
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Sports Act. Living , 05 July 2023
Sec. Biomechanics and Control of Human Movement
Volume 5 - 2023 | https://doi.org/10.3389/fspor.2023.1217009
Understanding neural control of movement necessitates a collaborative approach between many disciplines, including biomechanics, neuroscience, and motor control. Biomechanics grounds us to the laws of physics that our musculoskeletal system must obey. Neuroscience reveals the inner workings of our nervous system that functions to control our body. Motor control investigates the coordinated motor behaviours we display when interacting with our environment. The combined efforts across the many disciplines aimed at understanding human movement has resulted in a rich and rapidly growing body of literature overflowing with theories, models, and experimental paradigms. As a result, gathering knowledge and drawing connections between the overlapping but seemingly disparate fields can be an overwhelming endeavour. This review paper evolved as a need for us to learn of the diverse perspectives underlying current understanding of neuromuscular control. The purpose of our review paper is to integrate ideas from biomechanics, neuroscience, and motor control to better understand how we voluntarily control our muscles. As biomechanists, we approach this paper starting from a biomechanical modelling framework. We first define the theoretical solutions (i.e., muscle activity patterns) that an individual could feasibly use to complete a motor task. The theoretical solutions will be compared to experimental findings and reveal that individuals display structured muscle activity patterns that do not span the entire theoretical solution space. Prevalent neuromuscular control theories will be discussed in length, highlighting optimality, probabilistic principles, and neuromechanical constraints, that may guide individuals to families of muscle activity solutions within what is theoretically possible. Our intention is for this paper to serve as a primer for the neuromuscular control scientific community by introducing and integrating many of the ideas common across disciplines today, as well as inspire future work to improve the representation of neural control in biomechanical models.
A fundamental challenge facing biomechanists and neuroscientists alike is understanding neuromuscular control. For a given task, an individual can have several movement strategies available for facilitating performance (1). Each movement has a potentially infinite combination of feasible force solutions across muscles (2, 3). A single muscle can be composed of multiple anatomical regions (4–6), each with their own mechanical functions that are innervated by hundreds of sensory and motor neurons (7). How the neuromusculoskeletal system navigates this complex, abundant landscape of possibilities at several levels while producing smooth, voluntary movement with ease is remarkable. The complexity of sensorimotor control can be especially appreciated when considering the tremendous challenges that scientists and engineers encounter while attempting to design biologically inspired robots and machines (8–10). The upper limb is a particularly intricate system as it encompasses more than 70 muscles and 34 rotational degrees of freedom, enabling a wide range of movement and manual dexterity unparalleled by any other part of the human body (11, 12). A thorough understanding of neuromuscular control of the arm will aid in the design of robotic and prosthetic control, and perhaps more importantly, inform strategies for improving task performance, skill acquisition, and rehabilitative treatments for movement disorders.
Several theories and conceptual frameworks have been proposed to explain and understand neuromuscular control. Recently, Valero-Cuevas (13) and then Cohn et al. (2) presented “Feasibility Theory” as an approach grounded in biomechanical modelling that encompasses prior neuromuscular control theories (Figure 1). Feasibility Theory aims to understand neuromuscular control by defining the set of all theoretically feasible solutions for a task, exploring the structure of the solution space, and how individuals inhabit and navigate the space. When faced with a task, an individual's neuromusculoskeletal system is met with several constraints. These constraints include neural (e.g., hard-wire pathways) and mechanical factors (e.g., task demands, musculoskeletal limb properties) (14–18). Together, the constraints define the feasible solution space of muscle activations (i.e., red arrows and black polytope in Figure 1). An ongoing debate is whether muscle synergies, described as a functional unit of muscle groups with weighted co-activation, are an indirect consequence of constraints and optimality principles shaping the relationships across muscles (i.e., descriptive synergies) or are a specific constraint by the nervous system aiming to reduce control into a lower dimensional problem (i.e., prescriptive synergies) (13, 19–22). Nevertheless, the neuromusculoskeletal system can theoretically traverse the feasible solution space without any immediate impact on task performance. In other words, accounting for the neuromechanical properties of the limb and task demands, there are several ways to activate muscles with no difference in net joint torques, resulting in identical endpoint wrench (forces and torques) of the limb (13). The solution space is comparable to the uncontrolled manifold hypothesis, where the motor system can take advantage of the abundance of solutions by allowing elements (in this case, muscle activations) to freely vary within this subspace while keeping a select performance variable(s), such as endpoint forces and torques, constant (23, 24).
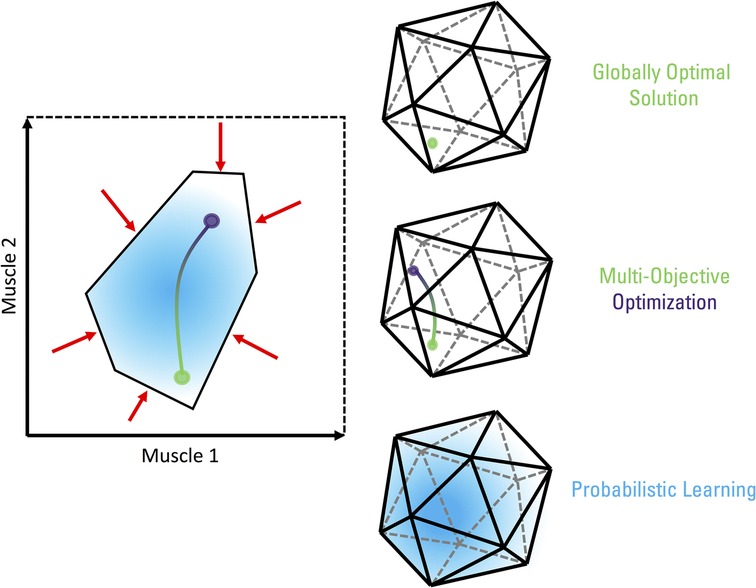
Figure 1. Conceptual visualization of a feasible solution space of muscle activations for a task at a single instant in time. The example on the left displays the activation of two muscles as part of a hypothesized limb with n muscles (n > 2) and m kinematic degrees of freedom, where n > m, as representative of human limbs. Unconstrained, the activation level for both these muscles can range from 0 (no activation) to 1 (maximum activation). Neural and mechanical factors constrain (red arrows) the activation patterns within a subset of these ranges (black polytope), as required to complete the task. The set of all muscle activation patterns within this polytope represent the feasible solution space. The feasible solution space will be a convex polytope in the case of linear constraints that do not produce a null set. Nonlinear constraints could produce nonconvex feasible solution spaces with curved boundaries. Without additional considerations, the neuromusculoskeletal system is free to vary muscle activations within this space as all feasible solutions are treated equally while keeping task performance constant. Certain solutions, however, may be preferred as guided by hypothesized sensorimotor control principles. Optimization aims to identify the single point within the feasible space that best attain the task performance under such principles. For example, most biomechanical models assume effort minimization, thus, solving for the muscle activation pattern that affords the lowest effort (green circle). In contrast, a system requiring maximum stiffness would necessitate greater muscle activations (and forces) as a means of minimizing displacements to perturbations, leading to a drastically different solution (purple circle). If both effort and stiffness are optimized (i.e., multi-objective optimization), a landscape of optimal solutions is formed depending on the relative weighting between effort and stiffness (green-purple line) as there is rarely a single feasible solution that globally optimizes multiple objectives. Note that we are assuming non-linear cost functions (as are typical in the biomechanics and motor control literature), where optimal solutions may lie inside or on the boundary of the feasible solution space. In the case of linear cost functions, optimal solutions (if they exist) will lie only on the boundary of the solution space. “Good-enough” solutions are suboptimal and are found in the neighbourhood of an optimal solution in the cost function landscape. In this case, a “good enough” solution would generate a combination of effort and stiffness costs that would be comparable to the globally optimal solution but is not the best combination that could be found. A probabilistic perspective to neuromuscular control is driven by exploring the feasible solution space with trial-and-error, such as Markov chains, simulated annealing, and genetic algorithms. If shaped by optimality principles and provided with sufficient exploration, the probability density of muscle activation patterns will converge towards optimal solutions with motor learning (blue gradient cloud). The examples on the right are visual depictions of the same neuromuscular control principles for a higher dimension feasible solution space.
It is hypothesized that all feasible solutions are not equivalent and certain solutions may be preferentially selected by overarching optimality principles. Traditionally in biomechanics and motor control, this is addressed as the degrees of freedom, redundancy, abundancy, underdetermined, indeterminacy, or load-sharing problem. It is commonly resolved using optimization (e.g., static optimization, optimal control) to determine a single solution within the feasible space that best meets a criterion of choice. Note that optimization methods using gradient-based search for minima (e.g., static optimization, optimal control) are a special case of machine learning. As described in detail by Valero-Cuevas et al. (25), computational methods that allow such explorations of feasible spaces are a combination of machine learning, control theory, and estimation detection theory. This combination has resulted in methodologies including reinforcement learning, unsupervised learning, optimal control, and many others being developed. However, as traditionally applied to neuromuscular control to find unique solutions, the choice of objective function to optimize varies but is often related to minimizing “effort” (i.e., green circle in Figure 1) (26–30). Others propose the use of multiple objective functions (31, 32), such as the additional consideration for limb stiffness (i.e., purple circle in Figure 1) (33, 34). In a multi-objective optimization, there is rarely a single global solution that will simultaneously optimize multiple objectives. This leads to a landscape of optimal solutions depending on the relative weights given to each objective (i.e., green-purple line in Figure 1). Optimal feedback control theory is a specific application of closed-loop optimal control, where a control policy (such as a gain matrix) is sought to determine the control signals (i.e., motor commands) that maximize task goals in addition to minimize effort (amongst other possible variables) while integrating state-dependent sensory (feedback) information with an internal feedforward model (35–38). What remains relatively less studied is evaluating the landscape of suboptimal solutions in the neighbourhood of the globally optimal solution (34, 39). Through exploration of the feasible solution space via trial-and-error, a probabilistic representation of motor patterns can be formed (40, 41). This probabilistic representation characterizes the degree of belief (or uncertainty) in which motor solutions may work for completing a given task. Such probabilistic representations are based on prior experiences (habitual behaviour) and can be further shaped by optimality principles. A probabilistic trial-and-error search guided by a fitness criterion functions as a Markov chain (42). Repeatedly exploring the solution space can lead to finding more rewarding solutions (e.g., less effortful solutions for accomplishing the same task) that are reinforced and exploited over time (i.e., blue gradient cloud of solutions in Figure 1). With motor learning, individuals may then converge upon the optimal solution, or alternatively, one of several approximately optimal solutions [or “good-enough” as coined in (39)] for a task at hand.
By identifying the set of all feasible motor solutions for a task, Feasibility Theory provides an elegant approach that embraces the complementary and competing perspectives of several neuromuscular control theories (e.g., muscle synergies, uncontrolled manifold hypothesis, optimization, probabilistic control). In the process, it naturally raises several fascinating questions:
• What are the specific neural and mechanical constraints that define the feasible solution space?
• Given these constraints, how do measured muscle activity patterns compare to feasible solution space of muscle activations? Do measured muscle activity patterns occupy a narrow or wide region within what is theoretically feasible?
• What hypothesized objective(s) does the neuromusculoskeletal system aim to optimize?
• Can different weightings across multiple objectives help explain within- and between-subject variability in motor patterns?
• With learning, do muscle activity patterns better approximate optimal solutions?
• Can variations in arriving at different “good enough” solutions help explain within- and between-subject variability in motor patterns?
Although the questions posed above are recognized and tackled in the literature, finding answers (especially for individuals starting out in this field) can be challenging because insights are often provided across several papers crossing many disciplines. As such, the purpose of this review is to explore many of these questions by integrating ideas from biomechanics, neuroscience, and motor control to understand how we voluntarily control our muscles. In particular, we aim to provide a broad presentation of the challenges and opportunities in this field, incorporating both modelling and experimental evidence, to allow others to make sense of the current debates and pitfalls, and help the community make progress in the future. We begin the paper by first formulating the degrees of problem using biomechanical modelling (Section 2). Motivated by the recently proposed Feasibility Theory (2) and the modelling foundations laid out in the textbook “Fundamentals of Neuromechanics” (13), we will use the concept of feasible solution spaces to develop an intuition and understanding of the degrees of freedom problem, as well as discuss earlier uses of solution space approaches in biomechanics and motor control (Section 3 and 4). The feasible solution space defines the set of all theoretical solutions that an individual's motor system has available to perform a task. The focus throughout this paper will be on the degrees of freedom problem at the muscle level, especially during static tasks, with particular emphasis on the upper extremity. The set of all theoretical possible solutions based on the feasible solution space will be compared with individuals' actual motor behaviour based on experimental data (Section 5). It will be highlighted that individuals exhibit structured patterns in their motor behaviour which inhabit only small volumes of the feasible solution space, raising the possibility of overarching neuromuscular control principles and/or neuromechanical constraints that may be governing motor behaviour. We will discuss dominant neuromuscular control theories, highlighting optimality, probabilistic principles, and neuromechanical constraints that are used to identify families of solutions in the feasible solution space (Section 6). The incompatibilities between current optimization-based model predictions and experimental data will be discussed and used as an impetus for future studies to account for additional neuromechanical factors and principles that may be shaping muscle activity patterns but are unaccounted for in current biomechanical models.
The equations of motion for a rigid body with m number of kinematic degrees of freedom can be expressed in linear algebra form as:
where is the m × 1 vector of the generalized joint forces (i.e., net joint forces and torques); q, and are the generalized joint positions, velocities, and accelerations, respectively; is the m × m inertia matrix; is the m × 1 vector of Coriolis forces that includes the velocity-related terms; is the m × 1 vector of gravitational forces; is the vector of forces and torques at the endpoint of the limb; and is the Jacobian matrix mapping endpoint to joint-level forces and torques.
The net joint forces and torques are balanced by the internal force carrying structures at the joints, which mainly include the musculotendon units, ligaments, and joint contact forces. As musculotendon units are the main contributors to net joint forces and torques, especially for postures in the mid range of motion typical of many tasks, most biomechanical models only include muscles. For a rigid body system with n number of muscles, the balance of net joint torques by musculotendon units can be expressed as:
where is the m × n matrix of muscle moment arms; is the n × n diagonal matrix of maximum musculotendon forces; and a is the n × 1 vector of muscle activations which is commonly assumed to range from 0 (no activation) to 1 (maximum activation). For the purposes of this paper, the term muscle activation will be used interchangeably to represent the theoretical neural commands sent to muscles and measurements made using electromyography (EMG), although caution is advised when interpreting neural commands through EMG (43, 44). Combining Equations 2, 3 allows us to map a transformation between muscle activations and the endpoint vector of forces and torques:
Human limbs have a greater number of muscles than kinematic degrees of freedom (n > m), which in turn are typically greater than the number of elements in the endpoint force and torque vector. As a result, Equations 2, 3 express an important concept that has challenged the area of biomechanical modelling for the past few decades and will be central to this paper. These equations represent a transformation from a higher dimensional space (muscle activations) to a lower dimensional space (net joint torques and endpoint force vector). In other words, there are more unknowns than knowns. Mathematically, Equations 2, 3 represent an underdetermined problem, allowing for several non-unique solutions (i.e., combinations of muscle activations), if they exist, for most tasks. In biomechanics and motor control, this is famously termed the degrees of freedom problem [often traced originally to the work by Nikolai Bernstein (1)] and is also otherwise known as the muscle/motor redundancy (or abundancy), statically indeterminate, or load-sharing problem.
