
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sports Act. Living, 15 July 2024
Sec. Physical Activity in the Prevention and Management of Disease
Volume 6 - 2024 | https://doi.org/10.3389/fspor.2024.1393332
This article is part of the Research TopicInnovative Approaches to Exercise Assessment and Prescription in Non-Communicable DiseasesView all 7 articles
 Kenneth A. Taylor1,2
Kenneth A. Taylor1,2 Megan K. Carroll3*
Megan K. Carroll3* Sarah A. Short3
Sarah A. Short3 Bettia E. Celestin4
Bettia E. Celestin4 Adam Gilbertson5
Adam Gilbertson5 Christoph B. Olivier6
Christoph B. Olivier6 Francois Haddad7,8,9
Francois Haddad7,8,9 Nicholas Cauwenberghs10
Nicholas Cauwenberghs10
Objectives: Physical performance tests are predictive of mortality and may screen for certain health conditions (e.g., sarcopenia); however, their diagnostic and/or prognostic value has primarily been studied in age-limited or disease-specific cohorts. Our objective was to identify the most salient characteristics associated with three lower quarter balance and strength tests in a cohort of community-dwelling adults.
Methods: We applied a stacked elastic net approach on detailed data on sociodemographic, health and health-related behaviors, and biomarker data from the first visit of the Project Baseline Health Study (N = 2,502) to determine which variables were most associated with three physical performance measures: single-legged balance test (SLBT), sitting-rising test (SRT), and 30-second chair-stand test (30CST). Analyses were stratified by age (<65 and ≥65).
Results: Female sex, Black or African American race, lower educational attainment, and health conditions such as non-alcoholic fatty liver disease and cardiovascular conditions (e.g., hypertension) were consistently associated with worse performance across all three tests. Several other health conditions were associated with either better or worse test performance, depending on age group and test. C-reactive protein was the only laboratory value associated with performance across age and test groups with some consistency.
Conclusions: Our results highlighted previously identified and several novel salient factors associated with performance on the SLBT, SRT, and 30CST. These tests could represent affordable, noninvasive biomarkers of prevalent and/or future disease in adult individuals; future research should validate these findings.
Clinical Trial Registration: ClinicalTrials.gov, identifier NCT03154346, registered on May 15, 2017.
A person's ability to perform daily living activities—physical function—can worsen by disease or injury and is affected by clinical and sociodemographic factors associated with unfavorable health-related behavior (1). Declines in physical function are often subtle and unnoticed until they reach a stage at which interventions may be less effective, hence the importance of identifying subclinical declines in a timely fashion (2–5).
Standardized clinical tests serve this purpose, enabling affordable and objective quantification of different aspects of physical function, including control of static posture and balance, musculoskeletal fitness and strength, and overall mobility. Several physical function tests can be indicators of prevalent health conditions and predictive of downstream health outcomes and all-cause mortality, particularly in older individuals (6–15).
To date, relationships between physical performance tests and specific clinical characteristics have primarily been studied in age-limited or diagnosis-specific cohorts, which are inherently limited to identify wide range relationships (16). Data from broadly-defined cohorts could reveal novel and comprehensive associations between physical function tests and individual characteristics. Therefore, we aimed to identify the most salient characteristics associated with the three lower quarter physical performance tests (single-legged balance [SLBT], sitting-rising [SRT], and 30-s chair-stand [30CST]), from sociodemographic and health features as recorded in a deeply phenotyped cohort of community-dwelling adults, stratified by age (≥65 years-old and <65 years-old).
In this study, we used data from The Project Baseline Health Study (PBHS). The PBHS is a multicenter longitudinal cohort study of adults in the United States (ClinicalTrials.gov identifier NCT03154346), approved by both a central Institutional Review Board (the WCG IRB; approval tracking number 20170163, work order number 1-1506365-1) and IRBs at each of the participating institutions: Stanford University (Palo Alto, CA), Duke University (Durham, NC; Kannapolis, NC), and the California Health and Longevity Institute (CHLI; Los Angeles, CA). Participants' deep phenotyping included data on demographic characteristics, socioeconomic status-related health behaviors, medical conditions, symptoms, and laboratory-collected biomarkers in addition to physical function markers and a range of patient-reported outcome measures. Full description of study procedures and design, inclusion and exclusion criteria, and IRB approval have been previously reported (17, 18).
This study used cross-sectional data from the first in-person study collection time point; self-reported data were either recorded in-person with assistance from study personnel, or collected via remote application. We limited data that were only collected remotely to participant responses within 200 days of participants' initial in-person data collection.
The SLBT assesses static postural and balance control with moderate-to-excellent test-retest reliability and excellent inter-rater reliability (19, 20). To perform the SLBT, participants were instructed to place their hands on their hips with their eyes open while focusing on a point straight ahead and stand on one leg while raising the other. The time was recorded from when one foot was lifted from the floor until whichever occurred first: the lifted foot touched the stance leg or the ground, the stance foot moved on the floor, either hand left the hips, or 60 s had passed. The test was performed on each leg one time and the SLBT time recorded was the mean of the left and right leg balance durations. Poor SLBT performance may help in predicting falls (21, 22) and inability to achieve at least 10 s has been linked to all-cause mortality (14).
The SRT was developed to assess muscle strength and power, flexibility, and balance components of physical fitness through evaluating an individual's ability to transfer from standing to sitting on the floor and then rise from the floor back to standing (23). The test has also been shown to predict all-cause mortality in a cohort of adults aged 51–80 years-old (15). To perform the SRT, participants were placed on a non-slippery flat surface in a minimum space of 2 × 2 m without wearing shoes. The basic movements of the test were explained to the participant before the participants were instructed, “without worrying about the speed of movement, try to sit then to rise from the floor using the minimum support that you believe is needed.” The test was scored based on performance, with a maximum of 10 points—5 points for transferring from standing to sitting on floor and 5 points transferring from sitting on the floor back to standing. Each participant started with a score of 5 for each movement from which 1.0 point was deducted for each time a hand, forearm, knee, side of the leg (but not the sides of the feet), or hand on the knee was used and 0.5 points deducted for each time a partial loss of balance was observed. Participants who did not obtain a full score on either the sitting or rising portions of the test were provided additional advice or demonstration of the action by site staff and allowed to perform additional attempts to improve their scores. Regardless of the number of attempts performed, only the best scores for each sitting and rising attempt were recorded. Despite the rater-based scoring, SRT has shown high inter-rater reliability (24).
