- 1Department of Neurology, University of Iowa Hospitals and Clinics, Iowa City, IA, United States
- 2Neuroscience, Clinical Effectiveness and Public Health Research Group, Universidad Científica del Sur, Lima, Peru
- 3Facultad de Medicina, Universidad de Valparaíso, Campus San Felipe, San Felipe, Chile
- 4Laboratorio de Neuroanatomía Microquirúrgica (LaNeMic) Facultad de Medicina, Universidad de Buenos Aires, Buenos Aires, Argentina
- 5Department of Neurology, Neurosurgery and Radiology, University of Iowa Hospitals and Clinics, Iowa City, IA, United States
Medium vessel occlusions (MeVOs) account for 25%−40% of acute ischemic stroke (AIS). While mechanical thrombectomy is the standard-of-care for selected patients with large vessel occlusion (LVO), there is currently a lack of level I evidence of the safety and efficacy of endovascular treatment (EVT) for MeVOs. Several randomized clinical trials (RCTs) have attempted to answer this relevant clinical question. However, several questions related to the EVT of MeVO stroke may remain unanswered even after successful completion of these trials: What is the optimal EVT approach for secondary MeVOs? Is EVT beneficial for posterior circulation MeVOs? Is pre-EVT intravenous thrombolysis better than EVT alone? What is the optimal first line thrombectomy technique for these lesions? Are the outcome assessment tools used for LVOs appropriate for MeVOs? Upcoming evidence and the natural evolution and development of new technologies will aid in overcoming these challenges.
1. Introduction
Medium vessel occlusions (MeVOs) account for 25% to 40% of acute ischemic stroke (AIS) (Saver et al., 2020). While mechanical thrombectomy is the standard-of-care for selected patients with large vessel occlusion (LVO) (Powers et al., 2019), there is currently no high-level evidence of the safety and efficacy of endovascular treatment (EVT) for MeVOs. Recently, several meta-analyses using nonrandomized data have assessed the benefit of EVT in MeVOs, suggesting promising safety and efficacy (Barchetti et al., 2020; Waqas et al., 2021; Bilgin et al., 2022; Loh et al., 2022, 2023; Rodriguez-Calienes et al., 2023; Toh et al., 2023). Nevertheless, despite the available evidence, some questions related to the EVT of MeVO stroke remain unanswered. Overcoming these challenges during or after conclusion of the ongoing randomized control trials (RCTs) on MeVO is of relevance given that EVT for MeVOs could be a promising next step forward in AIS treatment (Goyal et al., 2020).
2. MeVO definitions
There are different definitions for MeVOs in the literature. Among the ongoing RCTs, there are some differences between the trials regarding the definition of MeVOs (Figure 1). For example, the EnDovascular Therapy Plus Best Medical Treatment (BMT) vs. BMT Alone for MedIum VeSsel Occlusion sTroke (DISTAL) trial defines them as an occlusion of the co-/non-dominant M2, the M3/M4 segment of the middle cerebral artery (MCA), the A1/A2/A3 segment of the anterior cerebral artery (ACA) or the P1/P2/P3 segment of the posterior cerebral artery (PCA). The Evaluation of Mechanical Thrombectomy in Acute Ischemic Stroke Related to a Distal Arterial Occlusion (DISCOUNT) trial identifies MeVOs as an occlusion in one the following: distal M2 mainly above the mid-height of the insula, M3 segment, the A1/A2/A3 segment of the ACA or the P1/P2/P3 segment of the PCA. On the other hand, the EndovaSCular TreAtment to imProve outcomEs for Medium Vessel Occlusions (ESCAPE-MeVO) trial defines a MeVO as an occlusion in M2, M3 segment, A2, A3, P2 or P3 segment, while the Distal Ischemic Stroke Treatment With Adjustable Low-profile Stentriever (DISTALS) trial defines it as an occlusion within the territory of the ACA segments, a non-dominant or co-dominant M2 MCA segment, an M3 MCA, or the PCA segments. The definitions, inclusion criteria and primary endpoints of MeVOs are summarized in Table 1.