Before proceeding with the biomechanical modelling formulation presented above, a few remarks are required to set the context for the rest of the paper. Foremost, the biomechanical problem noted across Equations 1–3 traditionally focuses on determining muscle activity patterns to balance the net joint torques. However, human joints do not only exhibit rotational but also translational degrees of freedom. Furthermore, torques are not the only mechanical variable guiding muscle coordination. Motor behaviour can be driven by other mechanical factors. In particular, stability considerations may be essential from a task performance and injury prevention perspective (45, 46). In fact, the neuromechanical control of motion vs. force are distinct and can be incompatible with each other, with impedance control postulated by some researchers as an approach to bridge between the two (47–50). The flip side of the control of joint torques being underdetermined is that the control of movement driven by afferented muscles is overdetermined (13, 51). Specifically, the change in angles of a few joints determines the change in lengths of many musculotendon units. Finally, biomechanical models commonly assume musculotendon units to act mechanically and neurophysiologically independent of each other, with the prescribed activation and consequent forces generated in one muscle unaffected by other muscles. Our intention to make these caveats clear from the outset is to caution readers to keep these modelling assumptions and viewpoints in the back of their mind. For now, we begin this paper by adopting the traditional modelling framework (e.g., focusing on balancing joint torques, assuming independent control of musculotendon units) because it is the dominant approach in the field of biomechanics and allows us to be on the same starting page with the majority of biomechanical models and modelling programs currently available. Starting broadly also allows researchers to develop more detailed models as they see fit to answer their own neuromuscular control research questions of interest. Over the course of this paper, we will unravel many of the neuromechanical factors that are not always incorporated in traditional biomechanical models but may be essential for shaping muscle activity patterns.
The degrees of freedom problem fundamentally inquires about how the nervous system navigates and selects solutions from the set of all possible combinations of muscle activations (i.e., feasible solution space) that can theoretically be used to perform a task. For about half a century, since the 1970s, the standard approach in biomechanics has been to use optimization to solve for the single solution within the feasible solution space that best matches a hypothesized control principle approximating how the nervous system coordinates muscles (16, 18, 27, 30, 52–55). Before taking a closer look at the use of optimization, the feasible solution space of muscle activations itself warrants a detailed discussion as it lies at the heart of the degrees of freedom problem. Doing so will allow us to place experimental data of how humans behave within the context of what is theoretically possible based on biomechanical models. Similar approaches (uncontrolled manifold, goal-equivalent manifold, and tolerance-noise-covariation) have been undertaken in the field of motor control, where theoretical solution spaces of task execution variables (e.g., angle and velocity at release point during throwing) are compared to individual performance to reveal factors associated with motor learning (23, 24, 56–59). The feasible solution space can be used to generate null models to statistically test whether observed behaviour exhibit certain features that would otherwise be unexpected if individuals randomly navigated (i.e., sampled) the entire space (60). In the process, it enables researchers to evaluate competing hypotheses on which neuromechanical factors (e.g., constraints, optimality principles, probabilistic learning) are shaping the emergence of the specific muscle activity patterns from the broader landscape of all possible solutions, and how this may vary across different contexts, such as with learning, fatigue, neuromuscular impairments, or following surgical interventions (2, 16). In general, there are two approaches used to identify the feasible solution space of muscle activations: analytical and numerical. Both approaches have elegant graphical interpretations that further our understanding of the degrees of freedom problem (16, 61).
Analytical approaches characterize the exact feasible solution space. For example, from Equation 2, we can mathematically describe a system with 2 muscles and 1 kinematic degree of freedom as:
where , , and , refer to the moment arm, maximum musculotendon force, and activation of each muscle (n) respectively, which balance the net joint torque . Equation 4 represents a linear mechanical constraint to meet task demands (i.e., net joint torque torque) based on the musculoskeletal properties of the limb (i.e., moment arms and maximum musculotendon forces). Equation 5 represents the inequality constraints bounding muscle activations from 0 (no activation) to 1 (maximum activation). Thus, in this case, the feasible solution space of muscle activations is a line defined by the mechanical constraint (Equation 4) within a unit square representing the muscle activation boundaries (Figure 2A). Increasing the count to 3 muscles give us:
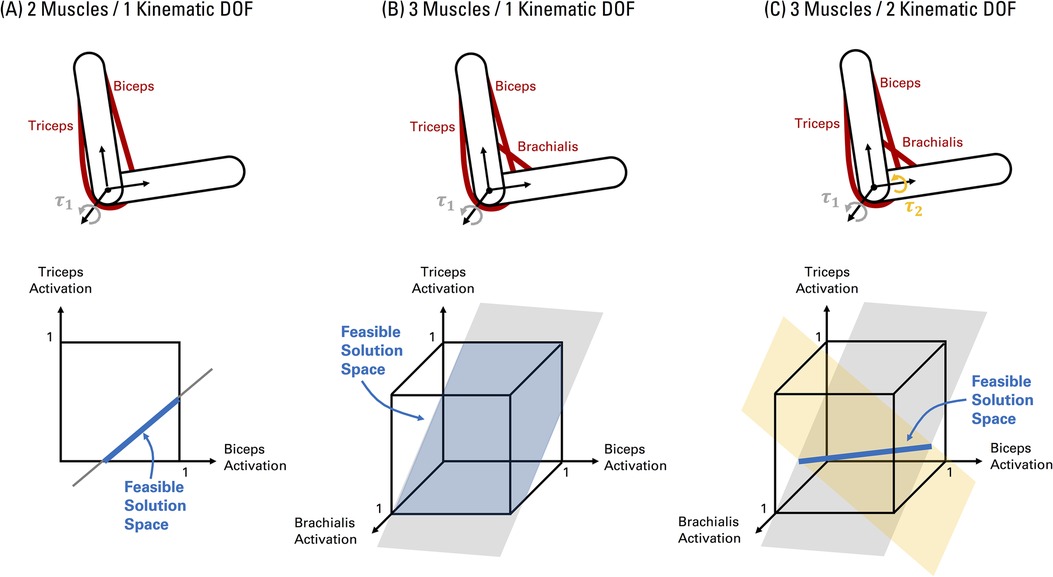
Figure 2. Visualizing the feasible solution space of muscle activations for simple elbow musculoskeletal models. (A) A system with 2 muscles and 1 kinematic degree of freedom. Given two muscles, the muscle activation space is two-dimensional as shown in the graph. The black border in the graph represents the bounds on muscle activations from 0 (no activation) to 1 (full activation) (Equation 5). The grey line represents the combination of muscle activations that can meet the net joint torque () requirement at the single kinematic degree of freedom (Equation 4). Note that the intercept and slope of the line is defined by the net joint torque, muscle moment arms, and maximum musculotendon forces. The feasible solution space of muscle activations is the segment of the line (shaded in blue) inside the muscle activation bounds. (B) The feasible solution space of a system with 3 muscles and 1 kinematic degree of freedom can be represented by the portion of a plane (Equation 6) within the boundaries of a 3D unit cube (Equation 7), as shaded by the blue area. (C) The feasible solution space of a system with 3 muscles and 2 kinematic degrees of freedom can be represented by the intersection of two planes, creating a line (shaded in blue) in 3D space. The shape and orientation of the planes in (B, C) are defined by the net joint torques , muscle moment arms, and maximum musculotendon forces.
In this case, the feasible solution space of muscle activations is visualized as a plane (Equation 6) within the boundaries of a unit cube (Equation 7) in 3D space (Figure 2B), as originally depicted by Crowninshield & Brand (26, 27) for the case of 3 muscles for an isometric elbow flexion task. If the number of kinematic degrees of freedom increase to 2 (e.g., elbow flexion-extension and forearm pronation-supination), the feasible solution space of muscle activations is then the intersection (if it exists) of two planes in 3D space, creating a line if the planes are not co-planar (62) (Figure 2C). Interestingly, Crowninshield & Brand (26, 27) illustrated changes in the selection of optimal solutions within the feasible solution space of muscle activations based on the nature of the cost function, particularly shifting towards the interior regions promoting higher muscle co-activation when using non-linear cost functions motivated by physiological bases. In this vein, analytical approaches are effectively applied to identify the force-sharing relationships across muscles and contexts when muscle co-activation can or cannot be predicted depending on the task conditions and/or structure of the musculoskeletal model (e.g., number of joints and kinematic degrees of freedom, uniarticular vs. biarticular groups of agonists and antagonist muscles) (16, 63–69). However, these investigations are primarily proof of principle focused on simple models. Generalizing the analytical approach to increasing complex models is challenging to interpret and visualize, as the feasible solution space is the intersection of multiple hyperplanes (each representing a single kinematic degree of freedom) imbedded in a hypercube with the number of dimensions equal to the number of muscles, resulting in a high-dimensional convex polytope (in the case of linear constraints, see Figure 1 caption) (13). Computational geometry tools, such as vertex enumeration algorithms, can be used to determine the vertices of the resulting polytope (17), and thus, the range of possible activation levels for each muscle in more complex models. As a result, researchers may then identify muscles that are “necessary” or “redundant” based on if a given task can be completed without activating a given muscle (20, 62). Nevertheless, in the former study, the model was reduced in complexity (from 44 to 14 muscles) to yield a solvable problem (20). Thus, analytical methods quickly become intractable when modelling human limbs.
Numerical approaches find approximations to the feasible solution space that help overcome the difficulties with applying analytical methods to complex models. Numerical methods can be broadly grouped into two categories: optimization and stochastic. Optimization methods find muscle activity patterns within the feasible solution space that minimize and maximize the activation of one particular muscle [see Figure 2 from (3)], thus identifying the range of activations possible for that muscle. Iterating this optimization process across all muscles will yield the activation range for each muscle within the feasible solution space. This method has been applied to quasi-static feline hindlimb standing balance (3, 34, 70), human walking (71), and static pedalling endpoint forces (72). Note that optimization in the context of identifying the feasible solution space differs from the traditional use of optimization in biomechanics used to find a single solution to solve the load-sharing problem (see Section 6). Although optimization can be effectively applied to determine the boundaries of the feasible solution space, it does not reveal any details on the internal structure of the feasible solution space. In contrast, stochastic solutions randomly sample specific points within the feasible solution space (2, 73, 74). Stochastic methods may not identify the true boundaries of the muscle activation range, but if enough points within the space are uniformly sampled, it allows for a glimpse at the internal structure of the feasible solution space by identifying how muscles would need to activate in relation with each other under the biomechanical constraints of the task and limb [see Figure 3 from (2)]. Alternatively, the solution space can be explored deterministically by first identifying the vertices and sampling interspersed points between the vertices (75, 76). Importantly, numerical approaches can be applied to approximate the feasible solution space in highly complex models, ranging from 7 to 92 musculotendon actuators with 4–23 kinematic degrees of freedom across the studies cited above.
From our formulation of the feasible solution space, we can immediately recognize that the structure of the space (i.e., number of dimensions, size, boundaries, orientation) will be fundamentally shaped by the interaction between the musculotendon mechanics, musculoskeletal geometry, and task demands. Musculotendon mechanics defines the capacity of our muscles to generate and transmit forces to bones, and is determined by properties of the muscle and tendon such as the maximum isometric force, pennation angle, and force-length-velocity relationship. Musculoskeletal geometry is the spatial relationship between the musculotendon units and the skeletal structure (i.e., muscle lines of action and moment arms) that map musculotendon forces into joint and endpoint forces/torques. The term limb mechanics will be used to refer to the collective effects of musculotendon mechanics and musculoskeletal geometry. Task demands refer to the magnitude and direction of the endpoint forces/torques required by the limb to perform a given task. In our simple case of a 2 muscle, 1 degree of freedom system (Equation 5 and Figure 2A), the feasible solution space is defined as a line, with its y-intercept and slope dependent on the maximum musculotendon force (musculotendon mechanics), moment arms (musculoskeletal geometry), and net joint torque (task demands). Altogether, limb mechanics and task requirements represent biomechanical constraints that shape the feasible solution space, and hence the muscle activity patterns required to perform a task. As a consequence, muscle coordination is not an arbitrary recruitment of muscles that will produce stochastic relationships in muscle activity patterns. Instead, the feasible solution space will have intrinsic structure. Multivariate relationships and correlations between muscles will naturally emerge due to the biomechanical properties of the limb meeting the specific task demands at hand (2, 75).
Studies directly evaluating the structure of the muscle activation solution space observe a complex dependency on tasks and limbs. When performing sub-maximal tasks, the feasible solution space is generally wide, and can range to the natural limits for many muscles (i.e., activations can range from 0%–100%) (71); however, this space tends to decrease (i.e., smaller range of muscle activation levels) as task intensity increases (2, 3). The increased feasibility at sub-maximal levels is possible because muscles have more ways to co-contract to compensate and balance joint moments. As most daily tasks are sub-maximal, this suggests that there can be substantial opportunity for within-individual variation in muscle activity patterns despite no changes in posture. In addition to the task intensity, the precision required for a task, such as during fine motor activities, will influence the feasible solution space. Kutch & Valero-Cuevas (62) demonstrated that the range of feasible activations for index finger muscles were practically limitless when generating sub-maximal fingertip radial forces without considering off-axis fingertip forces. Enforcing hard constraints to the off-axis forces, as simulating a well-directed precision key pinch task, drastically limited the possible muscle activity patterns to a small subset of solutions. The implication is that muscle impairments resulting in decreased musculotendon force capacity will predominantly affect the ability to perform tasks requiring greater intensity and/or precision.
Robustness to perform tasks with muscle dysfunction may vary across limbs. Comparing across models of varying complexities, Sohn et al. (72) reported that the feasible endpoint forces that can be generated at the endpoint of a human leg is more robust to muscle impairments in models of decreasing kinematic complexity (i.e., lower number of kinematic degrees of freedom) and increased number of muscles. It was further suggested that a ratio of muscles to kinematic degrees of freedom can provide an estimate of the musculoskeletal redundancy across different limbs. In other words, systems with more kinematic degrees of freedom add constraints which decrease the number of possible muscle activity solutions for a given task. In contrast, more independently controllable musculotendon units increase the possible number of muscle activity solutions. As such, limbs with a seemingly higher degree of musculoskeletal abundancy (based on model choices, see next paragraph) may have greater flexibility in feasible muscle activity patterns. Hence, relationships emerging from the feasible solution space may be less structured compared to limbs with a smaller ratio of muscle to kinematic degrees of freedom, indicating a fundamental difference in neuromuscular control of limbs of varying complexities.
The findings from Sohn et al. (72) concurrently reveal the importance of model choices [for a detailed review, see (77)]. As an example, the shoulder can exhibit up to nine rotational degrees of freedom with additional translational degrees of freedom at the glenohumeral joint (78). Muscle activity at the shoulder is known to be highly coordinated and is largely driven by the need to delicately balance all joint torque requirements along with enforcing joint stability (12, 79). Due to the challenges with measuring and modelling shoulder kinematics (78, 80, 81), many upper extremity models may simplify representation of the shoulder by reducing the number of degrees of freedom. However, these simplifications must be informed by the research question. If the aim is to better understand neuromuscular control of the shoulder, reducing the kinematic degrees of freedom can eliminate many of the mechanical functions that muscles enforce. For instance, the rotator cuff muscles are important stabilizers of the glenohumeral joint (82). Removing glenohumeral translational degrees of freedom or not enforcing glenohumeral joint reaction force constraints can underestimate the activation of rotator cuff muscles as the stabilizing requirement for activating these muscles is not modelled (83, 84). Improving anatomic fidelity by adding kinematic degrees of freedom, such as the toes for lower limb models (85) or the wrist for hand models (86), will require secondary moments to be balanced and generally lead to higher levels of muscle co-activation (69). Similarly, choice of muscle pathway representation can influence the mechanical functions of a muscle and affect predictions of neuromuscular control strategies. MacIntosh & Keir (87) reported improved predictions of antagonist muscle activity when incorporating intrinsic finger muscles and extensor mechanism representation for an index finger model. Thus, conclusions on neuromuscular control using biomechanical models can be sensitive to model choices.
Individual differences in limb mechanics will additionally alter the feasible solution space that can cause inherent between-individual variation in muscle activity patterns required to perform identical tasks. Although few studies have explicitly investigated how the solution space may vary across individuals, substantial sensitivity in predicting musculotendon forces and muscle activations is reported with alterations in the muscle model parameters (88, 89), muscle attachment points (82, 90, 91), and kinematic definition of the model (42) [for a review on investigating stochasticity in model parameters, see (92)]. Further complicating matters, posture will non-linearly affect limb mechanics properties (e.g., lines of action, moment arms, force-length, pennation angle, inertia), resulting in alterations in the mapping from musculotendon forces to endpoint forces. For instance, cadaveric studies report changes in the fingertip endpoint force vectors generated when an individual tendon is loaded with the same magnitude across different postures (93–97). As a result, muscle coordination patterns required to perform the identical task are expected to vary within-subject as an individual changes their posture and between-subjects when comparing individuals who adopt different postures due to anthropometric differences.