The 30CST is a measure of functional lower extremity strength with excellent criterion, interrater, and test-retest reliability (25). To perform the 30CST, participants were positioned in a 17-in. high straight-backed armless chair and instructed to keep their feet flat on the floor and arms crossed over their chest during the test. Upon hearing “go” participants were instructed to rise to a full standing position and then back down to sitting for 30 s. The test is scored by recording the number of full stands completed in 30 s. Being >50% to a full standing position at the end of the 30 s was counted as a full stand. Since the test is scored using stand count, the 30CST does not have the same floor effect as other standing tests that are scored by recording time-to-completion for a specific number of stands (e.g., 5-time sit-to-stand) (26).
Data collected from PHBS participants has been previously reported (17). We excluded variables with age-specific prevalence ≤1% from regression analyses. In participants aged <65 years, this removed 13 medical conditions from the list of candidate variables: atrial fibrillation, benign prostate hyperplasia, prostate cancer, breast cancer, hepatitis B, myocardial infarction, macular degeneration, melanoma, pulmonary embolism/deep vein thrombosis, peripheral vascular disease, stroke, transient ischemic attack, and goiter. In participants aged ≥65 years, we removed 6 conditions: bipolar disorder, type I diabetes mellitus, drug abuse, fibromyalgia, hepatitis C, and posttraumatic stress disorder. We applied Box-Cox transformations to each laboratory value to approximate Gaussian distributions. We present an ascertainment and definition summary of eligible variables used in our analyses in the Supplementary Material.
We summarized clinical and demographic characteristics for participants with completed physical performance tests. All analyses were stratified by age and models were estimated separately for younger (<65 years) and older (≥65 years) participants. The SLBT was dichotomized for adults aged <65 years as either achieving the maximum time (60 s) or not (<60 s) based on the high proportion of individuals in this subgroup hitting the 60-s ceiling. SLBT was kept as continuous for adults aged ≥65 years-old, which had a wider distribution in this subgroup. The SRT and 30CST did not demonstrate similar ceiling effects and were left as continuous variables across age subgroups. We used multiple imputation by chained equations to estimate values for missing data and fit regression models using elastic net (ENET) regularization methods. We employed ENET using a stacked objective function (sENET) with 5-fold cross-validation to penalize and select regression coefficients modeling each physical performance measure (27, 28). A more detailed description of the sENET methods we used can be found in the Supplementary Material. We performed a sensitivity analysis that added self-reported symptoms to the list of eligible covariate candidates. Additional details and reasoning are presented in the Supplementary Material.
This report follows STROBE guidelines and complies with the STROBE cohort checklist (29).
Of the 2,502 participants in the PBHS cohort, 2,339 (93.5%), 2,198 (87.9%), and 2,410 (96.3%) completed SLBT, SRT, and 30CST at the initial visit, respectively. Of these, 23.6% (SLBT), 22.2% (SRT), and 23.0% (30CST) were aged ≥65 years. For participants aged <65 years, mean (SD) physical performance test scores were 47 (20) seconds for SLBT, 7.7 (2.1) points for SRT, and 15 (5.2) stands for 30CST. For participants ≥65 years of age, mean (SD) test scores were 23 (20) seconds, 5.4 (2.3) points, and 14 (5.0) stands for SLBT, SRT, and 30CST, respectively (Figure 1 and Table 1).
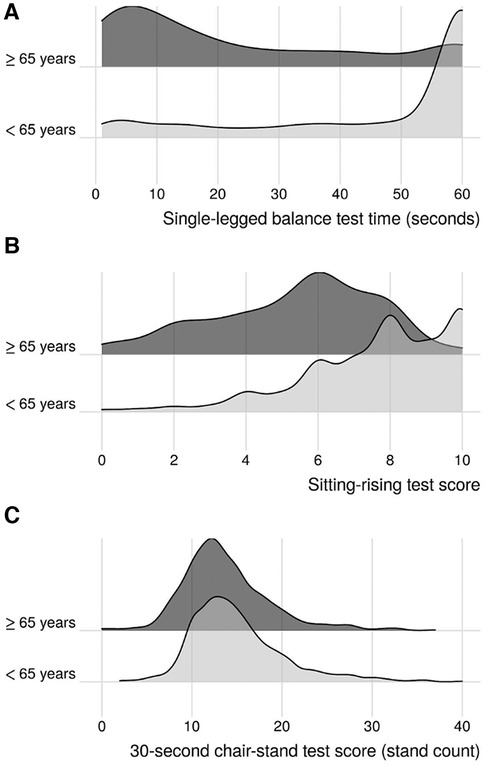
Figure 1 Kernel density ridgeline plots of age-stratified physical performance results. (A) Single-legged balance test; (B): sitting-rising test; (C): 30-s chair-stand test.
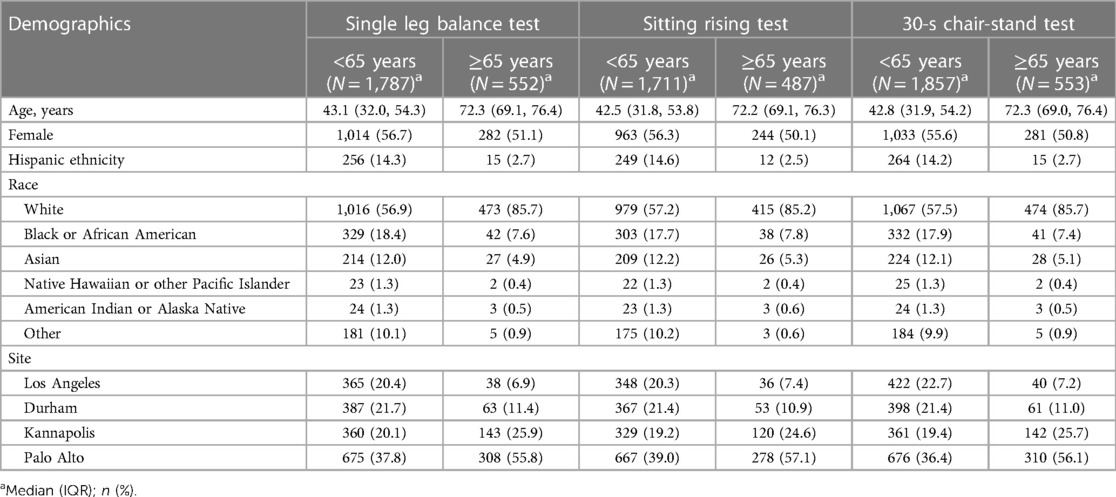
Table 1 Summary of age-stratified demographic characteristics by lower quarter physical performance test.