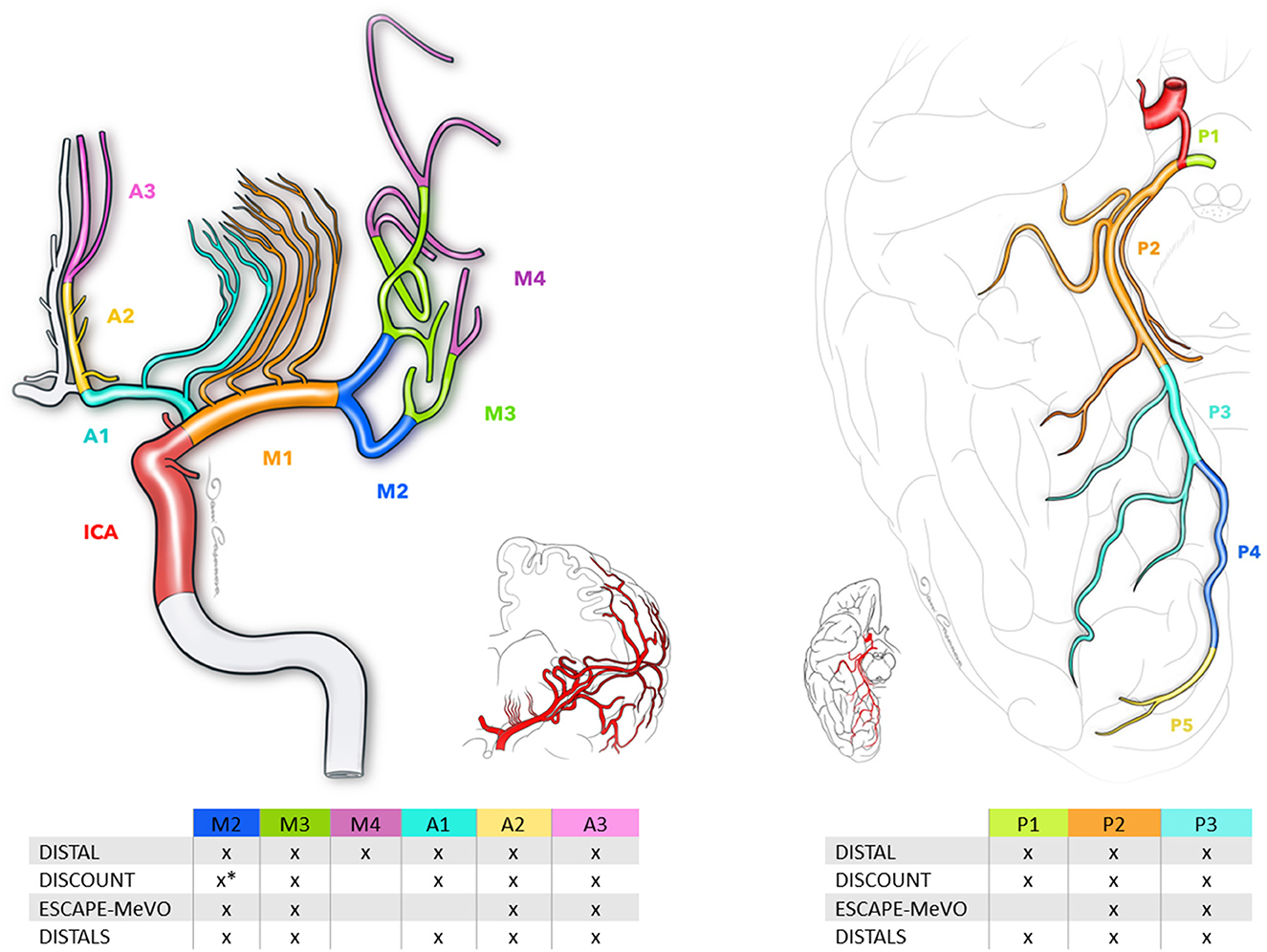
Figure 1. Schematic of medium vessel occlusions definitions, as defined by current randomized trials. The middle cerebral artery comprises three segments: M2, M3, and M4. The anterior cerebral artery consists of segments A1, A2, and A3, while the posterior cerebral artery includes segments P1, P2, and P3. *Distal portion of the M2 segment of the middle cerebral artery.
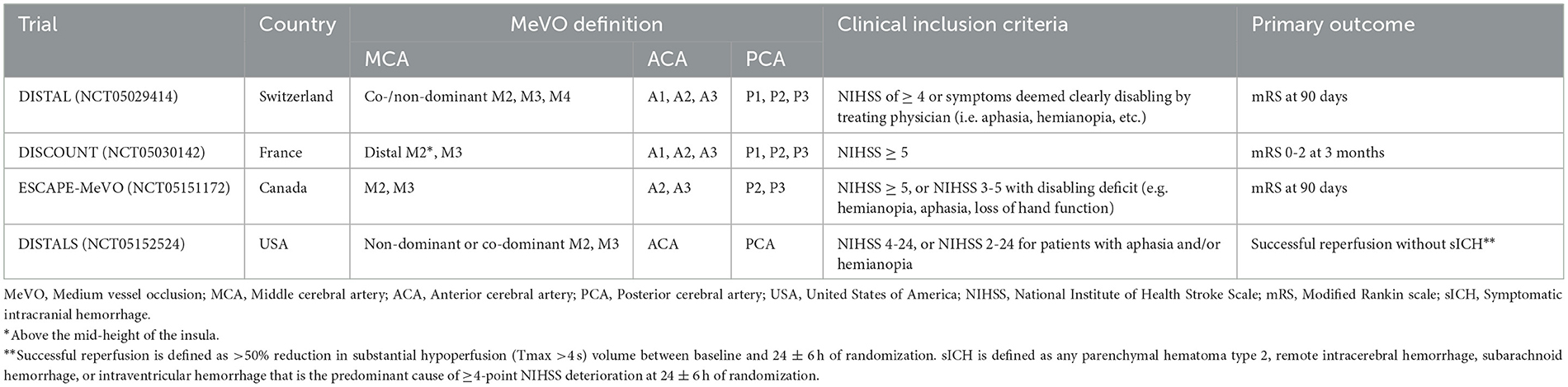
Table 1. Summary of ongoing MeVO trials including MeVO definitions, inclusion criteria, and primary study outcomes.
3. Primary and secondary medium vessel occlusions
MeVOs are not all identical and can be classified based on the underlying mechanism by which they occur (Goyal et al., 2020). Primary MeVOs arise “de novo” with underlying mechanisms very similar to LVOs. On the other hand, secondary MeVOs arise from LVOs mainly due to EVT-induced clot fragmentation or spontaneous clot migration. Secondary MeVOs that originate from more proximal occlusions can result in a larger infarct area and are associated with a worse 24-h Alberta Stroke Program Early Computed Tomography Score due to the ischemic infarct growth caused by the initial LVO (Goyal et al., 2021). Therefore, they are expected to initially present with more severe clinical presentations and with more neurological deficits (Goyal et al., 2021). Moreover, there is some evidence to support greater clot fragility in secondary spontaneous EVT MeVOs. Thus, it would be more challenging to treat secondary non-EVT MeVOs in comparison to primary MeVOs, given the increased risk of thrombus fragmentation following EVT (Goyal et al., 2021). On the contrary, EVT-related MeVOs are more often treated than primary MeVOs.