Taken together, relationships in activation levels across muscles are shaped by the constraints of limb mechanics and task demands, creating inherent structure in the feasible solution space. The exact nature of these relationships is dependent on the individual's anthropometrics, anatomy, posture, limb, and task at hand. For most sub-maximal tasks, there is a large feasible solution space of possible muscle activity patterns that allow for the possibility of substantial between- and within-individual variation in motor behaviour.
To this point, we have formally defined the degree of freedom problem and discussed theoretical solutions to the problem using the concept of the feasible solution space for muscle activations, which brings us to a key question: which solutions emerge when examining individuals in real life? That is, how do individuals inhabit and navigate the feasible solution space? Several studies have experimentally measured muscle activity patterns using EMG of the upper extremity during various static tasks. As expected due to changes in the feasible solution space, differences in EMG amplitude are observed with alterations in posture and task demands at the shoulder (98–102), elbow/forearm (103–107), and hand/wrist (17, 108–110). Variations in muscle activity patterns are also commonly noted within- and between-individuals. Interestingly, within-individual variability in EMG amplitude appears to increase with task intensity (111) despite the feasible solution space of muscle activations generally decreasing. This unintuitive finding is thought to arise due to the signal-dependent motor noise present within the nervous system (i.e., variability in EMG and force increases proportional to amplitude) (111, 112). The magnitude of between-individual variability is systematically larger than within-individuals (113), which is commonly hypothesized to represent individualized motor control strategies, partly due to differences in limb mechanics.
Upon further examination of the variability within- and between-individuals, despite the substantial magnitude of variability, muscles display relatively stereotypical coordination profiles as evidenced by extracting low-dimensional patterns (114–121). Although some structure in muscle coordination is to be expected due to mechanical constraints, the few studies making direct comparisons have found experimental data to exhibit more distinctive and less varying multivariate relationships across muscles than the set of all theoretically feasible solutions. During walking, the range of feasible activations for most lower limb muscles to balance net joint torques can range between natural limits (0%–100%). It should be noted that the feasible range of activations are likely to be reduced when considering the additional dynamical constraints that were not modelled (e.g., contraction-activation dynamics). Nevertheless, experimental muscle activity patterns display stereotypical patterns that fall within a relatively narrow band of activation levels (71). Similarly, experimental muscle activity patterns inhabited a small subspace of all feasible solutions during submaximal isometric finger pressing tasks (108). The small overlap in motor behaviour between what is observed experimentally in real-life vs. what is theoretically possible based on biomechanical modelling is incredibly meaningful and indicative of: (1) overarching neuromuscular control rules or principles governing the selection of certain muscle activity patterns and/or (2) additional neural and biomechanical constraints beyond just balancing net joint torques that are unaccounted for in current biomechanical models but may further shape muscle activity patterns.
Several neuromuscular control theories (muscle synergies, uncontrolled manifold hypothesis, optimality, probabilistic control) are proposed to explain and predict voluntary control of muscles from the large set of possibilities, each of them elegantly encompassed within the framework of feasible solution spaces (2, 13). The muscle synergies theory proposes that the central nervous system organizes muscles into functional groups (i.e., synergies) that are tuned to certain task-level goals (117, 121). As a consequence, instead of coordinating every individual muscle, the central nervous system simplifies neuromuscular control by operating within a lower-dimensional space by recruiting muscle synergies to perform a wide range of tasks. An alternative perspective is the uncontrolled manifold (UCM) hypothesis, which suggests that the sensorimotor system can take advantage of the abundance of solutions by allowing elements (e.g., muscle activations and joint angles) to freely vary as long as errors in the performance variable(s) of interest, such as endpoint fingertip forces during pressing, are kept to a minimum (23, 24). In other words, the central nervous system is free to explore the available solutions inside the feasible solution space since they are all equivalent with respect to the mechanical demands of the task. Unlike muscle synergies, the UCM hypothesis embraces the high dimensionality of the musculoskeletal system. Interestingly, both muscle synergies and UCM hypotheses can emerge naturally by simply applying optimality and probabilistic principles to neuromuscular control (2). In this context, synergies are not a hard-wired constraint by the central nervous system (i.e., prescriptive synergies) but arise inherently due to the biomechanical constraints and optimization processes shaping the relationship across muscles (i.e., descriptive synergies) (13, 20, 22, 122). Furthermore, if the system's goal is to optimize task performance while minimizing effort, any deviations in degrees of freedom that have negligible effects on task-relevant variables can be ignored, resulting in an uncontrolled manifold along task-irrelevant dimensions where elements are free to vary (36, 37). The following sections will detail optimality principles and probabilistic control for promoting the preference of certain muscle coordination patterns and examine the evidence for neuromechanical constraints in limiting independent control of muscles.
Optimality, as a neuromuscular control theory, proposes that sensorimotor behaviour is guided by overarching principles that aim to best meet neurophysiologically relevant criteria or goals. Fundamentally, it means that not every feasible solution is considered equal and that certain solutions are preferred. Optimality is an attractive idea, having parallels with many prevalent concepts across science (e.g., natural selection in biology, entropy maximization in chemistry). It would also be predictive of stereotypical patterns that fall within a narrow region of the feasible solution space, as consistent with experimental data of motor behaviour. In biomechanics and motor control, optimality principles have been the predominant approach over the past half-century for resolving the degrees of freedom problem, including the prediction of load-sharing across muscles and movement trajectories (123, 124). The two most common approaches are static optimization and optimal control. Static optimization is the partitioning of net joint moments, calculated using inverse dynamics, into individual muscle forces, and consequently muscle activations, independently at discrete timepoints (26, 27, 30, 52–54). Optimal control is a forward dynamics based method that finds the entire time history controls, typically muscle excitations, given an a priori cost function (consisting of 1 or more objectives) and a set of constraints (125, 126). Different optimal control methods exist [see (25) for a review of applications in neuromuscular control], with the majority of optimal control problems in biomechanics and motor control aimed at tracking or predicting movement (127–134). Constraints in these problems can be either incorporated indirectly through penalties in the cost function or directly enforced to be met. A less commonly used approach is inverse optimal control, which seeks to identify the cost function that best matches experimental data (typically movement trajectories) that is assumed to be optimal behaviour (31, 135). Optimal feedback control theory is a closed-loop application of optimal control that considers task goals and neurophysiological cost(s) while incorporating additive and/or multiplicative motor noise as well as delayed stochastic (i.e., noisy) sensory information with an internal feedforward model (35–37, 136). Integrating sensory information in a closed loop enables task performance despite model uncertainties and allows for online adaptations to instantaneous perturbations (e.g., motor/sensory noise, external environmental changes) (137) that can predict trial-to-trial motor variability as commonly observed in experimental data (37). The central challenge across these methods is identifying the one (or more) neurophysiological criteria that can be defined quantitatively as cost/objective function(s), which the system is presumably seeking to optimize for governing muscle and movement coordination. Although optimal control methods have successfully predicted many salient spatiotemporal features of movement trajectories using different cost functions, such as minimizing jerk (129, 138), torque (139), endpoint variance (140), and effort (37, 141), the focus here will be on predictions of muscle load-sharing.
Formulating a cost function to represent the sensorimotor system's goals has challenged researchers since early optimization models. Early work used linear optimization based on arbitrary, mathematically convenient objectives, such as minimizing muscle forces and stresses (force/cross-sectional area) (16, 52–54). The optimal solutions across these cost functions recruit muscles based on their moment arms and cross-sectional areas in a stepwise manner, with unrealistically large forces predicted in some muscles and minimal or no activity across several other muscles (28). The inherent limitations of linear optimization were also noted by Hardt (30), who found non-physiological results with the number of active muscles depending on the number of constraints modelled that are fundamentally unable to distribute forces across synergistic muscles. It was further suggested that physiological principles should be incorporated into the optimization process with the recommendation for a cost function based on muscle thermodynamics and minimizing the muscle energy requirements. Pedotti et al. (142) were able to predict forces distributed across muscles more reflective of experimental data using non-linear optimization (sum of square of muscle forces and normalized forces) but no physiological basis was provided for these cost functions. Combining the latter two approaches, Crowninshield & Brand (27) proposed minimizing the sum of muscle stresses raised to a power of 3 to reflect the sensorimotor system's possible goal of maximizing endurance during walking based on the non-linear inverse relationship between muscle stress and endurance. Similarly, Dul et al. (29) formulated a minimum fatigue criterion based on maximizing endurance accounting for muscle fiber type composition, reporting a better fit with experimental data compared to previously used cost functions. Since these initial modelling studies, non-linear effort-based cost functions (mainly quadratic and convex for ease of finding solutions) have remained the standard in biomechanics (123, 143). The definition of effort varies across studies, with most researchers electing for simple functions approximating what may be constituted as “effort”, such as muscle stresses, normalized muscle forces, or muscle activations (i.e., motor commands), while some studies include parameters underlying the metabolic processes of energy expenditure (127, 141, 144–146).
Coordinating muscles efficiently based on an effort criterion appears appealing and plausible. In addition to longer term evolutionary and developmental pressures (147, 148), several sources of evidence suggest that effort may drive motor behaviour. Direct evidence includes decreased muscle activity, co-activation, and metabolic expenditure (e.g., expired gas analysis) during learning of novel upper and lower extremity motor tasks (149–152). Indirectly, modelling simulations can successfully predict several spatiotemporal characteristics of reaching and locomotion kinematics using effort-based objectives (37, 127, 139, 141, 153). Studies making comparisons between the feasible solution space and EMG findings also report experimental muscle activity patterns to largely occupy the lower end of the feasible activation range for each muscle (71, 108). Although a causal link between effort and recruitment of muscles by the central nervous system is yet to be fully established, muscle coordination seems to be related (either directly or indirectly) with effort considerations. Nevertheless, the prediction of load-sharing across muscles by effort-based cost functions have proven controversial and unsatisfactory in many cases (123, 154, 155). Several studies observe a systematic failure to predict co-activation, even amongst the most advanced biomechanical models available today (156), particularly the under-prediction of antagonistic muscles by optimization-based models compared to experimental data. Based on the modelling framework presented, co-activation of muscles would simply increase the effort without altering the net joint moment, which would be “wasteful” mathematically using optimization. Caution should be made on whether the failure of optimization-based models to predict muscle co-activation may be due to choices made in model structure (16, 63–69) (see Section 4, paragraph 4). Nevertheless, the incompatibilities between effort-based optimization models and experimental observations for predicting muscle co-activation have been challenging to resolve but may be additionally explained by one or more of the following causes that will be highlighted in the subsequent sections:
a. Globally Optimal vs. “Good-Enough”: Is the central nervous system truly searching for the single best solution or are certain solutions deemed “good-enough”?
b. Multiple Objectives: Is the central nervous system simultaneously optimizing other objectives?
c. Model Assumptions: Are there additional neuromechanical constraints unaccounted for in the model?
While optimization may drive motor behaviour to explore more favourable solutions, the neuromuscular system may not identify the most favourable solution. The complexity of the high-dimensional feasible solution space limits the opportunity to exhaustively sample every solution and opens the possibility of considering “good-enough” solutions (39). “Good-enough” solutions generate costs that are comparable to the globally optimal solution, but not the best combination of costs that can be found (i.e., approximately optimal). A systematic exploration of the landscape of feasible solutions reveals that a diverse distribution of globally suboptimal muscle activity patterns can be found with similar costs (i.e., “functionally equivalent”) neighbouring the globally optimal solution in the cost function (i.e., fitness) landscape (34). Note that although different “good-enough” solutions may be “close” in the cost function space, they are not guaranteed to be “close” in the muscle activation space [see Figure 4b in (34)]. Convergence to different “good-enough” but functionally equivalent solutions may underlie individual variability in motor behaviour (34). The convergence to particular solutions may be determined by different initial states of the system (39), repeated exploration/sampling of the feasible solution space (157), motor variability (158), habitual behaviour (159, 160), and the diversity of adaptation strategies across people (161). Of interest, the exploration of the feasible solution space can be shaped by optimality principles. Greater variability in motor behaviour is commonly noted early on during motor learning, which may correspond with individuals exploring and searching for more rewarding solutions that are then exploited with decreasing variability over time (41, 152). As a result, individual-specific probabilistic representation of solutions are developed (i.e., multivariate probability distribution of muscle activities), characterizing the degree of belief (or uncertainty) in which motor solutions may work best for a given task (40, 41). Whether these solutions converge upon the optimal solution or one of several approximately optimal solutions, as well as how close to optimal constitutes “good-enough”, remain open questions (34, 39). Interestingly, the combination of “good-enough” solutions combined with strong prior probabilistic representations (40) may explain why muscle and movement coordination during some novel tasks remain similar to baseline motor tasks despite being suboptimal and individuals provided opportunities to practice the optimal solutions (see next section on multiple objectives as an alternative explanation) (159, 160, 162). In this vein, probabilistic methods can be a powerful approach for predicting muscle load-sharing that has yet to be fully exploited in biomechanics. Rather than finding the globally optimal solution with respect to effort, a range of possibilities can be predicted. These possibilities can be calculated using priors determined from the feasible solution space and adjusted based on the effort costs observed in the distribution of experimental muscle activity, allowing us to move past the shortcomings of simple optimization methods identifying only a single solution. For example, Markov Chain Monte Carlo methods with a prior weighed towards minimizing effort was recently used to find a distribution of plausible muscle activity patterns for a simple elbow flexion-extension motion (163). Probabilistic methods provide the benefit of capturing potential within- and between-individual variability in muscle activity patterns, uncertainty in motor control objectives, and identifying a range of possible outcomes when generalizing to novel experimental conditions (e.g., new motor tasks, neuromuscular impairments, rehabilitation, surgical outcomes).
The search for “good-enough” solutions and a probabilistic approach for predicting muscle load-sharing is especially promising in light of the growing evidence that the sensorimotor system optimizes multiple objectives. For example, optimal feedback control theory hypothesizes that sensorimotor behaviour balances the internal objective of minimizing effort with the external objective of maximizing task goals (i.e., reducing performance error) (36, 37). This can be evidenced by experimental studies observing greater muscle co-contraction during tasks requiring greater task accuracy (150). The central nervous system may also consider multiple external and internal objectives. One of the most well-known examples is the speed-accuracy trade-off that weighs two external task goals. More recently from the emerging field of neuroeconomics, decision-making during motor behaviour is found to weigh effort against rewards such that inefficient movements can be selected to elicit greater rewards (164, 165). Similar results are observed during decision-making weighing the relative costs of reaching and walking (i.e., preference for choosing paths requiring shorter reaching distances despite walking greater distances), which are found to be influenced by bottom-up biomechanical mechanisms (166, 167). Compared to any single measure of effort, multiple metrics of effort, including mechanical work and metabolic cost, were able to better estimate optimal strategy during split-belt adaptations (168). Using an inverse optimal control approach, a composite cost function combining minimizing effort and maximizing joint smoothness was able to better predict arm reaching trajectories compared to any single cost function alone (31). Likewise, a weighted cost function combining a number of objectives (cost of transport, muscle activity, head stability, foot-ground impact, and knee ligament use) resulted in closer agreement to healthy gait profiles (kinematics, kinetics, and electromyography) than any one objective (169). Although intuitively appealing, the implementation of multiple cost functions through a multi-objective optimization model is not trivial. Several cost functions may appear as appropriate neurophysiologically relevant objectives. A better fit between predicted and observed data may not necessarily reflect the explicit goals considered by the central nervous system and could be simple descriptors, but not necessarily causes, of motor behaviour (170). In fact, a multi-objective optimization model is expected to perform at least as well as single objectives since the weight of the additional objective can be zero if it adds no predictive value. Nevertheless, statistical comparisons between cost functions will allow us to start considering the likelihood of different objectives (170). An alternative approach to model-fitting with a priori cost functions is to design experimental paradigms that pit objectives against each other (171). Similarly, altering the cost landscape and identifying which solutions individuals converge towards during learning and adaptation may reveal how much weight (if any) the nervous system places on different objectives, which likely will vary across populations and tasks (158, 172, 173).