To focus results and avoid reporting on variables with small coefficients in the final models, we present the top 20 candidate characteristics from sENET results. Models for single-legged balance and sitting-rising test scores were optimized to LASSO regressions (ɑ = 1), while models for 30CST were optimized to ENET regression (ɑ = 0.5).
Across both age groups, sociodemographic factors positively associated with SLBT performance included identifying as Asian or being married. In participants aged <65, identifying as Black or African American or having high school or less as highest educational attainment was also associated with lower odds of achieving the 60-second SLBT ceiling. Among those >65 years-old, continuous age remained an important factor negatively associated with SLBT performance (Figures 2A,B).
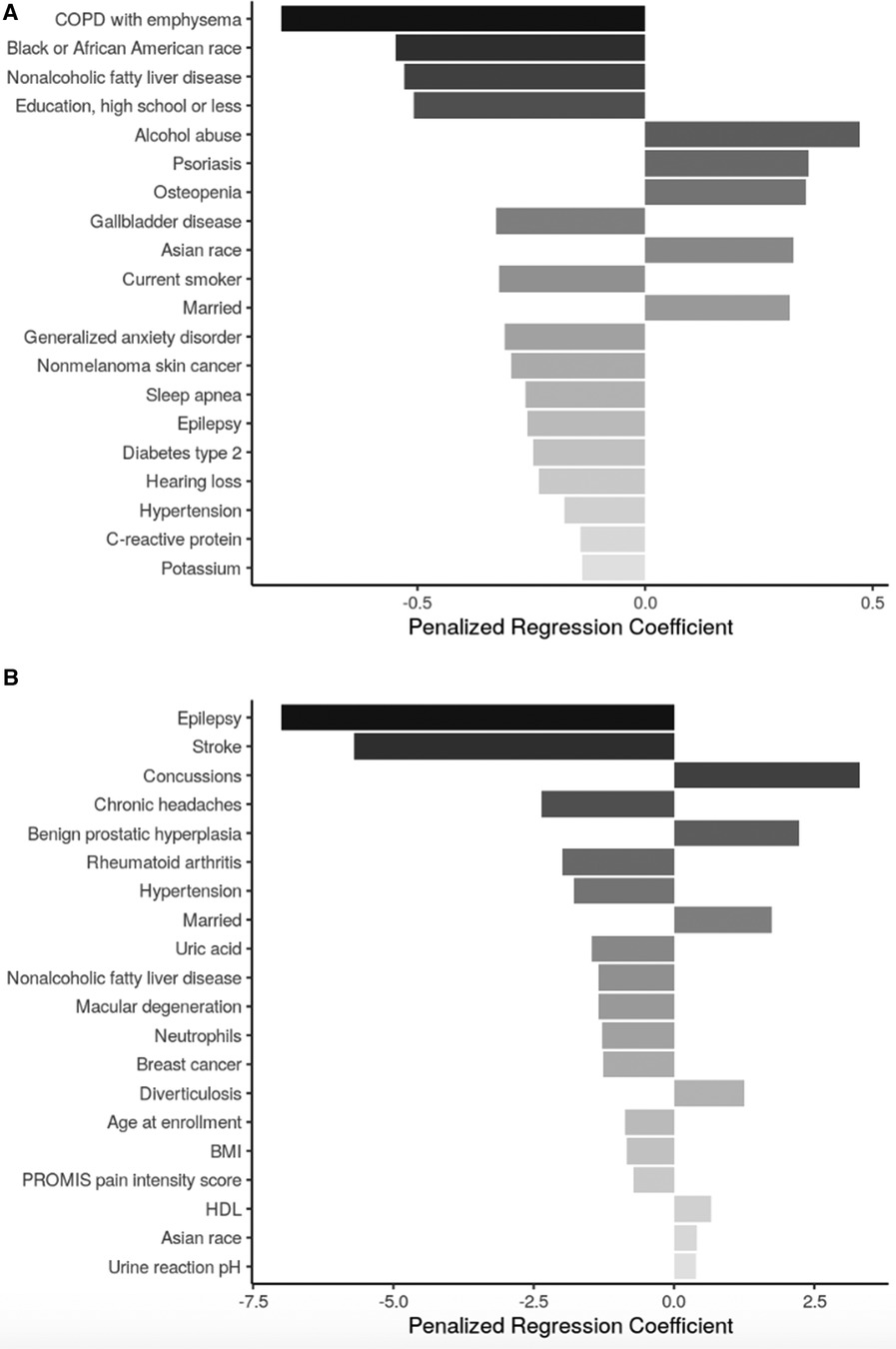
Figure 2 Top 20 regression coefficients of features selected from sENET regression model for single legged balance test. (A) Participants <65 years of age (ɑ = 1, λ = 0.0025); (B): participants ≥65 years of age (ɑ = 1, λ = 0.334). PTSD, posttraumatic stress disorder; BMI, body mass index; HDL, high density lipoprotein.
Health conditions and behaviors that were associated with worse SLBT performance across both age groups were non-alcoholic fatty liver disease (NAFLD), hypertension, and epilepsy. In adults <65 years-old, chronic obstructive pulmonary disease (COPD) with emphysema, gallbladder disease, being a current smoker, generalized anxiety disorder, and nonmelanoma skin cancer, sleep apnea, and type II diabetes were among the factors associated with lower odds of achieving the 60-s SLBT ceiling while alcohol abuse, psoriasis, and osteopenia were among those associated with increased odds of high SLBT performance. Among adults aged ≥65 years, stroke, chronic headaches, rheumatoid arthritis, macular degeneration, and breast cancer were all associated with lower SLBT times while concussions, benign prostatic hyperplasia, and diverticulosis were associated with higher SLBT times.
Biomarkers associated with SLBT performance in adults <65 years-old included C-reactive protein (CRP; associated with decreased odds of achieving 60 s). In adults aged ≥65 years, body mass index (BMI), uric acid, and neutrophil levels were negatively associated with SLBT time, while high-density lipoprotein (HDL) and urine pH levels were positively associated with SLBT time.