A recent systematic review and meta-analysis showed that EVT for primary and secondary MeVOs is efficient and safe (Rodriguez-Calienes et al., 2023). The majority of the available studies focused on primary MeVOs with a primary-to-secondary MeVO ratio of 3.3:1 (Rodriguez-Calienes et al., 2023). The reason is that it is challenging to enroll patients who experience secondary MeVOs, particularly those that are diagnosed during digital subtraction angiography after initial suspicion of LVO in non-invasive imaging, given the constraints of consent for randomizing a patient during an ongoing procedure (Rodriguez-Calienes et al., 2023). In addition, identifying secondary MeVOs that are not EVT-induced due to spontaneous or systemic thrombolysis may only be done following the repetition of vascular imaging prior to EVT, which is not practical and causes important treatment delays. The main features of the effects of EVT in secondary MeVOs are summarized in Table 2.
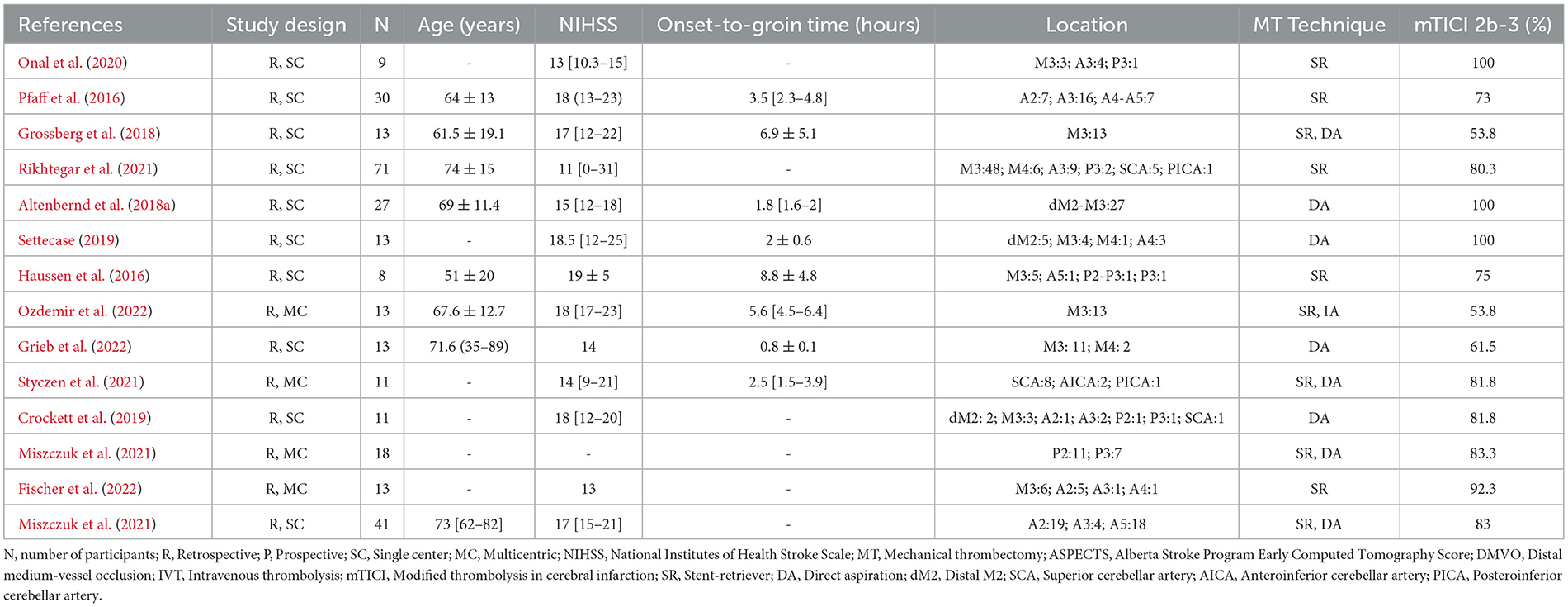
Table 2. Summary of studies on secondary medium vessel occlusion strokes treated with endovascular thrombectomy.
4. Presentation of posterior circulation occlusions
The presentation of isolated posterior circulation MeVOs may be vague, leading to late admission and consequent ineligibility to thrombolytic treatment, especially in patients with mild deficits (Sommer et al., 2017). Of note, some of the important eloquent brain regions, including the primary visual cortex and the thalami, can be affected by the lack of blood supply due to posterior circulation MeVOs, which can result in severe and detrimental effects on quality of life (Schmahmann, 2003; Ryan et al., 2010; Sand et al., 2016). Also, the use of the National Institutes of Health Stroke Scale (NIHSS) score for posterior circulation MeVOs is not representative and can result in lower NIHSS cutoff values as compared to anterior circulation strokes (Sato et al., 2008). As a result, the quest for more efficacious and safe clinical evaluation modalities for posterior circulation MeVOs is still ongoing.
Unlike anterior circulation MeVOs, there is limited evidence related to the efficacy of EVT for posterior circulation MeVOs. Moreover, EVT is usually avoided in patients who present with mild deficits and are eligible for thrombolytic treatment (Seners et al., 2020). A multicenter case-control study by Meyer et al. revealed promising results with successful revascularization rates of 87.4% and symptomatic intracranial hemorrhage (sICH) rates of 4% (Meyer et al., 2021). In addition, a recent meta-analysis performed by our group that included distal MeVOs (DMVOs) in the P2-P5 vascular territory showed high rates of successful revascularization (81%) and lower sICH rates (3%) (Rodriguez-Calienes et al., 2023). Although these results are encouraging, they are suggestive rather than conclusive, and there is still a need for larger RCTs for further investigation.
