
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sports Act. Living , 03 September 2024
Sec. Exercise Physiology
Volume 6 - 2024 | https://doi.org/10.3389/fspor.2024.1436742
This article is part of the Research Topic Exercise and Transplant Sport: The journey to a more active life View all 6 articles
 Marco Vecchiato1,2
Marco Vecchiato1,2 Federica Duregon1,2
Federica Duregon1,2 Emanuele Zanardo1,2*
Emanuele Zanardo1,2* Veronica Baioccato1,2
Veronica Baioccato1,2 Giulia Quinto1,2
Giulia Quinto1,2 Alberto Livio1,2
Alberto Livio1,2 Barbara Mazzucato1,2
Barbara Mazzucato1,2 Chiara Sarri1,2
Chiara Sarri1,2 Lia Bellis3
Lia Bellis3 Claudia Carella3
Claudia Carella3 Massimo Cardillo3
Massimo Cardillo3 Daniel Neunhaeuserer1,2
Daniel Neunhaeuserer1,2 Andrea Ermolao1,2
Andrea Ermolao1,2 Francesca Battista1,2
Francesca Battista1,2
Introduction: Tailored exercise prescription is a crucial intervention for kidney transplant recipients (KTRs). This longitudinal study investigates the impact on long-term effectiveness of exercise prescriptions over one year follow-up, implementing telehealth tools for exercise administration and adherence monitoring.
Materials and methods: KTRs were evaluated with clinical assessments including body composition, blood and urinary parameters, physical performance and quality of life at baseline (T0), after six (T6) and twelve (T12) months. The adherence to prescribed exercise training was monitored via video call interviews until T6 when the sample was divided into a group monitored via wearables (WG) and a group continuing video calls (VG) until T12.
Results: Twenty-six KTRs completed the study. No changes in body composition and kidney function were reported. KTRs showed an improvement in lipid profile, systolic blood pressure, cardiorespiratory fitness and quality of life. WG showed no clinical differences compared to VG except for reported higher quality of life.
Discussion: A good adherence to the exercise prescription was obtained with both monitoring methods (232 vs 253 min/week). This study reinforces the inclusion exercise training for KTRs to enhance physical fitness and reduce cardiovascular risk factors. These results emphasize the role of telehealth monitoring methods as motivators for adherence to long-term exercise prescriptions.
Kidney transplantation has significantly improved the survival and quality of life for individuals with end-stage kidney diseases. Despite advancements in medical care for kidney transplant recipients (KTRs), the risk of mortality from cardiac events remains significantly elevated, with an annual rate of fatal or nonfatal cardiovascular events up to 50 times higher compared to the general population (1, 2). This heightened cardiovascular risk is attributed to pre-existing conditions like hypertension and dyslipidemia, compounded by the effects of immunosuppressive therapy designed to prevent organ rejection (3).
Physical activity and exercise training have been demonstrated to counteract these mechanisms in patients with chronic kidney diseases and KTRs, mitigating inflammation, lowering blood pressure and cholesterol, and reducing endothelial disfunction (4, 5). For this reason, tailored exercise prescription has emerged in recent years as a promising intervention to address the physical deconditioning, enhance the functional capacity and improve overall quality of life of KTRs (6–8). Nevertheless, while exercise interventions are recognized as beneficial for global health, only few available studies have reported the effectiveness of this intervention over a prolonged period (9–11). Moreover, patient adherence to prescribed exercise is a critical concern, particularly in the context of KTRs, where routine exercise programs are not yet seamlessly integrated into standard clinical care. Telehealth tools to implement physical activity and exercise programs have been utilized in multiple studies due to their sustained benefits over time (12), with good results in increasing physical activity, improving body composition and fitness, across various age groups and both clinical and non-clinical populations (13), but poorly in KTRs and never to specifically monitor the efficiency and adherence to prescribed exercise (14, 15).
This longitudinal study primarily aims to investigate the impact of tailored exercise prescription and training on global health of KTRs over a one-year period with telehealth instruments implementation. A secondary aim is to verify the best method for improving adherence to exercise prescription.
The study is part of a project sponsored by the Italian National Institute for Insurance against Accidents at Work (INAIL) to promote a multidisciplinary network and web applications to facilitate the resumption of an active lifestyle and the reintegration into the work and social activities for KTRs. This study included patients aged 18–65 years old who had undergone kidney transplantation at least six months before the first assessment and agreed to participate in a 12-month monitoring study including adherence to exercise prescription. Participants with any acute or chronic medical conditions that contraindicate high-intensity exercise or cardiopulmonary exercise testing (CPET) as severe cardiovascular, respiratory, or musculoskeletal disorders, were excluded from the study. Others exclusion criteria were physical and cognitive limitations that impeded exercise testing and evidence of acute transplant rejection at the time of enrollment. Recruitment took place from October 2020 through May 2022 at the Sports and Exercise Medicine Division of the University Hospital of Padova. The study was coordinated by the National Transplantation Centre and approved by the Ethics Committee of the Italian National Institute of Health with the code PRE BIO CE n.16146, 24/07/2019 and by the Local Ethic Committee with the code 4777/AO/19 - AOP1893 - BRIC 01, 22/01/2020. Written informed consent was obtained by the patients before inclusion, according to the procedures approved by the Ethics Committee in compliance with the Helsinki Declaration and national rules regarding clinical trial management.
After eligibility evaluation, participants underwent a comprehensive global assessment including four different domains: body composition, blood and urinary parameters, physical performance and quality of life. During the monitoring period, the only changes in drug regimen were of immunosuppressive therapy.
Waist circumference was measured wrapping the measuring tape at the narrowest part of the abdomen, stand in front of the subject, between the iliac crest and the lowest rib. The body composition analysis was conducted using bioelectrical impedance analysis (Akern Srl, Florence, Italy). Impedance was measured through electrodes placed on the right hand and right foot of the participant in resting condition and under controlled room temperature and consistent hydration status. The resistance and reactance data acquired by the device were used to determine fat mass and fat-free mass using Bodygram 1.31 (Akern Srl, Florence, Italy).
Within one week prior to each assessment, patients underwent comprehensive blood and urine analyses, including the determination of kidney function, complete blood count, lipid profile, fasting glucose, C-reactive protein, and 24-h proteinuria. Standardized procedures were employed for the collection of blood and urine samples, conducted in the early morning following an overnight fasting period. Furthermore, patients refrained from engaging in exercise within the 48 h preceding samples collection.
Maximal 12-leads ECG-monitored CPET (Masterscreen CPX system Jaeger, Carefusion, Hoechberg, Germany) on a cycle-ergometer (Cycle-ergometer eBike, General Electrics) or a treadmill (T170 DE-med, h/p/cosmos, Nussdorf-Traunstein, Germany) was performed, with an individually adapted test protocol in accordance with international guidelines until patients reached a Borg rating of perceived exertion (RPE) ≥ 18/20 (16, 17). Systolic and diastolic blood pressure (SBP and DBP) were measured at rest, every three minutes during exercise until peak and during the recovery phase. Continuous monitoring of the electrocardiogram was performed throughout the test and the respiratory gas exchange were monitored breath by breath during the whole test. Peak oxygen uptake (VO2 peak) was defined as the highest value of VO2 attained in a 30-s interval at peak exercise. The first ventilatory threshold (VT1) was identified on the plots of the cardiopulmonary evaluation using the simplified V-Slope method (17) while the second ventilatory threshold (VT2) was identified by an increase of the ventilatory equivalent for carbon dioxide and a decrease in end-tidal pressure of carbon dioxide (18).