Sociodemographic factors negatively associated with SRT scores across both age subgroups were female sex and age, treated as a continuous measure beyond the stratification. Additional sociodemographic factors associated with lower SRT scores among participants aged <65 were annual income <$25,000 and identifying as Black or African American race. The only additional sociodemographic factor in the SRT model for participants aged ≥65 years was hispanic ethnicity, which was also associated with a lower SRT score (Figures 3A,B).
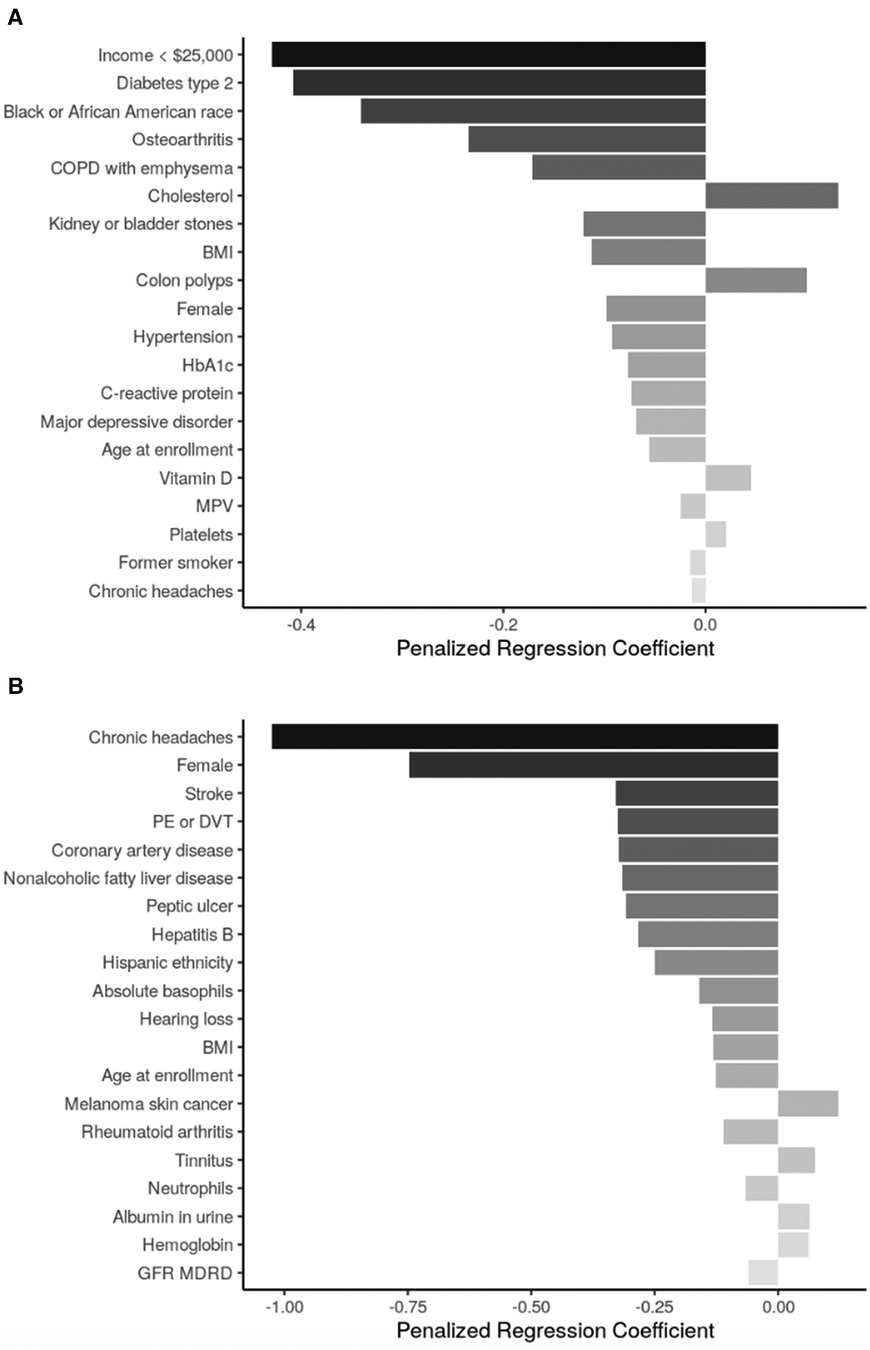
Figure 3 Top 20 regression coefficients of features selected from sENET regression model for sitting rising score. (A) Participants <65 years of age (ɑ = 1, λ = 0.023); (B): participants ≥65 years of age (ɑ = 1, λ = 0.038). BMI, body mass index; HbA1c, hemoglobin A1c; MPV = ; PE, pulmonary embolism; DVT, deep venous thrombosis; GFR MDRD, estimated glomerular filtration rate, modification of diet in renal disease study equation.
Health conditions and behaviors that were associated with worse SRT scores across both age groups were chronic headaches. Among participants aged <65 years, type 2 diabetes mellitus, osteoarthritis, COPD, hypertension, and major depressive disorder were associated with lower SRT score; conversely, colon polyps were associated with higher SRT score. Among participants aged ≥65 years, many health conditions including stroke, pulmonary embolism/deep venous thrombosis, coronary artery disease, NAFLD, peptic ulcer, and Hepatitis B were associated with lower SRT score while melanoma skin cancer and tinnitus were associated with higher SRT score.
BMI was negatively associated with SRT score in both age groups. Additional biomarkers negatively associated with SRT scores in participants <65 included HbA1c, CRP, and mean platelet volume, while cholesterol, Vitamin D, and platelet count had positive associations with SRT scores in this age group. Among participants ≥65 years, increased basophil count, neutrophil count, and glomerular filtration rate were associated with lower SRT scores, while increased urine albumin and hemoglobin were associated with higher SRT scores.
The only sociodemographic factor shared across both age subgroups in the models for the 30CST was being Black or African American (associated with lower stand count). Among adults aged <65 years, other sociodemographic characteristics in the model were Asian race (associated with higher stand count) and being unemployed (associated with lower stand count). Among adults aged ≥65 years, additional sociodemographic factors in the 30CST model were having high school or less as highest education attainment, which was associated with lower stand count, while being married was associated with higher stand count (Figures 4A,B).