A plausible candidate for the consideration of additional objectives to the muscle load-sharing problem is mechanical impedance. The issue of redundancy has been at the heart of this review but is not the only “computational” challenge encountered by the sensorimotor system. Several additional problems inherent to the system are thought to affect sensorimotor control: noise, delays, uncertainty, nonstationarity, and nonlinearity (174). Stochastic sensory and signal-dependent motor noise (112) as well as uncertainties, either through imperfect sensory processing, incomplete environmental information, and unpredictable situations, will especially challenge our ability to perform tasks with accuracy. Interestingly, emerging evidence suggests that the majority of signal-dependent motor noise may not arise at the motor unit level due to stochastic motor unit discharge rates and unfused twitches as commonly thought, but rather an emergent feature of closed-loop feedback control (175–177). Nevertheless, control challenges related with stochastic noise and uncertainties can be compounded by delays in our sensorimotor system. Neural feedback pathways through short-latency (20–50 ms), long-latency (50–100 ms), and voluntary responses (>100 ms) may affect our ability to respond to mechanical disturbances in time (178). To combat these challenges, it is thought that the sensorimotor system uses feedforward mechanisms to maintain stability by actively controlling mechanical impedance, defined as a resistance to perturbations (45, 46, 179). Particularly important for postural control during isometric tasks is stiffness. Stiffness is the component of impedance accounting for resistance to changes in position and is quantified as the ratio between force and displacement changes (179–182). Impedance can be altered through changes in muscle activity that can affect the viscoelastic properties of individual muscles as well as the concurrent forces applied by several muscles (i.e., co-activation of synergists and antagonists). The increased joint stiffness with greater muscle co-activation outweighs the negative effects of signal-dependent motor noise resulting in an overall decrease in movement variability (150, 183, 184). In fact, co-activation of antagonist muscles can emerge as the minimal effort solution when incorporating motor noise and time delays using stochastic optimal control simulations (185, 186). On a related note, in a redundant task execution space, individuals find and exploit error-tolerant (i.e., robust) solutions, presumably so that variations in execution due to sensorimotor noise or perturbations will have minimal effects on task performance (57–59).
A couple studies to date have combined effort and mechanical impedance in a multi-objective optimization. Of particular relevance is a recent study investigating trade-offs between effort and limb stability in an isometric postural control task using a cat hindlimb model (34). The authors reported that effort and stability are competing objectives, resulting in a landscape of optimal solutions based on how much weight (0–1) is given to each term between globally minimizing effort and maximizing stability. The landscape of optimal solutions is termed the Pareto optimal set, where no solution can be found without making at least one of the objectives worse. Interestingly, it was found that small increases in effort from the minimal effort solution resulted in rapid increases in limb stability, indicating that low amounts of muscle co-activation can be an effective feedforward mechanism for increasing the mechanical impedance of a limb. The study did not collect any EMG, so no conclusions were made on the relative weight between effort and stability in real-life motor behaviour and whether these weights are generalizable across individuals and tasks. During an isometric elbow flexion task with fatigue, predicted muscle activations from a multi-objective optimization between minimizing effort and maximizing joint stiffness was strongly associated with collected EMG (R2 = 0.94) (33). The effort term was weighed more than joint stiffness but with fatigue, an increase weighting towards joint stiffness was observed and suggestive of alterations in the objectives of the central nervous system with changes in task demands. Joint stiffness has been a common focus in the spinal literature (187, 188) but has garnered lesser attention across other body parts. Intuitively, the shoulder would be a natural location for the central nervous system to weigh effort and stiffness together given the lack of passive restraints and reliance on active musculature for minimizing glenohumeral joint translations (12). Studies have implemented joint reaction force constraints at the glenohumeral joint based on cadaveric data for joint dislocation to confer stiffness (84, 189). However, these constraints still under-predict muscle co-activation, with the minimal effort solutions typically directed at the rim of the glenohumeral joint that could easily dislocate with perturbations or motor noise (83). Thus, an open question is whether neuromechanical factors, such as joint stiffness, are to be treated as objectives that are optimized vs. constraints to be kept at or within a certain level. There is some evidence to suggest that individuals exhibit greater levels of stiffness than the minimum required for stability during mechanically unstable force production tasks (46). Overlaying the neuromechanical costs (e.g., joint stiffness) that individuals exhibit during real-world tasks (190, 191) with the feasible solution space can help future studies tease apart the consideration of objectives vs. constraints.
In addition to task performance and effort considerations, muscles have a key mechanical function in injury prevention and progression that may be guiding neuromuscular control strategies. The active role of musculature in enforcing postural and limb stability does not only facilitate task performance, as discussed above, but also minimizes the risk of acute injuries, such as the prevention of falls and joint dislocations (192, 193). Of equal importance, muscles impose substantial internal joint-level loads that can mechanically contribute to the development of chronic musculoskeletal disorders (e.g., osteoarthritis) (194–196). For instance, hip reaction forces during walking are modelled to range anywhere from 1 to 10× body weight when calculating the feasible muscle activation space when only considering joint torques (74, 75). Given the role these mechanical loads play in chronic injury development, neural control of muscles may be tuned to not only maximize task performance (i.e., satisfy joint torque requirements of the task), but to also minimize internal joint stresses. Perhaps the strongest evidence to date indicating that neuromuscular control strategies may (in part) be regulated by joint-level mechanical loads is the recent work by Matthew Tresch and colleagues (197–199). Across a series of three experiments, quadriceps muscle activity patterns (vastus lateralis, vastus medialis, vastus intermedius, rectus femoris) were measured in rats to investigate their role in regulating mediolateral patellofemoral loads. First, across all locomotor task conditions tested (different slopes and speeds), the vastus medialis and lateralis exhibit the strongest pairwise correlations in muscle activity patterns (between all quadriceps muscles), likely reflecting a concerted neuromuscular effort to generate knee torque while balancing net mediolateral patella force (197). Furthermore, induced lateral loads on the patella through a spring promoted an adaptive decrease in the ratio of vastus lateralis-medialis activity (198). Finally, paralysis of the vastus lateralis resulted in a maintenance of vastus medialis activity and an increase in rectus femoris activity during locomotor tasks (199). In the latter study, if the rats compensated for the lost vastus lateralis torque by increasing vastus medialis activity alone, it would be at the expense of minimizing joint stresses (due to unbalanced net mediolateral forces). Alternatively, if vastus medialis activity decreased, substantial increases in rectus femoris activity would be needed to compensate for the lost knee extension torques from both the vastus medialis and lateralis; however, this could come at the expense of task performance due to the bi-articular nature of the rectus femoris that would require complex compensations at other joints. Thus, the experimentally observed maintenance of vastus medialis activity and moderate increase in rectus femoris activity in response to vastus lateralis paralysis is hypothesized to reflect a central nervous system goal of balancing the multiple objectives of minimizing internal joint stresses and maximizing external task performance (199). Others have similarly thought that the central nervous system regulates its neuromuscular control strategies to limit mechanical loads across joint-level tissues (17). Notably, muscle activity patterns that minimize joint loads do not necessarily align with optimization-based predictions of minimizing muscular effort, which has promoted the development of neurorehabilitation strategies to identify and train muscle activity patterns that minimize mechanical joint loads to limit the progression of pathologies (200–203). As a result, evaluating the delicate balance between the multiple competing neuromechanical objectives related to task performance, effort, and injury does not only offer a deeper understanding of human movement, but can also inspire novel strategies at injury prevention and restoring function in the presence of neuro-musculoskeletal pathologies.
In our modelling framework so far and as consistent with most biomechanical models, muscles are assumed to act as independent actuators. However, neural and mechanical factors can lead to force production and transmission between muscles (15, 204, 205). Perhaps the clearest example in humans is in the hand. Fingertip force production and/or movement of one finger leads to involuntary fingertip force production and movement of other digits (206–210). The relative contributions of neural vs. mechanical factors to the lack of muscle independence is often debated. Regardless, strong dependencies between muscle activations and force transmission would act as a constraint to the feasible solution space, such that certain combinations of muscle activations and forces are unattainable (e.g., maximal activation of index finger flexors without activating the middle finger flexors). Failure to account for neuromechanical constraints could lead to activity patterns substantially different than what may be predicted using optimization. Assumptions of independent control are thought to explain the poor prediction of finger forces during multi-finger tasks compared to individual finger tasks (143, 210). In the following subsections, we will review the evidence of mechanical and neural constraints limiting independent control of muscles.
Mechanical constraints are attributed to passive tissue linkages between musculotendon structures [for a detailed recent review, see (211)]. The anatomical structures involved include connective tissue and neurovascular tract attachments between adjacent muscles and tendons, fascia surrounding muscle compartments, and intertendinous fascia (205, 211–213). As a result of these structures, force generation and length changes in one muscle can pull taut the connective tissue attachments, consequently leading to force transmission and altered force-generating capacity in neighbouring muscles.
Although the existence of inter-connections leading to interactions between musculotendon units is undoubted, their functional relevance remains uncertain. Early studies investigating musculotendon inter-connections in animal models observed differences in tendon forces between the origin and insertion sites, indicating force transmission outside the musculotendon unit. The tendon forces of a muscle can be further altered by changing the length and relative position of a neighbouring muscle (214–217). The functional contribution of these force transmission pathways during everyday behaviour is questioned (218), as manipulations to neighbouring muscles were beyond physiological ranges of motion. Follow-up experiments focused on manipulating limbs through the range of motion, particularly examining muscles crossing the knee and ankle. Within this experimental paradigm, knee flexion-extension angles were manipulated to produce length changes of biarticular muscles crossing both the knee and ankle (e.g., gastrocnemius). If forces are transmitted between the biarticular muscle and neighbouring uniarticular muscles crossing only the ankle (e.g., soleus), then different magnitudes of ankle moments across knee angles would be expected upon electrically stimulating the uniarticular ankle muscles. Yet, limited changes in ankle moments were found across knee angles among both cats and rats (219–221), with force transmission effects between muscles observed only when muscle groups were electrically stimulated at maximal levels (222, 223).
Conflicting evidence is also reported for the role of connective tissue linkages in limiting finger independence. Tensile forces applied to the tendon of one digit result in movement of adjacent digits (213). Removal of connective tissue linkages (e.g., intertendinous fascia, webbing) nearly eliminates movement at adjacent digits, supporting the mechanical function of intertendinous tissue in transmitting forces across fingers. Reinforcing these findings, passive motion of one digit at the metacarpophalangeal joint results in movement of other fingers (206), with similar magnitudes of involuntary movement observed during active motion (206) without any changes in motor unit activity (204, 207). In contrast, passive motion of the distal interphalangeal joint with anesthesia did not result in movement of other digits (204) and electrical stimulation of the extensor digitorum muscle compartments found relatively small magnitudes of force transmission to adjacent fingers (224).
Overall, the inconsistent evidence across studies suggests that assuming muscles are mechanically independent is non-trivial. Certainly, assuming that torque contributions from individual musculotendon units sum up linearly does not necessarily hold true. Several studies observe the phenomenon where electrically stimulating individual muscles and summing up their individual joint moment contributions does not equal the total joint moment when all muscles are simultaneously stimulated, indicating mechanical interactions between muscles (225–228). Furthermore, even if connective tissue attachments play a limited mechanical role in force transmission between muscles, spindle behaviour can be altered (229, 230), thereby affecting neural control of muscles through sensory pathways. Thus, a complex interplay between neural and mechanical factors, which are largely unaccounted for in current biomechanical models, emerges upon questioning whether muscles are independently controlled.
Hard-wired neural pathways can constrain the ability to independently control muscles. Within the motor cortex, corticomotoneuronal cells projecting onto different muscles have overlapping regions (231–234). In addition, neural circuits in the spinal cord can diverge onto multiple muscles (235–237). Using viral retrograding techniques, premotor interneurons across the spinal cord are found to have monosynaptic connections with motoneuron pools of multiple synergistic and antagonistic muscles (236). As such, excitatory (or inhibitory) inputs can be delivered simultaneously to different motoneuron pools, which may underlie common synaptic input and neural drive to muscles (238). These circuits may form the neural substrates limiting independent muscle control but allow for muscle co-activation and co-inhibition to generate coordinated movement across limbs or regulate limb stiffness. In support of these neuroanatomical observations, synchronous discharge rates between pairs of motor units are observed within and between muscles (239–241), although whether common synaptic input is causing this synchronicity is questioned (242, 243). The differences in divergent synaptic connections across descending pathways may act as a further neural constraint to task performance. For example, the reticulospinal tract is highly divergent and is hypothesized to play a larger role in gross motor actions at the hand (e.g., grasping), as opposed to the less divergent corticospinal tract that can promote individuated finger control essential for fine motor tasks (e.g., manipulation) (244–247). The different functional roles of these descending pathways are speculated to compete against each other when met with the interacting mechanical demands of multiple tasks (e.g., postural maintenance vs. force control vs. movement) (47, 248, 249), which may require integration of separate neural control strategies (250, 251). The control strategies can be further complicated by the emergence of stochastic signal-dependent noise from the closed-loop aspect of motor control (175–177). Moreover, while control of joint torques is underdetermined (i.e., many muscle activity solutions for a given combination of joint torques), movement is driven by afferented muscles via spindles, rendering the control of muscle excursions as overdetermined (13, 51). That is, the length changes of many musculotendon units are determined by changes in fewer joint angles, leaving at best one solution for the control of muscle excursions. Amongst this backdrop of multiple constraints arising from the neural circuitry, the feasible solution space of muscle activity patterns will be reduced.
As an extension of neural constraints limiting independent control of muscles, some scientists hypothesize that the central nervous system recruits muscles in groups known as synergies (117, 121). A synergy is defined here as a functional unit comprised of muscles with weighted activations to represent muscles that are co-activated (or reciprocally inhibited). Each synergy is thought to be tuned to a specific task-level goal (107, 252, 253). Hence, instead of coordinating muscles individually, the central nervous system functions within a lower-dimensional space by recruiting muscle synergies to perform a wide range of tasks. The concept of muscle synergies parallels that of neural manifolds, where cortical activity during motor behaviour is observed to occupy a lower-dimensional space (i.e., manifold) (254, 255). Evidence for muscle synergies is mainly derived from stimulation of the brain and spinal cord (256–261). In these experiments, electrical stimulation of the central nervous system is observed to generate multi-joint torques that produce functionally relevant actions (e.g., reach or grasp). Supporting these findings, dimensional reduction statistics (e.g., principal component analysis, non-negative matrix factorization) reveal robust structural variations in muscle activity patterns that can largely be described by a small number of synergies (114–121). For example, Roh et al. (107) accounted for greater than 90% of the variation in EMG amplitude across 8 arm muscles during a submaximal isometric hand task across 210 force directions using 4 synergies. However, whether these synergies are “prescribed” by the central nervous system as a control scheme or are simply “descriptive” of the relationships between muscles is debated (13, 19–22). Based upon the same task conditions from Roh et al. (107), simulations performed using a musculoskeletal model with static optimization were able to re-produce synergies matching those found experimentally (262). This finding would be consistent with the argument that muscle synergies are not encoded as a control scheme but are a by-product of biomechanical constraints and learning optimal policies within the neuromuscular system (2, 20, 122, 263–265). Furthermore, although behaviour may be largely explained by a few groupings (i.e., synergies), the remaining unexplained variance that is often disregarded as noise may be under volitional control and required for task performance, manipulating compliant object interactions, or explaining control differences in clinical populations (266–270).