Dominant and non-dominant handgrip strength was measured with a calibrated dynamometer (Baseline, Elmsford, NY, USA). Grip handle was adjusted if required and the elbow was flexed to 90° to guarantee the strongest grip strength measurement. For the analysis, an average of three standardized measurements was considered. The absolute handgrip strength values were then normalized for gender and body mass index (BMI) (19).
To assess lower limb strength, the 30 s Chair Stand Test (30CST), a component of the Senior Fitness Test battery, was employed. This test involves performing as many repetitions as possible within a predetermined 30-s period of rising from and sitting down on a chair. The starting position is seated with the upper limbs crossed over the chest, and a repetition is counted when the individual rises, completes the extension of the hip joint, and returns to the seated position (20).
The physical activity level was investigated with the International Physical Activity Questionnaire (IPAQ). This instrument is composed by a comprehensive set of domains including leisure time, domestic and gardening (yard) activities, work-related and transport-related activity. The items are structured to provide separate scores on walking, moderate-intensity, and vigorous-intensity activity, as well as a combined total score, expressed in MET/minutes/week, to describe overall level of activity (21).
Short Form 36 (SF−36) questionnaire estimates physical and mental health through thirty-six questions across eight categories. The results can then be summarised in two summary scales:
- Physical component summary (PCS) including physical functioning, bodily pain, general health perceptions and physical role functioning.
- Mental component summary (MCS) including vitality, emotional role functioning, social role functioning, mental health or emotional wellbeing.
The scores for each domain can vary between 0 and 100, where 100 represents the best possible perception of quality of life (22).
These assessments were conducted at baseline (T0), after six months (T6) and after twelve months (T12), as reported in Figure 1.
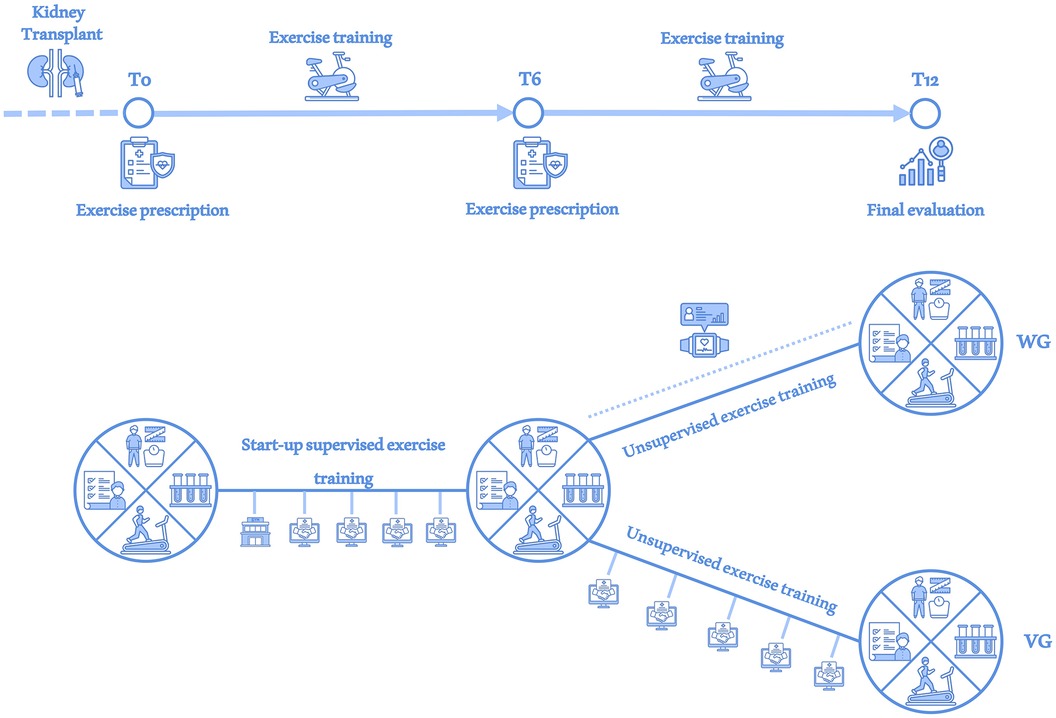
Figure 1. Study overview. At each assessment (T0, T6 and T12), investigations of the body composition data, blood and urinary parameters, physical performance and quality of life were performed (large circle divided into 4 sections). After a supervised exercise period during the first month, the entire study sample was monitored via monthly videocall interviews (handshake in the screen icon) until T6. At T6 the sample was divided into a WG group receiving physical activity monitoring via wearable device (smartwatch icon) until T12 and a VG group that continued to receive monitoring via videocall interviews until T12.
Based on the functional evaluation, a tailored exercise program was prescribed for each patient by a sports and exercise medicine physician, and all participants were invited to take part to a start-up supervised exercise training twice weekly, lasted one month and conducted by an exercise professional in the hospital gym. Exercise programs were tailored according to individual needs, particularly addressing any issues such as osteoarticular problems. Consequently, the intensity, volume, or type of exercises were adjusted to better meet each participant's requirements. Exercises targeted all major muscle groups, including squats, calf raises, seated rows, chest presses, and pull-downs, but were tailored for each participant based on their specific needs or limitations. Aerobic exercise intensities have been set according to the CPET results: - low intensity, under VT1; - moderate intensity, between VT1 and VT2; - high intensity, above VT2; as suggested by our working group and following international guidelines for exercise prescription (23, 24). Heart rates and RPE at VT1 and VT2 have been included in each patient's exercise prescription to clarify exercise intensity recommendations. Strength and flexibility exercise was planned based on previously described functional tests and following a standardized outline comprising breathing exercises and joint mobility warm-up at the beginning of the session, taking care not to exceed the level of discomfort. When possible, submaximal tests (10-Repetition Maximum) were also performed to determine the indicated percentage of intensity for each machine used. Where not possible, intensity in resistance training was based on RPE. The central part of the training comprised aerobic, resistance, proprioception and flexibility exercises targeted all major muscle groups. The prescribed exercise intensity was monitored using HR and the RPE scale, maintaining values corresponding to moderate intensity determined through CPET. Moreover, participants were encouraged to practice aerobic activity sessions independently; in this way, they were able to increase their weekly physical activity volume. Subsequently, at the end of the start-up training, exercise program was performed at home without a supervision, maintaining the same parts mentioned above (aerobic, resistance, flexibility training). At T6 adjustments in exercise prescriptions were made by the Sports and Exercise Medicine physician, as necessary, particularly regarding heart rates for aerobic exercise intensity; if no changes were needed, the initial T0 values were maintained. Further changes in exercise modalities have been implemented depending on the patient's clinical condition as already described for previous works of our group with serial CPETs (25).
