutr. Rev. 57, 84–88. doi: 10.1111/j.1753-4887.1999.tb06928.x
Szczuko, M., Kotlega, D., Palma, J., Zembron-Lacny, A., Tylutka, A., Golab-Janowska, M., et al. (2020). Lipoxins, RevD1 and 9, 13 HODE as the most important derivatives after an early incident of ischemic stroke. Sci. Rep. 10, 12849. doi: 10.1038/s41598-020-69831-0
Szpetnar, M., Hordyjewska, A., Malinowska, I., Golab, P., and Kurzepa, J. (2016). The fluctuation of free amino acids in serum during acute ischemic stroke. Curr. Issues Pharm. Med. Sci. 29, 151–154. doi: 10.1515/cipms-2016-0031
Taylor, R. M., Smith, R., Collins, C. E., Mossman, D., Wong-Brown, M. W., Chan, E. C., et al. (2018). Methyl-donor and cofactor nutrient intakes in the first 2-3 years and global DNA methylation at age 4: a prospective cohort study. Nutrients 10, 273. doi: 10.3390/nu10030273
Tyrovolas, S., Haro, J. M., Foscolou, A., Tyrovola, D., Mariolis, A., Bountziouka, V., et al. (2018). Anti-inflammatory nutrition and successful ageing in elderly individuals: the multinational MEDIS study. Gerontology 64, 3–10. doi: 10.1159/000479065
Urbanska, E. M., Luchowski, P., Luchowska, E., Pniewski, J., Wozniak, R., Chodakowska-Zebrowska, M., et al. (2006). Serum kynurenic acid positively correlates with cardiovascular disease risk factor, homocysteine: a study in stroke patients. Pharmacol. Rep. 58, 507–511.
van Wijk, N., Broersen, L. M., de Wilde, M. C., Hageman, R. J., Groenendijk, M., Sijben, J. W., et al. (2014). Targeting synaptic dysfunction in Alzheimer's disease by administering a specific nutrient combination. J. Alzheimers. Dis. 38, 459–479. doi: 10.3233/JAD-130998
van Wijk, N., Studer, B., van den Berg, C. A., Ripken, D., Lansink, M., Siebler, M., et al. (2023). Evident lower blood levels of multiple nutritional compounds and highly prevalent malnutrition in sub-acute stroke patients with or without dysphagia. Front. Neurol. 13, 1028991. doi: 10.3389/fneur.2022.1028991
Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. J. Statist. Softw. 36, 1–48. doi: 10.18637/jss.v036.i03
Wakeman, M., and Archer, D. T. (2020). Metformin and micronutrient status in type 2 diabetes: does polypharmacy involving acid-suppressing medications affect vitamin B12 levels? Diabetes Metab. Syndr. Obes. 13, 2093–2108. doi: 10.2147/DMSO.S237454
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135. doi: 10.1186/1471-2288-14-135
Wieloch, T., and Nikolich, K. (2006). Mechanisms of neural plasticity following brain injury. Curr. Opin. Neurobiol. 16, 258–264. doi: 10.1016/j.conb.2006.05.011
Wiesmann, M., Zinnhardt, B., Reinhardt, D., Eligehausen, S., Wachsmuth, L., Hermann, S., et al. (2017). A specific dietary intervention to restore brain structure and function after ischemic stroke. Theranostics 7, 493–512. doi: 10.7150/thno.17559
Williams, R. H., Maggiore, J. A., Reynolds, R. D., and Helgason, C. M. (2001). Novel approach for the determination of the redox status of homocysteine and other aminothiols in plasma from healthy subjects and patients with ischemic stroke. Clin. Chem. 47, 1031–1039. doi: 10.1093/clinchem/47.6.1031
Wurtman, R. J., Cansev, M., Sakamoto, T., and Ulus, I. (2010). Nutritional modifiers of aging brain function: use of uridine and other phosphatide precursors to increase formation of brain synapses. Nutr. Rev. 68(Suppl 2), S88–S101. doi: 10.1111/j.1753-4887.2010.00344.x
Keywords: stroke, malnutrition, neurorehabilitation, nutrient deficiency, blood levels
Citation: Broersen LM, Guida S, Cetinyurek-Yavuz A, van Wijk N, van Helvoort A, Michael-Titus AT and Lansink M (2023) Stroke patients have lower blood levels of nutrients that are relevant for recovery: a systematic review and meta-analysis. Front. Stroke 2:1274555. doi: 10.3389/fstro.2023.1274555
Received: 06 September 2023; Accepted: 17 November 2023;
Published: 13 December 2023.
Edited by:
Carmelo Chisari, Pisana University Hospital, ItalyReviewed by:
Rizaldy Taslim Pinzon, Duta Wacana Christian University, IndonesiaCopyright © 2023 Broersen, Guida, Cetinyurek-Yavuz, van Wijk, van Helvoort, Michael-Titus and Lansink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laus M. Broersen, bGF1cy5icm9lcnNlbkBkYW5vbmUuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.