Regardless of the neural origin of muscle synergies, it appears that the neural circuitry provides at least some level of constraint on the voluntary control of muscles. The advantage offered by such constraints are to simplify the ability to control our high-dimensional neuromusculoskeletal system. Neuromechanical constraints may further serve to uniformly distribute stress across musculotendon units, reducing injury risk (271). In contrast, constraints to muscle control would decrease the feasible forces that can be produced at the endpoint of a limb and limit the ability to generate rich, flexible behaviour, such as adapting to novel environments, learning new tasks, or regulating limb stiffness (13, 72, 255, 272, 273). It is possible that the nervous system may be operating across both high- and low-dimensional spaces (274). For instance, the ability to independently control individual motor units varies from remarkable flexibility (275, 276) to rigidly constrained (277). Similarly, coherence analyses of motor units spike trains indicate that the motoneuron pool for a single muscle may not all receive the same common synaptic input, while motoneuron pools from different muscle compartments or groups may be constrained by the same common synaptic input (278–280). These observations have led to the recent viewpoint that muscles may not be the fundamental building blocks of neuromuscular control but rather “clusters” of motor neurons, striking a balance between flexibility and simplified control (281).
We started this paper by formulating the degrees of freedom problem using biomechanical modelling. The degrees of freedom problem is described as finding a solution within a large number of possible solutions, termed the feasible solution space. The feasible solution space is constructed based on modelled neuromechanical constraints, accounting for task demands and limb properties, and will include muscle activity patterns that are both optimal and suboptimal based on any theoretical cost function. Defining the feasible solution space does not mean that individuals are traversing across all the solutions when performing and learning a task. Rather, it identifies that individuals must be somewhere on this landscape. In fact, real-world motor behaviour, specifically muscle activity patterns in the context of this paper, appears to occupy only small regions of the feasible solution space. Perhaps the most fascinating question, one that scientists from many disciplines have been grappling with over the past century, is why individuals are choosing certain solutions within this wide landscape of sensorimotor possibilities. Underpinning this question are a conglomeration of interacting neuromechanical factors that may be shaping muscle activity patterns through the considerations of task performance, effort, and injury prevention. Although it may be a daunting task to piece together this apparent muddle of puzzle pieces, the challenges and opportunities available make the goal of better understanding neuromuscular control an exciting endeavour. As a scientific community, it is clear that our path ahead requires multi-disciplinary teams to meet at the intersection of biomechanics, neuroscience, and motor control. At the same time, we want to encourage readers to not let scientific progress be tracked solely by intellectually stimulating conversations within academic circles. Equally important is the ability to translate knowledge of neuromuscular control into the real-world, whether it is to inform neurorehabilitation strategies, develop safe workplace practices, or train athletes to maximize performance. After all, the ultimate measure of scientific success in our viewpoint is defined by the difference we make in human lives.
DM and PK contributed to the conception of the review paper. DM wrote the first draft of the manuscript. DM and PK contributed to manuscript revision, as well as read and approved the submitted version. All authors contributed to the article and approved the submitted version.
This work was supported by research funding from the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery Grant RGPIN-2016-06460 to PJK and an NSERC CGS-D to DMM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Cohn BA, Szedlák M, Gärtner B, Valero-Cuevas FJ. Feasibility theory reconciles and informs alternative approaches to neuromuscular control. Front Comput Neurosci. (2018) 12:62. doi: 10.3389/fncom.2018.00062
3. Sohn MH, McKay JL, Ting LH. Defining feasible bounds on muscle activation in a redundant biomechanical task: practical implications of redundancy. J Biomech. (2013) 46(7):1363–8. doi: 10.1016/j.jbiomech.2013.01.020
4. Ackland DC, Pak P, Richardson M, Pandy MG. Moment arms of the muscles crossing the anatomical shoulder. J Anat. (2008) 213(4):383–90. doi: 10.1111/j.1469-7580.2008.00965.x
5. van der Helm FCT, Veenbaas R. Modelling the mechanical effect of muscles with large attachment sites: application to the shoulder mechanism. J Biomech. (1991) 24(12):1151–63. doi: 10.1016/0021-9290(91)90007-A
6. Webb JD, Blemker SS, Delp SL. 3D Finite element models of shoulder muscles for computing lines of actions and moment arms. Comput Methods Biomech Biomed Engin. (2014) 17(8):829–37. doi: 10.1080/10255842.2012.719605
7. Jenny A, Inukai J. Principles of motor organization of the monkey cervical spinal cord. J Neurosci. (1983) 3(3):567–75. doi: 10.1523/JNEUROSCI.03-03-00567.1983
8. Billard A, Kragic D. Trends and challenges in robot manipulation. Science. (2019) 364(6446):eaat8414. doi: 10.1126/science.aat8414
9. Kudithipudi D, Aguilar-Simon M, Babb J, Bazhenov M, Blackiston D, Bongard J, et al. Biological underpinnings for lifelong learning machines. Nat Mach Intell. (2022) 4(3):196–210. doi: 10.1038/s42256-022-00452-0
10. Marjaninejad A, Urbina-Meléndez D, Cohn BA, Valero-Cuevas FJ. Autonomous functional movements in a tendon-driven limb via limited experience. Nat Mach Intell. (2019) 1(3):144–54. doi: 10.1038/s42256-019-0029-0
11. Sobinov AR, Bensmaia SJ. The neural mechanisms of manual dexterity. Nat Rev Neurosci. (2021) 22(12):741–57. doi: 10.1038/s41583-021-00528-7
12. Veeger HEJ, van der Helm FCT. Shoulder function: the perfect compromise between mobility and stability. J Biomech. (2007) 40(10):2119–29. doi: 10.1016/j.jbiomech.2006.10.016
13. Valero-Cuevas FJ. Fundamentals of neuromechanics. London: Springer London (2016). (Biosystems & Biorobotics; vol. 8). Available at: https://link.springer.com/10.1007/978-1-4471-6747-1 (Cited 2023 Apr 27).
14. Mussa-Ivaldi F, Hogan N, Bizzi E. Neural, mechanical, and geometric factors subserving arm posture in humans. J Neurosci. (1985) 5(10):2732–43. doi: 10.1523/JNEUROSCI.05-10-02732.1985
15. Schieber MH, Santello M. Hand function: peripheral and central constraints on performance. J Appl Physiol. (2004) 96(6):2293–300. doi: 10.1152/japplphysiol.01063.2003
16. Chao EY, An KN. Graphical interpretation of the solution to the redundant problem in biomechanics. J Biomech Eng. (1978) 100(3):159–67. doi: 10.1115/1.3426207
17. Valero-Cuevas FJ, Zajac FE, Burgar CG. Large index-fingertip forces are produced by subject-independent patterns of muscle excitation. J Biomech. (1998) 31(8):693–703. doi: 10.1016/S0021-9290(98)00082-7
18. Spoor CW. Balancing a force on the fingertip of a two-dimensional finger model without intrinsic muscles. J Biomech. (1983) 16(7):497–504. doi: 10.1016/0021-9290(83)90064-7
19. Bizzi E, Cheung VCK. The neural origin of muscle synergies. Front Comput Neurosci. (2013) 7:51. doi: 10.3389/fncom.2013.00051
20. Kutch JJ, Valero-Cuevas FJ. Challenges and new approaches to proving the existence of muscle synergies of neural origin. PLoS Comput Biol. (2012) 8(5):e1002434. doi: 10.1371/journal.pcbi.1002434
21. Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol. (2009) 19(6):601–7. doi: 10.1016/j.conb.2009.09.002
22. Brock O, Valero-Cuevas F. Transferring synergies from neuroscience to robotics. Phys Life Rev. (2016) 17:27–32. doi: 10.1016/j.plrev.2016.05.011
23. Latash ML, Scholz JP, Schöner G. Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev. (2002) 30(1):26–31. doi: 10.1097/00003677-200201000-00006
24. Scholz JP, Schöner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res. (1999) 126(3):289–306. doi: 10.1007/s002210050738
25. Valero-Cuevas FJ, Hoffmann H, Kurse MU, Kutch JJ, Theodorou EA. Computational models for neuromuscular function. IEEE Rev Biomed Eng. (2009) 2:110–35. doi: 10.1109/RBME.2009.2034981
26. Crowninshield RD, Brand RA. The prediction of forces in joint structures; distribution of intersegmental resultants. Exerc Sport Sci Rev. (1981) 9:159–81. doi: 10.1249/00003677-198101000-00004
27. Crowninshield RD, Brand RA. A physiologically based criterion of muscle force prediction in locomotion. J Biomech. (1981) 14(11):793–801. doi: 10.1016/0021-9290(81)90035-X
28. Dul J, Townsend MA, Shiavi R, Johnson GE. Muscular synergism—I. On criteria for load sharing between synergistic muscles. J Biomech. (1984) 17(9):663–73. doi: 10.1016/0021-9290(84)90120-9
29. Dul J, Johnson GE, Shiavi R, Townsend MA. Muscular synergism—II. A minimum-fatigue criterion for load sharing between synergistic muscles. J Biomech. (1984) 17(9):675–84. doi: 10.1016/0021-9290(84)90121-0
30. Hardt DE. Determining muscle forces in the leg during normal human walking—an application and evaluation of optimization methods. J Biomech Eng. (1978) 100(2):72–8. doi: 10.1115/1.3426195
31. Berret B, Chiovetto E, Nori F, Pozzo T. Evidence for composite cost functions in arm movement planning: an inverse optimal control approach. PLoS Comput Biol. (2011) 7(10):e1002183. doi: 10.1371/journal.pcbi.1002183
32. Soechting J, Buneo C, Herrmann U, Flanders M. Moving effortlessly in three dimensions: does Donders’ law apply to arm movement? J Neurosci. (1995) 15(9):6271–80. doi: 10.1523/JNEUROSCI.15-09-06271.1995
33. Cashaback JGA, Cluff T. Increase in joint stability at the expense of energy efficiency correlates with force variability during a fatiguing task. J Biomech. (2015) 48(4):621–6. doi: 10.1016/j.jbiomech.2014.12.053
34. Sohn MH, Ting LH. The cost of being stable: trade-offs between effort and stability across a landscape of redundant motor solutions. bioRxiv. (2018). doi: 10.1101/477083
35. Diedrichsen J, Shadmehr R, Ivry RB. The coordination of movement: optimal feedback control and beyond. Trends Cogn Sci (Regul Ed). (2010) 14(1):31–9. doi: 10.1016/j.tics.2009.11.004
36. Scott SH. Optimal feedback control and the neural basis of volitional motor control. Nat Rev Neurosci. (2004) 5(7):532–45. doi: 10.1038/nrn1427
37. Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci. (2002) 5(11):1226–35. doi: 10.1038/nn963
38. Loeb GE, Levine WS, He J. Understanding sensorimotor feedback through optimal control. Cold Spring Harbor Symp Quant Biol. (1990) 55(0):791–803. doi: 10.1101/SQB.1990.055.01.074
39. Loeb GE. Optimal isn’t good enough. Biol Cybern. (2012) 106(11–12):757–65. doi: 10.1007/s00422-012-0514-6
40. Körding KP, Wolpert DM. Bayesian Integration in sensorimotor learning. Nature. (2004) 427(6971):244–7. doi: 10.1038/nature02169
41. Dhawale AK, Smith MA, Ölveczky BP. The role of variability in motor learning. Annu Rev Neurosci. (2017) 40(1):479–98. doi: 10.1146/annurev-neuro-072116-031548
42. Santos VJ, Bustamante CD, Valero-Cuevas FJ. Improving the fitness of high-dimensional biomechanical models via data-driven stochastic exploration. IEEE Trans Biomed Eng. (2009) 56(3):552–64. doi: 10.1109/TBME.2008.2006033
43. Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG: an update. J Appl Physiol. (2014) 117(11):1215–30. doi: 10.1152/japplphysiol.00162.2014
44. Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. (2004) 96(4):1486–95. doi: 10.1152/japplphysiol.01070.2003
45. McIntyre J, Mussa-Ivaldi FA, Bizzi E. The control of stable postures in the multijoint arm. Exp Brain Res. (1996) 110(2):248–64. doi: 10.1007/BF00228556
46. Rancourt D, Hogan N. Stability in force-production tasks. J Mot Behav. (2001) 33(2):193–204. doi: 10.1080/00222890109603150
47. Chib VS, Krutky MA, Lynch KM, Mussa-Ivaldi FA. The separate neural control of hand movements and contact forces. J Neurosci. (2009) 29(12):3939–47. doi: 10.1523/JNEUROSCI.5856-08.2009
48. Venkadesan M, Valero-Cuevas FJ. Neural control of motion-to-force transitions with the fingertip. J Neurosci. (2008) 28(6):1366–73. doi: 10.1523/JNEUROSCI.4993-07.2008
49. Hogan N. Impedance control: an approach to manipulation. 1984 American control conference. San Diego, CA, USA: IEEE (1984). p. 304–13. Available at: https://ieeexplore.ieee.org/document/4788393/ (Cited 2023 Jun 11).