Between T0 and T6, after the supervised one-month start-up period in the hospital gym, the patients were monitored for adherence by monthly video interviews conducted by exercise professionals via a user-free dedicated platform. These video interviews aimed to investigate adherence with the exercise prescription, the amount of at least moderate physical activity performed per week, the frequency of structured exercise sessions conducted, and any clinical or exercise-related problems.
At T6 the included sample has been casually divided in two subgroups, without a standardized randomization. Smartwatches to monitor physical activity (Fitbit Versa 4, Fitbit Inc., California, USA) were randomly assigned to the half of participants who formed the Wearable Group (WG), ensuring an unbiased distribution for sex, age and self-reported physical activity level across the study population. KTRs who did not receive the wearable device continued to receive the monthly video interviews and formed the videocall group (VG), as shown in Figure 1. Heart rate thresholds to determine exercise intensities (low, moderate and vigorous) were configured by exercise professionals based on the parameters individuated during CPET. Patients were instructed to wear the device as much as possible throughout the day to collect data on step count, exercise duration and intensity. After acquiring informed consent from all WG patients, data collected were monitored and analysed through Fitabase platform (Fitabase, San Diego, California, USA), a cloud-based data aggregation platform to extract and aggregate data from devices.
Continuous variables were described using means and standard deviations and categorical variables were described using absolute values (n) and percentages (%). The Shapiro-Wilk test was used as distribution test. Comparisons between the same population at T0, T6 and T12 were performed with the Friedman test and post hoc analysis with Bonferroni correction. The WG and VG groups at T12 were compared for differences between the variables at T6-T12 with Mann Whitney U test. A p-value of <0.05 was considered statistically significant. Analyses were carried out using SPSS software (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY, USA: IBM Corp).
Of the 40 initials patients enrolled in the study, 26 KTRs successfully completed the entire exercise intervention period and were included in the final analysis (Figure 2). Clinical characteristics of the included sample and the causes for kidney transplantation were reported in Table 1. The mean age was 42.1 ± 9.0 years with 14 males and 12 females. The global evaluations performed on KTRs who completed all three assessments at T0, T6 and T12 are shown in Table 2.
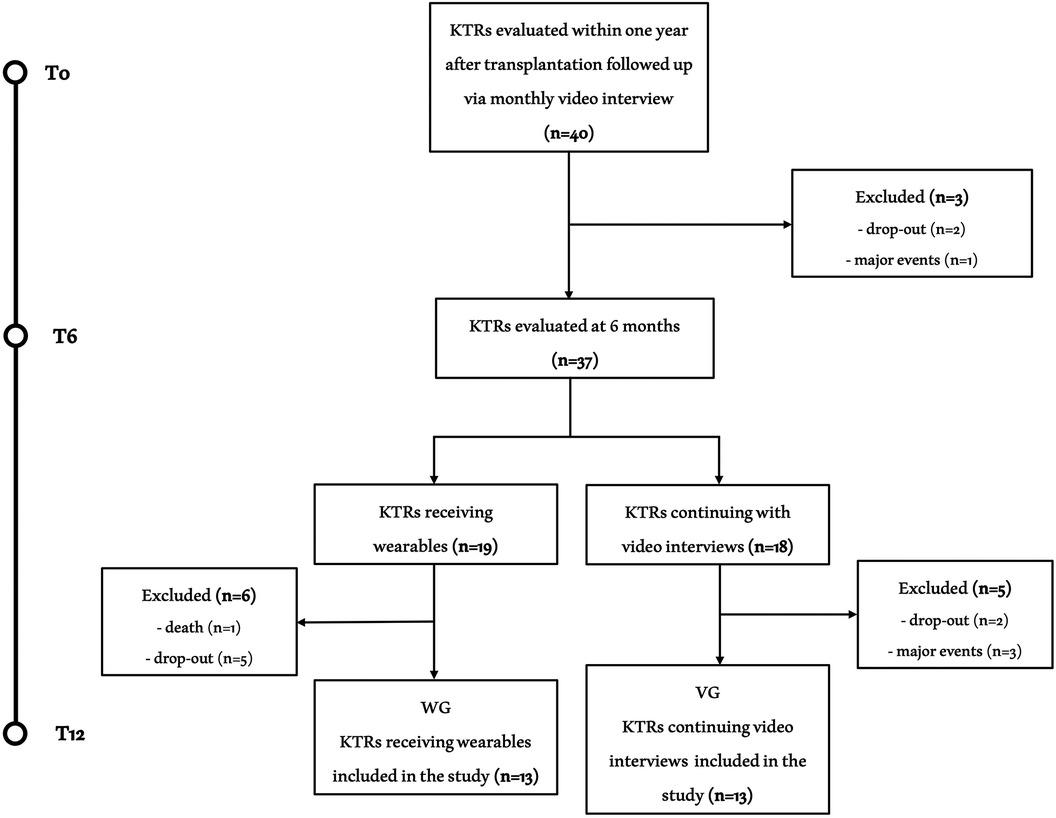
Figure 2. Study flow chart. Flow chart of the study. Of the 40 KTRs initially included, 26 completed all three assessments. The causes of exclusion were nine dropouts, four relevant clinical events (stroke, organ rejection, acute coronary artery disease and inguinal hernia with related inability to exercise) and one death (acute leukemia).
Comparing baseline body composition parameters with one year follow up values, no significant changes in BMI, waist circumference and fat mass were found. There were no differences in blood and urinary kidney functional values during the monitored period. Regarding the lipid profile, there was an improvement in HDL-cholesterol (from 55.50 to 60.42 mg/dl) and a non-significant decrease in LDL-cholesterol (from 124.27 to 109.38 mg/dl) with the total cholesterol value unchanged over the study period. No change in the glucose profile was detected. Three patients had C-reactive protein values compatible with inflammatory status at T0 (3.80 ± 1.35 mg/dl) and none at T6 and T12.
SBP at rest showed a significant decline at one year follow-up. SBP and DBP at exercise peak showed no significant changes. CPET revealed significant enhancements in both absolute and relative VO2 peak among participants. No included patients presented symptoms, desaturation, or direct/indirect stress-inducible ischaemia signs during CPET. No changes in handgrip strength have been observed at T6 and T12 compared to T0, while the performance in the 30CST showed improvement at 6 months that was maintained at 12 months. IPAQ showed increases at both T6 and T12 (1,619.61 vs. 2,454.53 vs. 2,908.23 METs/week, p = 0.001).
SF-36 MCS and PCS showed improvement at T6 and a maintenance at T12 (p = 0.003 and p = 0.001, respectively).
During T0-T6 period the referred volume of moderate/vigorous physical activity in all study sample was 245.91 ± 22.81 min per week. At T6, out of the 19 KTRs receiving the wearable device, 13 patients completed the third assessment at T12 and were include in WG, while of the remaining 18 KTRs continuing with monthly videocall interviews, 13 completes the study protocol and composed the VG (Figure 2). There were no differences in gender (both groups had seven men and six women) or age (WG: 39.85 ± 9.34 vs. VG: 44.38 ± 8.47 years; p = 0.223) between the groups.