5. Thrombectomy with or without intravenous thrombolysis
The role of intravenous thrombolysis (IVT) in LVOs in patients eligible for mechanical thrombectomy is a subject of debate. Theoretically, adding IVT may contribute to achieving early reperfusion of the ischemic territory before EVT (Desilles et al., 2015; Seners et al., 2016; Tsivgoulis et al., 2018; Ospel et al., 2021), increasing reperfusion rates with fewer recanalization attempts (Fischer et al., 2018), and may improve outcomes in patients with failed thrombectomy reperfusion attempts (Rozes et al., 2022). However, the theoretical risk of distal clot embolization (Ohara et al., 2021) and intracranial hemorrhage, the potential delays for arterial puncture, and the elevated cost are considerable disadvantages (Fischer et al., 2017; Ospel et al., 2022).
Regarding MeVOs, a multinational survey showed that more than 50% of physicians would perform EVT alone in M3, A2, and P2 occlusions if the patient is ineligible for IVT; however, if the patient is eligible for IVT, 40% would offer EVT in the A2 and P2 scenarios but only 18% would offer EVT in the M3 scenario (Kappelhof et al., 2022). This reflects that the willingness to use IVT in combination with EVT for MeVOs is low. Interestingly, pharmacologic fibrinolysis is more effective for the smaller clot burden of MeVOs than the large clot burdens of LVOs (Kim et al., 2015; Yoo et al., 2018); however, IVT alone recanalizes only one-third to one-half of visualized thrombi (Saver et al., 2020). In addition, since IVT may impact the risk of intraprocedural clot fragmentation, it is possible that the fear of causing an IVT-induced secondary MeVO may be considered as a reason to withhold IVT before EVT (Goyal et al., 2021). Moreover, the subtle and diverse clinical syndromes observed with MeVO stroke may contribute to delayed presentation times, which make these patients ineligible for IVT (Saver et al., 2020).
To date, no comparative analysis between EVT alone vs. IVT with EVT in MeVOs has been reported but some retrospective cohorts suggest that IVT before EVT can achieve high reperfusion rates with no risk of hemorrhage. In the study by Altenbernd et al., pre-EVT IVT was used in 91.4% of M2 and M3 occlusions. Successful reperfusion (modified thrombolysis in cerebral infarction [mTICI] 2b-3) was observed in 100% and complete reperfusion (mTICI 3) in 82.8%, while only 3.4% presented sICH (Altenbernd et al., 2018b). Similarly, in the study by Castro-Afonso et al., successful reperfusion (mTICI 2b-3) was observed in 89% of M2 occlusions treated with IVT before EVT (De Castro Afonso et al., 2019). In addition, Styczen et al. reported a successful reperfusion rate of 90% in a cohort of posterior inferior cerebellar artery, anterior inferior cerebellar artery, and superior cerebellar artery occlusions treated with IVT before EVT (Styczen et al., 2021).
6. Combined vs. single-device techniques
The recent introduction of new generation small caliber catheters and low-profile stent retrievers (SR) has allowed access to the sites of distal occlusions (Saver et al., 2020); however, the optimal specific endovascular technique for MeVOs remains unknown. A few years ago, the use of a primary combined approach (SR with direct aspiration [DA]) and advancing the system in a tri-axial manner was troublesome due the insufficient catheter length and diameter discrepancies (Ospel and Goyal, 2021). Thus, single-device thrombectomy approaches (SR or DA) were traditionally used for this subgroup of patients. The meta-analysis of DMVOs performed by our group found higher rates of successful reperfusion (mTICI 2b-3) and favorable functional outcomes with DA techniques compared to SR techniques (Rodriguez-Calienes et al., 2023). In addition, the pooled rates of sICH and 90-day mortality were lower in the DA group (Rodriguez-Calienes et al., 2023). On the other hand, the meta-analysis of proximal and DMVOs by Loh et al. found higher odds of functional independence and lower odds of mortality in the SR/primary combined group compared to DA alone group, while reperfusion and sICH rates were similar between the two groups (Toh et al., 2023). Nevertheless, when they compared SR alone with DA alone, there were no differences in the odds of functional independence, sICH, or mortality (Toh et al., 2023). Therefore, we can infer that for certain outcomes the superior effect observed in the SR/primary combined group compared to the DA group may be primarily influenced by the subpopulation of the combined approach.
Compared to single-device approaches, combined techniques can ensure the capture of thromboembolic clots from both sides via a SR inserted distally and an aspiration catheter placed proximally, thus enhancing clot removal (Massari et al., 2016; McTaggart et al., 2017; Maus et al., 2018). In addition, the “pinning technique” (deployment of a SR through an intermediate catheter engaging the clot while exerting local aspiration) minimizes deformation of the tortuous distal vessels (Yoo and Andersson, 2017). Thus, a combined approach can provide advantages in the treatment of MeVOs by minimizing the risk of distal clot embolization and device-withdrawal risks for subarachnoid hemorrhage caused by small vessel size and tortuosity (Haussen et al., 2020; Pérez-Garciá et al., 2020).