50. Hogan N. Impedance control: an approach to manipulation: part II—implementation. J Dyn Syst Meas Control. (1985) 107(1):8–16. doi: 10.1115/1.3140713
51. Hagen DA, Valero-Cuevas FJ. Similar movements are associated with drastically different muscle contraction velocities. J Biomech. (2017) 59:90–100. doi: 10.1016/j.jbiomech.2017.05.019
52. Penrod DD, Davy DT, Singh DP. An optimization approach to tendon force analysis. J Biomech. (1974) 7(2):123–9. doi: 10.1016/0021-9290(74)90050-5
53. Seireg A, Arvikar RJ. The prediction of muscular load sharing and joint forces in the lower extremities during walking. J Biomech. (1975) 8(2):89–102. doi: 10.1016/0021-9290(75)90089-5
54. Seireg A, Arvikar RJ. A mathematical model for evaluation of forces in lower extremeties of the musculo-skeletal system. J Biomech. (1973) 6(3):313–26. doi: 10.1016/0021-9290(73)90053-5
55. Chao EY, An KN. Determination of internal forces in human hand. J Engrg Mech Div. (1978) 104(1):255–72. doi: 10.1061/JMCEA3.0002325
56. John J, Dingwell JB, Cusumano JP. Error correction and the structure of inter-trial fluctuations in a redundant movement task. PLoS Comput Biol. (2016) 12(9):e1005118. doi: 10.1371/journal.pcbi.1005118
57. Sternad D. It’s not (only) the mean that matters: variability, noise and exploration in skill learning. Curr Opin Behav Sci. (2018) 20:183–95. doi: 10.1016/j.cobeha.2018.01.004
58. Sternad D, Abe MO, Hu X, Müller H. Neuromotor noise, error tolerance and velocity-dependent costs in skilled performance. PLoS Comput Biol. (2011) 7(9):e1002159. doi: 10.1371/journal.pcbi.1002159
59. Cohen RG, Sternad D. Variability in motor learning: relocating, channeling and reducing noise. Exp Brain Res. (2009) 193(1):69–83. doi: 10.1007/s00221-008-1596-1
60. Váša F, Mišić B. Null models in network neuroscience. Nat Rev Neurosci. (2022) 23(8):493–504. doi: 10.1038/s41583-022-00601-9
61. Ait-Haddou R, Jinha A, Herzog W, Binding P. Analysis of the force-sharing problem using an optimization model. Math Biosci. (2004) 191(2):111–22. doi: 10.1016/j.mbs.2004.05.003
62. Kutch JJ, Valero-Cuevas FJ. Muscle redundancy does not imply robustness to muscle dysfunction. J Biomech. (2011) 44(7):1264–70. doi: 10.1016/j.jbiomech.2011.02.014
63. Ait-Haddou R, Binding P, Herzog W. Theoretical considerations on cocontraction of sets of agonistic and antagonistic muscles. J Biomech. (2000) 33(9):1105–11. doi: 10.1016/S0021-9290(00)00085-3
64. Binding P, Jinha A, Herzog W. Analytic analysis of the force sharing among synergistic muscles in one- and two-degree-of-freedom models. J Biomech. (2000) 33(11):1423–32. doi: 10.1016/S0021-9290(00)00108-1
65. Herzog W, Binding P. Cocontraction of pairs of antagonistic muscles: analytical solution for planar static nonlinear optimization approaches. Math Biosci. (1993) 118(1):83–95. doi: 10.1016/0025-5564(93)90034-8
66. Herzog W, Binding P. Predictions of antagonistic muscular activity using nonlinear optimization. Math Biosci. (1992) 111(2):217–29. doi: 10.1016/0025-5564(92)90071-4
67. Herzog W, Leonard TR. Validation of optimization models that estimate the forces exerted by synergistic muscles. J Biomech. (1991) 24:31–9. doi: 10.1016/0021-9290(91)90375-W
68. Jinha A, Ait-Haddou R, Binding P, Herzog W. Antagonistic activity of one-joint muscles in three-dimensions using non-linear optimisation. Math Biosci. (2006) 202(1):57–70. doi: 10.1016/j.mbs.2006.03.018
69. Jinha A, Ait-Haddou R, Herzog W. Predictions of co-contraction depend critically on degrees-of-freedom in the musculoskeletal model. J Biomech. (2006) 39(6):1145–52. doi: 10.1016/j.jbiomech.2005.03.001
70. Sohn MH, Ting LH. Suboptimal muscle synergy activation patterns generalize their motor function across postures. Front Comput Neurosci. (2016) 10:7. doi: 10.3389/fncom.2016.00007
71. Simpson CS, Sohn MH, Allen JL, Ting LH. Feasible muscle activation ranges based on inverse dynamics analyses of human walking. J Biomech. (2015) 48(12):2990–7. doi: 10.1016/j.jbiomech.2015.07.037
72. Sohn MH, Smith DM, Ting LH. Effects of kinematic complexity and number of muscles on musculoskeletal model robustness to muscle dysfunction. PLoS ONE. (2019) 14(7):e0219779. doi: 10.1371/journal.pone.0219779
73. Martelli S, Calvetti D, Somersalo E, Viceconti M. Stochastic modelling of muscle recruitment during activity. Interface Focus. (2015) 5(2):20140094. doi: 10.1098/rsfs.2014.0094
74. Martelli S, Calvetti D, Somersalo E, Viceconti M, Taddei F. Computational tools for calculating alternative muscle force patterns during motion: a comparison of possible solutions. J Biomech. (2013) 46(12):2097–100. doi: 10.1016/j.jbiomech.2013.05.023
75. Stanev D, Moustakas K. Modeling musculoskeletal kinematic and dynamic redundancy using null space projection. PLoS ONE. (2019) 14(1):e0209171. doi: 10.1371/journal.pone.0209171
76. Stanev D, Moustakas K. Stiffness modulation of redundant musculoskeletal systems. J Biomech. (2019) 85:101–7. doi: 10.1016/j.jbiomech.2019.01.017
77. Hicks JL, Uchida TK, Seth A, Rajagopal A, Delp SL. Is my model good enough? Best practices for verification and validation of musculoskeletal models and simulations of movement. J Biomech Eng. (2015) 137(2):020905. doi: 10.1115/1.4029304
78. Bolsterlee B, Veeger DHEJ, Chadwick EK. Clinical applications of musculoskeletal modelling for the shoulder and upper limb. Med Biol Eng Comput. (2013) 51(9):953–63. doi: 10.1007/s11517-013-1099-5
79. Lippitt SB, Vanderhooft JE, Harris SL, Sidles JA, Harryman DT, Matsen FA. Glenohumeral stability from concavity-compression: a quantitative analysis. J Shoulder Elbow Surg. (1993) 2(1):27–35. doi: 10.1016/S1058-2746(09)80134-1
80. Duprey S, Naaim A, Moissenet F, Begon M, Chèze L. Kinematic models of the upper limb joints for multibody kinematics optimisation: an overview. J Biomech. (2017) 62:87–94. doi: 10.1016/j.jbiomech.2016.12.005
81. Seth A, Matias R, Veloso AP, Delp SL. A biomechanical model of the scapulothoracic joint to accurately capture scapular kinematics during shoulder movements. PLoS ONE. (2016) 11(1):e0141028. doi: 10.1371/journal.pone.0141028
82. Mulla DM, Hodder JN, Maly MR, Lyons JL, Keir PJ. Glenohumeral stabilizing roles of the scapulohumeral muscles: implications of muscle geometry. J Biomech. (2020) 100:109589. doi: 10.1016/j.jbiomech.2019.109589
83. Blache Y, Begon M, Michaud B, Desmoulins L, Allard P, Dal Maso F. Muscle function in glenohumeral joint stability during lifting task. PLoS ONE. (2017) 12(12):e0189406. doi: 10.1371/journal.pone.0189406
84. Dickerson CR, Chaffin DB, Hughes RE. A mathematical musculoskeletal shoulder model for proactive ergonomic analysis. Comput Methods Biomech Biomed Engin. (2007) 10(6):389–400. doi: 10.1080/10255840701592727
85. Falisse A, Afschrift M, De Groote F. Modeling toes contributes to realistic stance knee mechanics in three-dimensional predictive simulations of walking. PLoS ONE. (2022) 17(1):e0256311. doi: 10.1371/journal.pone.0256311
86. Paclet F, Quaine F. Motor control theories improve biomechanical model of the hand for finger pressing tasks. J Biomech. (2012) 45(7):1246–51. doi: 10.1016/j.jbiomech.2012.01.038
87. MacIntosh AR, Keir PJ. An open-source model and solution method to predict co-contraction in the finger. Comput Methods Biomech Biomed Engin. (2017) 20(13):1373–81. doi: 10.1080/10255842.2017.1364732
88. Carbone V, van der Krogt MM, Koopman HFJM, Verdonschot N. Sensitivity of subject-specific models to Hill muscle–tendon model parameters in simulations of gait. J Biomech. (2016) 49(9):1953–60. doi: 10.1016/j.jbiomech.2016.04.008
89. Carbone V, van der Krogt MM, Koopman HFJM, Verdonschot N. Sensitivity of subject-specific models to errors in musculo-skeletal geometry. J Biomech. (2012) 45(14):2476–80. doi: 10.1016/j.jbiomech.2012.06.026
90. Mulla DM, Hodder JN, Maly MR, Lyons JL, Keir PJ. Modeling the effects of musculoskeletal geometry on scapulohumeral muscle moment arms and lines of action. Comput Methods Biomech Biomed Engin. (2019) 22(16):1311–22. doi: 10.1080/10255842.2019.1661392
91. Valero-Cuevas FJ, Anand VV, Saxena A, Lipson H. Beyond parameter estimation: extending biomechanical modeling by the explicit exploration of model topology. IEEE Trans Biomed Eng. (2007) 54(11):1951–64. doi: 10.1109/TBME.2007.906494
92. Laz PJ, Browne M. A review of probabilistic analysis in orthopaedic biomechanics. Proc Inst Mech Eng H. (2010) 224(8):927–43. doi: 10.1243/09544119JEIM739
93. Goehler CM, Murray WM. The sensitivity of endpoint forces produced by the extrinsic muscles of the thumb to posture. J Biomech. (2010) 43(8):1553–9. doi: 10.1016/j.jbiomech.2010.01.032
94. Lee SW, Chen H, Towles JD, Kamper DG. Estimation of the effective static moment arms of the tendons in the index finger extensor mechanism. J Biomech. (2008) 41(7):1567–73. doi: 10.1016/j.jbiomech.2008.02.008
95. Pearlman JL, Roach SS, Valero-Cuevas FJ. The fundamental thumb-tip force vectors produced by the muscles of the thumb. J Orthop Res. (2004) 22(2):306–12. doi: 10.1016/j.orthres.2003.08.001
96. Qiu D, Lee SW, Amine M, Kamper DG. Intersegmental kinetics significantly impact mapping from finger musculotendon forces to fingertip forces. J Biomech. (2017) 65:82–8. doi: 10.1016/j.jbiomech.2017.10.004
97. Wohlman SJ, Murray WM. Bridging the gap between cadaveric and in vivo experiments: a biomechanical model evaluating thumb-tip endpoint forces. J Biomech. (2013) 46(5):1014–20. doi: 10.1016/j.jbiomech.2012.10.044
98. Antony NT, Keir PJ. Effects of posture, movement and hand load on shoulder muscle activity. J Electromyogr Kinesiol. (2010) 20(2):191–8. doi: 10.1016/j.jelekin.2009.04.010
99. Hodder JN, Keir PJ. Targeted gripping reduces shoulder muscle activity and variability. J Electromyogr Kinesiol. (2012) 22(2):186–90. doi: 10.1016/j.jelekin.2011.11.011
100. McDonald A, Picco BR, Belbeck AL, Chow AY, Dickerson CR. Spatial dependency of shoulder muscle demands in horizontal pushing and pulling. Appl Ergon. (2012) 43(6):971–8. doi: 10.1016/j.apergo.2012.01.005
101. McDonald AC, Tsang C, Meszaros KA, Dickerson CR. Shoulder muscle activity in off-axis pushing and pulling tasks. Int J Ind Ergon. (2020) 75:102892. doi: 10.1016/j.ergon.2019.102892
102. McDonald AC, Brenneman EC, Cudlip AC, Dickerson CR. The spatial dependency of shoulder muscle demands for seated lateral hand force exertions. J Appl Biomech. (2014) 30(1):1–11. doi: 10.1123/jab.2012-0221
103. Buchanan TS, Rovai GP, Rymer WZ. Strategies for muscle activation during isometric torque generation at the human elbow. J Neurophysiol. (1989) 62(6):1201–12. doi: 10.1152/jn.1989.62.6.1201
104. Buchanan TS, Almdale DP, Lewis JL, Rymer WZ. Characteristics of synergic relations during isometric contractions of human elbow muscles. J Neurophysiol. (1986) 56(5):1225–41. doi: 10.1152/jn.1986.56.5.1225
105. Flanders M, Soechting JF. Arm muscle activation for static forces in three-dimensional space. J Neurophysiol. (1990) 64(6):1818–37. doi: 10.1152/jn.1990.64.6.1818
106. Mogk J, Keir P. The effects of posture on forearm muscle loading during gripping. Ergonomics. (2003) 46(9):956–75. doi: 10.1080/0014013031000107595
107. Roh J, Rymer WZ, Beer RF. Robustness of muscle synergies underlying three-dimensional force generation at the hand in healthy humans. J Neurophysiol. (2012) 107(8):2123–42. doi: 10.1152/jn.00173.2011
108. Lee SW, Qiu D, Fischer HC, Conrad MO, Kamper DG. Modulation of finger muscle activation patterns across postures is coordinated across all muscle groups. J Neurophysiol. (2020) 124(2):330–41. doi: 10.1152/jn.00088.2020
109. Valero-Cuevas FJ. Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. J Neurophysiol. (2000) 83(3):1469–79. doi: 10.1152/jn.2000.83.3.1469
110. Weiss EJ, Flanders M. Muscular and postural synergies of the human hand. J Neurophysiol. (2004) 92(1):523–35. doi: 10.1152/jn.01265.2003
111. Jones KE, Hamilton AF, Wolpert DM. Sources of signal-dependent noise during isometric force production. J Neurophysiol. (2002) 88(3):1533–44. doi: 10.1152/jn.2002.88.3.1533
112. Faisal AA, Selen LPJ, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. (2008) 9(4):292–303. doi: 10.1038/nrn2258
113. Mathiassen SE, Möller T, Forsman M. Variability in mechanical exposure within and between individuals performing a highly constrained industrial work task. Ergonomics. (2003) 46(8):800–24. doi: 10.1080/0014013031000090125
114. d’Avella A, Portone A, Lacquaniti F. Superposition and modulation of muscle synergies for reaching in response to a change in target location. J Neurophysiol. (2011) 106(6):2796–812. doi: 10.1152/jn.00675.2010
115. d’Avella A, Fernandez L, Portone A, Lacquaniti F. Modulation of phasic and tonic muscle synergies with reaching direction and speed. J Neurophysiol. (2008) 100(3):1433–54. doi: 10.1152/jn.01377.2007
116. d’Avella A, Portone A, Fernandez L, Lacquaniti F. Control of fast-reaching movements by muscle synergy combinations. J Neurosci. (2006) 26(30):7791–810. doi: 10.1523/JNEUROSCI.0830-06.2006
117. d’Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci. (2003) 6(3):300–8. doi: 10.1038/nn1010
118. Overduin SA, d’Avella A, Roh J, Bizzi E. Modulation of muscle synergy recruitment in primate grasping. J Neurosci. (2008) 28(4):880–92. doi: 10.1523/JNEUROSCI.2869-07.2008
119. Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol. (2005) 93(1):609–13. doi: 10.1152/jn.00681.2004
120. Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol. (2006) 96(3):1530–46. doi: 10.1152/jn.00810.2005
121. Tresch MC, Saltiel P, Bizzi E. The construction of movement by the spinal cord. Nat Neurosci. (1999) 2(2):162–7. doi: 10.1038/5721
122. Steele KM, Tresch MC, Perreault EJ. Consequences of biomechanically constrained tasks in the design and interpretation of synergy analyses. J Neurophysiol. (2015) 113(7):2102–13. doi: 10.1152/jn.00769.2013
123. Erdemir A, McLean S, Herzog W, van den Bogert AJ. Model-based estimation of muscle forces exerted during movements. Clin Biomech. (2007) 22(2):131–54. doi: 10.1016/j.clinbiomech.2006.09.005
124. Todorov E. Optimality principles in sensorimotor control. Nat Neurosci. (2004) 7(9):907–15. doi: 10.1038/nn1309
125. Kirk DE. Optimal control theory: An Introduction. Englewood Cliffs, New Jersey: Prentice-Hall (1970).
126. Bryson AE, Ho YC. Applied optimal control: Optimization, estimation, and control. New York: Taylor & Francis (1975).
127. Anderson FC, Pandy MG. Dynamic optimization of human walking. J Biomech Eng. (2001) 123(5):381–90. doi: 10.1115/1.1392310
128. Davy DT, Audu ML. A dynamic optimization technique for predicting muscle forces in the swing phase of gait. J Biomech. (1987) 20(2):187–201. doi: 10.1016/0021-9290(87)90310-1
129. Flash T, Hogan N. The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci. (1985) 5(7):1688–703. doi: 10.1523/JNEUROSCI.05-07-01688.1985
130. Hatze H. The complete optimization of a human motion. Math Biosci. (1976) 28(1–2):99–135. doi: 10.1016/0025-5564(76)90098-5
131. Kuo AD. An optimal control model for analyzing human postural balance. IEEE Trans Biomed Eng. (1995) 42(1):87–101. doi: 10.1109/10.362914
132. Dembia CL, Bianco NA, Falisse A, Hicks JL, Delp SL. Opensim Moco: musculoskeletal optimal control. PLoS Comput Biol. (2020) 16(12):e1008493. doi: 10.1371/journal.pcbi.1008493
133. Van Wouwe T, Ting LH, De Groote F. An approximate stochastic optimal control framework to simulate nonlinear neuro-musculoskeletal models in the presence of noise. PLoS Comput Biol. (2022) 18(6):e1009338. doi: 10.1371/journal.pcbi.1009338
134. Van Den Bogert AJ, Blana D, Heinrich D. Implicit methods for efficient musculoskeletal simulation and optimal control. Procedia IUTAM. (2011) 2:297–316. doi: 10.1016/j.piutam.2011.04.027
135. Mombaur K, Truong A, Laumond JP. From human to humanoid locomotion—an inverse optimal control approach. Auton Robot. (2010) 28(3):369–83. doi: 10.1007/s10514-009-9170-7
136. Todorov E. Stochastic optimal control and estimation methods adapted to the noise characteristics of the sensorimotor system. Neural Comput. (2005) 17(5):1084–108. doi: 10.1162/0899766053491887
137. Brunton SL, Kutz JN. Data driven science & engineering: Machine learning, dynamical systems, and control. New York: Cambridge University Press (2019).