Among WG, continuous monitoring showed consistent engagement in physical activity and exercise training throughout the study period in 9 of the 13 patients received wearable monitoring with an average number of days of use of 143.38 ± 51.10. The recorded volume of moderate/vigorous physical activity was 231.98 ± 28.44 min per week with 9,584.47 ± 4,286.81 number of steps per day reached. Among VG, 11 of 13 patients self-reported regular physical activity throughout T6-T12 with an average of 1.42 ± 0.35 exercise sessions per week. Eight out of 13 patients reported mixed aerobic and strength activity and three reported predominantly aerobic activity. The average referred volume of moderate/vigorous physical activity was 252.85 ± 31.35 min per week. Four patients reported issues in the videocall interviews (two patients reported SBP increases, one patient reported musculoskeletal impairments in the upper limbs and one in the lower limbs), which resulted in an adaptation of the planned training schedule. The average data of physical activity level monitored during the study were shown in Figure 3.
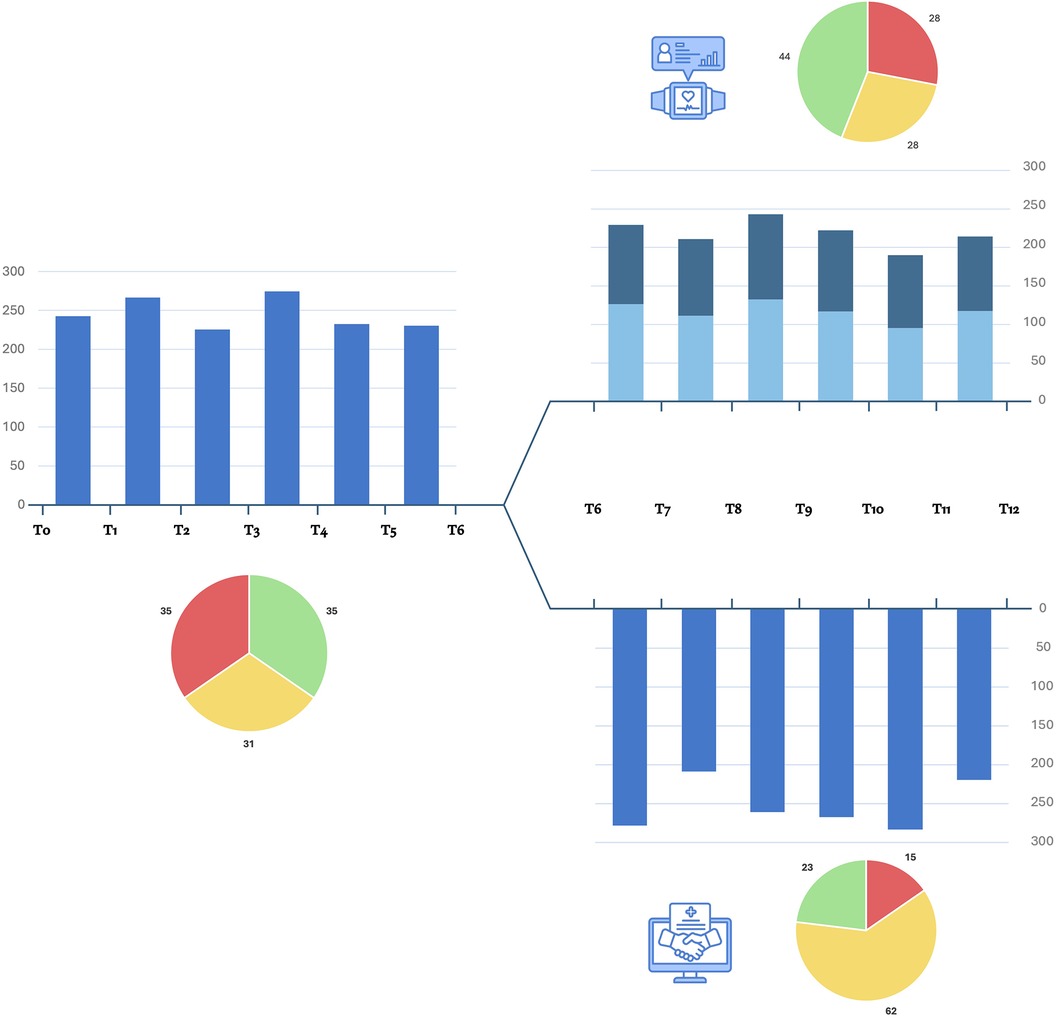
Figure 3. Monitoring of physical activity during the study. Blue bars show the average monthly level of moderate/vigorous activity per week (expressed as minutes per week) self-reported by video interviews during T0-T6 period and for the VG during T6-T12 period. For WG, moderate (light blue) and vigorous (dark blue) average monthly registered minutes of physical activity are shown as distinct. The cake diagrams show the percentage of patients who met the required minutes per week of moderate/vigorous physical activity: red, <150 min/week; yellow, 150–300 min/week; green, >300 min/week.
No statistically significant differences in body composition, blood and urinary markers, and functional parameters were shown between the WG and VG. WG participants showed a higher value on the MCS (p = 0.003) and PCS (p = 0.010) compared to VG, as reported in Table 3.
Findings from the current study contribute to the growing body of evidence supporting the benefits of exercise prescription and interventions in KTRs. The observed improvements in the lipid profile, blood pressure and cardiorespiratory fitness underscore the potential of exercise as an integral component of post-transplant care. In addition, both monitoring methods allowed us to assess an overall good adherence to the prescribed exercise programme with the wearable device appearing to provide a greater impact on perceived quality of life. This appears as good news for patients and prescribers because whatever is the method, telehealth monitoring sustains positive effects for health in KTRs undergoing exercise training programs.
Exercise intervention therapy, as an approach focused on functional exercise, has gained prominence in recent years as an adjunctive treatment strategy for various surgical and non-surgical procedures, including solid organ transplantation (26). Furthermore, the application of exercise intervention programs has been investigated in the post-transplantation setting, with notable findings in different organ transplant scenarios (27). Four recent systematic reviews with meta-analysis conducted exploring exercise as an intervention in KTRs showed as it can improve kidney function, cardiopulmonary fitness, dyslipidaemia, physical performance, and quality of life in KTRs (6, 7, 28, 29). Our results are in agreement with these reviews adding an interesting insight into exercise prescription and adherence with different telehealth monitoring methods.
Our study did not show significant changes in BMI, waist circumference, or percentage of lean and fat mass during the monitored period. This is in line with previous findings suggesting the complex relationship between exercise and body composition in transplant and specifically KTRs (6, 28). Individual variability, different exercise modalities and potential interactions with immunosuppressive medications may contribute to the observed stability. Indeed, the only study on KTRs that showed a relevant effect of exercise training on BMI was conducted on patients with severe obesity (11).
The observed improvement in HDL levels over the one-year intervention period aligns with well-established evidence linking regular exercise to favourable lipid profiles. Exercise has been consistently associated with increased HDL cholesterol, a key component of cardiovascular health, known for its role in reverse cholesterol transport and anti-inflammatory properties, not necessarily with significant reductions in total cholesterol, supporting the potential cardioprotective effects of exercise in KTRs (30). Some studies have shown a protective effect on the atherogenic risk of exercise on KTRs (31, 32) but others did not report substantial changes in the lipid profile, probably influenced by the role of immunosuppressive therapy in altering the overall lipid balance (28, 33).