Recently, several studies have suggested the superiority of the combined approach vs. the single-technique approach. The meta-analysis of DMVOs by Loh et al. compared first-line combined techniques with single-device techniques and found higher odds of reperfusion at first pass and lower odds of sICH with the combined approach, but no differences in final reperfusion, functional independence, or mortality (Loh et al., 2023). Similarly, the systematic review by Biling et al. described higher rates of successful reperfusion and functional independence with a combined treatment technique compared to DA or SR alone (Bilgin et al., 2022). Finally, despite the limitations of the current generation of thrombectomy devices in treating MeVOs, advances in technology and techniques may result in new tools specifically suited for MeVOs, which will allow the identification of an optimal endovascular technique to achieve better outcomes.
7. Outcomes assessment
Given that the occlusion location is more distal in MeVOs and that these MeVOs usually result in a smaller ischemic area, the outcomes would be expected to be better in MeVOs as compared to LVO strokes (Ospel and Goyal, 2021). Nonetheless, the results of the INTERRSeCT and PRove-IT trials revealed that almost one out of four MeVO patients do not attain functional independence with the standard treatment. Additionally, only half of the patients with MeVOs end up with an excellent outcome (Ospel et al., 2020). Therefore, the use of EVT in MeVOs might be plausible. The use of EVT has gained a lot of attention lately and was shown to be associated with decent efficacy and safety (Rodriguez-Calienes et al., 2023). The evidence is still in its infancy though, given the lack of randomized trials to prove this. To better evaluate the efficacy of EVT for MeVOs, especially since the outcomes of MeVOs are expected to be milder than LVO, it is more justified to use more restrictive outcomes and outcomes that are tailored to MeVOs. Instead of using good outcomes (i.e. modified Rankin scale [mRS 0-2]), which were adopted by the majority of the studies in the literature, it appears more reasonable to opt for excellent outcomes (i.e. mRS 0-1) or shift analysis (Ospel and Goyal, 2021). Despite that, the mRS and NIHSS are still not fully representative of MeVOs and, thus, do not grasp the whole clinical picture of the patient. Clinical deficits including isolated abulia, alexia and agraphia are not caught in these prior scorings, which really questions the suitability of these scoring systems for MeVOs, and calls for studies to develop outcomes that are tailored to MeVOs (Ospel and Goyal, 2021). Similarly, angiographic outcomes using the TICI score are not deemed reflective for MeVOs as the majority of patients have TICI 2b at baseline (Ospel and Goyal, 2021). As a result, there is a need to develop a comprehensive angiographic scoring system for MeVOs.
8. Future directions
Although the clinical syndromes associated with MeVOs are heterogenous and fractionated, the natural history of the ischemic lesions they cause is poor and frequently disabling. The safety and efficacy profile of EVT is favored by the continual evolution and development of imaging technologies, devices, and techniques. However, the development and acceptance of new outcome measurement tools are needed to objectively quantify the safety and efficacy of performing EVT in these lesions. Currently, there are 4 ongoing RCTs on primary MeVO (Table 1): (1) NCT05029414, DISTAL is a multicenter, parallel assignment, open-label, superiority trial based in Europe that expects to enroll 526 patients by December 2024, and in which all EVT techniques are allowed; (2) NCT05030142, DISCOUNT is a multicenter, parallel assignment, open-label trial based in France that aims to enroll 488 participants by February 2024; EVT will be performed with a specific selection of SRs; (3) NCT05151172 ESCAPE-MeVO is a multicenter, open-label trail based in Canada that aims to enroll 530 participants by December 2025; all the first attempts of EVT will be performed with the Solitaire X (Medtronic, USA) SR; and (4) NCT05152524, DISTALS is an international (United States and Europe) multicenter, open-label trial that aims to enroll 168 participants by January 2024, and in which all EVTs must be performed with Tigertriever 13 (Rapid Medical, Yoqneam, Israel).
While we await the results of the current ongoing clinical trials, several interventionalists are already routinely treating primary and secondary MeVOs. Hopefully, the randomized results provide additional evidence to standardize the best selection of imaging protocols, treatment indication criteria, and techniques that favor the best clinical outcomes.
Author contributions
AR-C, JV-S, and MD wrote the sections of the manuscript. DC designed the illustrations. MG-C and MF contributed to manuscript revision. SO-G contributed to concept and design of study and approved the submitted version. All authors contributed to the article and approved the submitted version.