138. Hogan N. An organizing principle for a class of voluntary movements. J Neurosci. (1984) 4(11):2745–54. doi: 10.1523/JNEUROSCI.04-11-02745.1984
139. Uno Y, Kawato M, Suzuki R. Formation and control of optimal trajectory in human multijoint arm movement. Biol Cybern. (1989) 61(2):89–101. doi: 10.1007/BF00204593
140. Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. (1998) 394(6695):780–4. doi: 10.1038/29528
141. Alexander RM. A minimum energy cost hypothesis for human arm trajectories. Biol Cybern. (1997) 76(2):97–105. doi: 10.1007/s004220050324
142. Pedotti A, Krishnan VV, Stark L. Optimization of muscle-force sequencing in human locomotion. Math Biosci. (1978) 38(1–2):57–76. doi: 10.1016/0025-5564(78)90018-4
143. Prilutsky BI, Zatsiorsky VM. Optimization-based models of muscle coordination. Exerc Sport Sci Rev. (2002) 30(1):32–8. doi: 10.1097/00003677-200201000-00007
144. Alexander RM. Energy-minimizing choices of muscles and patterns of movement. Motor Control. (2000) 4(1):45–7. doi: 10.1123/mcj.4.1.45
145. Kaufman KR, Au KN, Litchy WJ, Chao EYS. Physiological prediction of muscle forces—II. Application to isokinetic exercise. Neuroscience. (1991) 40(3):793–804. doi: 10.1016/0306-4522(91)90013-E
146. Praagman M, Chadwick EKJ, van der Helm FCT, Veeger HEJ. The relationship between two different mechanical cost functions and muscle oxygen consumption. J Biomech. (2006) 39(4):758–65. doi: 10.1016/j.jbiomech.2004.11.034
148. Sockol MD, Raichlen DA, Pontzer H. Chimpanzee locomotor energetics and the origin of human bipedalism. Proc Natl Acad Sci USA. (2007) 104(30):12265–9. doi: 10.1073/pnas.0703267104
149. Finley JM, Bastian AJ, Gottschall JS. Learning to be economical: the energy cost of walking tracks motor adaptation: split-belt adaptation reduces metabolic power. J Physiol (Lond). (2013) 591(4):1081–95. doi: 10.1113/jphysiol.2012.245506
150. Gribble PL, Mullin LI, Cothros N, Mattar A. Role of cocontraction in arm movement accuracy. J Neurophysiol. (2003) 89(5):2396–405. doi: 10.1152/jn.01020.2002
151. Huang HJ, Kram R, Ahmed AA. Reduction of metabolic cost during motor learning of arm reaching dynamics. J Neurosci. (2012) 32(6):2182–90. doi: 10.1523/JNEUROSCI.4003-11.2012
152. Abram SJ, Poggensee KL, Sánchez N, Simha SN, Finley JM, Collins SH, et al. General variability leads to specific adaptation toward optimal movement policies. Curr Biol. (2022) 32(10):2222–32.e5. doi: 10.1016/j.cub.2022.04.015
153. Srinivasan M, Ruina A. Computer optimization of a minimal biped model discovers walking and running. Nature. (2006) 439(7072):72–5. doi: 10.1038/nature04113
154. Buchanan TS, Shreeve DA. An evaluation of optimization techniques for the prediction of muscle activation patterns during isometric tasks. J Biomech Eng. (1996) 118(4):565–74. doi: 10.1115/1.2796044
155. Collins JJ. The redundant nature of locomotor optimization laws. J Biomech. (1995) 28(3):251–67. doi: 10.1016/0021-9290(94)00072-C
156. McFarland DC, Binder-Markey BI, Nichols JA, Wohlman SJ, de Bruin M, Murray WM. A musculoskeletal model of the hand and wrist capable of simulating functional tasks. IEEE Trans Biomed Eng. (2023) 70(5):1424–35. doi: 10.1109/TBME.2022.3217722
157. Adolph KE, Cole WG, Komati M, Garciaguirre JS, Badaly D, Lingeman JM, et al. How do you learn to walk? Thousands of steps and dozens of falls per day. Psychol Sci. (2012) 23(11):1387–94. doi: 10.1177/0956797612446346
158. Cashaback JGA, Lao CK, Palidis DJ, Coltman SK, McGregor HR, Gribble PL. The gradient of the reinforcement landscape influences sensorimotor learning. PLoS Comput Biol. (2019) 15(3):e1006839. doi: 10.1371/journal.pcbi.1006839
159. de Rugy A, Loeb GE, Carroll TJ. Muscle coordination is habitual rather than optimal. J Neurosci. (2012) 32(21):7384–91. doi: 10.1523/JNEUROSCI.5792-11.2012
160. Ganesh G, Haruno M, Kawato M, Burdet E. Motor memory and local minimization of error and effort, not global optimization, determine motor behavior. J Neurophysiol. (2010) 104(1):382–90. doi: 10.1152/jn.01058.2009
161. Cluff T, Crevecoeur F, Scott SH. Tradeoffs in optimal control capture patterns of human sensorimotor control and adaptation. bioRxiv. (2019). doi: 10.1101/730713
162. Kistemaker DA, Wong JD, Gribble PL. The central nervous system does not minimize energy cost in arm movements. J Neurophysiol. (2010) 104(6):2985–94. doi: 10.1152/jn.00483.2010
163. Johnson RT, Lakeland D, Finley JM. Using Bayesian inference to estimate plausible muscle forces in musculoskeletal models. J NeuroEngineering Rehabil. (2022) 19(1):34. doi: 10.1186/s12984-022-01008-4
164. Shadmehr R, Huang HJ, Ahmed AA. A representation of effort in decision-making and motor control. Curr Biol. (2016) 26(14):1929–34. doi: 10.1016/j.cub.2016.05.065
165. Shadmehr R, Ahmed AA. Vigor: neuroeconomics of movement control. Cambridge, MA: The MIT Press (2020). Available at: http://direct.mit.edu/books/book/4845/VigorNeuroeconomics-of-Movement-Control (Cited 2023 Apr 27).
166. Cappelletto JAM. Mediators of decision making in action planning: assessing the functional costs of alternative movement strategies. Hamilton, ON: McMaster University (2019). Available at: http://hdl.handle.net/11375/24936
167. Rosenbaum DA, Brach M, Semenov A. Behavioral ecology meets motor behavior: choosing between walking and reaching paths. J Mot Behav. (2011) 43(2):131–6. doi: 10.1080/00222895.2010.548423
168. Sánchez N, Park S, Finley JM. Evidence of energetic optimization during adaptation differs for metabolic, mechanical, and perceptual estimates of energetic cost. Sci Rep. (2017) 7(1):7682. doi: 10.1038/s41598-017-08147-y
169. Veerkamp K, Waterval NFJ, Geijtenbeek T, Carty CP, Lloyd DG, Harlaar J, et al. Evaluating cost function criteria in predicting healthy gait. J Biomech. (2021) 123:110530. doi: 10.1016/j.jbiomech.2021.110530
170. Berniker M, O’Brien MK, Kording KP, Ahmed AA. An examination of the generalizability of motor costs. PLoS ONE. (2013) 8(1):e53759. doi: 10.1371/journal.pone.0053759
171. McDonald KA, Cusumano JP, Hieronymi A, Rubenson J. Humans trade off whole-body energy cost to avoid overburdening muscles while walking. Proc R Soc B. (2022) 289(1985):20221189. doi: 10.1098/rspb.2022.1189
172. Selinger JC, Wong JD, Simha SN, Donelan JM. How people initiate energy optimization and converge on their optimal gaits. J Exp Biol. (2019) 222(19):jeb.198234. doi: 10.1242/jeb.198234
173. Selinger JC, O’Connor SM, Wong JD, Donelan JM. Humans can continuously optimize energetic cost during walking. Curr Biol. (2015) 25(18):2452–6. doi: 10.1016/j.cub.2015.08.016
174. Franklin DW, Wolpert DM. Computational mechanisms of sensorimotor control. Neuron. (2011) 72(3):425–42. doi: 10.1016/j.neuron.2011.10.006
175. Laine CM, Nagamori A, Valero-Cuevas FJ. The dynamics of voluntary force production in afferented muscle influence involuntary tremor. Front Comput Neurosci. (2016) 10:86. doi: 10.3389/fncom.2016.00086
176. Nagamori A, Laine CM, Valero-Cuevas FJ. Cardinal features of involuntary force variability can arise from the closed-loop control of viscoelastic afferented muscles. PLoS Comput Biol. (2018) 14(1):e1005884. doi: 10.1371/journal.pcbi.1005884
177. Nagamori A, Laine CM, Loeb GE, Valero-Cuevas FJ. Force variability is mostly not motor noise: theoretical implications for motor control. PLoS Comput Biol. (2021) 17(3):e1008707. doi: 10.1371/journal.pcbi.1008707
178. Reschechtko S, Pruszynski JA. Stretch reflexes. Curr Biol. (2020) 30(18):R1025–30. doi: 10.1016/j.cub.2020.07.092
179. Hogan N. Adaptive control of mechanical impedance by coactivation of antagonist muscles. IEEE Trans Automat Contr. (1984) 29(8):681–90. doi: 10.1109/TAC.1984.1103644
180. Burdet E, Osu R, Franklin DW, Milner TE, Kawato M. The central nervous system stabilizes unstable dynamics by learning optimal impedance. Nature. (2001) 414(6862):446–9. doi: 10.1038/35106566
181. Hu X, Murray WM, Perreault EJ. Biomechanical constraints on the feedforward regulation of endpoint stiffness. J Neurophysiol. (2012) 108(8):2083–91. doi: 10.1152/jn.00330.2012
182. Hu X, Murray WM, Perreault EJ. Muscle short-range stiffness can be used to estimate the endpoint stiffness of the human arm. J Neurophysiol. (2011) 105(4):1633–41. doi: 10.1152/jn.00537.2010
183. Selen LPJ, Beek PJ, van Dieën JH. Impedance is modulated to meet accuracy demands during goal-directed arm movements. Exp Brain Res. (2006) 172(1):129–38. doi: 10.1007/s00221-005-0320-7
184. Selen LPJ, Beek PJ, van Dieën JH. Can co-activation reduce kinematic variability? A simulation study. Biol Cybern. (2005) 93(5):373–81. doi: 10.1007/s00422-005-0015-y
185. Koelewijn AD, Van Den Bogert AJ. Antagonistic co-contraction can minimize muscular effort in systems with uncertainty. PeerJ. (2022) 10:e13085. doi: 10.7717/peerj.13085
186. Berret B, Jean F. Stochastic optimal open-loop control as a theory of force and impedance planning via muscle co-contraction. PLoS Comput Biol. (2020) 16(2):e1007414. doi: 10.1371/journal.pcbi.1007414
187. Cholewicki J, McGill S. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech. (1996) 11(1):1–15. doi: 10.1016/0268-0033(95)00035-6
188. Potvin JR, Brown SHM. An equation to calculate individual muscle contributions to joint stability. J Biomech. (2005) 38(5):973–80. doi: 10.1016/j.jbiomech.2004.06.004
189. McFarland DC, Brynildsen AG, Saul KR. Sensitivity of neuromechanical predictions to choice of glenohumeral stability modeling approach. J Appl Biomech. (2020) 36(4):249–58. doi: 10.1123/jab.2019-0088
190. Nicolozakes CP, Ludvig D, Baillargeon EM, Perreault EJ, Seitz AL. Muscle contraction has a reduced effect on increasing glenohumeral stability in the apprehension position. Med Sci Sports Exerc. (2021) 53(11):2354–62. doi: 10.1249/MSS.0000000000002708
191. Lipps DB, Baillargeon EM, Ludvig D, Perreault EJ. Quantifying the multidimensional impedance of the shoulder during volitional contractions. Ann Biomed Eng. (2020) 48(9):2354–69. doi: 10.1007/s10439-020-02509-w
192. Sawers A, Pai YCC, Bhatt T, Ting LH. Neuromuscular responses differ between slip-induced falls and recoveries in older adults. J Neurophysiol. (2017) 117(2):509–22. doi: 10.1152/jn.00699.2016
193. Labriola JE, Lee TQ, Debski RE, McMahon PJ. Stability and instability of the glenohumeral joint: the role of shoulder muscles. J Shoulder Elbow Surg. (2005) 14(1):S32–8. doi: 10.1016/j.jse.2004.09.014
194. Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. (2004) 32(3):447–57. doi: 10.1023/B:ABME.0000017541.82498.37
195. Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. (2009) 42(14):2294–300. doi: 10.1016/j.jbiomech.2009.06.019
196. Hug F, Tucker K. Muscle coordination and the development of musculoskeletal disorders. Exerc Sport Sci Rev. (2017) 45(4):201–8. doi: 10.1249/JES.0000000000000122
197. Alessandro C, Barroso FO, Prashara A, Tentler DP, Yeh HY, Tresch MC. Coordination amongst quadriceps muscles suggests neural regulation of internal joint stresses, not simplification of task performance. Proc Natl Acad Sci USA. (2020) 117(14):8135–42. doi: 10.1073/pnas.1916578117
198. Barroso FO, Alessandro C, Tresch MC. Adaptation of muscle activation after patellar loading demonstrates neural control of joint variables. Sci Rep. (2019) 9(1):20370. doi: 10.1038/s41598-019-56888-9
199. Alessandro C, Rellinger BA, Barroso FO, Tresch MC. Adaptation after vastus lateralis denervation in rats demonstrates neural regulation of joint stresses and strains. eLife. (2018) 7:e38215. doi: 10.7554/eLife.38215
200. Meireles S, Reeves ND, Jones RK, Smith CR, Thelen DG, Jonkers I. Patients with medial knee osteoarthritis reduce medial knee contact forces by altering trunk kinematics, progression speed, and stepping strategy during stair ascent and descent: a pilot study. J Appl Biomech. (2019) 35(4):280–9. doi: 10.1123/jab.2017-0159
201. Uhlrich SD, Jackson RW, Seth A, Kolesar JA, Delp SL. Muscle coordination retraining inspired by musculoskeletal simulations reduces knee contact force. Sci Rep. (2022) 12(1):9842. doi: 10.1038/s41598-022-13386-9
202. Van Veen B, Montefiori E, Modenese L, Mazzà C, Viceconti M. Muscle recruitment strategies can reduce joint loading during level walking. J Biomech. (2019) 97:109368. doi: 10.1016/j.jbiomech.2019.109368
203. DeMers MS, Pal S, Delp SL. Changes in tibiofemoral forces due to variations in muscle activity during walking: tibiofemoral forces and muscle activity. J Orthop Res. (2014) 32(6):769–76. doi: 10.1002/jor.22601
204. Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. J Physiol (Lond). (1994) 479(3):487–97. doi: 10.1113/jphysiol.1994.sp020312
205. Leijnse JNAL, Walbeehm ET, Sonneveld GJ, Hovius SER, Kauer JMG. Connections between the tendons of the musculus flexor digitorum profundus involving the synovial sheaths in the carpal tunnel. Cells Tissues Organs. (1997) 160(2):112–22. doi: 10.1159/000148003
206. Lang CE, Schieber MH. Human finger independence: limitations due to passive mechanical coupling versus active neuromuscular control. J Neurophysiol. (2004) 92(5):2802–10. doi: 10.1152/jn.00480.2004
207. Mirakhorlo M, Maas H, Veeger DHEJ. Timing and extent of finger force enslaving during a dynamic force task cannot be explained by EMG activity patterns. PLoS ONE. (2017) 12(8):e0183145. doi: 10.1371/journal.pone.0183145
208. Sanei K, Keir PJ. Independence and control of the fingers depend on direction and contraction mode. Hum Mov Sci. (2013) 32(3):457–71. doi: 10.1016/j.humov.2013.01.004
209. Zatsiorsky VM, Li ZM, Latash ML. Enslaving effects in multi-finger force production. Exp Brain Res. (2000) 131(2):187–95. doi: 10.1007/s002219900261
210. Zatsiorsky VM, Li ZM, Latash ML. Coordinated force production in multi-finger tasks: finger interaction and neural network modeling. Biol Cybern. (1998) 79(2):139–50. doi: 10.1007/s004220050466
211. Finni T, de Brito Fontana H, Maas H. Force transmission and interactions between synergistic muscles. J Biomech. (2023) 111575. doi: 10.1016/j.jbiomech.2023.111575