The consistent maintenance of blood glucose levels within the normal range throughout the study supports the notion that exercise interventions in KTRs may contribute to glycaemic control still mitigating the potentially hyperglycaemic role of immunosuppressive therapies. The study by Morales Febles and colleagues emphasized the role of exercise in improving insulin sensitivity and glycaemic regulation in KTRs, corroborating our findings (34).
The modest decrease in creatinine and urea, although not statistically significant, suggests a potential trend toward improved kidney function with exercise. Previous literature has shown that exercise may enhance kidney blood flow and mitigate its dysfunction in various populations with kidney diseases (35). In KTRs, the evidence is conflicting, with several studies showing an improvement in kidney function markers (11, 32, 36) and as many finding no significant difference after the exercise interventions (10, 37, 38).
The reduction in inflammatory markers among participants with initially elevated levels highlights a potential anti-inflammatory effect of exercise. Chronic inflammation is a known contributor to graft dysfunction and cardiovascular complications post-transplant (39). Aerobic and resistance training at moderate intensity may exert anti-inflammatory effects in KTRs with a reduction in tumour necrosis factor alpha levels and modulating interleukin-6 blood level (38, 40).
The significant increase in absolute and relative VO2 peak underscores the positive impact of the exercise intervention on cardiorespiratory fitness in KTRs (6, 7, 28, 29, 41, 42). The observed benefits in cardiorespiratory fitness are crucial given their association with reduced cardiovascular risk and improved overall mortality in all patients with chronic diseases, including KTRs. Indeed, a recent exploratory analysis showed as each increase in VO2 peak of 1 ml/kg/min was associated with a 0.5% decrease in 7-year risk of major adverse cardiovascular events and 1% decrease in 7-year risk of mortality in KTRs (43). Moreover, our study group showed as low cardiorespiratory fitness may be considered as a modifiable predictor of long-term severe infectious events in KTRs stressing the importance of CPET in cardiovascular evaluation of this specific population (44). Finally, assessing ventilatory thresholds through CPET allows the formulation of a tailored exercise prescription aimed at improving the functional capacity of KTRs (4, 45).
Otherwise than cardiorespiratory fitness, handgrip strength did not exhibit a significant increase, confirming the heterogeneous findings on strength in KTRs from recent evidence (36, 37). The non-significant change and the small decrease in strength in the dominant upper limb between T6 and T12 may be influenced by individual variations in response to exercise and the tendency to engage in predominantly aerobic rather than strength activities when training was unsupervised.
The increase in repetitions during the 30SCT at T6, sustained at T12, suggests an improvement in muscular endurance. This finding resonates with previous studies indicating that exercise interventions positively impact functional capacity, including chair stand performance, in KTRs (37, 38, 46).
The increase in IPAQ scores at 6 months, followed by a further rise at 12 months, suggests a sustained improvement in self-reported physical activity levels. These findings align with the concurrent increase of the cardiorespiratory fitness in our study and with existing literature emphasizing the positive impact of structured exercise interventions on promoting and maintaining increased physical activity in KTRs (42).
Ensuring long-term exercise adherence is one of the major issues inherent in exercise prescription (47, 48). During the first half of the study, patients were initiated into supervised exercise in our hospital gym and then monitored monthly with videocall interviews until T6. The subdivision of the initial cohort at T6 into a WG, employing continuous objective monitoring, and a VG, relying on monthly video interviews, provided valuable insights into the different monitoring approaches on exercise-related outcomes. The lack of significant differences in most parameters, except for higher PCS and MSC scores in WG, showed how the method itself does not bring a direct clinical benefit to the KTRs but leads to a better quality of life perception by the patients. Different studies indicated that wearable device-based monitoring is associated with improved health-related quality of life indices in various clinical populations (49, 50). The ability of wearables to provide real-time feedback and personalized insights may contribute to enhanced motivation and engagement in prescribed exercise regimens, translating into improved mental well-being also in KTRs (51). Objective monitoring offers advantages over self-report methods by providing accurate data on volume and intensity levels accomplished during exercise training (52, 53). This supports the growing consensus that incorporating objective monitoring into post-transplant exercise interventions enhances precision and reliability in assessing patient responses (54). On the other hand, VG reported even higher levels of physical activity. Probably, the possibility of being able to see and talk to an exercise professional on a regular basis provided more specific benefits, such as gaining information about the type of exercise practised, and any problems associated with the exercise performed with the health condition. This interaction modality belongs to the larger world of telehealth using digital interventions to improve physical fitness, which has proven its worth for various chronic diseases (55–60), including KTRs (14, 15).
A notable focus is the exceptional adherence observed in both groups, where most patients consistently adhered to the exercise prescription over an extended period. We used the moderate to vigorous exercise intensity model (MVPA) for quantifying adherence. A patient was considered adherent to the prescribed exercise if they engaged in at least 150 min per week of moderate-intensity physical activity or 75 min per week of high-intensity physical activity (or a combination of both), as recommended by WHO guidelines. Our patient reported more than 200 min per week in mean of moderate exercise, more than the minimum recommended. This aligns with current literature emphasizing the positive impact of health information technology on exercise adherence in KTRs, regardless of the intervention tools (61). Both videocalls and wearable devices interventions can improve self-management in KTRs, with different pros and cons (Figure 4), tracking progress and contributing to the sustained adherence observed in the study sample.
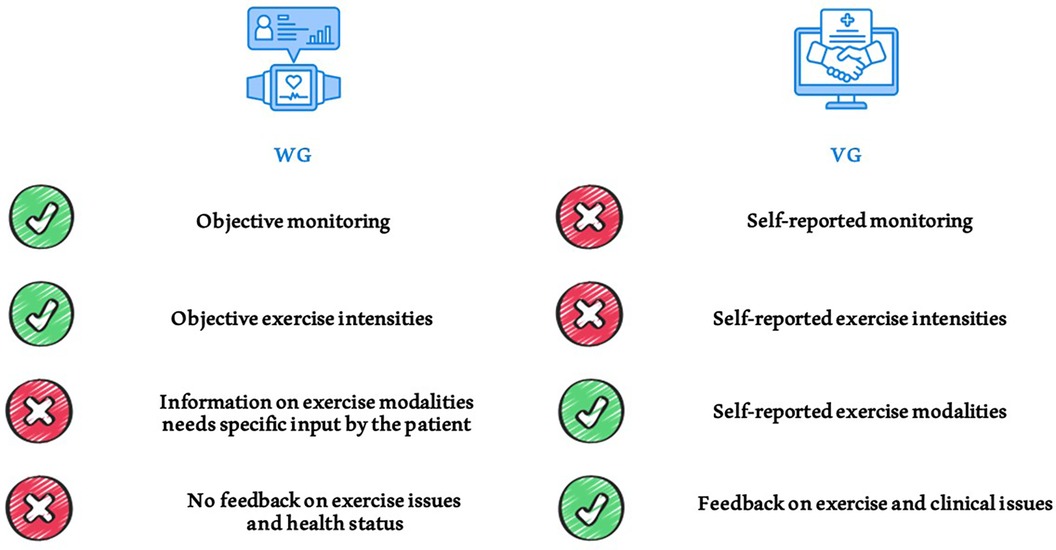
Figure 4. Comparison of different exercise monitoring methods used in the study. Pros and cons of using telehealth tools such as wearables and videocall interviews for monitoring physical activity and exercise training in patients.