Conflict of interest
SO-G has previously received grants from NIH-NINDS (R01NS127114-01), Stryker, Medtronic, Microvention, Methinks, IschemiaView, Viz.ai, and Siemens. They have received consulting fees from Medtronic, Stryker Neurovascular.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors SO-G and MF declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Altenbernd, J., Kuhnt, O., Hennigs, S., Hilker, R., and Loehr, C. (2018a). Frontline ADAPT therapy to treat patients with symptomatic M2 and M3 occlusions in acute ischemic stroke: initial experience with the Penumbra ACE and 3MAX reperfusion system. J. Neurointerv. Surg. 10, 434–439. doi: 10.1136/neurintsurg-2017-013233
Altenbernd, J., Kuhnt, O., Hennigs, S., Hilker, R., and Loehr, C. (2018b). Frontline ADAPT therapy to treat patients with symptomatic M2 and M3 occlusions in acute ischemic stroke: Initial experience with the Penumbra ACE and 3MAX reperfusion system. J. Neurointerv. Surg. 10, 435–440. doi: 10.1136/neurintsurg-2017-SNIS.9
Barchetti, G., Cagnazzo, F., Raz, E., Barbagallo, G., Toccaceli, G., and Peschillo, S. (2020). Mechanical thrombectomy of distal occlusions using a direct aspiration first pass technique compared with new generation of mini-0.017 microcatheter compatible-stent retrievers: a meta-analysis. World Neurosurg. 134, 111–119. doi: 10.1016/j.wneu.2019.10.030
Bilgin, C., Hardy, N., Hutchison, K., Pederson, J. M., Mebane, A., Olaniran, P., et al. (2022). First-line thrombectomy strategy for distal and medium vessel occlusions: a systematic review. J. Neurointerv. Surg, 15, 558–565. doi: 10.1136/neurintsurg-2022-SNIS.363
Crockett, M. T., Phillips, T. J., and Chiu, A. H. Y. (2019). Dual suction Headway27 microcatheter thrombectomy for the treatment of distal intracranial arterial occlusion strokes: initial experience with the micro-ADAPT technique. J. Neurointerv. Surg. 11, 714–718. doi: 10.1136/neurintsurg-2018-014385
De Castro Afonso, L. H., Borghini Pazuello, G., Seizem Nakiri, G., Monsignore, L. M., Antunes Dias, F., Pontes-Neto, O. M., et al. (2019). Thrombectomy for M2 occlusions and the role of the dominant branch. Interv. Neuroradiol. 25, 697–704. doi: 10.1177/1591019919847693
Desilles, J. P., Loyau, S., Syvannarath, V., Gonzalez-Valcarcel, J., Cantier, M., Louedec, L., et al. (2015). Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke 46, 3241–3248. doi: 10.1161/STROKEAHA.115.010721
Fischer, S., Will, L., Phung, T., Weber, W., Maus, V., and Nordmeyer, H. (2022). The Tigertriever 13 for mechanical thrombectomy in distal and medium intracranial vessel occlusions. Neuroradiology 64, 775–783. doi: 10.1007/s00234-021-02792-x
Fischer, U., Kaesmacher, J., Mendes Pereira, V., Chapot, R., Siddiqui, A. H., Froehler, M. T., et al. (2017). Direct mechanical thrombectomy vs. combined intravenous and mechanical thrombectomy in large-artery anterior circulation stroke: a topical review. Stroke 48, 2912–2918. doi: 10.1161/STROKEAHA.117.017208
Fischer, U., Kaesmacher, J., Molina, C. A., Selim, M. H., Alexandrov, A. V., and Tsivgoulis, G. (2018). Primary thrombectomy in tPA (tissue-type plasminogen activator) eligible stroke patients with proximal intracranial occlusions. Stroke 49, 265–269. doi: 10.1161/STROKEAHA.117.018564
Goyal, M., Kappelhof, M., Mcdonough, R., and Ospel, J. M. (2021). Secondary medium vessel occlusions: when clots move north. Stroke 52, 1147–1153. doi: 10.1161/STROKEAHA.120.032799
Goyal, M., Ospel, J. M., Menon, B. K., and Hill, M. D. (2020). MeVO: the next frontier? J. Neurointerv. Surg. 12, 545–547. doi: 10.1136/neurintsurg-2020-015807
Grieb, D., Greling, B., Schulz, K., Boxberg, F., Melber, K., Abu-Fares, O., et al. (2022). Endovascular treatment of distal medium vessel occlusions using microcatheter aspiration thrombectomy. Interv. Neuroradiol. 15910199221133470. doi: 10.1177/15910199221133470
Grossberg, J. A., Rebello, L. C., Haussen, D. C., Bouslama, M., Bowen, M., Barreira, C. M., et al. (2018). Beyond large vessel occlusion strokes: distal occlusion thrombectomy. Stroke 49, 1662–1668. doi: 10.1161/STROKEAHA.118.020567
Haussen, D. C., Al-Bayati, A. R., Eby, B., Ravindran, K., Rodrigues, G. M., Frankel, M. R., et al. (2020). Blind exchange with mini-pinning technique for distal occlusion thrombectomy. J. Neurointerv. Surg. 12, 392–395. doi: 10.1136/neurintsurg-2019-015205
Haussen, D. C., Lima, A., and Nogueira, R. G. (2016). The Trevo XP 3x20 mm retriever ('Baby Trevo') for the treatment of distal intracranial occlusions. J. Neurointerv. Surg. 8, 295–299. doi: 10.1136/neurintsurg-2014-011613
Kappelhof, M., Ospel, J., Kashani, N., Cimflova, P., Singh, N., Almekhlafi, M. A., et al. (2022). Influence of intravenous alteplase on endovascular treatment decision-making in acute ischemic stroke due to primary medium-vessel occlusion: a case-based survey study. J. Neurointerv. Surg. 14, 439–443. doi: 10.1136/neurintsurg-2021-017471
Kim, Y. D., Nam, H. S., Kim, S. H., Kim, E. Y., Song, D., Kwon, I., et al. (2015). Time-dependent thrombus resolution after tissue-type plasminogen activator in patients with stroke and mice. Stroke 46, 1877–1882. doi: 10.1161/STROKEAHA.114.008247
Loh, E., Kwok, G., Toh, K., Koh, M. Y., Teo, Y. H., Teo, Y. N., et al. (2023). Thrombectomy for distal medium vessel occlusion stroke: combined vs. single-device techniques - a systematic review and meta-analysis. Front. Stroke 2. doi: 10.3389/fstro.2023.1126130
Loh, E. D. W., Toh, K. Z. X., Kwok, G. Y. R., Teo, Y. H., Teo, Y. N., Goh, C., et al. (2022). Endovascular therapy for acute ischemic stroke with distal medium vessel occlusion: a systematic review and meta-analysis. J. Neurointerv. Surg. doi: 10.1136/jnis-2022-019717. [Epub ahead of print].