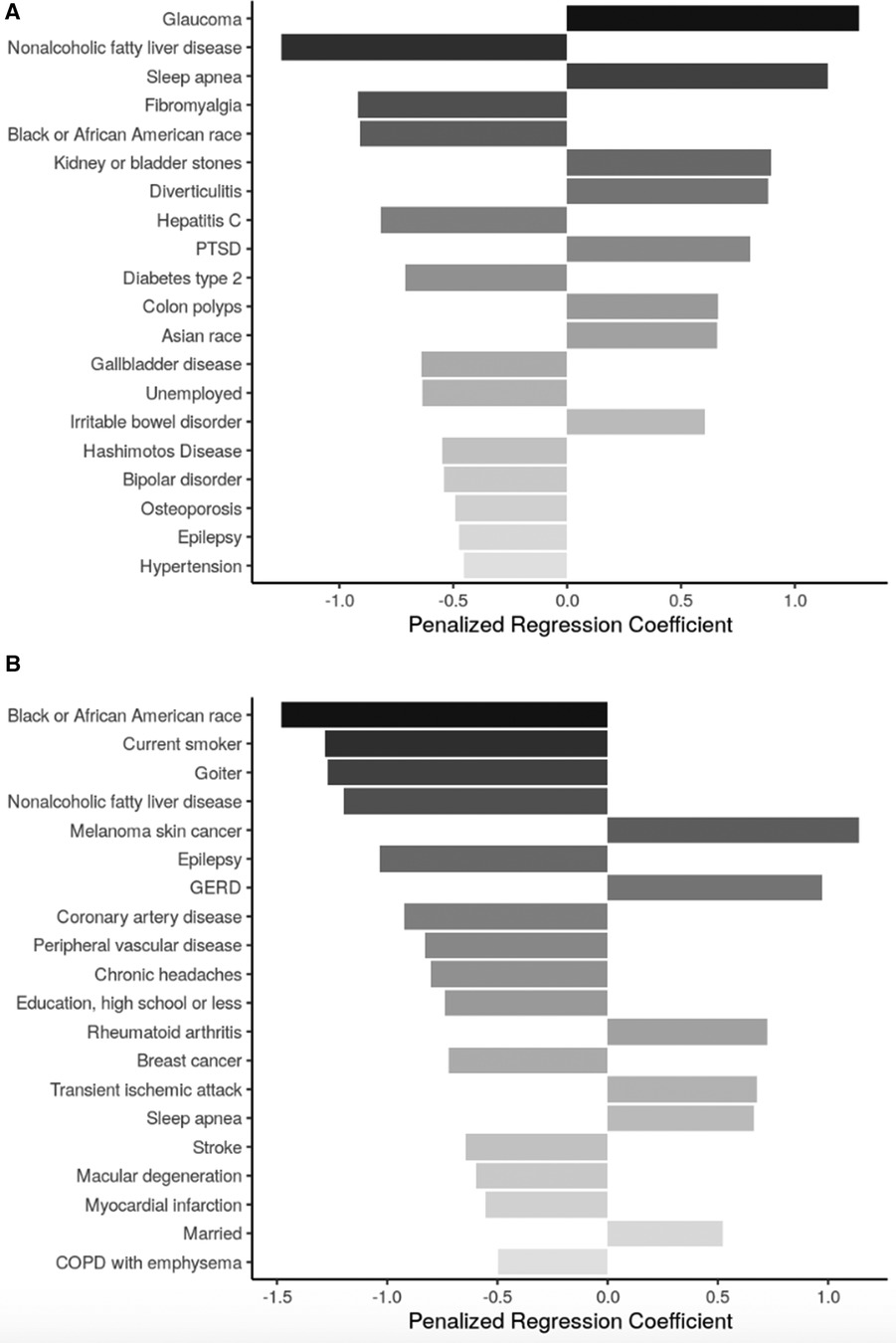
Figure 4 Top 20 regression coefficients of features selected from sENET regression model for 30-s chair stand test. (A) Participants <65 years of age (ɑ = 0.5, λ = 0.0238); (B): participants ≥65 years of age (ɑ = 0.5, λ = 0.0752). PTSD, posttraumatic stress disorder; GERD, gastroesophageal reflux disease; COPD, chronic obstructive pulmonary disease.
Health conditions and behaviors that were associated with lower 30CST count across both age groups were NALFD and epilepsy. Sleep apnea was also present in the models for both age subgroups, but was associated with improved 30CST performance. Among participants aged <65 years, glaucoma, kidney or bladder stones, diverticulitis, PTSD, colon polyps, and IBD were also associated with a higher stand count while fibromyalgia, hepatitis C, type 2 diabetes mellitus, gallbladder disease, and Hashimoto's disease were among the conditions associated with lower stand count. Among participants aged ≥65 years, melanoma skin cancer, GERD, rheumatoid arthritis, and transient ischemic attack were associated with higher stand count while a variety of conditions, including being a current smoker, goiter, epilepsy, coronary artery disease, peripheral vascular disease, breast cancer, and stroke were associated with lower stand count.
Neither of the models for 30CST identified any biomarkers as one of the top 20 features selected.
We present results from sensitivity analyses in the Supplementary Material.
This study used a deeply phenotyped cohort to identify variables possibly associated with three physical performance tests. We found associations that stood out across performance tests and age-stratification, including sociodemographic characteristics, health conditions/behaviors, and biomarker values. After selecting top-five features across tests and age groups, being Black or African American was a top-five candidate feature across performance tests among participants aged <65 years, and NAFLD was a consistently top-five feature among participants aged ≥65.
In general, physical function measures were slightly higher in the PBHS cohort than age-based reference values for SLBT, SRT and 30CST in previous reports based on community-dwelling adults (primarily aged ≥60 years) and in disease-based populations. More specifically, the SLBT time was higher across both age groups compared to previously published data (20, 29). For the 30CST, the mean stand count among adults aged ≥65 was only slightly higher than a prior reference (30). Prior data are scarce for comparisons in the <65 year group, but the distributions of the 30CST results were relatively similar across age-stratified groups. While age-based median SRT values have been reported, direct comparability with our is limited (23).
We found several sociodemographic characteristics associated with physical performance, for example: race and ethnicity, income level, and educational attainment. These could be considered consistent with previous research, particularly if we assume that general physical activity is closely related to physical performance measures. For instance, there are prior reports of higher educational attainment associated with physically active lifestyles (31–33). Substantial physical activity disparities by race and income have also been previously reported for adolescents and young adults (34). Taken together, this research underlines the complex relationship between physical performance, sociodemographics and social determinants of health. Additional research is warranted to understand the impact, if any, of targeted physical activity interventions on the associations between physical performance and sociodemographic factors.