Healthcare system should have a key role in supporting KTRs to become more physically active with the application of different available tools to monitor and improve patients' adherence to therapeutic strategies, including exercise training.
It is essential to acknowledge the limitations of this study, including the relatively small sample size and the potential for selection bias due to the one-year exercise program with three complete assessments. Indeed, of the initial enrolled cohort only 65% of the participants completed the intervention phase up to the final evaluation. Long-term studies with larger cohorts are needed to confirm the durability of the observed improvements and to account for potential confounding variables. A potential limitation dictated by non-regular use of the wearable by patients was minimised by the high compliance of the patients. Future research endeavours should focus on expanding the understanding of the optimal exercise prescription parameters, including intensity, duration, and modality. Additionally, investigations into the long-term impact of exercise on graft survival and transplant-related comorbidities will further inform clinical practice.
This study supports the clinical relevance of integrating tailored exercise training into standard kidney transplant care due to the improvement of physical fitness, cardiovascular risk factors and quality of life. Moreover, these findings underscore the importance of using telehealth monitoring methods as an incentive for maintaining good adherence to the exercise prescription in the long-term. Solutions are needed to overcome barriers to equal access to these instruments so that all KTRs can benefit from high-quality exercise interventions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the National Transplantation Centre and approved by the Ethics Committee of the Italian National Institute of Health with the code PRE BIO CE n.16146, 24/07/2019 and by the Local Ethic Committee with the code 4777/AO/19 - AOP1893 - BRIC 01, 22/01/2020. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MV: Conceptualization, Formal Analysis, Software, Writing – original draft. FD: Writing – original draft. EZ: Conceptualization, Data curation, Formal Analysis, Software, Writing – original draft, Writing – review & editing. VB: Data curation, Investigation, Methodology, Writing – review & editing. GQ: Data curation, Investigation, Methodology, Writing – review & editing. AL: Data curation, Investigation, Writing – review & editing. BM: Data curation, Investigation, Methodology, Writing – original draft. CS: Writing – review & editing, Data curation, Investigation, Validation. LB: Funding acquisition, Resources, Visualization, Writing – review & editing. CC: Funding acquisition, Resources, Visualization, Writing – review & editing. MC: Writing – review & editing, Funding acquisition, Resources, Visualization. DN: Writing – review & editing. AE: Writing – review & editing, Project administration, Resources, Supervision, Validation. FB: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The POST project received funds in the framework of BRIC-INAIL 2018 funding programme. Open Access funding provided by Università degli Studi di Padova University of Padua, Open Science Committee.
Thanks to all the members of the POST Project Group: Paola Di Ciaccio, Silvia Pisanu, Manuela Trerotola, Valentina Totti (Centro Nazionale Trapianti, Istituto Superiore di Sanità, Rome, Italy); Riccardo Melloni, Lucia Botti, Maniva Oliva, Simone Mosconi, Claudia Canali [Università degli studi di Modena e Reggio Emilia – Centro di Ricerca Interdipartimentale sulla Sicurezza e Prevenzione dei Rischi (CRIS)]; Giovanni Mosconi (Azienda Unità Sanitaria Locale della Romagna - Dipartimento di Nefrologia e Dialisi Forlì), Gianluigi Sella (Azienda Unità Sanitaria Locale della Romagna - Dipartimento di Medicina dello Sport Ravenna) Matteo Scarpa, (UOC Sports and Exercise Medicine, Public Health Department, AUSL of Imola, Italy); Carlo De Cillia, Gabriela Sangiorgi, (IRCCS St Orsola-Malpighi Polyclinic, University of Bologna, Bologna, Italy) Mariacristina Morelli, Vittoria Vero (Internal Medicine Unit for the Treatment of Severe Organ Failure, IRCCS St Orsola-Malpighi Polyclinic, University of Bologna, Bologna, Italy); Bruno Papaleo, Mariarosa Marchetti [Istituto nazionale per l'assicurazione contro gli infortuni sul lavoro (INAIL) – DiMEILA – Laboratorio di Sorveglianza sanitaria e promozione della salute, Rome, Italy]. This research project is part of the Italian initiative of Exercise is Medicine. The figures have been designed using icons from Flaticon.com.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jardine AG, Gaston RS, Fellstrom BC, Holdaas H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet. (2011) 378:1419–27. doi: 10.1016/S0140-6736(11)61334-2
2. Liefeldt L, Budde K. Risk factors for cardiovascular disease in renal transplant recipients and strategies to minimize risk. Transpl Int. (2010) 23:1191–204. doi: 10.1111/J.1432-2277.2010.01159.X
3. Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. (2004) 351:2715–29. doi: 10.1056/NEJMRA033540
4. De Smet S, Van Craenenbroeck AH. Exercise training in patients after kidney transplantation. Clin Kidney J. (2021) 14:II15–24. doi: 10.1093/CKJ/SFAB022
5. Kanbay M, Copur S, Yildiz AB, Tanriover C, Mallamaci F, Zoccali C. Physical exercise in kidney disease: a commonly undervalued treatment modality. Eur J Clin Invest. (2024) 54:e14105. doi: 10.1111/eci.14105
6. Zhang D, Yu L, Xia B, Zhang X, Liang P, Hu X. Systematic review and meta-analysis of the efficacy of exercise intervention in kidney transplant recipients. World J Urol. (2023) 41(12):3449–69. doi: 10.1007/S00345-023-04673-9
7. Oguchi H, Tsujita M, Yazawa M, Kawaguchi T, Hoshino J, Kohzuki M, et al. The efficacy of exercise training in kidney transplant recipients: a meta-analysis and systematic review. Clin Exp Nephrol. (2019) 23:275–84. doi: 10.1007/S10157-018-1633-8
8. Roi GS, Stefoni S, Mosconi G, Brugin E, Burra P, Ermolao A, et al. Physical activity in solid organ transplant recipients: organizational aspects and preliminary results of the Italian project. Transplant Proc. (2014) 46:2345–9. doi: 10.1016/J.TRANSPROCEED.2014.07.055
9. O’Connor EM, Koufaki P, Mercer TH, Lindup H, Nugent E, Goldsmith D, et al. Long-term pulse wave velocity outcomes with aerobic and resistance training in kidney transplant recipients – a pilot randomised controlled trial. PLoS One. (2017) 12:e0171063. doi: 10.1371/JOURNAL.PONE.0171063