Massari, F., Henninger, N., Lozano, J. D., Patel, A., Kuhn, A. L., Howk, M., et al. (2016). ARTS (Aspiration-Retriever Technique for Stroke): initial clinical experience. Interv. Neuroradiol. 22, 325–332. doi: 10.1177/1591019916632369
Maus, V., Behme, D., Kabbasch, C., Borggrefe, J., Tsogkas, I., Nikoubashman, O., et al. (2018). Maximizing first-pass complete reperfusion with SAVE. Clin. Neuroradiol. 28, 327–338. doi: 10.1007/s00062-017-0566-z
McTaggart, R. A., Tung, E. L., Yaghi, S., Cutting, S. M., Hemendinger, M., Gale, H. I., et al. (2017). Continuous aspiration prior to intracranial vascular embolectomy (CAPTIVE): a technique which improves outcomes. J. Neurointerv. Surg. 9, 1154–1159. doi: 10.1136/neurintsurg-2016-012838
Meyer, L., Stracke, C. P., Jungi, N., Wallocha, M., Broocks, G., Sporns, P. B., et al. (2021). Thrombectomy for primary distal posterior cerebral artery occlusion stroke: the TOPMOST study. JAMA Neurol. 78, 434–444. doi: 10.1001/jamaneurol.2021.0001
Miszczuk, M., Bauknecht, H. C., Kleine, J. F., Kabbasch, C., Liebig, T., Bohner, G., et al. (2021). Mechanical thrombectomy of acute distal posterior cerebral artery occlusions. J. Clin. Neurosci. 88, 57–62. doi: 10.1016/j.jocn.2021.03.027
Ohara, T., Menon, B. K., Al-Ajlan, F. S., Horn, M., Najm, M., Al-Sultan, A., et al. (2021). Thrombus migration and fragmentation after intravenous alteplase treatment. Stroke 52, 203–212. doi: 10.1161/STROKEAHA.120.029292
Onal, Y., Velioglu, M., Demir, U., Celikoglu, E., and Karakas, H. M. (2020). Feasibility of distal mechanical thrombectomy in M3, A3 and P3 segments via a 0.013-inch delivery system: preliminary experience. Turk. Neurosurg. 30, 614–620. doi: 10.5137/1019-5149.JTN.30083-20.2
Ospel, J. M., and Goyal, M. (2021). A review of endovascular treatment for medium vessel occlusion stroke. J. Neurointerv. Surg. 13, 623–630. doi: 10.1136/neurintsurg-2021-017321
Ospel, J. M., Mcdonough, R., Kunz, W. G., and Goyal, M. (2022). Is concurrent intravenous alteplase in patients undergoing endovascular treatment for large vessel occlusion stroke cost-effective even if the cost of alteplase is only US$1? J. Neurointerv. Surg. 14, 568–572. doi: 10.1136/neurintsurg-2021-017817
Ospel, J. M., Menon, B. K., Demchuk, A. M., Almekhlafi, M. A., Kashani, N., Mayank, A., et al. (2020). Clinical course of acute ischemic stroke due to medium vessel occlusion with and without intravenous alteplase treatment. Stroke 51, 3232–3240. doi: 10.1161/STROKEAHA.120.030227
Ospel, J. M., Singh, N., Almekhlafi, M. A., Menon, B. K., Butt, A., Poppe, A. Y., et al. (2021). Early Recanalization with alteplase in stroke because of large vessel occlusion in the ESCAPE trial. Stroke 52, 304–307. doi: 10.1161/STROKEAHA.120.031591
Ozdemir, G., Eren, A., Aygul, R., Eren, F., Kizildag, N., Kocaturk, I., et al. (2022). Endovascular treatment for M3 occlusions. Interv. Neuroradiol. 15910199221127357. doi: 10.1177/15910199221127357
Pérez-Garciá, C., Moreu, M., Rosati, S., Simal, P., Egido, J. A., Gomez-Escalonilla, C., et al. (2020). Mechanical thrombectomy in medium vessel occlusions: blind exchange with mini-pinning technique vs. mini stent retriever alone. Stroke 3224–3231. doi: 10.1161/STROKEAHA.120.030815
Pfaff, J., Herweh, C., Pham, M., Schieber, S., Ringleb, P. A., Bendszus, M., et al. (2016). Mechanical thrombectomy of distal occlusions in the anterior cerebral artery: recanalization rates, periprocedural complications, and clinical outcome. AJNR Am. J. Neuroradiol. 37, 673–678. doi: 10.3174/ajnr.A4594
Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., Bambakidis, N. C., Becker, K., et al. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 50, e344–e418. doi: 10.1161/STR.0000000000000211
Rikhtegar, R., Mosimann, P. J., Weber, R., Wallocha, M., Yamac, E., Mirza-Aghazadeh-Attari, M., et al. (2021). Effectiveness of very low profile thrombectomy device in primary distal medium vessel occlusion, as rescue therapy after incomplete proximal recanalization or following iatrogenic thromboembolic events. J. Neurointerv. Surg. 13, 1067–1072. doi: 10.1136/neurintsurg-2020-017035
Rodriguez-Calienes, A., Vivanco-Suarez, J., Sequeiros, J. M., Galecio-Castillo, M., Zevallos, C. B., Farooqui, M., et al. (2023). Mechanical thrombectomy for the treatment of primary and secondary distal medium-vessel occlusion stroke: systematic review and meta-analysis. J. Neurointerv. Surg. doi: 10.1136/jnis-2022-019975