Our results confirm previous findings associating reduced physical function with health status, which highlight the potential to identify subclinical disease in patients at risk. For example, the associations of both SLBT and SRT with hypertension (age <65) and with history of stroke or coronary artery disease (age ≥65) indicate a potential value in predicting cardiovascular disease conditions or events.
NAFLD was notably associated with worse performance on the SLBT, SRT, and 30CST and was a top associated factor across both age groups. NAFLD is over two-times more prevalent among adults who are overweight (75%) or obese (76%) (35). Future research could validate the role of SLBT, SRT, and 30CST in low-cost screening prior to NAFLD full diagnostic testing.
Among those aged ≥65, each of the physical performance measures was negatively associated with experiencing chronic headaches. Although previous research indicates chronic headaches tend to have negative effects on physical activity (36), it is unclear why this consistent relationship was limited to those ≥65 years-old, pending future research.
Biomarkers associated with each of the three physical performance tests had limited commonalities across tests and age-groups. While only associated with two physical performance tests in the younger population (SLBT and SRT), CRP was shown to be negatively associated with each. CRP is known as a marker of inflammation (37), either acute due to infectious disease or chronic due to other disease states, such as atherosclerotic cardiovascular disease (38), both of which can impact physical function.
We also found a negative association between BMI and SLBT scores (in the older population) and SRT scores (in both age groups). Prior research has similarly reported on an inverse relationship between body composition and physical function measures (39–41).
First, this is an exploratory study, cross-sectional in nature, aimed at identifying associations without implying causality (42). Follow-up studies could determine and validate the individual diagnostic or prognostic predictive value SLBT, SRT, and 30CST related to these novel associations, but determining causal effects will require an alternative methodological approach (43). Second, while the PBHS participants are overall representative of U.S. adult age, sex, race, and ethnicity (18), recruitment sites were limited to two states (California and North Carolina) which may limit generalizability. Third, analyses were stratified into two age-based groups (<65 and ≥65 years) only, due to sample size limitations; further research is warranted to identify associations specific to more granular age subgroups.
In this study, we identified several novel salient factors with the SLBT, SRT, and 30CST when stratifying by age—many of them common across the three tests. Our results support the need for further investigation of the potential for these physical performance tests as affordable, noninvasive biomarkers of prevalent conditions and their predictive utility for incident health conditions in later life (e.g., cardiovascular disease and/or events) in community-dwelling adults. Further research would also be warranted to estimate the direction and magnitude of any causal relationships between these physical function tests and novel factors identified where biologically plausible.
The de-identified Project Baseline Health Study (PBHS) data corresponding to this study are available upon request for the purpose of examining its reproducibility. Requests are subject to approval by PBHS governance. Requests to access the datasets should be directed tobWVnYW5rY2Fycm9sbEB2ZXJpbHkuY29t.
The studies involving humans were approved by both a central Institutional Review Board (the WCG IRB; approval tracking number 20170163, work order number 1-1506365-1) and IRBs at each of the participating institutions: Stanford University (Palo Alto, CA), Duke University (Durham, NC; Kannapolis, NC), and the California Health and Longevity Institute (CHLI; Los Angeles, CA). ClinicalTrials.gov identifier NCT03154346. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
KT: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. MC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. SS: Data curation, Methodology, Validation, Writing – review & editing. BC: Resources, Writing – review & editing. AG: Writing – review & editing. CO: Writing – review & editing. FH: Writing – review & editing. NC: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The Project Baseline Health Study and this analysis are funded by Verily Life Sciences, San Francisco, CA. This analysis was funded by Verily Life Sciences (South San Francisco, CA) in partnership with Stanford University and Duke University. The employees from the funding source contributed to the data analyses, interpretation, editing of the manuscript and are co-authors. The final decision to submit the manuscript was made by the academic authors.
Project Baseline Health Study Team: American Society of Clinical Oncology, Alexandria, VA, USA: Richard L. Schilsky. Duke University, School of Medicine, Durham, NC, USA: Jennifer Allen, MaryAnn Anderson, Kevin Anstrom, Lucus Araujo, Kristine Arges, Kaveh Ardalan, Bridget Baldwin, Suresh Balu, Mustafa R. Bashir, Manju Bhapkar, Robert Bigelow, Tanya Black, Rosalia Blanco, Gerald Bloomfield, Durga Borkar, Leah Bouk, Ebony Boulware, Nikki Brugnoni, Erin Campbell, Paul Campbell, Larry Carin, Tammy Jo Cassella, Tina Cates, Ranee Chatterjee Montgomery, Victoria Christian, John Choong, Michael Cohen-Wolkowiez, Elizabeth Cook, Scott Cousins, Ashley Crawford, Nisha Datta, Melissa Daubert, James Davis, Jillian Dirkes, Isabelle Doan, Marie Dockery, P. Murali Doraiswamy, Pamela S. Douglas, Shelly Duckworth, Ashley Dunham, Gary Dunn, Ryan Ebersohl, Julie Eckstrand, Vivienne Fang, April Flora, Emily Ford, Lucia Foster, Elizabeth Fraulo, John French, Geoffrey S. Ginsburg, Cindy Green, Latoya Greene, Jeffrey Guptill, Donna Hamel, Jennifer Hamill, Chris Harrington, Rob Harrison, Lauren Hedges, Brooke Heidenfelder, Adrian F. Hernandez, Cindy Heydary, Tim