212. von Schroeder HP, Botte MJ, Gellman H. Anatomy of the juncturae tendinum of the hand. J Hand Surg Am. (1990) 15(4):595–602. doi: 10.1016/S0363-5023(09)90021-1
213. von Schroeder HP, Botte MJ. The functional significance of the long extensors and juncturae tendinum in finger extension. J Hand Surg Am. (1993) 18(4):641–7. doi: 10.1016/0363-5023(93)90309-Q
214. Maas H, Meijer HJM, Huijing PA. Intermuscular interaction between synergists in rat originates from both intermuscular and extramuscular myofascial force transmission. Cells Tissues Organs. (2005) 181(1):38–50. doi: 10.1159/000089967
215. Maas H, Baan GC, Huijing PA. Muscle force is determined also by muscle relative position: isolated effects. J Biomech. (2004) 37(1):99–110. doi: 10.1016/S0021-9290(03)00235-5
216. Maas H, Jaspers RT, Baan GC, Huijing PA. Myofascial force transmission between a single muscle head and adjacent tissues: length effects of head III of rat EDL. J Appl Physiol. (2003) 95(5):2004–13. doi: 10.1152/japplphysiol.00220.2003
217. Maas H, Baan GC, Huijing PA. Intermuscular interaction via myofascial force transmission: effects of tibialis anterior and extensor hallucis longus length on force transmission from rat extensor digitorum longus muscle. J Biomech. (2001) 34(7):927–40. doi: 10.1016/S0021-9290(01)00055-0
218. Herbert RD, Hoang PD, Gandevia SC. Are muscles mechanically independent? J Appl Physiol. (2008) 104(6):1549–50. doi: 10.1152/japplphysiol.90511.2008
219. Maas H, Sandercock TG. Are skeletal muscles independent actuators? Force transmission from soleus muscle in the cat. J Appl Physiol. (2008) 104(6):1557–67. doi: 10.1152/japplphysiol.01208.2007
220. Tijs C, van Dieën JH, Maas H. Limited mechanical effects of intermuscular myofascial connections within the intact rat anterior crural compartment. J Biomech. (2016) 49(13):2953–9. doi: 10.1016/j.jbiomech.2016.07.005
221. Tijs C, van Dieën JH, Maas H. No functionally relevant mechanical effects of epimuscular myofascial connections between rat ankle plantar flexors. J Exp Biol. (2015) 218(18):jeb.122747. doi: 10.1242/jeb.122747
222. Bernabei M, van Dieën JH, Baan GC, Maas H. Significant mechanical interactions at physiological lengths and relative positions of rat plantar flexors. J Appl Physiol. (2015) 118(4):427–36. doi: 10.1152/japplphysiol.00703.2014
223. Tijs C, van Dieën JH, Baan GC, Maas H. Synergistic co-activation increases the extent of mechanical interaction between rat ankle plantar-flexors. Front Physiol. (2016) 7:414. doi: 10.3389/fphys.2016.00414
224. Keen DA, Fuglevand AJ. Role of intertendinous connections in distribution of force in the human extensor digitorum muscle. Muscle Nerve. (2003) 28(5):614–22. doi: 10.1002/mus.10481
225. de Brito Fontana H, de Campos D, Sawatsky A, Han SW, Herzog W. Why do muscles lose torque potential when activated within their agonistic group? J Exp Biol. (2019) 223(1):jeb.213843. doi: 10.1242/jeb.213843
226. de Brito Fontana H, Han SW, Sawatsky A, Herzog W. The mechanics of agonistic muscles. J Biomech. (2018) 79:15–20. doi: 10.1016/j.jbiomech.2018.07.007
227. Han SW, Sawatsky A, de Brito Fontana H, Herzog W. Contribution of individual quadriceps muscles to knee joint mechanics. J Exp Biol. (2019) 222(6):jeb.188292. doi: 10.1242/jeb.188292
228. Tijs C, van Dieën JH, Baan GC, Maas H. Three-dimensional ankle moments and nonlinear summation of rat triceps surae muscles. PLoS ONE. (2014) 9(10):e111595. doi: 10.1371/journal.pone.0111595
229. Maas H, Noort W. Knee movements cause changes in the firing behaviour of muscle spindles located within the mono-articular ankle extensor soleus in the rat. Exp Physiol. (2023):EP090764. doi: 10.1113/EP090764
230. Smilde HA, Vincent JA, Baan GC, Nardelli P, Lodder JC, Mansvelder HD, et al. Changes in muscle spindle firing in response to length changes of neighboring muscles. J Neurophysiol. (2016) 115(6):3146–55. doi: 10.1152/jn.00937.2015
231. Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. (2008) 31(1):195–218. doi: 10.1146/annurev.neuro.31.060407.125547
232. Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci USA. (2006) 103(21):8257–62. doi: 10.1073/pnas.0602933103
233. Sanes JN, Donoghue JP, Thangaraj V, Edelman RR, Warach S. Shared neural substrates controlling hand movements in human motor cortex. Science. (1995) 268(5218):1775–7. doi: 10.1126/science.7792606
234. Schieber MH, Hibbard LS. How somatotopic is the motor cortex hand area? Science. (1993) 261(5120):489–92. doi: 10.1126/science.8332915
235. Eccles RM, Lundberg A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol (Lond). (1958) 144(2):271–98. doi: 10.1113/jphysiol.1958.sp006101
236. Ronzano R, Lancelin C, Bhumbra GS, Brownstone RM, Beato M. Proximal and distal spinal neurons innervating multiple synergist and antagonist motor pools. eLife. (2021) 10:e70858. doi: 10.7554/eLife.70858
237. Takei T, Seki K. Spinal interneurons facilitate coactivation of hand muscles during a precision grip task in monkeys. J Neurosci. (2010) 30(50):17041–50. doi: 10.1523/JNEUROSCI.4297-10.2010
238. Farina D, Negro F, Dideriksen JL. The effective neural drive to muscles is the common synaptic input to motor neurons: effective neural drive to muscles. J Physiol. (2014) 592(16):3427–41. doi: 10.1113/jphysiol.2014.273581
239. De Luca CJ, Erim Z. Common drive in motor units of a synergistic muscle pair. J Neurophysiol. (2002) 87(4):2200–4. doi: 10.1152/jn.00793.2001
240. Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle. J Neurophysiol. (2004) 91(1):57–62. doi: 10.1152/jn.00650.2003
241. McIsaac TL, Fuglevand AJ. Motor-unit synchrony within and across compartments of the human flexor digitorum superficialis. J Neurophysiol. (2007) 97(1):550–6. doi: 10.1152/jn.01071.2006
242. De Luca CJ, Kline JC. Statistically rigorous calculations do not support common input and long-term synchronization of motor-unit firings. J Neurophysiol. (2014) 112(11):2729–44. doi: 10.1152/jn.00725.2013
243. Kline JC, De Luca CJ. Synchronization of motor unit firings: an epiphenomenon of firing rate characteristics not common inputs. J Neurophysiol. (2016) 115(1):178–92. doi: 10.1152/jn.00452.2015
244. Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol (Lond). (2011) 589(23):5603–12. doi: 10.1113/jphysiol.2011.215160
245. Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. (2009) 29(15):4993–9. doi: 10.1523/JNEUROSCI.3720-08.2009
246. Glover IS, Baker SN. Both corticospinal and reticulospinal tracts control force of contraction. J Neurosci. (2022) 42(15):3150–64. doi: 10.1523/JNEUROSCI.0627-21.2022
247. Zaaimi B, Dean LR, Baker SN. Different contributions of primary motor cortex, reticular formation, and spinal cord to fractionated muscle activation. J Neurophysiol. (2018) 119(1):235–50. doi: 10.1152/jn.00672.2017
248. Rácz K, Brown D, Valero-Cuevas FJ. An involuntary stereotypical grasp tendency pervades voluntary dynamic multifinger manipulation. J Neurophysiol. (2012) 108(11):2896–911. doi: 10.1152/jn.00297.2012
249. Keenan KG, Santos VJ, Venkadesan M, Valero-Cuevas FJ. Maximal voluntary fingertip force production is not limited by movement speed in combined motion and force tasks. J Neurosci. (2009) 29(27):8784–9. doi: 10.1523/JNEUROSCI.0853-09.2009
250. Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nat Neurosci. (2005) 8(4):498–504. doi: 10.1038/nn1420
251. Albert ST, Hadjiosif AM, Jang J, Zimnik AJ, Soteropoulos DS, Baker SN, et al. Postural control of arm and fingers through integration of movement commands. eLife. (2020) 9:e52507. doi: 10.7554/eLife.52507
252. Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH. Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. J Neurophysiol. (2011) 106(2):999–1015. doi: 10.1152/jn.00549.2010
253. Oliveira AS, Gizzi L, Farina D, Kersting UG. Motor modules of human locomotion: influence of EMG averaging, concatenation, and number of step cycles. Front Hum Neurosci. (2014) 8:335. doi: 10.3389/fnhum.2014.00335
254. Gallego JA, Perich MG, Miller LE, Solla SA. Neural manifolds for the control of movement. Neuron. (2017) 94(5):978–84. doi: 10.1016/j.neuron.2017.05.025
255. Sadtler PT, Quick KM, Golub MD, Chase SM, Ryu SI, Tyler-Kabara EC, et al. Neural constraints on learning. Nature. (2014) 512(7515):423–6. doi: 10.1038/nature13665
256. Giszter S, Mussa-Ivaldi F, Bizzi E. Convergent force fields organized in the frog’s spinal cord. J Neurosci. (1993) 13(2):467–91. doi: 10.1523/JNEUROSCI.13-02-00467.1993
257. Graziano MSA, Aflalo TNS, Cooke DF. Arm movements evoked by electrical stimulation in the motor Cortex of monkeys. J Neurophysiol. (2005) 94(6):4209–23. doi: 10.1152/jn.01303.2004
258. Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral Cortex. Neuron. (2002) 34(5):841–51. doi: 10.1016/S0896-6273(02)00698-0
259. Overduin SA, d’Avella A, Carmena JM, Bizzi E. Muscle synergies evoked by microstimulation are preferentially encoded during behavior. Front Comput Neurosci. (2014) 8:20. doi: 10.3389/fncom.2014.00020
260. Overduin SA, d’Avella A, Carmena JM, Bizzi E. Microstimulation activates a handful of muscle synergies. Neuron. (2012) 76(6):1071–7. doi: 10.1016/j.neuron.2012.10.018
261. Saltiel P, Wyler-Duda K, D’Avella A, Tresch MC, Bizzi E. Muscle synergies encoded within the spinal cord: evidence from focal intraspinal NMDA iontophoresis in the frog. J Neurophysiol. (2001) 85(2):605–19. doi: 10.1152/jn.2001.85.2.605
262. Steele KM, Tresch MC, Perreault EJ. The number and choice of muscles impact the results of muscle synergy analyses. Front Comput Neurosci. (2013) 7:105. doi: 10.3389/fncom.2013.00105
263. Burkholder TJ, van Antwerp KW. Practical limits on muscle synergy identification by non-negative matrix factorization in systems with mechanical constraints. Med Biol Eng Comput. (2013) 51(1–2):187–96. doi: 10.1007/s11517-012-0983-8
264. Kutch JJ, Kuo AD, Bloch AM, Rymer WZ. Endpoint force fluctuations reveal flexible rather than synergistic patterns of muscle cooperation. J Neurophysiol. (2008) 100(5):2455–71. doi: 10.1152/jn.90274.2008
265. Valero-Cuevas FJ, Venkadesan M, Todorov E. Structured variability of muscle activations supports the minimal intervention principle of motor control. J Neurophysiol. (2009) 102(1):59–68. doi: 10.1152/jn.90324.2008
266. Barradas VR, Kutch JJ, Kawase T, Koike Y, Schweighofer N. When 90% of the variance is not enough: residual EMG from muscle synergy extraction influences task performance. J Neurophysiol. (2020) 123(6):2180–90. doi: 10.1152/jn.00472.2019
267. Bartsch-Jimenez A, Błażkiewicz M, Azadjou H, Novotny R, Valero-Cuevas FJ. “Fine synergies” describe motor adaptation in people with drop foot in a way that supplements traditional “coarse synergies”. Front Sports Act Living. (2023) 5:1080170. doi: 10.3389/fspor.2023.1080170
268. de Rugy A, Loeb GE, Carroll TJ. Are muscle synergies useful for neural control? Front Comput Neurosci. (2013) 7:19. doi: 10.3389/fncom.2013.00019
269. Yan Y, Goodman JM, Moore DD, Solla SA, Bensmaia SJ. Unexpected complexity of everyday manual behaviors. Nat Commun. (2020) 11(1):3564. doi: 10.1038/s41467-020-17404-0
270. Deimel R, Brock O. A novel type of compliant and underactuated robotic hand for dexterous grasping. Int J Rob Res. (2016) 35(1–3):161–85. doi: 10.1177/0278364915592961
271. Maas H, Finni T. Mechanical coupling between muscle-tendon units reduces peak stresses. Exerc Sport Sci Rev. (2018) 46(1):26–33. doi: 10.1249/JES.0000000000000132
272. Inouye JM, Valero-Cuevas FJ. Muscle synergies heavily influence the neural control of arm endpoint stiffness and energy consumption. PLoS Comput Biol. (2016) 12(2):e1004737. doi: 10.1371/journal.pcbi.1004737
273. Latash ML. The bliss (not the problem) of motor abundance (not redundancy). Exp Brain Res. (2012) 217(1):1–5. doi: 10.1007/s00221-012-3000-4
274. Humphries MD. Strong and weak principles of neural dimension reduction. (2020). Available at: https://arxiv.org/abs/2011.08088 (Cited 2023 Apr 27).
275. Marshall NJ, Glaser JI, Trautmann EM, Amematsro EA, Perkins SM, Shadlen MN, et al. Flexible neural control of motor units. Nat Neurosci. (2022) 25(11):1492–504. doi: 10.1038/s41593-022-01165-8
276. Patel K, Katz CN, Kalia SK, Popovic MR, Valiante TA. Volitional control of individual neurons in the human brain. Brain. (2021) 144(12):3651–63. doi: 10.1093/brain/awab370
277. Bräcklein M, Barsakcioglu DY, Ibáñez J, Eden J, Burdet E, Mehring C, et al. The control and training of single motor units in isometric tasks are constrained by a common input signal. eLife. (2022) 11:e72871. doi: 10.7554/eLife.72871
278. Aeles J, Sarcher A, Hug F. Common synaptic input between motor units from the lateral and medial posterior soleus compartments does not differ from that within each compartment. J Appl Physiol. (2023) 134(1):105–15. doi: 10.1152/japplphysiol.00587.2022
279. Del Vecchio A, Marconi Germer C, Kinfe TM, Nuccio S, Hug F, Eskofier B, et al. The forces generated by agonist muscles during isometric contractions arise from motor unit synergies. J Neurosci. (2023) 43(16):2860–73. doi: 10.1523/JNEUROSCI.1265-22.2023
280. Rossato J, Tucker K, Avrillon S, Lacourpaille L, Holobar A, Hug F. Less common synaptic input between muscles from the same group allows for more flexible coordination strategies during a fatiguing task. J Neurophysiol. (2022) 127(2):421–33. doi: 10.1152/jn.00453.2021
Keywords: neuromechanics, optimization, synergy, musculoskeletal modelling, simulation, muscle, sensorimotor
Citation: Mulla DM and Keir PJ (2023) Neuromuscular control: from a biomechanist's perspective. Front. Sports Act. Living 5:1217009. doi: 10.3389/fspor.2023.1217009
Received: 4 May 2023; Accepted: 21 June 2023;
Published: 5 July 2023.
Edited by:
Jaap Van Dieen, VU Amsterdam, NetherlandsReviewed by:
Eric Jon Perreault, Northwestern University, United States© 2023 Mulla and Keir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter J. Keir cGprZWlyQG1jbWFzdGVyLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.