Rozes, C., Maier, B., Gory, B., Bourcier, R., Kyheng, M., Labreuche, J., et al. (2022). Influence of prior intravenous thrombolysis on outcome after failed mechanical thrombectomy: ETIS registry analysis. J. Neurointerv. Surg. 14, 688–692. doi: 10.1136/neurintsurg-2021-017867
Ryan, D., Murphy, S. M., and Hennessey, M. J. (2010). Bilateral posterior cerebral artery infarction. BMJ Case Rep. 2010. doi: 10.1136/bcr.03.2010.2798
Sand, K. M., Wilhelmsen, G., Naess, H., Midelfart, A., Thomassen, L., and Hoff, J. M. (2016). Vision problems in ischaemic stroke patients: effects on life quality and disability. Eur. J. Neurol. 23, 1–7. doi: 10.1111/ene.12848
Sato, S., Toyoda, K., Uehara, T., Toratani, N., Yokota, C., Moriwaki, H., et al. (2008). Baseline NIH Stroke Scale Score predicting outcome in anterior and posterior circulation strokes. Neurology 70, 2371–2377. doi: 10.1212/01.wnl.0000304346.14354.0b
Saver, J. L., Chapot, R., Agid, R., Hassan, A., Jadhav, A. P., Liebeskind, D. S., et al. (2020). Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke 51, 2872–2884. doi: 10.1161/STROKEAHA.120.028956
Schmahmann, J. D. (2003). Vascular syndromes of the thalamus. Stroke 34, 2264–2278. doi: 10.1161/01.STR.0000087786.38997.9E
Seners, P., Perrin, C., Lapergue, B., Henon, H., Debiais, S., Sablot, D., et al. (2020). Bridging therapy or IV thrombolysis in minor stroke with large vessel occlusion. Ann. Neurol. 88, 160–169. doi: 10.1002/ana.25756
Seners, P., Turc, G., Maïer, B., Mas, J. L., Oppenheim, C., and Baron, J. C. (2016). Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta-analysis. Stroke 47, 2409–2412. doi: 10.1161/STROKEAHA.116.014181
Settecase, F. (2019). 3MAX catheter for thromboaspiration of downstream and new territory emboli after mechanical thrombectomy of large vessel occlusions: initial experience. Interv. Neuroradiol. 25, 277–284. doi: 10.1177/1591019918811802
Sommer, P., Seyfang, L., Posekany, A., Ferrari, J., Lang, W., Fertl, E., et al. (2017). Prehospital and intra-hospital time delays in posterior circulation stroke: results from the Austrian Stroke Unit Registry. J. Neurol. 264, 131–138. doi: 10.1007/s00415-016-8330-x
Styczen, H., Fischer, S., Yeo, L. L., Yong-Qiang Tan, B., Maurer, C. J., Berlis, A., et al. (2021). Approaching the boundaries of endovascular treatment in acute ischemic stroke: multicenter experience with mechanical thrombectomy in vertebrobasilar artery branch occlusions. Clin. Neuroradiol. 31, 791–798. doi: 10.1007/s00062-020-00970-7
Toh, K. Z. X., Koh, M. Y., Loh, E. D. W., Kwok, G. Y. R., Teo, Y. H., Teo, Y. N., et al. (2023). Distal medium vessel occlusions in acute ischaemic stroke–stent retriever vs. direct aspiration: A systematic review and meta-analysis. Eur. Stroke J. 8, 434–447. doi: 10.1177/23969873231151262
Tsivgoulis, G., Katsanos, A. H., Schellinger, P. D., Köhrmann, M., Varelas, P., Magoufis, G., et al. (2018). Successful reperfusion with intravenous thrombolysis preceding mechanical thrombectomy in large-vessel occlusions. Stroke 49, 232–235. doi: 10.1161/STROKEAHA.117.019261
Waqas, M., Kuo, C. C., Dossani, R. H., Monteiro, A., Baig, A. A., Alkhaldi, M., et al. (2021). Mechanical thrombectomy vs. intravenous thrombolysis for distal large-vessel occlusion: a systematic review and meta-analysis of observational studies. Neurosurg. Focus 51, E5. doi: 10.3171/2021.4.FOCUS21139
Yoo, A. J., and Andersson, T. (2017). Thrombectomy in acute ischemic stroke: challenges to procedural success. J Stroke 19, 121–130. doi: 10.5853/jos.2017.00752
Keywords: stroke, endovascular, thrombectomy, medium vessel occlusion, MeVO
Citation: Rodriguez-Calienes A, Vivanco-Suarez J, Dibas M, Casanova D, Galecio-Castillo M, Farooqui M and Ortega-Gutierrez S (2023) Current challenges in the endovascular treatment of medium vessel occlusions. Front. Stroke 2:1242961. doi: 10.3389/fstro.2023.1242961
Received: 20 June 2023; Accepted: 24 July 2023;
Published: 15 August 2023.
Edited by:
Ana Catarina Fonseca, University of Lisbon, PortugalReviewed by:
Waldo Rigoberto Guerrero, University of South Florida, United StatesCopyright © 2023 Rodriguez-Calienes, Vivanco-Suarez, Dibas, Casanova, Galecio-Castillo, Farooqui and Ortega-Gutierrez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Santiago Ortega-Gutierrez, c2FudHktb3J0ZWdhQHVpb3dhLmVkdQ==
 Aaron Rodriguez-Calienes
Aaron Rodriguez-Calienes Juan Vivanco-Suarez
Juan Vivanco-Suarez Mahmoud Dibas1
Mahmoud Dibas1 Mudassir Farooqui
Mudassir Farooqui Santiago Ortega-Gutierrez
Santiago Ortega-Gutierrez