Hicks, Lina Hight, Deborah Hopkins, Erich S. Huang, Grace Huh, Jillian Hurst, Kelly Inman, Gemini Janas, Glenn Jaffee, Janace Johnson, Tiffanie Keaton, Michel Khouri, Daniel King, Jennifer Korzekwinski, Lynne H. Koweek, Anthony Kuo, Lydia Kwee, Dawn Landis, Rachele Lipsky, Desiree Lopez, Carolyn Lowry, Kelly Marcom, Keith Marsolo, Paige McAdams, Shannon McCall, Robert McGarrah, John McGugan, Dani Mee, Sabrena Mervin-Blake, Prithu Mettu, Mathias Meyer, Justin Meyers, Calire N. Miller, Rebecca Moen, Lawrence H. Muhlbaier, Michael Murphy, Ben Neely, L. Kristin Newby, Jayne Nicoldson, Hoang Nguyen, Maggie Nguyen, Lori O’Brien, Sumru Onal, Jeremey O'Quinn, David Page, Neha J. Pagidipati, Kishan Parikh, Sarah R. Palmer, Bray Patrick-Lake, Brenda Pattison, Michael Pencina, Eric D. Peterson, Jon Piccini, Terry Poole, Tom Povsic, Alicia Provencher, Dawn Rabineau, Annette Rich, Susan Rimmer, Fides Schwartz, Angela Serafin, Nishant Shah, Svati Shah, Kelly Shields, Steven Shipes, Peter Shrader, Jon Stiber, Lynn Sutton, Geeta Swamy, Betsy Thomas, Sandra Torres, Debara Tucci, Anthony Twisdale, Susan A. Whitney, Robin Williamson, Lauren Wilverding, Charlene A. Wong, Lisa Wruck. Ellen Young Gemini Group, USA: Jane Perlmutter. Health Collaboratory and Cancer 101, New York, NY, USA: Sarah Krug. Rare Dots, Inc., USA: S. Whitney Bowman-Zatzkin. Society of Participatory Medicine, USA: Sarah Krug. Stanford University, School of Medicine, Stanford, CA, USA: Themistocles Assimes, Vikram Bajaj, Maxwell Cheong, Millie Das, Manisha Desai, Alice C. Fan, Dominik Fleischmann, Sanjiv S. Gambhir, Garry Gold, Francois Haddad, David Hong, Curtis Langlotz, Yaping J. Liao, Rong Lu, Kenneth W. Mahaffey, David Maron, Rebecca McCue, Rajan Munshi, Fatima Rodriguez, Sumana Shashidhar, George Sledge, Susie Spielman, Ryan Spitler, Sue Swope, Donna Williams, Julio C. Nunes. University of Florida, College of Medicine, Gainesville, FL, USA: Carl J Pepine. University of Missouri, Children's Mercy Hospital, Kansas City, MO, USA: John D Lantos. University of Texas, Dell Medical School, Austin, TX, USA: Michael Pignone. University of Washington, Department of Biostatistics, Seattle, WA, USA: Patrick Heagerty. Vanderbilt University, School of Medicine, Nashville, TN, USA: Laura Beskow, Gordon Bernard. Verily Inc., South San Francisco, CA, USA: Kelley Abad, Giulia Angi, Robert M. Califf, Lawrence Deang, Joy Huynh, Manway Liu, Cherry Mao, Michael Magdaleno, William J. Marks, Jr., Jessica Mega, David Miller, Nicole Ong, Darshita Patel, Vanessa Ridaura, Scarlet Shore, Sarah Short, Michelle Tran, Veronica Vu, Celeste Wong. Harvard University, School of Medicine, Boston, MA, USA: Robert C. Green. Google Inc., Mountain View, CA, USA: John Hernandez. California Health and Longevity Institute, Westlake Village, CA, USA: Jolene Benge, Gislia Negrete, Gelsey Sierra, Terry Schaack.
MC and SS are employees of Verily Life Sciences.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fspor.2024.1393332/full#supplementary-material
1. Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. (1983) 31:721–7. doi: 10.1111/j.1532-5415.1983.tb03391.x
2. Sciamanna CN, Danilovich MK. A call for screening for physical dysfunction in clinical practice. Prev Med Rep. (2020) 20:101258. doi: 10.1016/j.pmedr.2020.101258
3. Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. (2014) 311:2387–96. doi: 10.1001/jama.2014.5616
4. Araujo de Carvalho I, Epping-Jordan J, Pot AM, Kelley E, Toro N, Thiyagarajan JA, et al. Organizing integrated health-care services to meet older people’s needs. Bull World Health Organ. (2017) 95:756–63. doi: 10.2471/BLT.16.187617
5. Tavassoli N, de Souto Barreto P, Berbon C, Mathieu C, de Kerimel J, Lafont C, et al. Implementation of the WHO integrated care for older people (ICOPE) programme in clinical practice: a prospective study. Lancet Healthy Longev. (2022) 3:e394–404. doi: 10.1016/S2666-7568(22)00097-6
6. Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, et al. Added value of physical performance measures in predicting adverse health-related events: results from the health, aging and body composition study. J Am Geriatr Soc. (2009) 57:251–9. doi: 10.1111/j.1532-5415.2008.02126.x
7. Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. (2007) 55:1727–34. doi: 10.1111/j.1532-5415.2007.01413.x
8. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. (1994) 49:M85–94. doi: 10.1093/geronj/49.2.m85
9. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. (1995) 332:556–61. doi: 10.1056/NEJM199503023320902
10. Cesari M, Kritchevsky SB, Penninx BWHJ, Nicklas BJ, Simonsick EM, Newman AB, et al. Prognostic value of usual gait speed in well-functioning older people–results from the health, aging and body composition study. J Am Geriatr Soc. (2005) 53:1675–80. doi: 10.1111/j.1532-5415.2005.53501.x
11. Cesari M, Penninx BWJH, Pahor M, Lauretani F, Corsi AM, Williams GR, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. (2004) 59:242–8. doi: 10.1093/gerona/59.3.m242
12. Tinetti ME. Shared risk factors for falls, incontinence, and functional dependence. JAMA. (1995) 273:1348. doi: 10.1001/jama.1995.03520410042024
13. Cesari M, Onder G, Russo A, Zamboni V, Barillaro C, Ferrucci L, et al. Comorbidity and physical function: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study). Gerontology. (2006) 52:24–32. doi: 10.1159/000089822
14. Araujo CG, de Souza e Silva CG, Laukkanen JA, Fiatarone Singh M, Kunutsor SK, Myers J, et al. Successful 10-second one-legged stance performance predicts survival in middle-aged and older individuals. Br J Sports Med. (2022) 56:975–80. doi: 10.1136/bjsports-2021-105360
15. Brito LBB de, Ricardo DR, Araújo DSMS de, Ramos PS, Myers J, Araújo CGS de. Ability to sit and rise from the floor as a predictor of all-cause mortality. Eur J Prev Cardiol. (2014) 21:892–8. doi: 10.1177/2047487312471759
16. Garber CE, Greaney ML, Riebe D, Nigg CR, Burbank PA, Clark PG. Physical and mental health-related correlates of physical function in community dwelling older adults: a cross sectional study. BMC Geriatr. (2010) 10:6. doi: 10.1186/1471-2318-10-6