10. Painter PL, Hector L, Ray K, Lynes L, Paul SM, Dodd M, et al. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am J Kidney Dis. (2003) 42:362–9. doi: 10.1016/S0272-6386(03)00673-5
11. Tzvetanov I, West-Thielke P, D’Amico G, Johnsen M, Ladik A, Hachaj G, et al. A novel and personalized rehabilitation program for obese kidney transplant recipients. Transplant Proc. (2014) 46:3431–7. doi: 10.1016/J.TRANSPROCEED.2014.05.085
12. Brown RCC, Coombes JS, Jungbluth Rodriguez K, Hickman IJ, Keating SE. Effectiveness of exercise via telehealth for chronic disease: a systematic review and meta-analysis of exercise interventions delivered via videoconferencing. Br J Sports Med. (2022) 56:1042–52. doi: 10.1136/BJSPORTS-2021-105118
13. Ferguson T, Olds T, Curtis R, Blake H, Crozier AJ, Dankiw K, et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digit Health. (2022) 4:e615–26. doi: 10.1016/S2589-7500(22)00111-X
14. Hezer B, Massey EK, Reinders MEJ, Tielen M, van de Wetering J, Hesselink DA, et al. Telemedicine for kidney transplant recipients: current state, advantages, and barriers. Transplantation. 108(2):409–20. doi: 10.1097/TP.0000000000004660
15. Biancone L, Minetti E, De Rosa P, Rigotti P, Stallone G, Volpe M, et al. Telemedicine monitoring in the follow-up of kidney transplant recipients: consensus indications from an Italian panel of surgeons and nephrologists after the COVID-19 experience. J Nephrol. (2022) 35:725–33. doi: 10.1007/S40620-021-01193-W/FIGURES/1
16. Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. (2013) 128:873–934. doi: 10.1161/CIR.0B013E31829B5B44
17. Vecchiato M, Neunhaeuserer D, Quinto G, Bettini S, Gasperetti A, Battista F, et al. Cardiopulmonary exercise testing in patients with moderate-severe obesity: a clinical evaluation tool for OSA? Sleep Breath. (2021) 1:1–9. doi: 10.1007/S11325-021-02475-0
18. Vecchiato M, Neunhaeuserer D, Zanardo E, Quinto G, Battista F, Aghi A, et al. Publisher Correction: Respiratory exchange ratio overshoot during exercise recovery: a promising prognostic marker in HfrEF. Clin Res Cardiol. (2024) doi: 10.1007/s00392-024-02416-3. [Epub ahead of print]. Erratum for: Clin Res Cardiol. (2024). doi: 10.1007/s00392-024-02391-9
19. Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. (1985) 66:69–74.3970660
20. Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. (1999) 70:113–9. doi: 10.1080/02701367.1999.10608028
21. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
22. Brazier JE, Harper R, Jones NMB, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Br Med J. (1992) 305:160–4. doi: 10.1136/BMJ.305.6846.160
23. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0B013E318213FEFB
24. Faggian S, Centanini A, Quinto G, Vecchiato M, Ermolao A, Battista F, et al. The many faces of exercise intensity: a call to agree on definitions and provide standardized prescriptions. Eur J Prev Cardiol. (2024):zwae034. doi: 10.1093/eurjpc/zwae034. [Epub ahead of print]38271589
25. Vecchiato M, Mazzucato B, Battista F, Neunhaeuserer D, Quinto G, Aghi A, et al. Serial cardiopulmonary exercise testing in young patients after one-and-half ventricle repair and Fontan procedure: a comparative study. Eur Heart J Qual Care Clin Outcomes. (2024):qcae041. doi: 10.1093/EHJQCCO/QCAE041. [Epub ahead of print]38782728
26. Janaudis-Ferreira T, Mathur S, Deliva R, Howes N, Patterson C, Räkel A, et al. Exercise for solid organ transplant candidates and recipients: a joint position statement of the Canadian society of transplantation and CAN-RESTORE. Transplantation. (2019) 103:E220–38. doi: 10.1097/TP.0000000000002806
27. Didsbury M, McGee RG, Tong A, Craig JC, Chapman JR, Chadban S, et al. Exercise training in solid organ transplant recipients: a systematic review and meta-analysis. Transplantation. (2013) 95:679–87. doi: 10.1097/TP.0B013E31827A3D3E
28. Chen G, Gao L, Li X. Effects of exercise training on cardiovascular risk factors in kidney transplant recipients: a systematic review and meta-analysis. Ren Fail. (2019) 41:408–18. doi: 10.1080/0886022X.2019.1611602
29. Wilkinson TJ, Bishop NC, Billany RE, Lightfoot CJ, Castle EM, Smith AC, et al. The effect of exercise training interventions in adult kidney transplant recipients: a systematic review and meta-analysis of randomised control trials. Phys Ther Rev. (2021) 27:114–34. doi: 10.1080/10833196.2021.2002641
30. Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. (2017) 16(1):132. doi: 10.1186/S12944-017-0515-5
31. Pooranfar S, Shakoor E, Shafahi MJ, Salesi M, Karimi MH, Roozbeh J, et al. The effect of exercise training on quality and quantity of sleep and lipid profile in renal transplant patients: a randomized clinical trial. Int J Organ Transplant Med. (2014) 5:157.25426284
32. Juskowa J, Lewandowska M, Bartłomiejczyk I, Foroncewicz B, Korabiewska I, Niewczas M, et al. Physical rehabilitation and risk of atherosclerosis after successful kidney transplantation. Transplant Proc. (2006) 38:157–60. doi: 10.1016/J.TRANSPROCEED.2005.12.077
33. Karelis AD, Hébert MJ, Rabasa-Lhoret R, Räkel A. Impact of resistance training on factors involved in the development of new-onset diabetes after transplantation in renal transplant recipients: an open randomized pilot study. Can J Diabetes. (2016) 40:382–8. doi: 10.1016/j.jcjd.2015.08.014
34. Morales Febles R, Marrero Miranda D, Jiménez Sosa A, González Rinne A, Cruz Perera C, Rodríguez-Rodríguez AE, et al. Exercise and prediabetes after renal transplantation (EXPRED-I): a prospective study. Sports Med Open. (2023) 9(1):32. doi: 10.1186/S40798-023-00574-8
35. Baker LA, March DS, Wilkinson TJ, Billany RE, Bishop NC, Castle EM, et al. Clinical practice guideline exercise and lifestyle in chronic kidney disease. BMC Nephrol. (2022) 23:1–36. doi: 10.1186/S12882-021-02618-1
36. Lima PS, De Campos AS, De Faria Neto O, Ferreira TCA, Amorim CEN, Stone WJ, et al. Effects of combined resistance plus aerobic training on body composition, muscle strength, aerobic capacity, and renal function in kidney transplantation subjects. J Strength Cond Res. (2021) 35:3243–50. doi: 10.1519/JSC.0000000000003274
37. Hernández Sánchez S, Carrero JJ, Morales JS, Ruiz JR. Effects of a resistance training program in kidney transplant recipients: a randomized controlled trial. Scand J Med Sci Sports. (2021) 31:473–9. doi: 10.1111/SMS.13853
38. Greenwood SA, Koufaki P, Mercer TH, Rush R, O’Connor E, Tuffnell R, et al. Aerobic or resistance training and pulse wave velocity in kidney transplant recipients: a 12-week pilot randomized controlled trial (the exercise in renal transplant [ExeRT] trial). Am J Kidney Dis. (2015) 66:689–98. doi: 10.1053/j.ajkd.2015.06.016