17. Arges K, Assimes T, Bajaj V, Balu S, Bashir MR, Beskow L, et al. The project baseline health study: a step towards a broader mission to map human health. NPJ Digit Med. (2020) 3:84. doi: 10.1038/s41746-020-0290-y
18. Califf RM, Wong C, Doraiswamy PM, Hong DS, Miller DP, Mega JL. Biological and clinical correlates of the patient health questionnaire-9: exploratory cross-sectional analyses of the baseline health study. BMJ Open. (2022) 12:e054741. doi: 10.1136/bmjopen-2021-054741
19. Birmingham TB. Test-retest reliability of lower extremity functional instability measures. Clin J Sport Med. (2007) 10:264–8. doi: 10.1097/00042752-200010000-00007
20. Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative values for the unipedal stance test with eyes open and closed. J Geriatr Phys Ther. (2007) 30:8–15. doi: 10.1519/00139143-200704000-00003
21. Blodgett JM, Hardy R, Davis D, Peeters G, Kuh D, Cooper R. One-legged balance performance and fall risk in mid and later life: longitudinal evidence from a British birth cohort. Am J Prev Med. (2022) 63:997–1006. doi: 10.1016/j.amepre.2022.07.002
22. Beck Jepsen D, Robinson K, Ogliari G, Montero-Odasso M, Kamkar N, Ryg J, et al. Predicting falls in older adults: an umbrella review of instruments assessing gait, balance, and functional mobility. BMC Geriatr. (2022) 22:615. doi: 10.1186/s12877-022-03271-5
23. Araújo CGS, Castro CLB, Franca JFC, Araújo DS. Sitting-rising test: sex- and age-reference scores derived from 6141 adults. Eur J Prev Cardiol. (2020) 27:888–90. doi: 10.1177/2047487319847004
24. Lira VA, Araújo DSMS, Coelho CW, Araújo CGS. Sitting-rising test—inter-observer reliability results. Med Sci Sports Exerc. (1999) 31(Supplement):S78. doi: 10.1097/00005768-199905001-00228
25. Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. (1999) 70:113–9. doi: 10.1080/02701367.1999.10608028
26. Lee YC, Chang SF, Kao CY, Tsai HC. Muscle strength, physical fitness, balance, and walking ability at risk of fall for prefrail older people. Biomed Res Int. (2022) 2022:4581126. doi: 10.1155/2022/4581126
27. Du J, Boss J, Han P, Beesley LJ, Kleinsasser M, Goutman SA, et al. Variable selection with multiply-imputed datasets: choosing between stacked and grouped methods. J Comput Graph Stat. (2022) 31:1063–75. doi: 10.1080/10618600.2022.2035739
28. Rix A, Du J. miselect: Variable Selection for Multiply Imputed Data. CRAN: Contributed Packages (2020). doi: 10.32614/cran.package.miselect
29. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
30. Chen HT, Lin CH, Yu LH. Normative physical fitness scores for community-dwelling older adults. J Nurs Res. (2009) 17:30–41. doi: 10.1097/JNR.0b013e3181999d4c
31. Kari JT, Viinikainen J, Böckerman P, Tammelin TH, Pitkänen N, Lehtimäki T, et al. Education leads to a more physically active lifestyle: evidence based on Mendelian randomization. Scan J Med Sci Sports. (2020) 30:1194–204. doi: 10.1111/sms.13653
32. Droomers M, Schrijvers CT, Mackenbach JP. Educational level and decreases in leisure time physical activity: predictors from the longitudinal GLOBE study. J Epidemiol Community Health. (2001) 55:562–8. doi: 10.1136/jech.55.8.562
33. Davies NM, Dickson M, Davey Smith G, van den Berg GJ, Windmeijer F. The causal effects of education on health outcomes in the UK biobank. Nat Hum Behav. (2018) 2:117–25. doi: 10.1038/s41562-017-0279-y
34. Armstrong S, Wong CA, Perrin E, Page S, Sibley L, Skinner A. Association of physical activity with income, race/ethnicity, and sex among adolescents and young adults in the United States: findings from the national health and nutrition examination survey, 2007–2016. JAMA Pediatr. (2018) 172:732–40. doi: 10.1001/jamapediatrics.2018.1273
35. Quek J, Chan KE, Wong ZY, Tan C, Tan B, Lim WH, et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2023) 8:20–30. doi: 10.1016/S2468-1253(22)00317-X
36. González de la Flor Á, García Pérez de Sevilla G, Domíngez Balmaseda D, Martín Vera D, Montero Martínez M, Del Blanco Muñiz JÁ. Relationship between self-efficacy and headache impact, anxiety, and physical activity levels in patients with chronic tension-type headache: an observational study. Behav Neurol. (2022) 2022:8387249. doi: 10.1155/2022/8387249
37. Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. (2011) 10:319–29. doi: 10.1016/j.arr.2010.11.002
38. Nathan C, Ding A. Nonresolving inflammation. Cell. (2010) 140:871–82. doi: 10.1016/j.cell.2010.02.029
39. Klukowska AM, Staartjes VE, Vandertop WP, Schröder ML. Five-repetition sit-to-stand test performance in healthy individuals: reference values and predictors from 2 prospective cohorts. Neurospine. (2021) 18:760–9. doi: 10.14245/ns.2142750.375
40. Woo J, Leung J, Kwok T. BMI, body composition, and physical functioning in older adults. Obesity. (2007) 15:1886–94. doi: 10.1038/oby.2007.223
41. Blodgett JM, Cooper R, Davis DHJ, Kuh D, Hardy R. Associations between factors across life and one-legged balance performance in mid and later life: evidence from a British birth cohort study. Front Sports Act Living. (2020) 2020:00028. doi: 10.3389/fspor.2020.00028
42. Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. (2013) 177(4):292–8. doi: 10.1093/aje/kws412
Keywords: project baseline health study, biomarkers, physical functional performance, single-legged balance test, sitting-rising test, 30-second chair-stand test, community-dwelling adults
Citation: Taylor KA, Carroll MK, Short SA, Celestin BE, Gilbertson A, Olivier CB, Haddad F and Cauwenberghs N (2024) Factors associated with lower quarter performance-based balance and strength tests: a cross-sectional analysis from the project baseline health study. Front. Sports Act. Living 6:1393332. doi: 10.3389/fspor.2024.1393332
Received: 28 February 2024; Accepted: 27 June 2024;
Published: 15 July 2024.
Edited by:
Beatrice Cairo, University of Milan, ItalyReviewed by:
Jorge Lopes Storniolo, University of Milan, Italy© 2024 Taylor, Carroll, Short, Celestin, Gilbertson, Olivier, Haddad and Cauwenberghs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Megan K. Carroll, bWVnYW5rY2Fycm9sbEB2ZXJpbHkuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.