39. Dahle DO, Mjøen G, Öqvist B, Scharnagl H, Weihrauch G, Grammer T, et al. Inflammation-associated graft loss in renal transplant recipients. Nephrol Dial Transplant. (2011) 26:3756–61. doi: 10.1093/NDT/GFR163
40. Hemmati N, Kazemi S, Jamshidian-Tehrani N, Roozbeh J, Koushkie Jahromi M, Salesi M, et al. Effects of exercise training on immunological factors in kidney transplant recipients; a randomized controlled trial. Res Sports Med. (2022) 30:80–91. doi: 10.1080/15438627.2021.1906671
41. Calella P, Hernández-Sánchez S, Garofalo C, Ruiz JR, Carrero JJ, Bellizzi V. Exercise training in kidney transplant recipients: a systematic review. J Nephrol. (2019) 32:567–79. doi: 10.1007/S40620-019-00583-5
42. Roi GS, Mosconi G, Totti V, Angelini ML, Brugin E, Sarto P, et al. Renal function and physical fitness after 12-mo supervised training in kidney transplant recipients. World J Transplant. (2018) 8:13. doi: 10.5500/WJT.V8.I1.13
43. Billany RE, Mubaarak Z, Bishop N, Smith A, Graham-Brown M. MO605: exploring the relationship between cardiorespiratory fitness and cardiovascular risk in kidney transplant recipients. Nephrol Dial Transplant. (2022) 37:gfac075.018. doi: 10.1093/ndt/gfac075.018
44. Ortolan S, Neunhaeuserer D, Battista F, Patti A, Gobbo S, Di Bella C, et al. Physical fitness as a prognostic marker for infectious events in kidney transplant recipients. Am J Nephrol. (2022) 53:1–9. doi: 10.1159/000520758
45. Patti A, Neunhaeuserer D, Gasperetti A, Baioccato V, Vecchiato M, Battista F, et al. Overshoot of the respiratory exchange ratio during recovery from maximal exercise testing in kidney transplant recipients. Int J Environ Res Public Health. (2021) 18(17):9236. doi: 10.3390/ijerph18179236
46. Zhang P, Liu S, Zhu X, Liu H, Zeng L, Yan J, et al. The effects of a physical exercise program in Chinese kidney transplant recipients: a prospective randomised controlled trial. Clin Kidney J. (2023) 16:1316–29. doi: 10.1093/CKJ/SFAD065
47. Findorff MJ, Wyman JF, Gross CR. Predictors of long-term exercise adherence in a community-based sample of older women. J Womens Health. (2009) 18:1769. doi: 10.1089/JWH.2008.1265
48. Collado-Mateo D, Lavín-Pérez AM, Peñacoba C, Del Coso J, Leyton-Román M, Luque-Casado A, et al. Key factors associated with adherence to physical exercise in patients with chronic diseases and older adults: an Umbrella review. Int J Environ Res Public Health. (2021) 18:1–24. doi: 10.3390/IJERPH18042023
49. Kim BYB, Lee J. Smart devices for older adults managing chronic disease: a scoping review. JMIR Mhealth Uhealth. (2017) 5(5):e69. doi: 10.2196/MHEALTH.7141
50. Fan K, Zhao Y. Mobile health technology: a novel tool in chronic disease management. Intell Med. (2022) 2:41–7. doi: 10.1016/J.IMED.2021.06.003
51. O’Brien T, Meyer T. A feasibility study for teaching older kidney transplant recipients how to wear and use an activity tracker to promote daily physical activity. Nephrol Nurs J. (2020) 47:47. doi: 10.37526/1526-744x.2020.47.1.47
52. Cadmus-Bertram L, Marcus BH, Patterson RE, Parker BA, Morey BL. Use of the fitbit to measure adherence to a physical activity intervention among overweight or obese, postmenopausal women: self-monitoring trajectory during 16 weeks. JMIR Mhealth Uhealth. (2015) 3(4):e96. doi: 10.2196/MHEALTH.4229
53. Davergne T, Pallot A, Dechartres A, Fautrel B, Gossec L. Use of wearable activity trackers to improve physical activity behavior in patients with rheumatic and musculoskeletal diseases: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). (2019) 71:758–67. doi: 10.1002/ACR.23752
54. Serper M, Barankay I, Chadha S, Shults J, Jones LS, Olthoff KM, et al. A randomized, controlled, behavioral intervention to promote walking after abdominal organ transplantation: results from the LIFT study. Transpl Int. (2020) 33:632. doi: 10.1111/TRI.13570
55. Goode AD, Reeves MM, Eakin EG. Telephone-delivered interventions for physical activity and dietary behavior change: an updated systematic review. Am J Prev Med. (2012) 42:81–8. doi: 10.1016/j.amepre.2011.08.025
56. Goode AD, Lawler SP, Brakenridge CL, Reeves MM, Eakin EG. Telephone, print, and web-based interventions for physical activity, diet, and weight control among cancer survivors: a systematic review. J Cancer Surviv. (2015) 9:660–82. doi: 10.1007/S11764-015-0442-2
57. Foccardi G, Vecchiato M, Neunhaeuserer D, Mezzaro M, Quinto G, Battista F, et al. Effectiveness of text messaging as an incentive to maintain physical activity after cardiac rehabilitation: a randomized controlled pilot study. Int J Environ Res Public Health. (2021) 18:6645. doi: 10.3390/IJERPH18126645
58. Yu T, Xu H, Sui X, Zhang X, Pang Y, Yu T, et al. Effectiveness of eHealth interventions on moderate-to-vigorous intensity physical activity among patients in cardiac rehabilitation: systematic review and meta-analysis. J Med Internet Res. (2023) 25. doi: 10.2196/42845
59. Deng L, Wu Q, Ding F, Liu Y, Shen J, Lin Y, et al. The effect of telemedicine on secondary prevention of atherosclerotic cardiovascular disease: a systematic review and meta-analysis. Front Cardiovasc Med. (2022) 9:1020744. doi: 10.3389/FCVM.2022.1020744
60. Turner J, Clanchy K, Vincze L. Telehealth interventions for physical activity and exercise participation in postpartum women: a quantitative systematic review. Prev Med (Baltim). (2023) 167:107413. doi: 10.1016/J.YPMED.2022.107413
Keywords: KTR, exercise, physical activity, transplantation, telemedicine, device, tele-health
Citation: Vecchiato M, Duregon F, Zanardo E, Baioccato V, Quinto G, Livio A, Mazzucato B, Sarri C, Bellis L, Carella C, Cardillo M, Neunhaeuserer D, Ermolao A and Battista F (2024) Tailored exercise with telehealth monitoring improves adherence and global health in kidney transplant recipients. Front. Sports Act. Living 6:1436742. doi: 10.3389/fspor.2024.1436742
Received: 22 May 2024; Accepted: 12 August 2024;
Published: 3 September 2024.
Edited by:
David Michael Bellar, University of West Florida, United StatesReviewed by:
Jared M. Gollie, United States Department of Veterans Affairs, United StatesCopyright: © 2024 Vecchiato, Duregon, Zanardo, Baioccato, Quinto, Livio, Mazzucato, Sarri, Bellis, Carella, Cardillo, Neunhaeuserer, Ermolao and Battista. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emanuele Zanardo, ZW1hbnVlbGUuemFuYXJkb0BzdHVkZW50aS51bmlwZC5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.